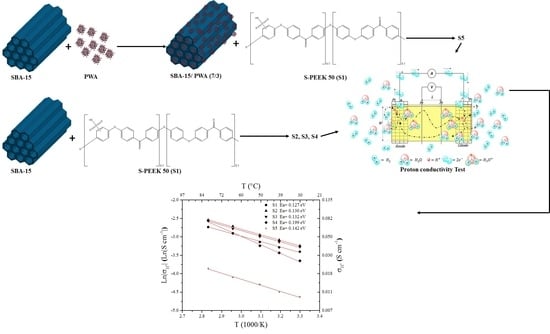
Composite Sulfonated Polyether-Ether Ketone Membranes with SBA-15 for Electrochemical Energy Systems
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of the Inorganic Filler
2.2. Sulfonation of the Poly-Ether-Ether-Ketone (PEEK)
2.3. Membranes Preparation
2.4. MEAs Fabrication
2.5. Inorganic Filler and Membranes Characterization
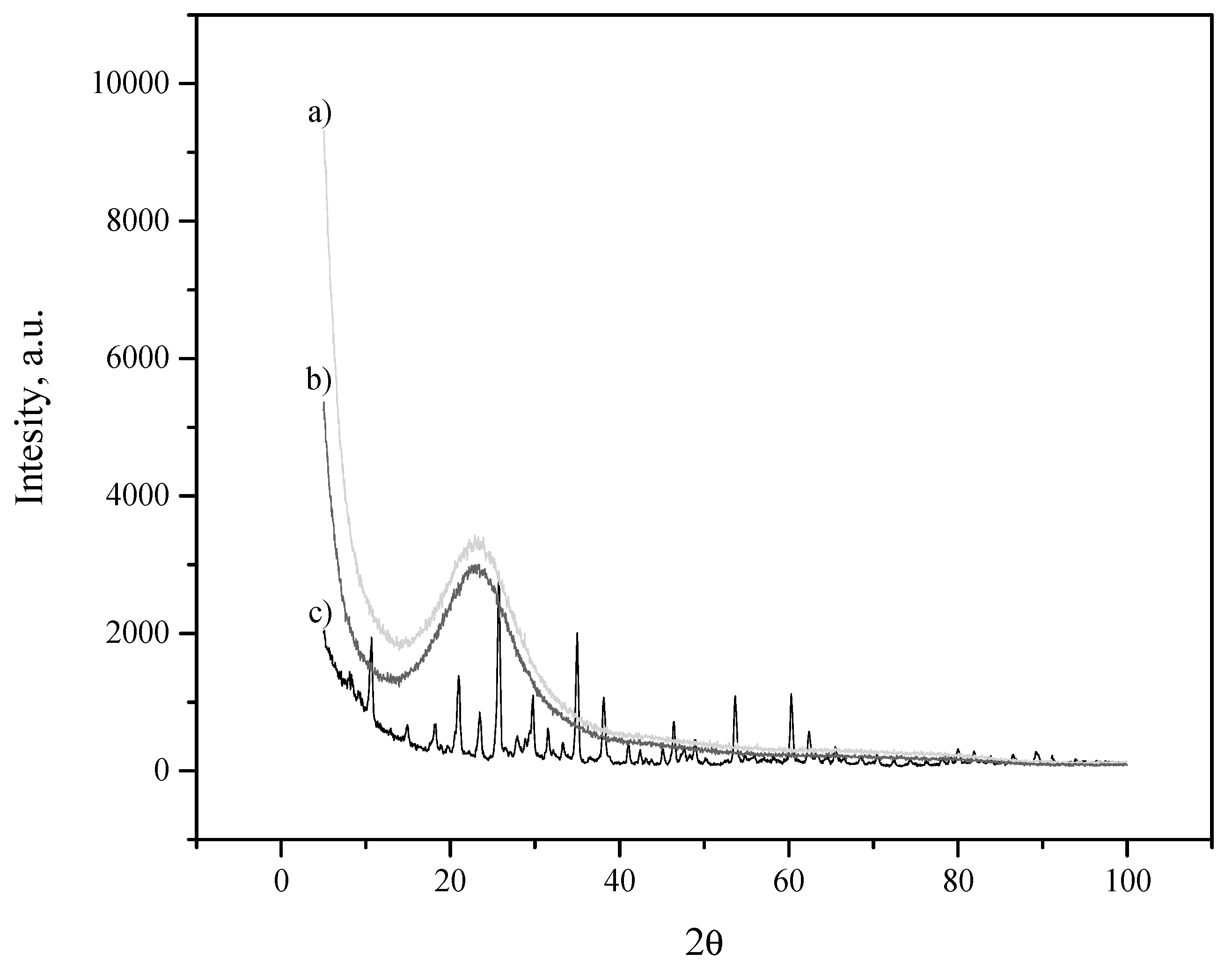
2.5.1. Inorganic Filler Characterization
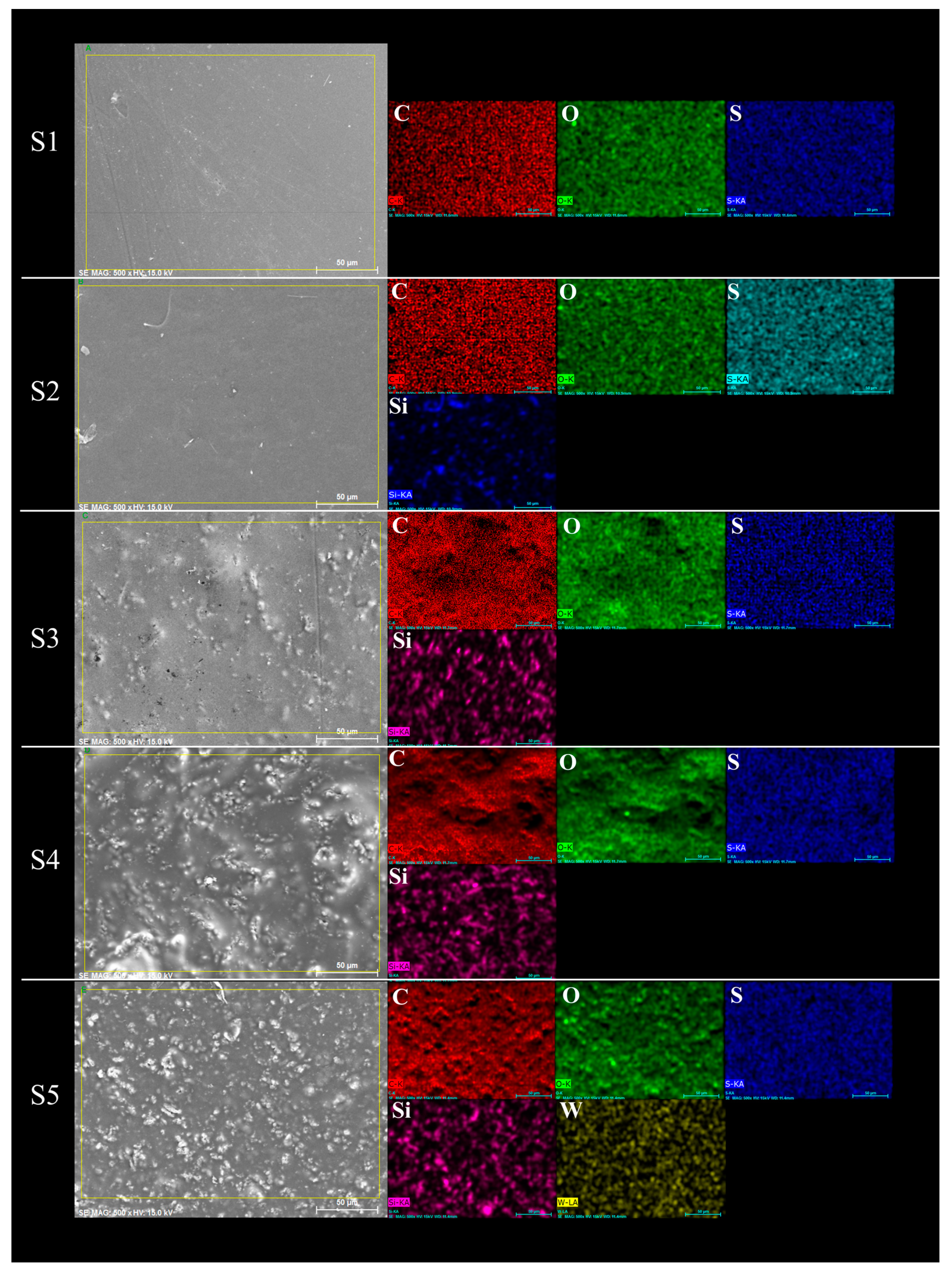
2.5.2. Composite Membranes Morphology
2.5.3. Water uptake (Wup), Swelling, Ion Exchange Capacity (IEC), Proton Conductivity () and Methanol Permeability
2.6. Electrochemical Characterization
2.6.1. Proton Exchange Membrane Fuel Cell
2.6.2. Electrochemical Hydrogen Pumping
3. Results
3.1. Filler Characterization
3.2. Membranes Characterization
3.3. Electrochmical Characterization
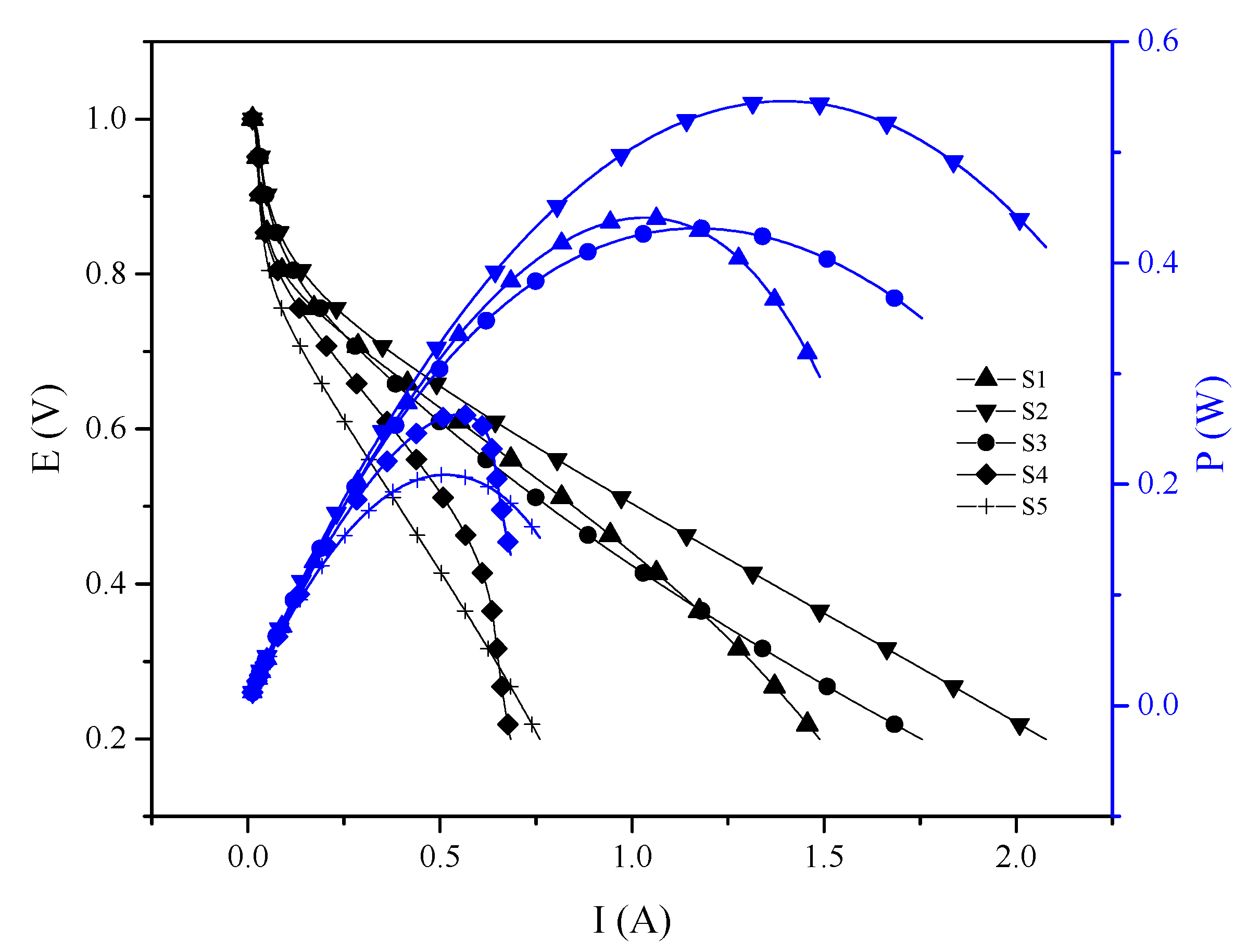
3.3.1. Proton Exchange Membrane Fuel Cell
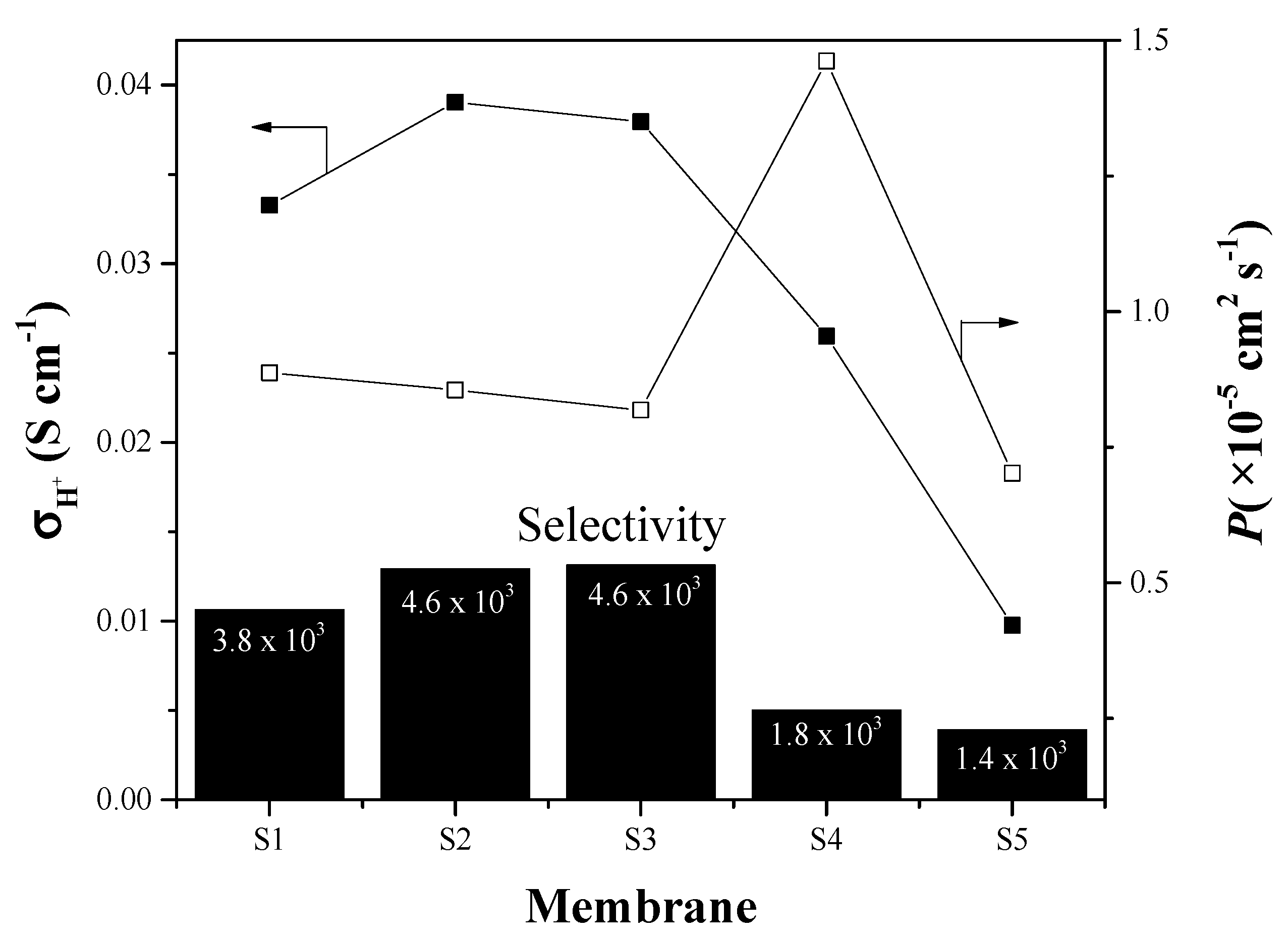
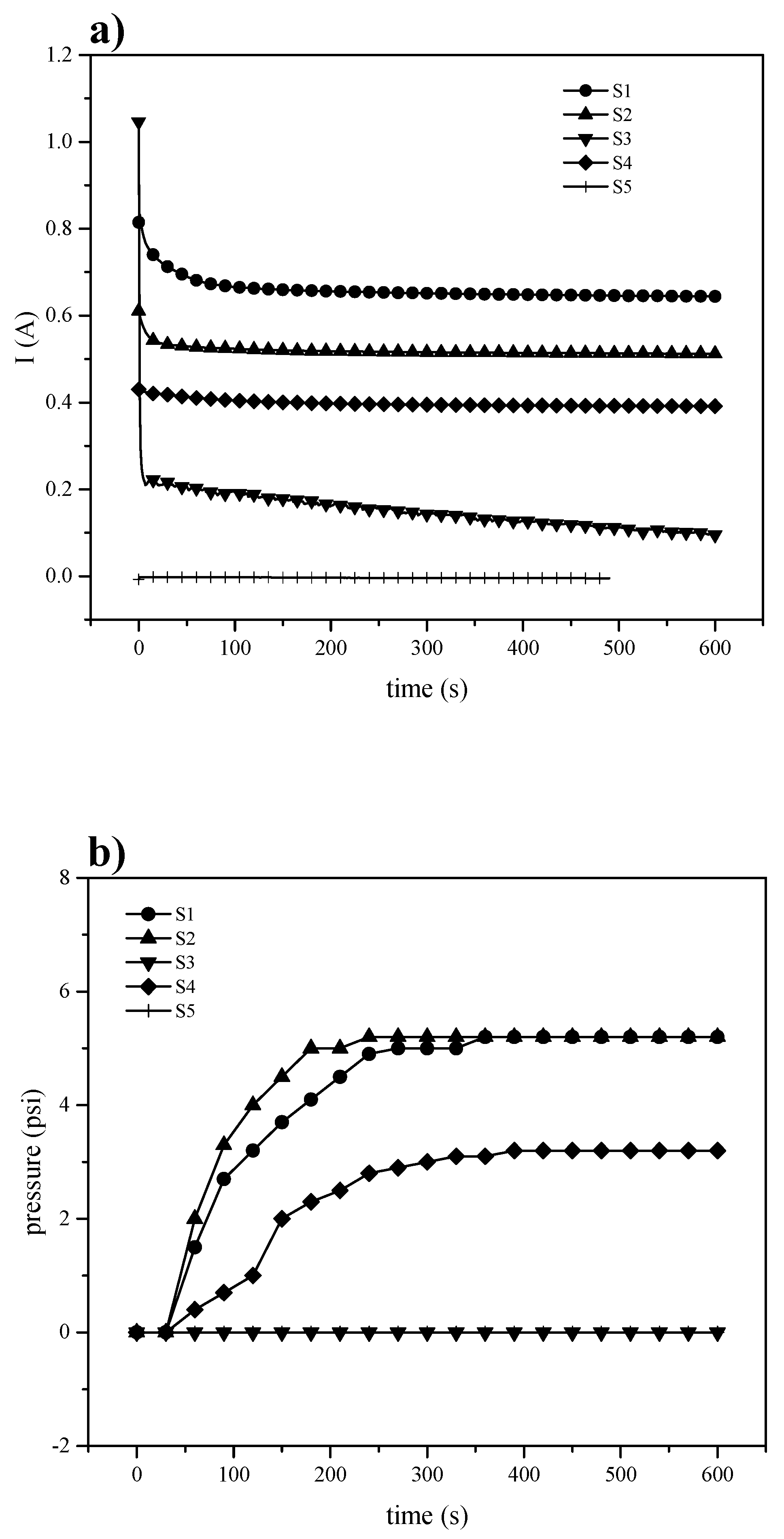
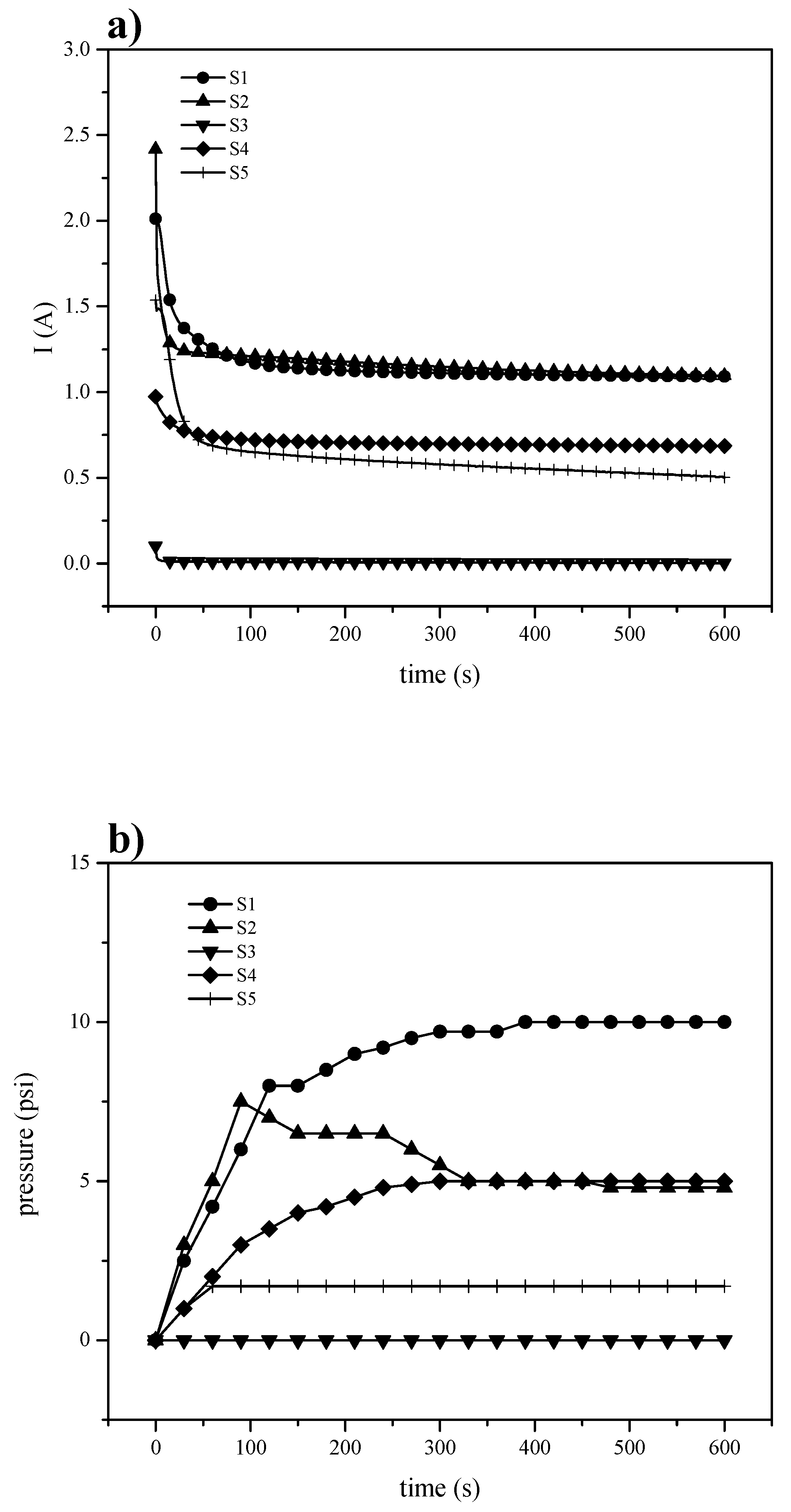
3.3.2. Electrochemical Hydrogen Pumping
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stambouli, A.B.; Traversa, E. Solid oxide fuel cells (SOFCs): A review of an environmentally clean and efficient source of energy. Renew. Sustain. Energy Rev. 2002, 6, 433–455. [Google Scholar] [CrossRef]
- Smitha, B.; Sridhar, S.; Khan, A.A. Solid polymer electrolyte membranes for fuel cell applications—A review. J. Memb. Sci. 2005, 259, 10–26. [Google Scholar] [CrossRef]
- Rohland, B.; Eberle, K.; Ströbel, R.; Scholta, J.; Garche, J. Electrochemical hydrogen compressor. Electrochim. Acta 1998, 43, 3841–3846. [Google Scholar] [CrossRef]
- Ströbel, R.; Oszcipok, M.; Fasil, M.; Rohland, B.; Jörissen, L.; Garche, J. The compression of hydrogen in an electrochemical cell based on a PE fuel cell design. J. Power Sources 2002, 105, 208–215. [Google Scholar] [CrossRef]
- Barbir, F.; Görgün, H. Electrochemical hydrogen pump for recirculation of hydrogen in a fuel cell stack. J. Appl. Electrochem. 2007, 37, 359–365. [Google Scholar] [CrossRef]
- Nguyen, M.T.D.; Grigoriev, S.A.; Kalinnikov, A.A.; Filippov, A.A.; Millet, P.; Fateev, V.N. Characterisation of a electrochemical hydrogen pump using electrochemical impedance spectroscopy. J. Appl. Electrochem. 2011, 41, 1383. [Google Scholar] [CrossRef][Green Version]
- Peighambardoust, S.J.; Rowshanzamir, S.; Amjadi, M. Review of the proton exchange membranes for fuel cell applications. Int. J. Hydrogen Energy 2010, 35, 9349–9384. [Google Scholar] [CrossRef]
- Gao, Y.; Robertson, G.P.; Guiver, M.D.; Mikhailenko, S.D.; Li, X.; Kaliaguine, S. Low-swelling proton-conducting copoly(aryl ether nitrile)s containing naphthalene structure with sulfonic acid groups meta to the ether linkage. Polymer 2006, 47, 808–816. [Google Scholar] [CrossRef]
- Yaroslavtsev, A.B.; Stenina, I.A.; Golubenko, D.V. Membrane materials for energy production and storage. Pure Appl. Chem 2020. (published online ahead of print). [Google Scholar] [CrossRef]
- Bouwman, P. Electrochemical Hydrogen Compression (EHC) solutions for hydrogen infrastructure. Fuel Cells Bull. 2014, 2014, 12–16. [Google Scholar] [CrossRef]
- Kreuer, K.D. On the development of proton conducting materials for technological applications. Solid State Ionics 1997, 97, 1–15. [Google Scholar] [CrossRef]
- Iojoiu, C.; Chabert, F.; Mar, M.; el Kissi, N. From polymer chemistry to membrane elaboration A global approach of fuel cell polymeric electrolytes. J. Power Sources 2006, 153, 198–209. [Google Scholar] [CrossRef]
- Iulianelli, A.; Basile, A. Sulfonated PEEK-based polymers in PEMFC and DMFC applications: A review. Int. J. Hydrogen Energy 2012, 37, 15241–15255. [Google Scholar] [CrossRef]
- Zhang, L.; Chae, S.; Hendren, Z.; Park, J.; Wiesner, M.R. Recent advances in proton exchange membranes for fuel cell applications. Chem. Eng. J. 2012, 204–206, 87–97. [Google Scholar] [CrossRef]
- Bardin, B.B.; Bordawekar, S.V.; Neurock, M.; Davis, R.J. Acidity of keggin-type heteropolycompounds evaluated by catalytic probe reactions, sorption microcalorimetry, and density functional quantum chemical calculations. J. Phys. Chem. B 1998, 102, 10817–10825. [Google Scholar] [CrossRef]
- Tripathi, B.P.; Shahi, V.K. Organic-inorganic nanocomposite polymer electrolyte membranes for fuel cell applications. Prog. Polym. Sci. 2011, 36, 945–979. [Google Scholar] [CrossRef]
- Mahreni, A.; Mohamad, A.B.; Kadhum, A.A.H.; Daud, W.R.W.; Iyuke, S.E. Nafion/silicon oxide/phosphotungstic acid nanocomposite membrane with enhanced proton conductivity. J. Memb. Sci. 2009, 327, 32–40. [Google Scholar] [CrossRef]
- Cui, Z.; Xing, W.; Liu, C.; Liao, J.; Zhang, H. Chitosan/heteropolyacid composite membranes for direct methanol fuel cell. J. Power Sources 2009, 188, 24–29. [Google Scholar] [CrossRef]
- Filippov, A.; Petrova, D.; Falina, I.; Kononenko, N.; Ivanov, E.; Lvov, Y.; Vinokurov, V. Transport Asymmetry of Novel Bi-Layer Hybrid Perfluorinated Membranes on the Base of MF-4SC Modified by Halloysite Nanotubes with Platinum. Polymers. 2018, 10, 366. [Google Scholar] [CrossRef]
- Filippov, A.N.; Kononenko, N.A.; Falina, I.V.; Titskaya, E.V.; Petrova, D.A. Electrodiffusion Characteristics of Halloysite-Modified Bilayer Membranes. Colloid J. 2020, 82, 81–92. [Google Scholar] [CrossRef]
- Carbone, A.; Pedicini, R.; Saccà, A.; Gatto, I.; Passalacqua, E. Composite S-PEEK membranes for medium temperature polymer electrolyte fuel cells. J. Power Sources 2008, 178, 661–666. [Google Scholar] [CrossRef]
- Zhang, H.; Ma, C.; Wang, J.; Wang, X.; Bai, H.; Liu, J. Enhancement of proton conductivity of polymer electrolyte membrane enabled by sulfonated nanotubes. Int. J. Hydrogen Energy 2014, 39, 974–986. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzmán, C.; Carbone, A.; Saccà, A.; Gatto, I.; Passalacqua, E.; Nava, R.; Ornelas, R.; Ledesma-García, J.; Arriaga, L.G. Influence of silica morphology in composite Nafion membranes properties. Int. J. Hydrogen Energy 2011, 36, 14725–14733. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzman, C.; Peza-Ledesma, C.; Godinez, L.A.; Nava, R.; Duron-Torres, S.M.; Ledesma-Garcia, J.; Arriaga, L.G. Silica-based Composite Membranes for Methanol Fuel Cells Operating at High Temperature. J. New Mater. Electrochem. Syst. 2011, 14, 87–91. [Google Scholar] [CrossRef]
- Casati, C.; Longhi, P.; Zanderighi, L.; Bianchi, F. Some fundamental aspects in electrochemical hydrogen purification/compression. J. Power Sources 2008, 180, 103–113. [Google Scholar] [CrossRef]
- Saccà, A.; Carbone, A.; Pedicini, R.; Marrony, M.; Barrera, R.; Elomaa, M.; Passalacqua, E. Phosphotungstic acid supported on a nanopowdered ZrO2 as a filler in nafion-based membranes for polymer electrolyte fuel cells. Fuel Cells 2008, 8, 225–235. [Google Scholar] [CrossRef]
- Bardin, B.B.; Davis, R.J. Effect of water on silica-supported phosphotungstic acid catalysts for 1-butene double bond shift and alkane skeletal isomerization. Appl. Catal. A Gen. 2000, 200, 219–231. [Google Scholar] [CrossRef]
- Feng, Z.; Guo, J.; Yan, Y.; Sun, J.; Zhang, S.; Wang, W.; Gu, X.; Li, H. Modi fi cation of mesoporous silica with phosphotungstic acid and its effects on the combustion and thermal behavior of polylactic acid composites. Polym. Degrad. Stab. 2019, 160, 24–34. [Google Scholar] [CrossRef]
- Alvarez, A.; Guzmán, C.; Carbone, A.; Saccá, A.; Gatto, I.; Pedicini, R.; Passalacqua, E.; Nava, R.; Ornelas, R.; Ledesma-García, J.; et al. Composite membranes based on micro and mesostructured silica: A comparison of physicochemical and transport properties. J. Power Sources 2011, 196, 5394–5401. [Google Scholar] [CrossRef]
- Gurrola, M.P.; Guerra-balcázar, M.; Álvarez-contreras, L.; Nava, R.; Ledesma-garcía, J. High surface electrochemical support based on Sb-doped SnO2. J. Power Sources 2013, 243, 826–830. [Google Scholar] [CrossRef]
- Rico-Zavala, A.; Gurrola, M.P.; Arriaga, L.G.; Bañuelos, J.A.; Álvarez-Contreras, L.; Carbone, A.; Saccà, A.; Matera, F.V.; Pedicini, R.; Álvarez, A.; et al. Synthesis and characterization of composite membranes modified with Halloysite nanotubes and phosphotungstic acid for electrochemical hydrogen pumps. Renew. Energy 2018, 122, 163–172. [Google Scholar] [CrossRef]
- Antonucci, P.; Aricò, A.; Cretì, P.; Ramunni, E.; Antonucci, V. Investigation of a direct methanol fuel cell based on a composite Nafion®-silica electrolyte for high temperature operation. Solid State Ionics 1999, 125, 431–437. [Google Scholar] [CrossRef]
- Jin, Y.G.; Qiao, S.Z.; da Costa, J.C.D.; Wood, B.J.; Ladewig, B.P.; Lu, G.Q. Hydrolytically Stable Phosphorylated Hybrid Silicas for Proton Conduction. Adv. Funct. Mater. 2007, 17, 3304–3311. [Google Scholar] [CrossRef]
- Liu, X.; He, S.; Song, G.; Jia, H.; Shi, Z.; Liu, S.; Zhang, L.; Lin, J.; Nazarenko, S. Proton conductivity improvement of sulfonated poly(ether ether ketone) nanocomposite membranes with sulfonated halloysite nanotubes prepared via dopamine-initiated atom transfer radical polymerization. J. Memb. Sci. 2016, 504, 206–219. [Google Scholar] [CrossRef]
- Peckham, T.J.; Schmeisser, J.; Rodgers, M.; Holdcroft, S. Main-chain, statistically sulfonated proton exchange membranes: The relationships of acid concentration and proton mobility to water content and their effect upon proton conductivity. J. Mater. Chem. 2007, 17, 3255. [Google Scholar] [CrossRef]
- Colomban, P.; Novak, A. Proton transfer and superionic conductivity in solids and gels. J. Mol. Struct. 1988, 177, 277–308. [Google Scholar] [CrossRef]
- Munakata, H.; Chiba, H.; Kanamura, K. Enhancement on proton conductivity of inorganic–organic composite electrolyte membrane by addition of sulfonic acid group. Solid State Ionics 2005, 176, 2445–2450. [Google Scholar] [CrossRef]
- Li, G.; Zhao, C.; Cui, Y.; Rong, T.; Na, H. RSC Advances poly (ether ether ketone) membranes with excellent mechanical properties and selectivity for direct. RSC Adv. 2016, 6, 23025–23032. [Google Scholar] [CrossRef]
- Rodgers, M.P.; Shi, Z.; Holdcroft, S. Transport properties of composite membranes containing silicon dioxide and Nafion (R). J. Memb. Sci. 2008, 325, 346–356. [Google Scholar] [CrossRef]
- Grigoriev, S.A.; Shtatniy, I.G.; Millet, P.; Porembsky, V.I.; Fateev, V.N. Description and characterization of an electrochemical hydrogen compressor/concentrator based on solid polymer electrolyte technology. Int. J. Hydrogen Energy 2011, 36, 4148–4155. [Google Scholar] [CrossRef]



| Inorganic Filler | Surface Area (m2/g) | Pore Diameter (nm) |
|---|---|---|
| SBA-15 | 782 | 9.4 ± 2 |
| PWA | 5 | 2.7 ± 1 |
| PWA/SBA-15 | 289 | 7.7 ± 2 |
| PWA/SBA-15 Washed | 852 | 9.3 ± 2 |
| Membrane | IEC | Wup % | Swelling | @30 °C | ||
|---|---|---|---|---|---|---|
| meq g−1 (±0.097) | @30 °C (±2) | @80 °C (±3) | @30 °C (±0.15) | @80 °C (±0.2) | (±2) | |
| (S1) S-PEEK | 1.63 | 46 | 79 | 1.2 | 1.5 | 16 |
| (S2) S-PEEK+SBA15 5% | 1.53 | 40 | 68 | 1.2 | 1.4 | 14 |
| (S3) S-PEEK+SBA15 10% | 1.36 | 45 | 58 | 1.3 | 1.3 | 18 |
| (S4) S-PEEK+SBA15 15% | 1.47 | 42 | 75 | 1.0 | 1.4 | 16 |
| (S5) S-PEEK+SBA15 10% +PWA | 1.42 | 40 | 52 | 1.0 | 1.2 | 16 |
| Nafion 115 | 1.01 | 26 | 42 | 1.3 | 1.6 | 14 |
| Membrane | Ea (eV) | σ0 (S cm−1) |
|---|---|---|
| S1 | 0.127 | 4.26 |
| S2 | 0.130 | 4.76 |
| S3 | 0.132 | 4.81 |
| S4 | 0.199 | 8.05 |
| S5 | 0.142 | 2.18 |
| Membrane | R (Ohm) by EIS at HFR |
|---|---|
| S1 | 0.185 |
| S2 | 0.251 |
| S3 | 0.412 |
| S4 | 0.6 |
| S5 | 0.476 |
| Membrane | × 10−6 | Pressure (psi) | Thickness (μm) | × 10−4 | |||
|---|---|---|---|---|---|---|---|
| 300 mV | 600 mV | 300 mV | 600 mV | 300 mV | 600 mV | ||
| S1 | 3.338 | 5.66 | 5.2 | 10 | 73 | 4.57 | 7.75 |
| S2 | 2.654 | 5.67 | 5.2 | 7.5 | 80 | 3.32 | 7.09 |
| S3 | 0.492 | 0.014 | 0 | 0 | 70 | 0.703 | 0.02 |
| S4 | 2.03 | 3.554 | 3.2 | 5 | 90 | 2.26 | 3.95 |
| S5 | - | 2.59 | 0 | 1.7 | 95 | - | 2.73 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rico-Zavala, A.; Pineda-Delgado, J.L.; Carbone, A.; Saccà, A.; Passalacqua, E.; Gurrola, M.P.; Alvarez, A.; Rivas, S.; Ledesma-García, J.; Arriaga, L.G. Composite Sulfonated Polyether-Ether Ketone Membranes with SBA-15 for Electrochemical Energy Systems. Materials 2020, 13, 1570. https://doi.org/10.3390/ma13071570
Rico-Zavala A, Pineda-Delgado JL, Carbone A, Saccà A, Passalacqua E, Gurrola MP, Alvarez A, Rivas S, Ledesma-García J, Arriaga LG. Composite Sulfonated Polyether-Ether Ketone Membranes with SBA-15 for Electrochemical Energy Systems. Materials. 2020; 13(7):1570. https://doi.org/10.3390/ma13071570
Chicago/Turabian StyleRico-Zavala, A., J. L. Pineda-Delgado, A. Carbone, A. Saccà, E. Passalacqua, M.P. Gurrola, A. Alvarez, S. Rivas, J. Ledesma-García, and L.G. Arriaga. 2020. "Composite Sulfonated Polyether-Ether Ketone Membranes with SBA-15 for Electrochemical Energy Systems" Materials 13, no. 7: 1570. https://doi.org/10.3390/ma13071570
APA StyleRico-Zavala, A., Pineda-Delgado, J. L., Carbone, A., Saccà, A., Passalacqua, E., Gurrola, M. P., Alvarez, A., Rivas, S., Ledesma-García, J., & Arriaga, L. G. (2020). Composite Sulfonated Polyether-Ether Ketone Membranes with SBA-15 for Electrochemical Energy Systems. Materials, 13(7), 1570. https://doi.org/10.3390/ma13071570