4.1. Composition of Miscanthus and Switchgrass Biomass as a Feedstock for Carbonization
Porous structure and other characteristics of activated carbon are determined by the properties of the feedstock and conditions of carbonization and activation. In turn, the chemical composition of biomass depends on many factors such as plant species, type of organ and tissue, growth conditions including climate, year of cultivation and harvesting time, etc. [
31]. In the study, the winter-harvested biomass was used, as it is usually used for practical purposes and also contains higher levels of structural biopolymers, mainly holocellulose and lignin.
Here, holocellulose content in miscanthus corresponded with data recorded for plantations in the temperate climate zone [
19,
32,
33]. Lower holocellulose contents in switchgrass biomass were also observed earlier [
34,
35]. Regarding cellulose, the main holocellulose component, the determined contents for
M. ×
giganteus,
M. sacchariflorus and
M. sinensis (
Table 2), also fell within the ranges for these species cultivated in other temperate regions [
19,
32,
33,
36,
37]. In turn, Gismatulina and Budaeva [
38] reported higher cellulose contents in biomass of
M. sinensis, but cultivated under the severe continental climate of Siberia. In comparison to miscanthus, cellulose content in switchgrass was significantly lower (
Table 2), which corresponds to data reported by other authors [
18,
33,
34,
35].
Unlike cellulose, the hemicellulose fraction in the tested raw materials did not differ significantly (
Table 2). Yet, a slightly higher hemicellulose content was found in the species characterized by a lower cellulose fraction. Again, the observed amount of these low-polymerized carbohydrates corresponded with data reported for miscanthus and switchgrass cultivated in the temperate climate [
32,
33,
34,
35,
36,
37]. The content of pentosans corresponded to that for hemicellulose, as these glycans constitute a major fraction of the latter. Analogously, their content did not vary between the tested materials, similarly to an earlier report [
19]. A larger discrepancy in the content of pentosans, between genotypes and also plant parts, was reported only for
M. sinensis cultivated in the continental climate [
38,
39].
In contrast to hemicellulose, lignin content varied between the investigated grasses; yet this differentiation did not correspond to their genera as it was the case with cellulose. Biomass of
M. ×
giganteus contained significantly the highest amount of lignin, while in the
M. sinensis biomass it was the lowest (
Table 2). This range of values is approx. 1.5–2 times higher in comparison to data from regions of maritime or warmer climates [
33,
36,
37], while it was similar to that for miscanthus or switchgrass cultivated under the continental climate [
19,
33,
35,
38]. Interestingly, the biomass of
M. sacchariflorus and switchgrass, characterized by an intermediate lignin content, slightly above 20%, after processing yielded ACs of the most developed porous structure (
Table 5). However, this cannot be related to the cellulose-lignin ratio, as this parameter is determined by two other traits.
In contrast to structural cell wall biopolymers, switchgrass DM biomass, in comparison with miscanthus, contained more extractives, which include free sugars, proteins, dyes, waxes and other soluble compounds (
Table 3). In particular, substances soluble in cold and hot water, were much more abundant, but the contents of other extractives were also significantly higher in switchgrass. Our results are comparable with data from previous reports, mostly regarding miscanthus and switchgrass coming from the transitional or continental climate [
19,
32,
34,
36,
38,
40].
Compared to woody biomass, grasses are typically characterized by a higher content of ash, i.e., mineral compounds [
41]. Low ash content (
Table 3) in the tested miscanthus species was comparable to previously reported lower contents (<3%) of mineral compounds in miscanthus biomass [
19,
32,
33,
42]. In the case of switchgrass, determined ash content can be classified as low in comparison to the reported 2.1%–8.8% [
18,
35]. However, most authors reported higher ash contents, showing a considerable effect of growing conditions, including year of cultivation, location, type of soil or fertilization [
18,
33,
35,
37,
38,
39,
40,
42,
43,
44]. In this study, the biomass of
M. sacchariflorus and switchgrass contained significantly lower amounts of ash than the other miscanthus species. It cannot be excluded that a low content of mineral compounds was reflected in the formation of a more developed porous structure in ACs (
Table 5).
4.2. Properties of Activated Carbons Derived from Miscanthus and Switchgrass Biomass
The biomass of all tested energy grasses was carbonized at 600 °C according to the commonly adopted technique. All ACs derived from crude carbonizates were characterized by a rich chemical structure of the surface and substantial porosity (
Table 4 and
Table 5). The effect of used activators, i.e., KOH or NaOH, was much more evident than that of the precursor and it was statistically significant in the case of some AC parameters. Regardless of the type of precursor and hydroxide used, mainly acidic groups were generated (
Table 4). They were predominantly phenolic groups, followed by lactonic and carboxyl groups. However, for all precursors, significantly more groups of the two latter types were identified on the surface of the carbons obtained after activation with KOH. When NaOH was used as the activator, the number of basic oxygen groups significantly increased. Hence, based on the obtained results of the Boehm analysis, it may be concluded that the mechanisms of KOH and NaOH action on carbon precursors are not identical, which directly affects the surface structure of carbon materials.
The mechanisms of KOH and NaOH action on carbonizate precursors has been described (see chemical Equations (3)–(10) below) by many scientists [
45,
46,
47,
48,
49,
50]. It has been found that hydrogen is the product of the reaction for both these hydroxides with a carbon matrix (Equations (3) and (4), where Me is a potassium or sodium ion) [
48,
50]:
The reaction of the alkaline activator within the temperature range of 400–700 °C leads to increased CO
2 production (Equations (5) and (6)) [
47,
50]:
During the heating of carbons also the CO emission occurs. Carbon monoxide is the product of carbon with sodium or potassium oxides and carbonates (Equations (7) and (8)) [
50].
In turn, the release of water vapor is primarily associated with the physical state of the activators used, as well as the physicochemical properties of hydroxides. The course of water release curves is different. In the carbon activation process using KOH, water is released at temperatures from 300 to 500 °C, whereas with the use of NaOH it is only above 600 °C. It is assumed that metallic hydroxide activators react with carbon, volatile oxidation products or decompose with the release of water (Equations (6), (9) and (10)) [
47,
50].
Activation with NaOH leads to the release of larger volumes of reaction gases at higher temperatures than when activated with KOH. Thus, above 600 °C, Equations (6) and (9), leading to the release of water vapor and carbon dioxide, are more likely for carbon activation with NaOH. Additionally, the emission of hydrogen generated in the reaction of NaOH with a carbon matrix appears in the reaction space also at temperatures higher than at the application of KOH. This may indicate a lower share of sodium carbonate in activation processes than in the case of potassium carbonate, which is formed at higher temperatures of carbon activation using KOH. At temperatures above 700 °C, the emission of carbon monoxide is evidently more intensive when NaOH is used [
48,
49]. On this basis, it can be concluded that the formation of acid surface groups is influenced not only by the oxygen content in carbonizate, but also by the amount of oxygen supplied from products of the reaction between an activator and carbon. Having in mind the thermal stability of individual functional groups, it should be stated that lower concentrations of acid groups in carbons obtained after NaOH activation are associated with more difficult oxidation of the carbon surface than in the case of KOH activation.
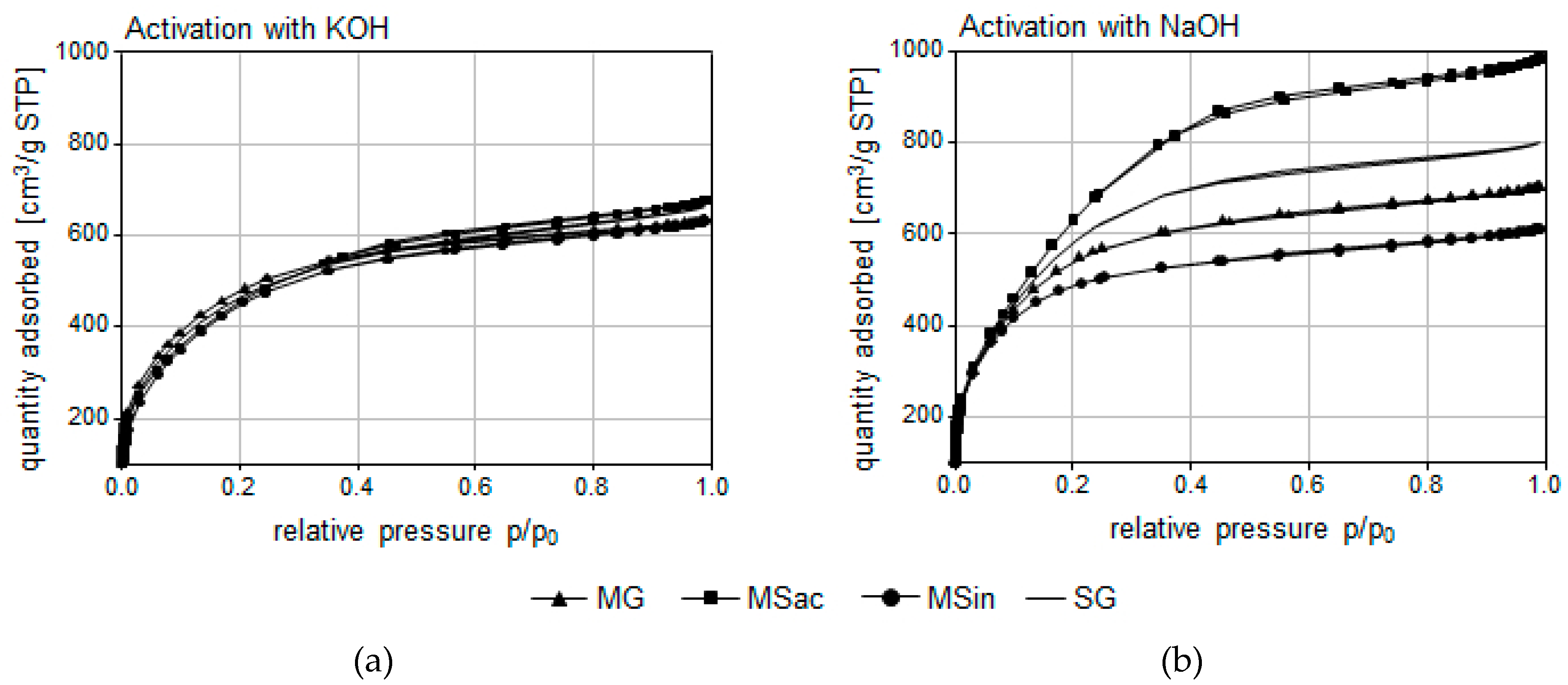
All ACs obtained from miscanthus and switchgrass biomass were characterized by a developed porous structure, as indicated by the high iodine numbers and direct assays (
Table 5). As IN is an indicator of microporosity (pores < 1 nm in diameter), higher INs reflect better development of the microporous structure, on which greater adsorption abilities for low-molar-mass solutes depend to a large extent [
51]. This should definitely be a subject of the next phase of research. At this moment, it can be stated that an extensive specific surface area and large pore volume were found for virtually all ACs. However, higher values, especially of S
BET, V
micro (both also with the determined significant effect of activator), as well as V
T and IN, were found for ACs obtained after the use of NaOH. Thus, analyses of AC porosity also confirmed that the mechanism of action for KOH and NaOH hydroxides is different.
Structural and surface parameters were similar for all ACs, yet the best were found for MSac/NaOH followed by SG/NaOH, i.e., those obtained by NaOH activation of carbonizates from
M. sacchariflorus or switchgrass biomass, respectively (
Table 5). It is difficult to comprehensively explain the reason for this fact, especially since the chemical composition of all used biomass feedstocks was similar (
Table 2 and
Table 3). However, certain selected common characteristics of MSac and SG, significantly different from the other feedstocks, may be indicated. These were the above-mentioned intermediate lignin contents and low amounts of ash, which synergistically might have exerted some positive impact on the porous structure of obtained ACs. This possible effect, expanded to cover the lignin subunit composition and ultrastructure, could be a subject of further detailed research. Literature studies also indicate that each natural material requires an individual approach and the mechanism of pyrolysis is specific to a particular raw material. It is related to the diversity in the structure of lignocellulosic biomass, i.e., different chemical composition and diverse anatomical structure [
52].
The values of S
BET and other parameters of porous structure for ACs derived from miscanthus and switchgrass are comparable with those of other lignocellulosic materials obtained using the same activation methods (
Table 7). The only exceptions to that rule are clearly higher S
BET values for ACs produced by KOH activation from walnut shells and plum stones composed of sclereid cells with strongly lignified and thickened cell walls.
The results of TG analysis based on changes in the mass of respective samples at consecutive stages of thermolysis (
Table 6) indicate varied thermal properties of the obtained ACs. Depending on the temperature, particular oxygen surface groups decompose. It is assumed that, in the range of 200–500 °C, stronger acidic groups (e.g., carboxyl) are degraded, whereas at 500–700 °C, weakly acidic groups (e.g., phenolic) disintegrate. However, at temperatures above 700 °C, groups of basic characters are broken down [
56]. Therefore, the percentage share of functional groups in the AC structure corresponds to the thermal properties of the tested materials.
ACs obtained with the use of KOH as an activator contained more carboxyl groups of strongly acidic character, as well as a higher number of lactonic groups, which may be broken down and release carboxyl groups (
Table 4). In the temperature range associated with the degradation of these groups (200–700 °C), the above-mentioned ACs showed greater mass losses as well as a significant effect of activator in the part of the range (
Table 6). As shown above, the use of NaOH as the activator caused an increase in the number of basic oxygen groups (
Table 4), which was also reflected in the results of TG analysis. These ACs showed greater mass losses and partially also a significant effect of activator (
Table 6) in the temperature range of 700–1200 °C, i.e., specific to the decomposition of basic functional groups. For the tested ACs, mass losses were also determined in the whole range of thermal analysis, i.e., from 20 to 1200 °C. The values of these mass losses may be treated as a measure of the thermal stability of the obtained ACs. However, for particular precursors, the effect of the activators on thermal stability was generally opposite, which further confirms their different activation mechanisms. In addition, for most temperature ranges, ACs obtained by NaOH activation were slightly more stable. Overall, MG/NaOH, MSin/NaOH and MSac/KOH may be considered the most thermally resistant ACs. Nonetheless, it may be assumed that the above-mentioned ACs with the most developed porous structure, i.e., SG/NaOH and especially MSac/NaOH, have the highest potential applicability, e.g., in decontamination or purification, to replace carbons obtained so far from
M. ×
giganteus [
9,
27].