Supercritical Phase Inversion: A Powerful Tool for Generating Cellulose Acetate-AgNO3 Antimicrobial Membranes
Abstract
:1. Introduction
2. Materials and Methods
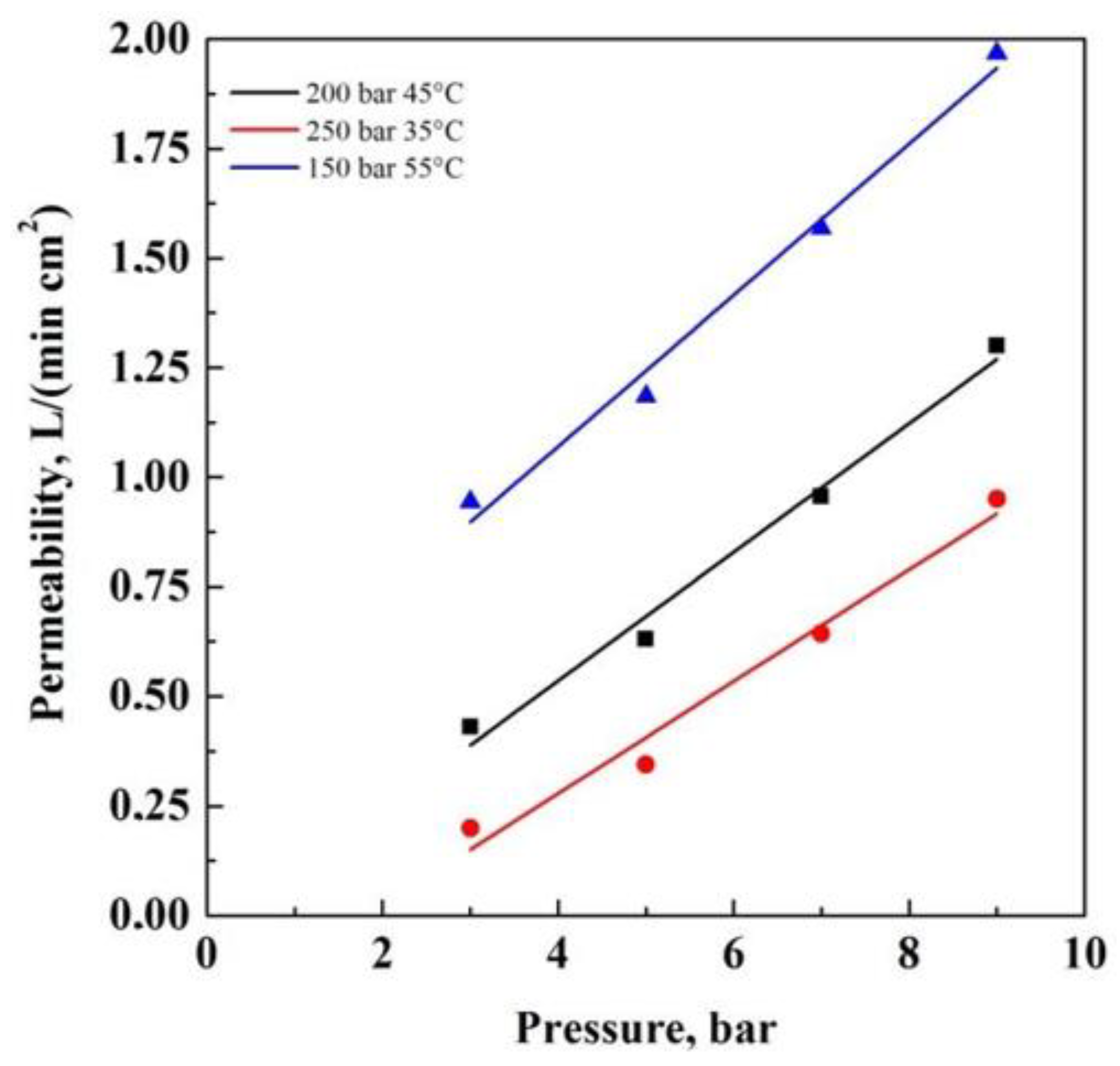
3. Results and Discussion
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Honarvar, Z.; Hadian, Z.; Mashayekh, M. Nanocomposites in food packaging applications and their risk assessment for health. Electron. Phys. 2016, 8, 2531–2538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, M.; Jiménez, A.; Garrigós, M.C. Active nanocomposites in food contact materials. Nanosci. Food Agric. 2017, 4, 1–44. [Google Scholar]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyphert, E.L.; von Recum, H.A. Emerging technologies for long-term antimicrobial device coatings: Advantages and limitations. Exp. Biol. Med. 2017, 242, 788–798. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, L.; Pompa, P.P. Nanosilver-based antibacterial drugs and devices: Mechanisms, methodological drawbacks, and guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef]
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Higueras, L.; López-Carballo, G.L.; Hernández-Muñoz, P.; Gavara, R.; Rollini, M. Development of a novel antimicrobial film based on chitosan with LAE (ethyl-Nα-dodecanoyl-l-arginate) and its application to fresh chicken. Int. J. Food Microbiol. 2013, 165, 339–345. [Google Scholar] [CrossRef]
- Sacco, P.; Travan, A.; Borgogna, M.; Paoletti, S.; Marsich, E. Silver-containing antimicrobial membrane based on chitosan-TPP hydrogel for the treatment of wounds. J. Mater. Sci. Mater. Med. 2015, 26, 128. [Google Scholar] [CrossRef]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Sreedhara Reddy, P. Biogenic nano-scale silver particles by Tephrosia purpurea leaf extract and their inborn antimicrobial activity. Spectrochim. Acta Part A 2014, 121, 164–172. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver I: Its antibacterial properties and mechanism of action. J. Wound Care 2013, 11, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.H.; Nguyen, V.Q.; Le, A.-T. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001–033021. [Google Scholar] [CrossRef] [Green Version]
- Lara, H.H.; Ayala-Núñez, N.V.; del Carmen Ixtepan Turrent, L.; Padilla, C.R. Bactericidal effect of silver nanoparticles against multidrug-resistant bacteria. World J. Microbiol. Biotechnol. 2010, 26, 615–621. [Google Scholar] [CrossRef]
- Sawada, I.; Fachrul, R.; Ito, T.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Development of a hydrophilic polymer membrane containing silver nanoparticles with both organic antifouling and antibacterial properties. J. Membr. Sci. 2012, 387–388, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Durán, N.; Durán, M.; de Bispo Jesus, M.; Seabra, A.B.; Fávaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomed. Nanotechnol. 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Dallas, P.; Sharm, V.K.; Zboril, R. Silver polymeric nanocomposites as advanced antimicrobial agents: Classification, synthetic paths, applications, and perspectives. Adv. Colloid Interface Sci. 2011, 166, 119–135. [Google Scholar] [CrossRef]
- Miranda, M.; Fernández, A.; Díaz, M.; Esteban-Tejeda, L.; López-Esteban, S.; Malpartida, F.; Torrecillas, R.; Moya, J.S. Silver-hydroxyapatite nanocomposites as bactericidal and fungicidal materials. Int. J. Mater. Res. 2010, 101, 122–127. [Google Scholar] [CrossRef] [Green Version]
- Shuai, C.; Xu, Y.; Feng, P.; Wang, G.; Xiong, S.; Peng, S. Antibacterial polymer scaffold based on mesoporous bioactive glass loaded with in situ grown silver. Chem. Eng. J. 2019, 374, 304–315. [Google Scholar] [CrossRef]
- Ito, K.; Saito, A.; Fujie, T.; Miyazaki, H.; Kinoshita, M.; Saitoh, D.; Ohtsubo, S.; Takeoka, S. Development of a ubiquitously transferrable silver-nanoparticle-loaded polymer nanosheet as an antimicrobial coating. J. Biomed. Mater. Res. Part B 2016, 104, 585–593. [Google Scholar] [CrossRef]
- Girdthep, S.; Worajittiphon, P.; Molloy, R.; Leejarkpai, T.; Punyodom, W. Formulation and characterization of compatibilized poly (lactic acid)-based blends and their nanocomposites with silver-loaded kaolinite. Polym. Int. 2015, 64, 203–211. [Google Scholar] [CrossRef]
- Shao, W.; Liu, H.; Liu, X.; Wang, S.; Wu, J.; Zhang, R.; Min, H.; Huang, M. Development of silver sulfadiazine loaded bacterial cellulose/sodium alginate composite films with enhanced antibacterial property. Carbohydr. Polym. 2015, 132, 351–358. [Google Scholar] [CrossRef]
- Deng, A.; Chen, A.; Wang, S.; Li, Y.; Liu, Y.; Cheng, X.; Zhao, Z.; Lin, D. Porous nanostructured poly-l-lactide scaffolds prepared by phase inversion using supercritical CO2 as a nonsolvent in the presence of ammonium bicarbonate particles. J. Supercrit. Fluids 2013, 77, 110–116. [Google Scholar] [CrossRef]
- Duarte, A.R.C.; Mano, J.F.; Reis, R.L. The role of organic solvent on the preparation of chitosan scaffolds by supercritical assisted phase inversion. J. Supercrit. Fluids 2012, 72, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Sizov, V.E.; Kondratenko, M.S.; Gallyamov, M.O.; Stevenson, K.J. Advanced porous polybenzimidazole membranes for vanadium redox batteries synthesized via a supercritical phase-inversion method. J. Supercrit. Fluids 2018, 137, 111–117. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Biodegradable membranes loaded with curcumin to be used as engineered independent devices in active packaging. J. Taiwan Inst. Chem. Eng. 2017, 71, 518–526. [Google Scholar] [CrossRef]
- Tabernero, A.; Baldino, L.; González-Garcinuño, Á.; Cardea, S.; Del Valle, E.M.M.; Reverchon, E. Supercritical CO2 assisted formation of composite membranes containing an amphiphilic fructose-based polymer. J. CO2 Utiliz. 2019, 34, 274–281. [Google Scholar] [CrossRef]
- Baldino, L.; Aragón, J.; Mendoza, G.; Irusta, S.; Cardea, S.; Reverchon, E. Production, characterization and testing of antibacterial PVA membranes loaded with HA-Ag3PO4 nanoparticles, produced by SC-CO2 phase inversion. J. Chem. Technol. Biotechnol. 2019, 94, 98–108. [Google Scholar] [CrossRef] [Green Version]
- Chou, W.-L.; Yu, D.-G.; Yang, M.-C. The preparation and characterization of silver-loading cellulose acetate hollow fiber membrane for water treatment. Polym. Adv. Technol. 2005, 16, 600–607. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimi-crobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interfaces Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Alt, V.; Bechert, T.; Steinrücke, P.; Wagener, M.; Seidel, P.; Din-geldein, E.; Domann, E.; Schnettler, R. An in vitro assessment of the antibacterial properties and cytotoxicity of nanoparticu-late silver bone cement. Biomaterials 2004, 25, 4383–4391. [Google Scholar] [CrossRef]
- Miller, I.; Freund, J.F. Probability and Statistics for Engineering, 3rd ed.; Prentice-Hall: Englewood Cliffs, NJ, USA, 1985. [Google Scholar]
- Xu, X.; Zhou, M. Antimicrobial Gelatin Nanofibers Containing Silver Nanoparticles. Fibers Polym. 2008, 9, 685–690. [Google Scholar] [CrossRef]
- Reverchon, E.; Cardea, S. Formation of cellulose acetate membranes using a supercritical fluid assisted process. J. Membr. Sci. 2004, 240, 187–195. [Google Scholar] [CrossRef]
- Zaherzadeh, A.; Karimi-Sabet, J.; Mousavian, S.M.A.; Ghorbanian, S. Optimization of flat sheet hydrophobic membranes synthesis via supercritical CO2 induced phase inversion for direct contact membrane distillation by using response surface methodology (RSM). J. Supercrit. Fluids 2015, 103, 105–114. [Google Scholar] [CrossRef]
- Cardea, S.; Baldino, L.; Reverchon, E. Comparative study of PVDF-HFP-curcumin porous structures produced by supercritical assisted processes. J. Supercrit. Fluids 2018, 133, 270–277. [Google Scholar] [CrossRef]
- Baldino, L.; Sarno, M.; Cardea, S.; Irusta, S.; Ciambelli, P.; Santamaria, J.; Reverchon, E. Formation of cellulose acetate-graphene oxide nanocomposites by supercritical CO2 assisted phase inversion. Ind. Eng. Chem. Res. 2015, 54, 8147–8156. [Google Scholar] [CrossRef] [Green Version]
- Hubadillah, S.K.; Harun, Z.; Dzarfan Othman, M.H.; Ismail, A.F.; Gani, P. Effect of kaolin particle size and loading on the characteristics of kaolin ceramic support prepared via phase inversion technique. J. Asian Ceram. Soc. 2016, 4, 164–177. [Google Scholar] [CrossRef]
- Basri, H.; Ismail, A.F.; Aziz, M. Polyethersulfone (PES)–silver composite UF membrane: Effect of silver loading and PVP molecular weight on membrane morphology and antibacterial activity. Desalination 2011, 273, 72–80. [Google Scholar] [CrossRef]
- Tatykaev, B.B.; Burkitbayev, M.M.; Uralbekov, B.M.; Urakaev, F.K. Mechanochemical synthesis of silver chloride nanoparticles by a dilution method in the system NH4Cl-AgNO3-NH4NO3. Acta Phys. Pol. A 2014, 126, 1044–1048. [Google Scholar] [CrossRef]
- Rodríguez, F.J.; Galotto, M.J.; Guarda, A.; Bruna, J.E. Modification of cellulose acetate films using nanofillers based on organoclays. J. Food Eng. 2012, 110, 262–268. [Google Scholar] [CrossRef]
- Baldino, L.; Cardea, S.; Reverchon, E. Production of antimicrobial membranes loaded with potassium sorbate using a supercritical phase separation process. Innov. Food Sci. Emerg. Technol. 2016, 34, 77–85. [Google Scholar] [CrossRef]
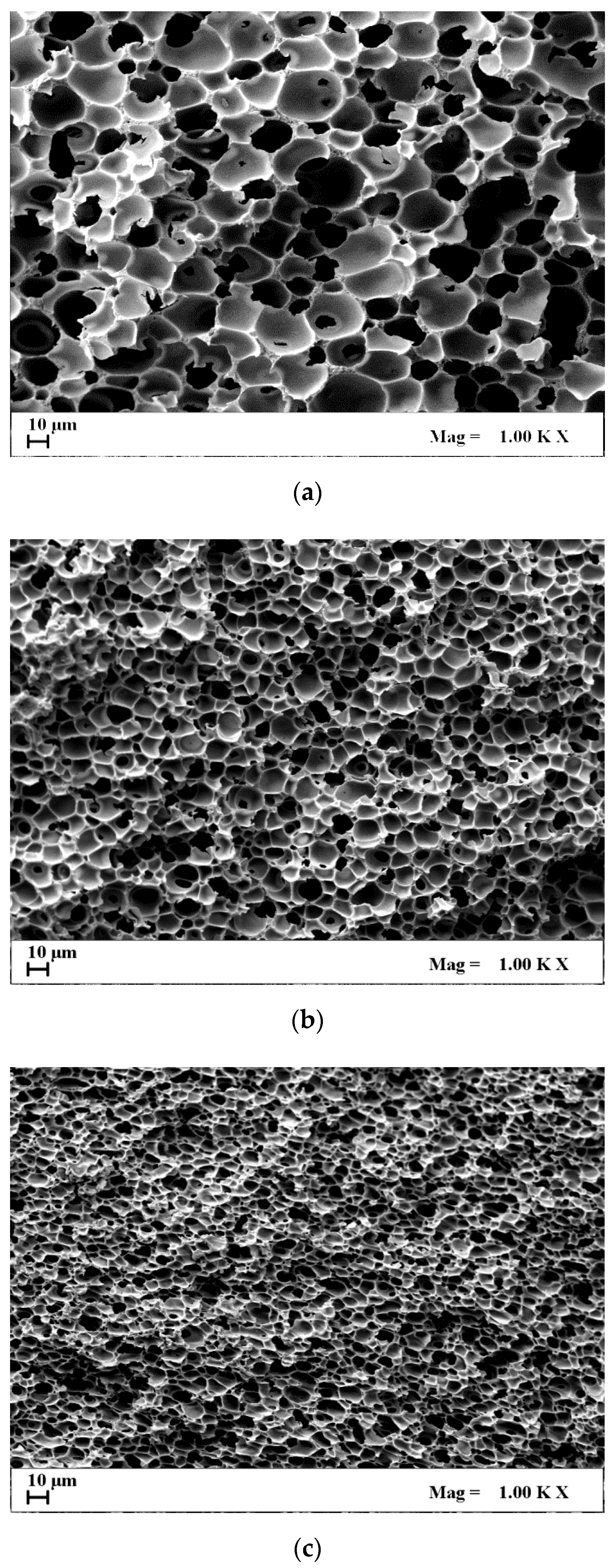
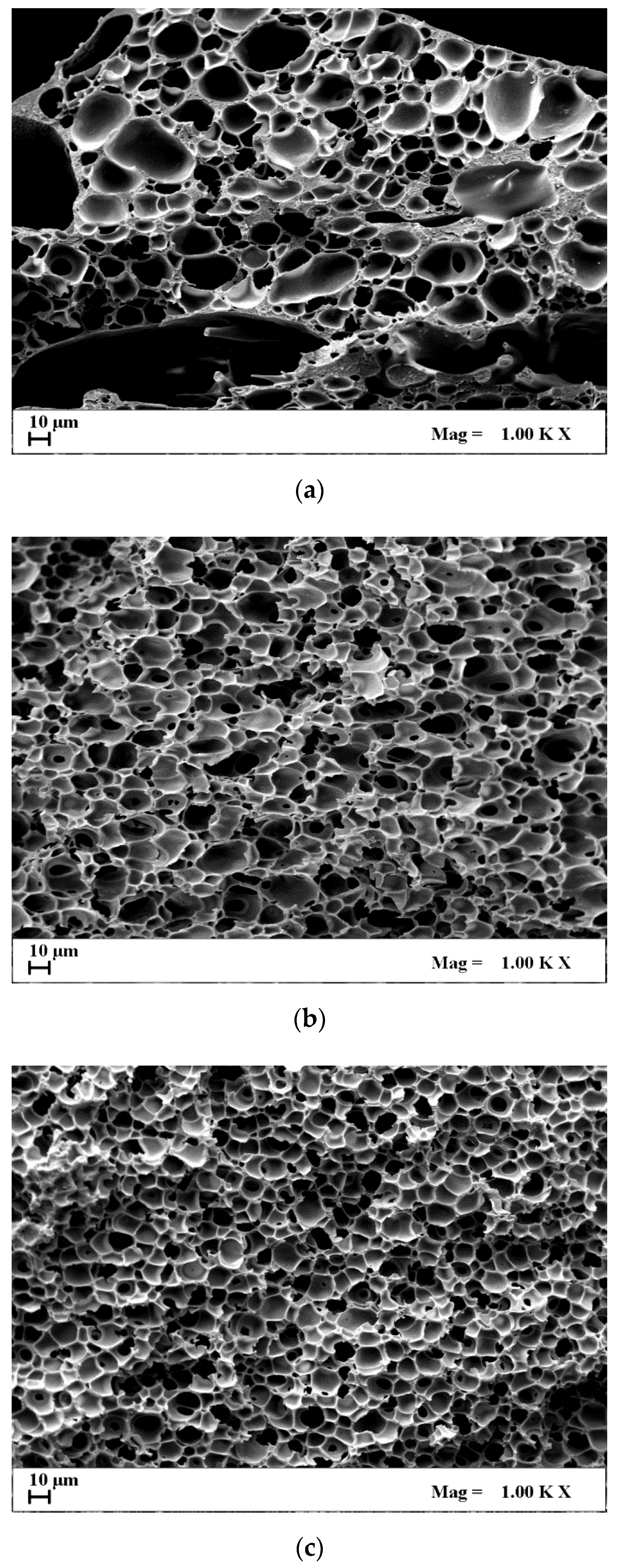
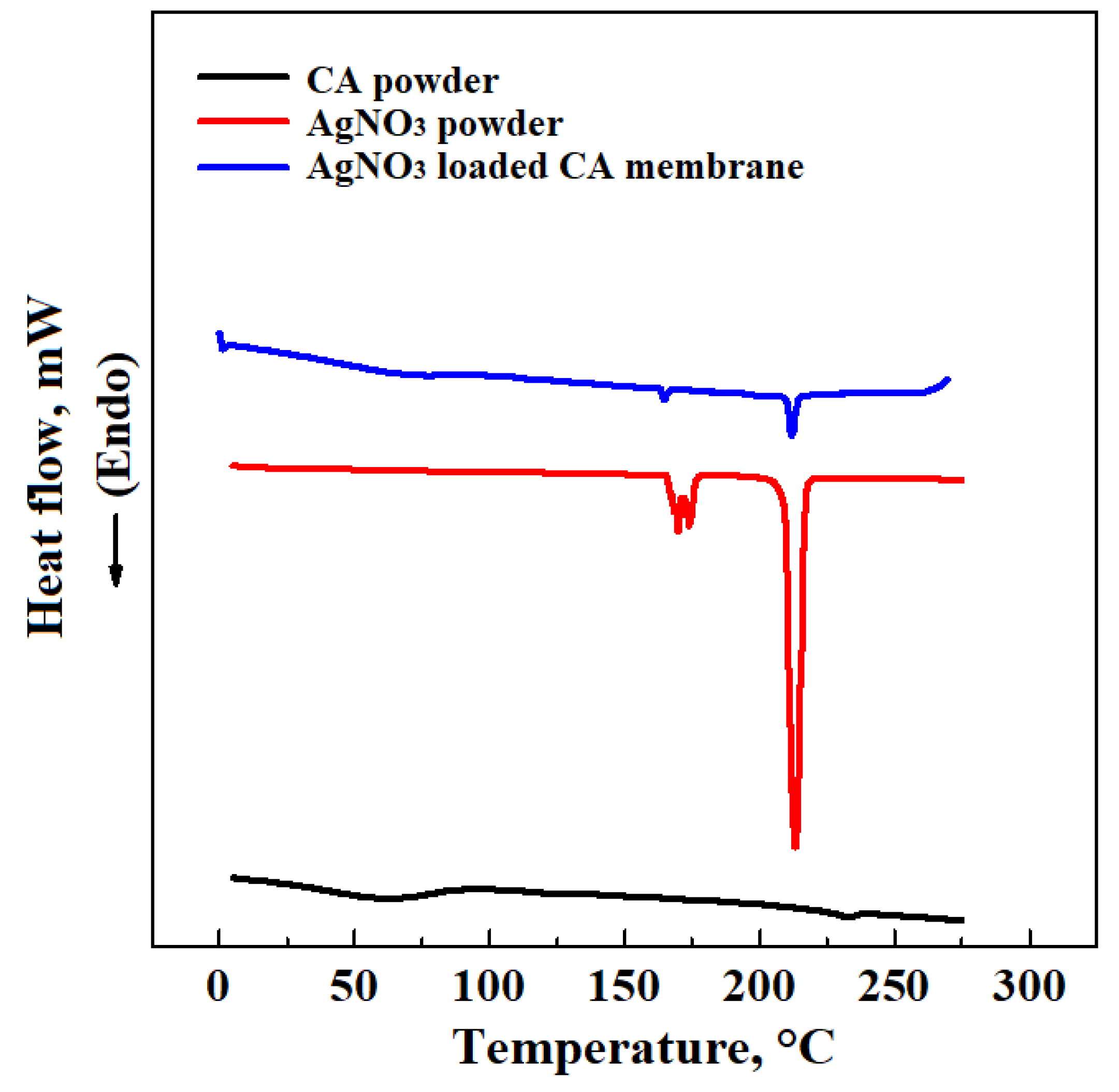
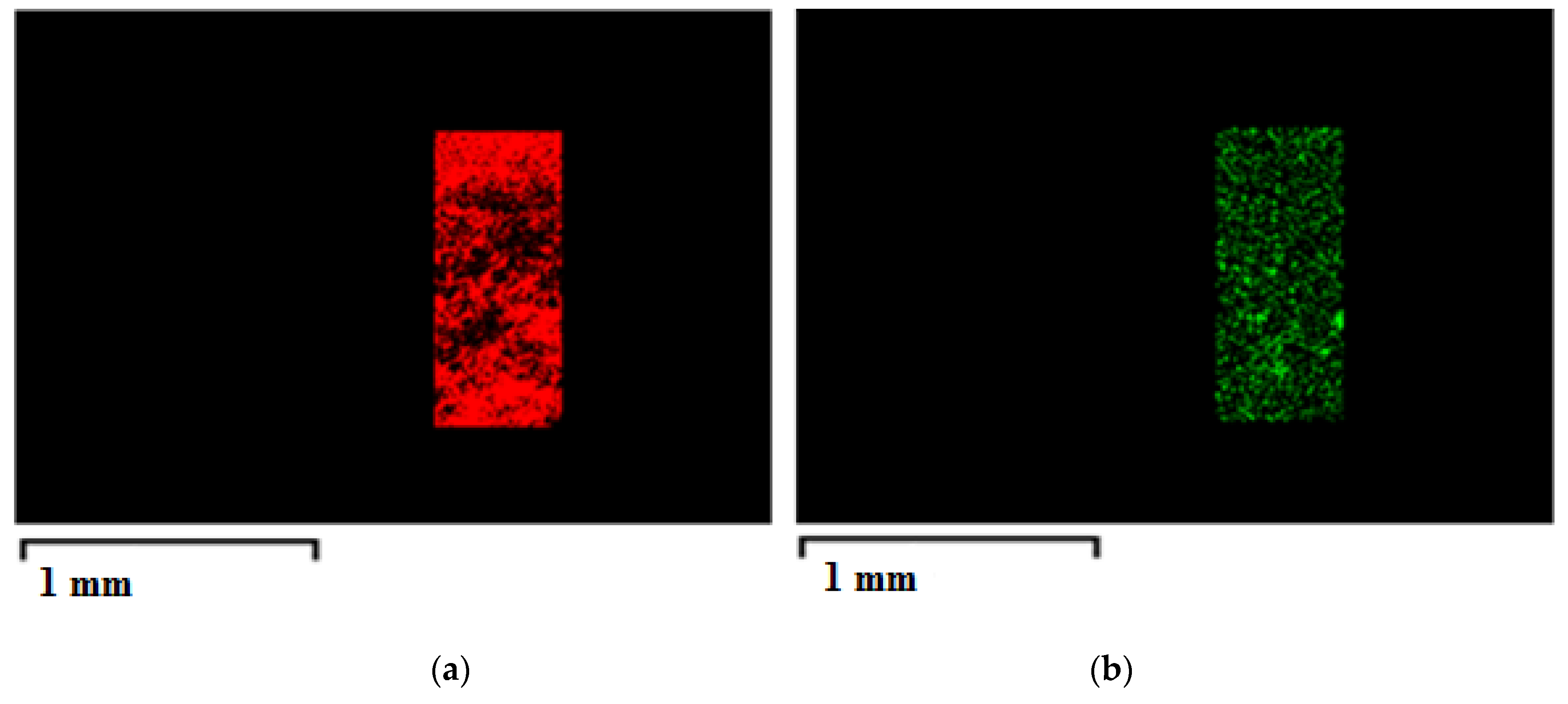

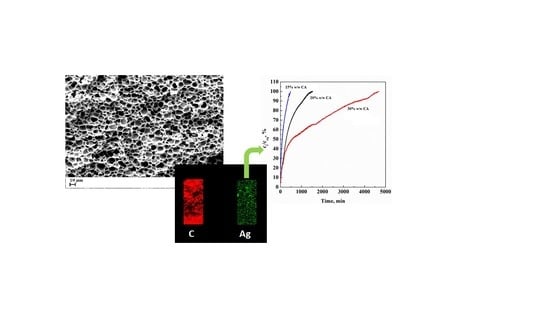
| CA Concentration (AgNO3 0.1%(w/w) CA) | Mean Pore Diameter, μm | Porosity, % |
|---|---|---|
| 150 bar 55 °C | ||
| 15%(w/w) | 14.36 ± 4.05 | 89.7 ± 6.2 |
| 20%(w/w) | 10.93 ± 2.54 | 85.6 ± 5.4 |
| 30%(w/w) | 10.06 ± 2.19 | 84.1 ± 4.7 |
| 200 bar 45 °C | ||
| 15%(w/w) | 12.67 ± 3.66 | 87.4 ± 5.8 |
| 20%(w/w) | 9.84 ± 2.93 | 84.2 ± 4.9 |
| 30%(w/w) | 8.42 ± 1.78 | 82.4 ± 4.2 |
| 250 bar 35 °C | ||
| 15%(w/w) | 8.73 ± 2.14 | 83.7 ± 4.6 |
| 20%(w/w) | 7.99 ± 1.79 | 82.3 ± 4.1 |
| 30%(w/w) | 6.86 ± 1.45 | 81.2 ± 3.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baldino, L.; Cardea, S.; Reverchon, E. Supercritical Phase Inversion: A Powerful Tool for Generating Cellulose Acetate-AgNO3 Antimicrobial Membranes. Materials 2020, 13, 1560. https://doi.org/10.3390/ma13071560
Baldino L, Cardea S, Reverchon E. Supercritical Phase Inversion: A Powerful Tool for Generating Cellulose Acetate-AgNO3 Antimicrobial Membranes. Materials. 2020; 13(7):1560. https://doi.org/10.3390/ma13071560
Chicago/Turabian StyleBaldino, Lucia, Stefano Cardea, and Ernesto Reverchon. 2020. "Supercritical Phase Inversion: A Powerful Tool for Generating Cellulose Acetate-AgNO3 Antimicrobial Membranes" Materials 13, no. 7: 1560. https://doi.org/10.3390/ma13071560
APA StyleBaldino, L., Cardea, S., & Reverchon, E. (2020). Supercritical Phase Inversion: A Powerful Tool for Generating Cellulose Acetate-AgNO3 Antimicrobial Membranes. Materials, 13(7), 1560. https://doi.org/10.3390/ma13071560