The Preparation of High-Purity Iron (99.987%) Employing a Process of Direct Reduction–Melting Separation–Slag Refining
Abstract
1. Introduction
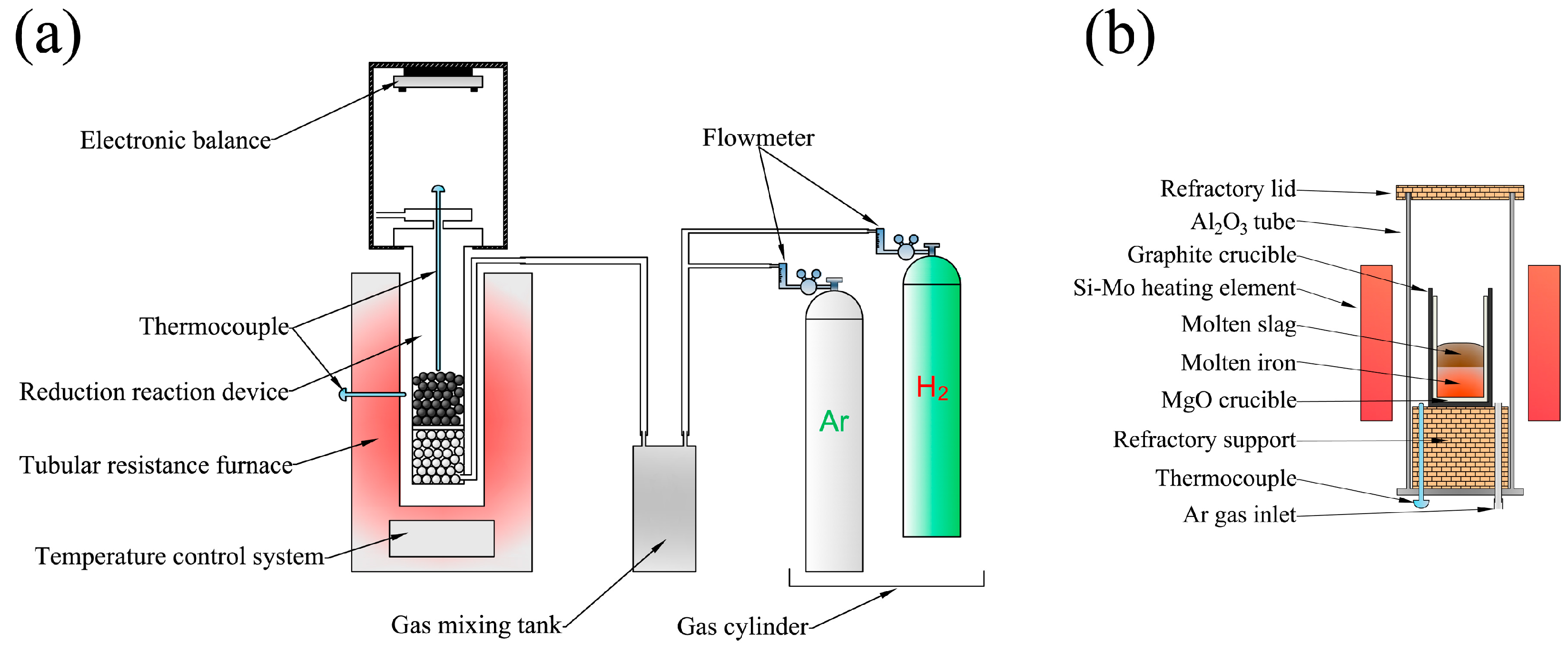
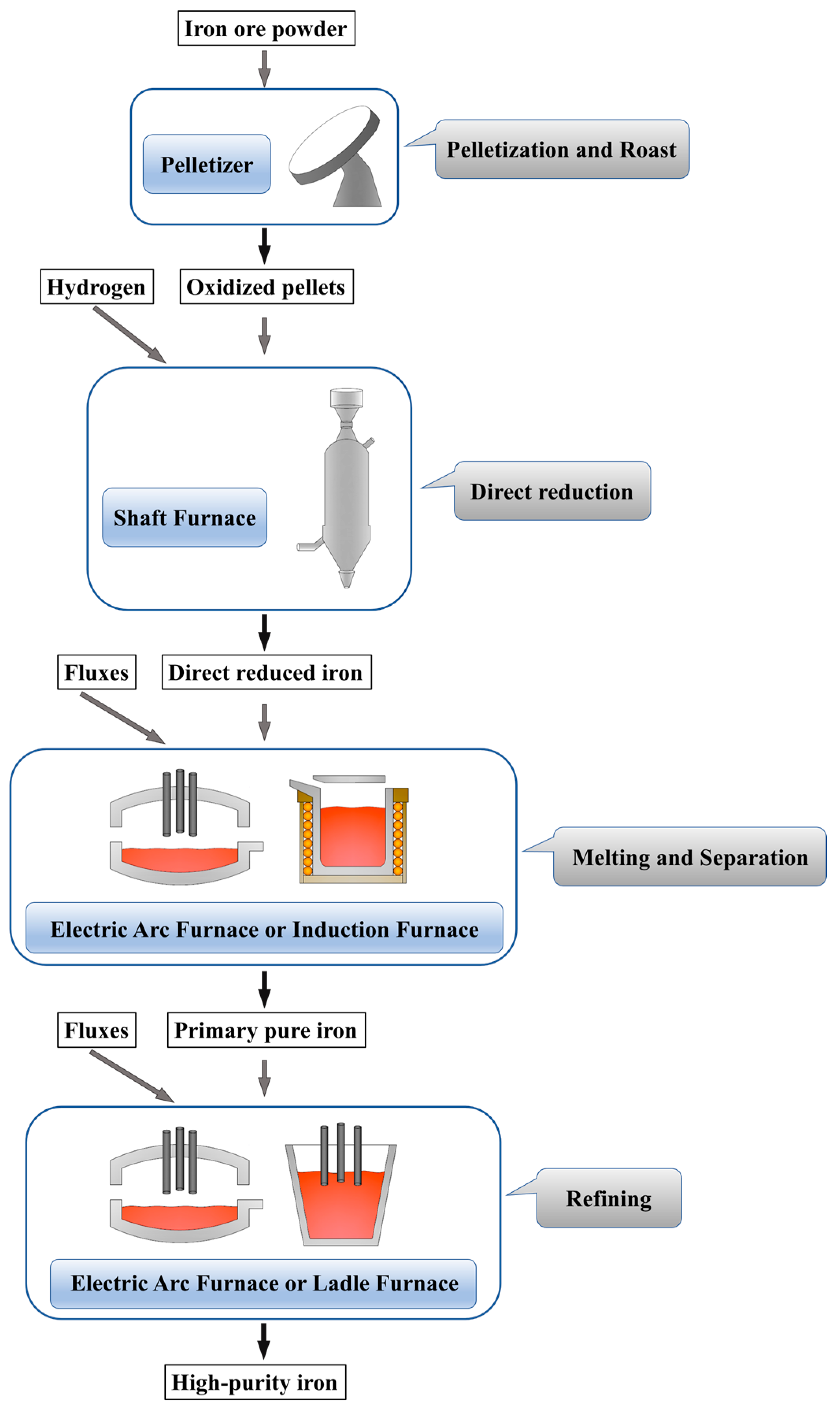
2. Experimental
3. Results and Discussion
3.1. Removal of General Elements and the Selective Reduction of Oxides in Direct Reduction Process
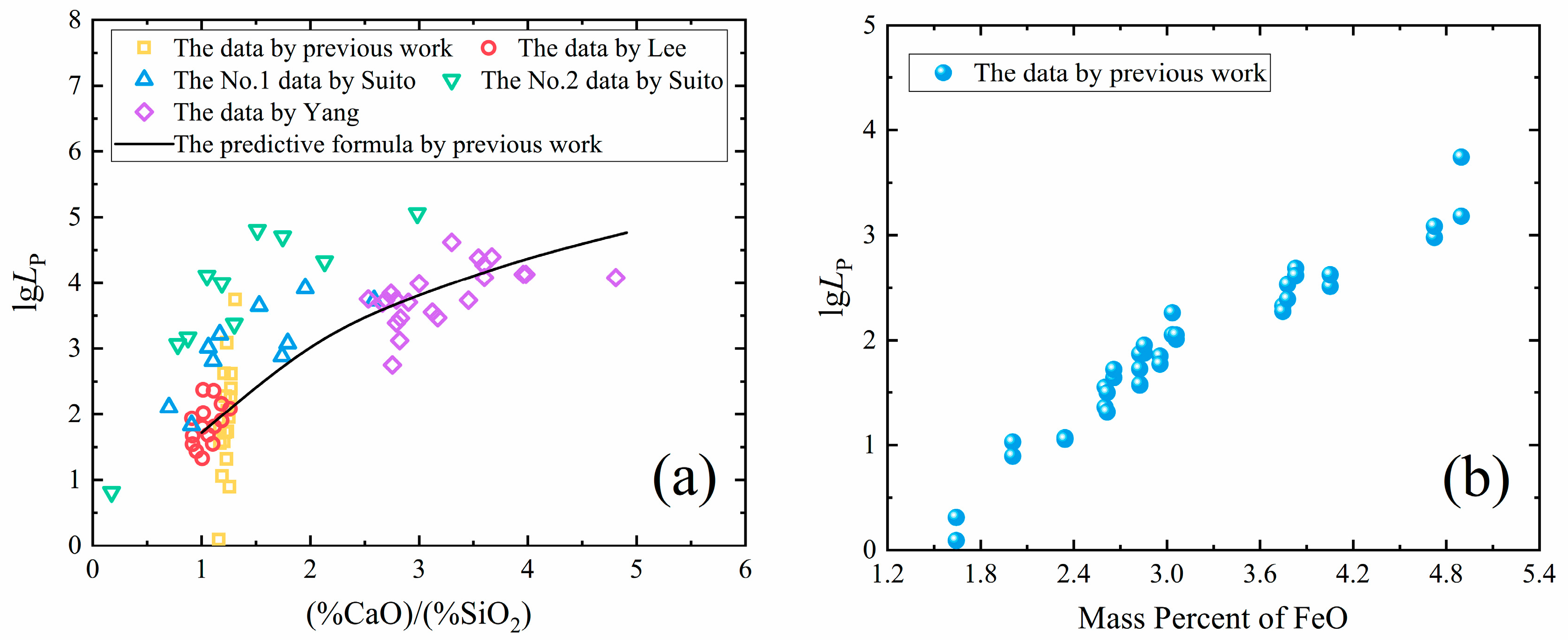
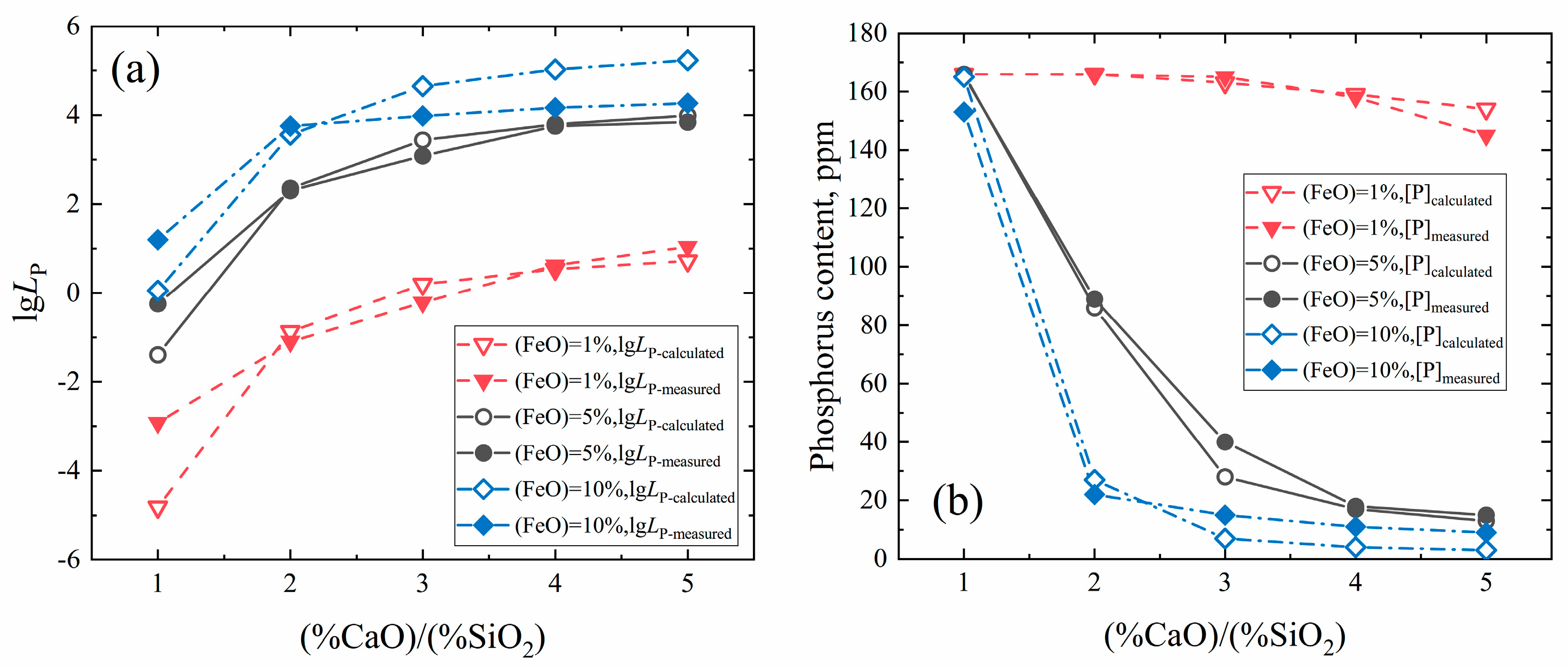
3.2. Dephosphorization in the Melting Separation Process
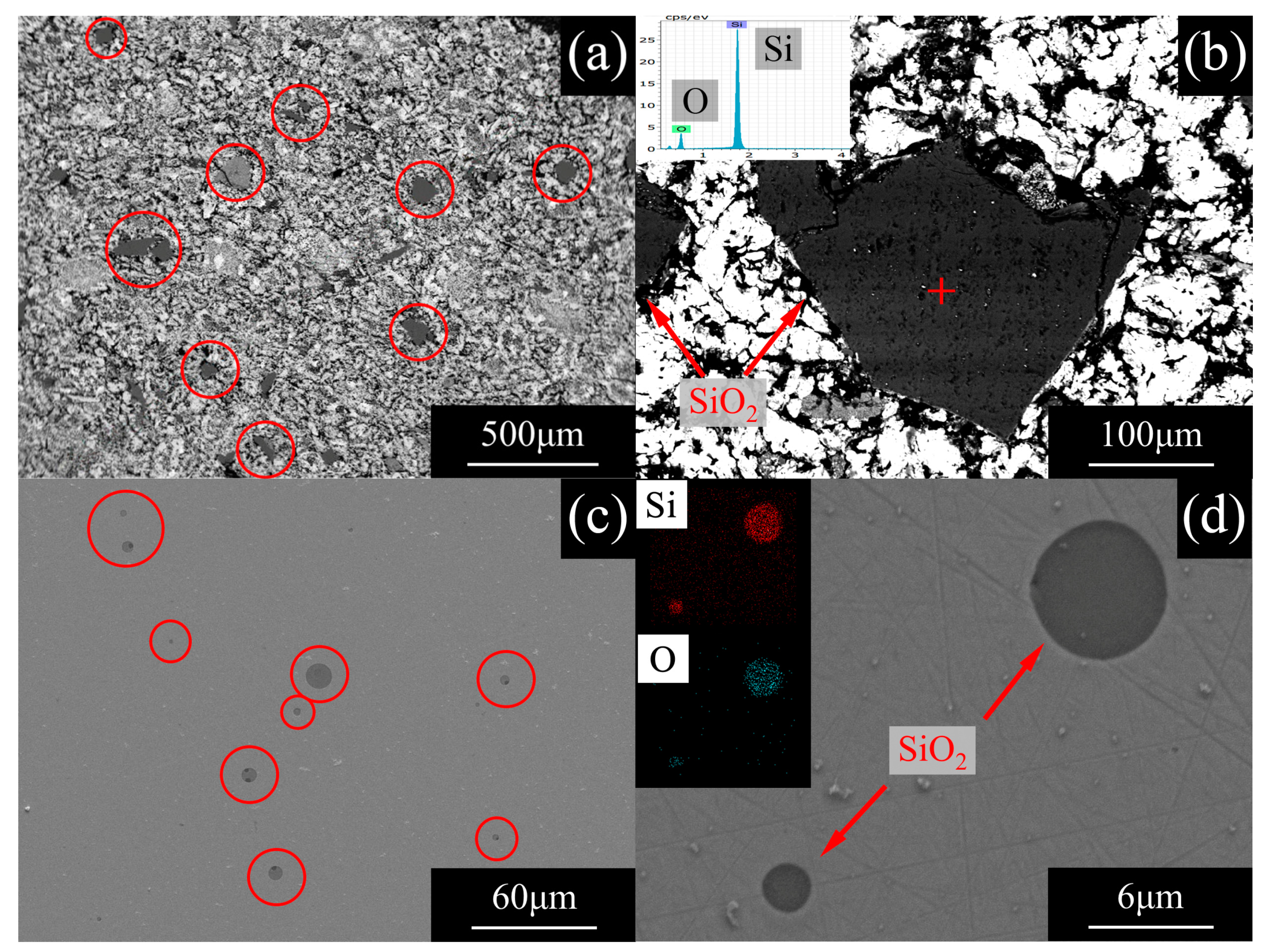
3.3. Deoxidation by Secondary Refining
3.4. Feasibility of Industrialization and Simple Estimation of Cost
4. Conclusions
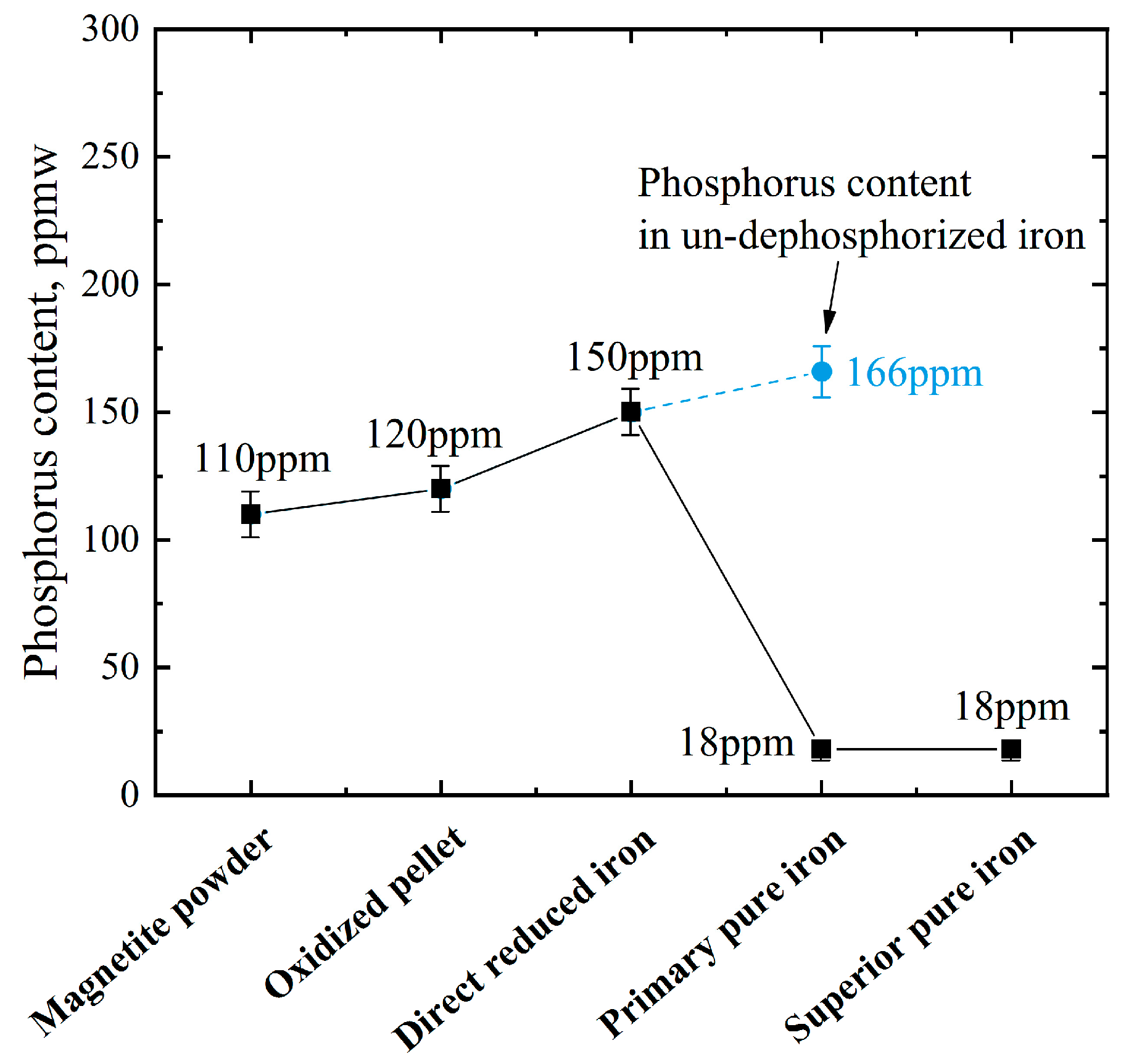
- The sulfur in the iron ore pellets was removed during the roasting step. Direct reduction by hydrogen produced carbon-free direct reduced iron (DRI) while avoiding the reduction of impurities such as Si, Mn, Ti, Al and V.
- Dephosphorization was implemented simultaneously during the melting separation step by making use of the FeO contained in direct reduced iron, and the phosphorus content in primary pure iron dropped to 18 mass ppm.
- The problem of deoxidization for pure iron was solved. Oxygen in pure iron existed as inclusions. The oxygen content of superior pure iron was reduced to 10 mass ppm by refining with a high basicity slag.
- The cost of producing high-purity iron by this method is about $690 US dollars per tonne. Compared with electrolytic iron, the pure iron by this method has tremendous advantages in cost and scale, and has more outstanding quality than technically pure iron, which makes it possible to produce high-purity iron on a large scale.
Author Contributions
Funding
Conflicts of Interest
References
- Faudot, F.; Rouchaud, J.C.; Debove, L.; Fedoroff, M.; Bigot, J. Elaboration of high purity iron (RRR4000) by horizontal zone melting—Attempts for chemical characterization. J. Phys. Chem. Solids 1987, 48, 761–765. [Google Scholar] [CrossRef]
- Takaki, S.; Kimura, H. Preparation of high-purity iron by electron-beam floating-zone melting under ultra-high vacuum. Scr. Met. 1976, 10, 1095–1100. [Google Scholar] [CrossRef]
- Abiko, K.; Takaki, S. Ultra-purification of iron by ultra-high vacuum melting. Vacuum 1999, 53, 93–96. [Google Scholar] [CrossRef]
- Takaki, S.; Abiko, K. Ultra-purification of iron by three types of melting furnaces constructed using ultra-high vacuum technology. Vacuum 1999, 53, 97–100. [Google Scholar] [CrossRef]
- Takaki, S.; Abiko, K. Ultra-purification of electrolytic iron by cold-crucible induction melting and induction-heating floating-zone melting in ultra-high vacuum. Mater. Trans. JIM 2000, 41, 2–6. [Google Scholar] [CrossRef]
- Uchikoshi, M.; Imaizumi, J.; Shibuya, H.; Kekesi, T.; Mimura, K.; Isshiki, M. Production of semiconductor grade high-purity iron. Thin Solid Films 2004, 461, 94–98. [Google Scholar] [CrossRef]
- Uchikoshi, M.; Shibuya, H.; Kekesi, T.; Mimura, K.; Isshiki, M. Mass production of high-purity iron using anion-exchange separation and plasma arc melting. Met. Mater. Trans. B 2009, 40, 615–618. [Google Scholar] [CrossRef]
- Appa Rao, G.; Srinivas, M.; Sarma, D.S. Effect of oxygen content of powder on microstructure and mechanical properties of hot isostatically pressed superalloy Inconel 718. Mat. Sci. Eng. A-Struct. 2006, 435, 84–89. [Google Scholar]
- Qi, J.H. Research on Producing Ultraclean Industrial Pure Iron. Master’s Thesis, Wuhan University of Science and Technology, Wuhan, Hubei, China, 2006. [Google Scholar]
- Li, T.T.; Zhang, J.B.; Jin, Y.; Yang, W.K. Investigation on high purity iron producing. Angang Technol. 1998, 9, 41–44. [Google Scholar]
- Mo, S.C.; Liang, W.G.; Li, D.X. Smelting pure iron with sponge iron in 0.5 t medium frequency induction furnace. Ferro-Alloys 2001, 6, 31–35. [Google Scholar]
- Barin, I. Thermochemical Data of Pure Substances; VCH: Weinheim, Germany, 1989. [Google Scholar]
- Heo, J.H.; Park, J.H. Effect of direct reduced iron (DRI) on dephosphorization of molten steel by electric arc furnace slag. Met. Mater. Trans. B 2018, 49, 3381–3389. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Zhang, H.; Li, Z.E.; Zhang, A.M.; Zhang, X.H.; Qing, S. Impact of slag composition activity on the behavior of phosphorus in the smelting reduction process of high-phosphorus iron ores. Int. J. Hydrogen. Energy 2017, 42, 24487–24494. [Google Scholar] [CrossRef]
- Lee, C.M.; Fruehan, R.J. Phosphorus equilibrium between hot metal and slag. Ironmak. Steelmak. 2005, 32, 503–508. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R. Phosphorus distribution between MgO saturated CaO–FetO–SiO2–P2O5–MnO slags and liquid iron. Tetsu-to-Hagane 1984, 70, 186–193. [Google Scholar] [CrossRef]
- Suito, H.; Inoue, R.; Takada, M.I. Phosphorus distribution between liquid iron and MgO saturated slags of the system CaO–MgO–FeOx–SiO2. Tetsu-to-Hagane 1981, 67, 2645–2654. [Google Scholar] [CrossRef]
- Yang, X.M.; Duan, J.P.; Shi, C.B.; Zhang, M.; Zhang, Y.L.; Wang, J.C. A thermodynamic model of phosphorus distribution ratio between CaO–SiO2–MgO–FeO–Fe2O3–MnO–Al2O3–P2O5 slags and molten steel during a top-bottom combined blown converter steelmaking process based on the ion and molecule coexistence theory. Met. Mater. Trans. A 2011, 42, 738–770. [Google Scholar] [CrossRef]
- Li, B.; Li, L.; Guo, H.J.; Guo, J.; Duan, S.C.; Sun, W.X. A phosphorus distribution prediction model for CaO–SiO2–MgO–FeO–Fe2O3–Al2O3–P2O5 slags based on the IMCT. Ironmak. Steelmak. 2019. [Google Scholar] [CrossRef]
- Turkdogan, E. Nucleation, growth, and flotation of oxide inclusions in liquid steel. Iron Steel Inst. 1966, 204, 914. [Google Scholar]
- Valdez, M.; Shannon, G.S.; Sridhar, S. The ability of slags to absorb solid oxide inclusions. ISIJ Int. 2006, 46, 450–457. [Google Scholar] [CrossRef]
- Park, J.S.; Park, J.H. Effect of physicochemical properties of slag and flux on the removal rate of oxide inclusion from molten steel. Met. Mater. Trans. B 2016, 47, 3225–3230. [Google Scholar] [CrossRef]
- Ren, Y.; Zhang, L.F.; Fang, W.; Shao, S.J.; Yang, J.; Mao, W.D. Effect of Slag Composition on Inclusions in Si-Deoxidized 18Cr-8Ni Stainless Steels. Met. Mater. Trans. B 2016, 47, 1024–1034. [Google Scholar] [CrossRef]
- Zhang, J. Computational Thermodynamics of Metallurgical Melts and Solutions; Metallurgical Industry Press: Beijing, China, 2007. [Google Scholar]
- Yang, X.M.; Shi, C.B.; Zhang, M.; Chai, G.M.; Wang, F. A thermodynamic model of sulfur distribution ratio between CaO–SiO2–MgO–FeO–MnO–Al2O3 slags and molten steel during LF refining process based on the ion and molecule coexistence theory. Met. Mater. Trans. B 2011, 42, 1150–1180. [Google Scholar] [CrossRef]
- Karakaya, E.; Nuur, C.; Assbring, L. Potential transitions in the iron and steel industry in Sweden: Towards a hydrogen-based future. J. Clean. Prod. 2018, 195, 651–663. [Google Scholar] [CrossRef]



| Element | Pure Iron in This Work | Typical Technically Pure Iron | Typical Commercial Electrolytic Iron |
|---|---|---|---|
| C | 0.0024 | 0.0030 | 0.0057 |
| Si | 0.0011 | 0.0100 | 0.0030 |
| Mn | 0.0003 | 0.0400 | 0.0003 |
| P | 0.0018 | 0.0021 | 0.0005 |
| S | 0.0005 | 0.0016 | 0.0018 |
| Cr | 0.0009 | 0.0100 | 0.0003 |
| Ni | 0.0005 | 0.0100 | 0.0032 |
| Al | 0.0015 | 0.0150 | 0.0011 |
| Ti | 0.0003 | 0.0011 | 0.0009 |
| Cu | 0.0001 | 0.0100 | 0.0015 |
| Mo | 0.0007 | 0.0020 | 0.0004 |
| V | 0.0005 | 0.0020 | 0.0003 |
| N | 0.0015 | 0.0025 | 0.0038 |
| H | 0.0001 | 0.0001 | 0.0013 |
| O | 0.0010 | 0.0030 | 0.0574 |
| Purity of Fe | 99.9868 | 99.8876 | 99.9185 |
| TFe | FeO | Fe2O3 | SiO2 | CaO | MnO | V2O5 | P2O5 | S |
|---|---|---|---|---|---|---|---|---|
| 67.37 | 30.48 | 62.37 | 6.62 | 0.26 | 0.11 | 0.04 | 0.03 | 0.09 |
| No. | CaO/SiO2 | FeO | CaO | SiO2 | Al2O3 | MgO | wslag |
|---|---|---|---|---|---|---|---|
| Slag 1 | 1 | 1% | 34.5% | 34.5% | 25% | 5% | 26.09 g |
| Slag 2 | 2 | 1% | 46% | 23% | 25% | 5% | 39.13 g |
| Slag 3 | 3 | 1% | 51.75% | 17.25% | 25% | 5% | 52.17 g |
| Slag 4 | 4 | 1% | 55.2% | 13.8% | 25% | 5% | 65.22 g |
| Slag 5 | 5 | 1% | 57.5% | 11.5% | 25% | 5% | 78.26 g |
| Slag 6 | 1 | 5% | 32.5% | 32.5% | 25% | 5% | 27.69 g |
| Slag 7 | 2 | 5% | 43.33% | 21.67% | 25% | 5% | 41.53 g |
| Slag 8 | 3 | 5% | 48.75% | 16.25% | 25% | 5% | 55.38 g |
| Slag 9 | 4 | 5% | 52% | 13% | 25% | 5% | 69.23 g |
| Slag 10 | 5 | 5% | 54.17% | 10.83% | 25% | 5% | 83.1 g |
| Slag 11 | 1 | 10% | 30% | 30% | 25% | 5% | 30 g |
| Slag 12 | 2 | 10% | 40% | 20% | 25% | 5% | 45 g |
| Slag 13 | 3 | 10% | 45% | 15% | 25% | 5% | 60 g |
| Slag 14 | 4 | 10% | 48% | 12% | 25% | 5% | 75 g |
| Slag 15 | 5 | 10% | 50% | 10% | 25% | 5% | 90 g |
| CaO | SiO2 | Al2O3 | CaF2 | MgO | CaO/SiO2 | Completely Melting Temperature | Viscosity |
|---|---|---|---|---|---|---|---|
| 46.2% | 6.6% | 23.1% | 20% | 4.1% | 7 | 1736.1 K | 0.0404 |
| Average Diameter | Amount | Average Composition | |
|---|---|---|---|
| 3.05 μm | 56.06 /mm2 | Si | 33.98 atom % |
| O | 66.02 atom % | ||
| Raw Material Cost | Energy Cost | Running Cost | Sum | |||||
|---|---|---|---|---|---|---|---|---|
| Ore | Reductant | Flux | Power | Gas | Water | Labor | Maintenance | 690 |
| 170 | 210 | 70 | 120 | 20 | 20 | 50 | 30 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Sun, G.; Li, S.; Guo, H.; Guo, J. The Preparation of High-Purity Iron (99.987%) Employing a Process of Direct Reduction–Melting Separation–Slag Refining. Materials 2020, 13, 1839. https://doi.org/10.3390/ma13081839
Li B, Sun G, Li S, Guo H, Guo J. The Preparation of High-Purity Iron (99.987%) Employing a Process of Direct Reduction–Melting Separation–Slag Refining. Materials. 2020; 13(8):1839. https://doi.org/10.3390/ma13081839
Chicago/Turabian StyleLi, Bin, Guanyong Sun, Shaoying Li, Hanjie Guo, and Jing Guo. 2020. "The Preparation of High-Purity Iron (99.987%) Employing a Process of Direct Reduction–Melting Separation–Slag Refining" Materials 13, no. 8: 1839. https://doi.org/10.3390/ma13081839
APA StyleLi, B., Sun, G., Li, S., Guo, H., & Guo, J. (2020). The Preparation of High-Purity Iron (99.987%) Employing a Process of Direct Reduction–Melting Separation–Slag Refining. Materials, 13(8), 1839. https://doi.org/10.3390/ma13081839







