Retrieval Analysis of Modern Knee Tumor Megaendoprosthesis Shows Considerable Volumetric Metal Wear Generated at the Rotating Hinge
Abstract
:1. Introduction
2. Materials and Methods
2.1. Retrievals
2.2. Semi-Quantitative Wear Analysis by Scoring System
2.3. Quantitative Wear Analysis by Coordinate Measurements
2.4. Statistical Analysis
3. Results
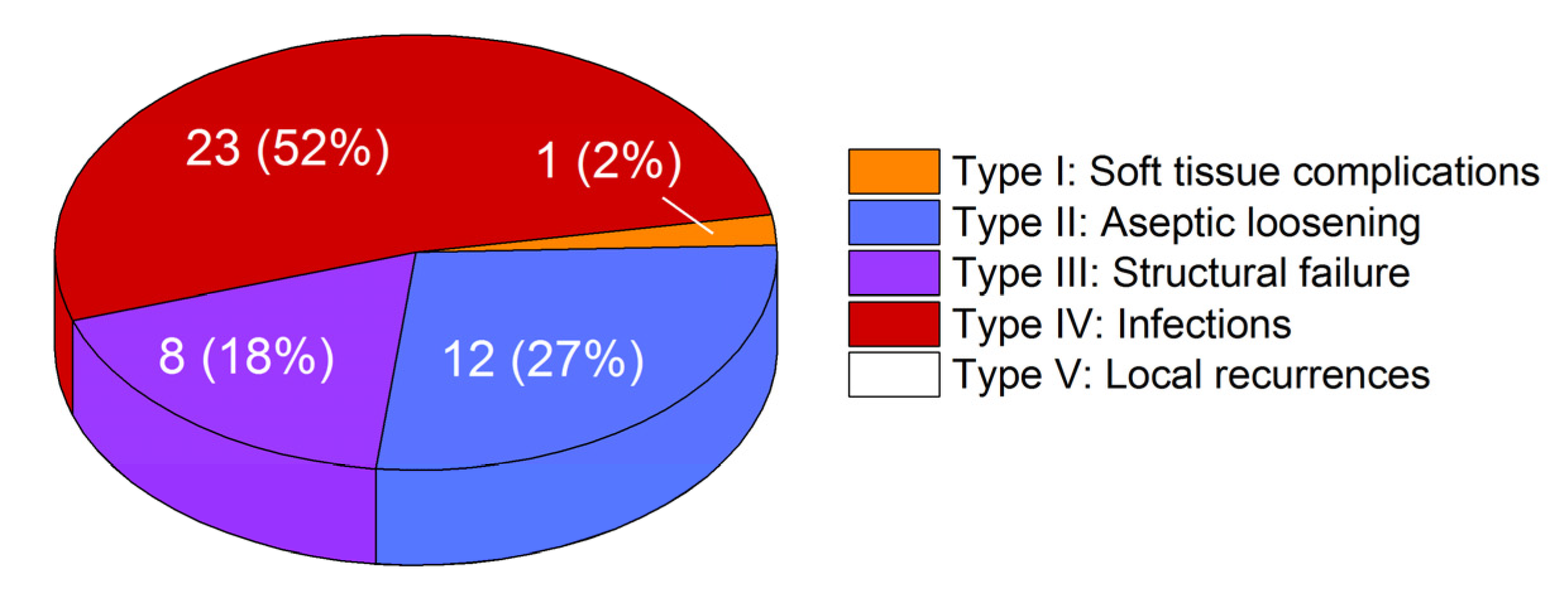
3.1. Failure Modes of Retrieved Implants
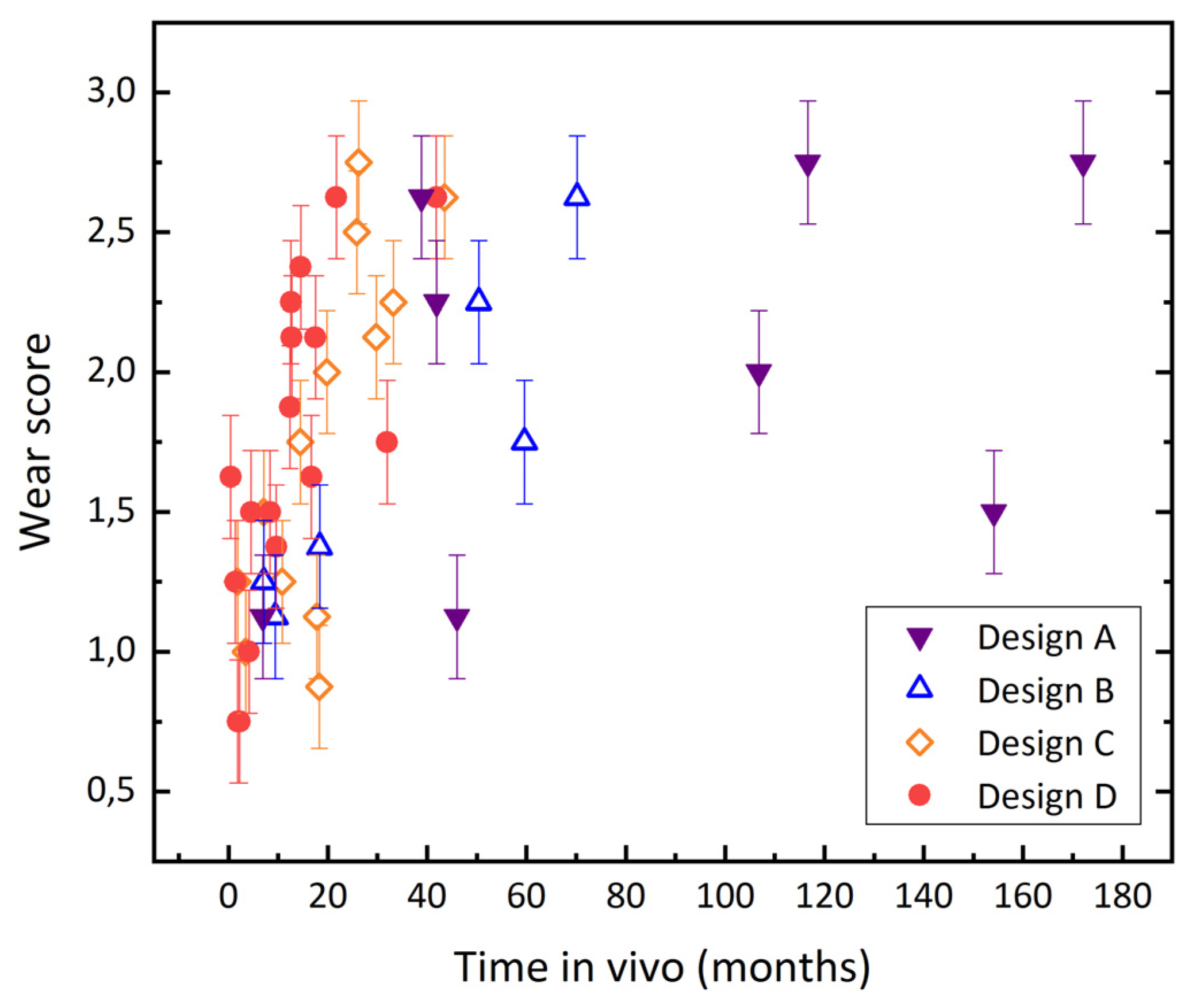
3.2. Evaluation of Wear by Damage Score
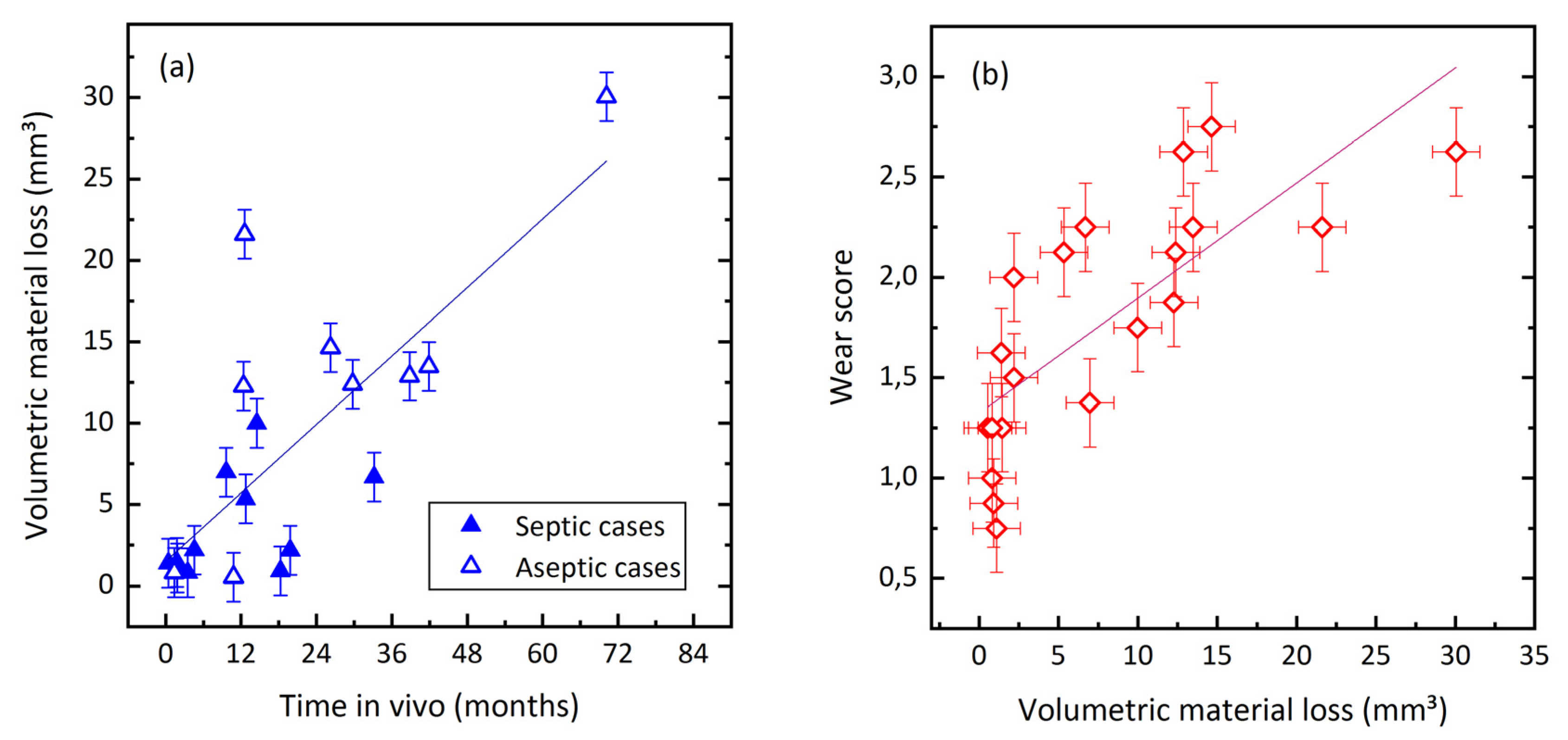
3.3. Quantification of Wear by Coordinate Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gosheger, G.M.D.; Gebert, C.M.D.; Ahrens, H.M.D.; Streitbuerger, A.M.D.; Winkelmann, W.M.D.; Hardes, J.M.D. Endoprosthetic reconstruction in 250 patients with sarcoma. Clin. Orthop. Relat. Rese. 2006, 450, 164–171. [Google Scholar] [CrossRef]
- Myers, G.J.C.; Abudu, A.T.; Carter, S.R.; Tillman, R.M.; Grimer, R.J. Endoprosthetic replacement of the distal femur for bone tumours. J. Bone Jt. Surg. Br. Vol. 2007, 89, 521–526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heisel, C.; Kinkel, S.; Bernd, L.; Ewerbeck, V. Megaprostheses for the treatment of malignantbone tumours of the lower limbs. Int. Orthop. 2006, 30, 452–457. [Google Scholar] [CrossRef] [Green Version]
- Toepfer, A.; Harrasser, N.; Schwarz, P.-R.; Pohlig, F.; Lenze, U.; Mühlhofer, H.M.L.; Gerdesmeyer, L.; von Eisenhart-Rothe, R.; Suren, C. Distal femoral replacement with the MML system: A single center experience with an average follow-up of 86 months. BMC Musculoskelet. Disord. 2017, 18, 206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Australian Orthopaedic Association National Joint Replacement Registry (AOANJRR). Hip, Knee & Shoulder Arthroplasty: 2019 Annual Report; AOANJRR: Adelaide, Australia, 2019. [Google Scholar]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure mode classification for tumor endoprostheses: Retrospective review of five institutions and a literature review. JBJS 2011, 93, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Kinkel, S.; Lehner, B.; Kleinhans, J.A.; Jakubowitz, E.; Ewerbeck, V.; Heisel, C. Medium to long-term results after reconstruction of bone defects at the knee with tumor endoprostheses. J. Surg. Oncol. 2010, 101, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Theil, C.; Röder, J.; Gosheger, G.; Deventer, N.; Dieckmann, R.; Schorn, D.; Hardes, J.; Andreou, D. What is the likelihood that tumor endoprostheses will experience a second complication after first revision in patients with primary malignant bone tumors and what are potential risk factors? Clin. Orthop. Relat. Res. 2019, 477, 2705–2714. [Google Scholar] [CrossRef]
- Hardes, J.; Henrichs, M.-P.; Gosheger, G.; Guder, W.; Nottrott, M.; Andreou, D.; Bormann, E.; Eveslage, M.; Hauschild, G.; Streitbürger, A. Tumour endoprosthesis replacement in the proximal tibia after intra-articular knee resection in patients with sarcoma and recurrent giant cell tumour. Int. Orthop. 2018, 42, 2475–2481. [Google Scholar] [CrossRef]
- Bus, M.P.A.; van de Sande, M.A.J.; Fiocco, M.; Schaap, G.R.; Bramer, J.A.M.; Dijkstra, P.D.S. What are the long-term results of MUTARS® modular endoprostheses for reconstruction of tumor resection of the distal femur and proximal tibia? Clin. Orthop. Relat. Res. 2017, 475, 708–718. [Google Scholar] [CrossRef] [Green Version]
- Hardes, J.; Henrichs, M.P.; Hauschild, G.; Nottrott, M.; Guder, W.; Streitbuerger, A. Silver-coated megaprosthesis of the proximal tibia in patients with sarcoma. J. Arthroplast. 2017, 32, 2208–2213. [Google Scholar] [CrossRef] [Green Version]
- Laitinen, M.; Nieminen, J.; Reito, A.; Pakarinen, T.-K.; Suomalainen, P.; Pamilo, K.; Parkkinen, J.; Lont, T.; Eskelinen, A. High blood metal ion levels in 19 of 22 patients with metal-on-metal hinge knee replacements. Acta Orthop. 2017, 88, 269–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lützner, J.; Günther, K.-P.; Postler, A.; Morlock, M. Metallionenfreisetzung nach Hüft- und Kniegelenkendoprothetik—Mechanismen, biologische Wirkungen und notwendige Diagnostik. ZS Orthounfall 2019. [Google Scholar] [CrossRef]
- Paulus, A.C.; Ebinger, K.; Cheng, X.; Haßelt, S.; Weber, P.; Kretzer, J.P.; Bader, R.; Utzschneider, S. Local biological reactions and pseudotumor-like tissueformation in relation to metal wear in a murine in vivo model. BioMed Res. Int. 2019, 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Grammatopoulos, G.; Pandit, H.; Kamali, A.; Maggiani, F.; Glyn-Jones, S.; Gill, H.S.; Murray, D.W.; Athanasou, N. The correlation of wear with histological features after failed hip resurfacing arthroplasty. JBJS 2013, 95, e81. [Google Scholar] [CrossRef] [Green Version]
- German Standard DIN ISO 5832-4. Implants for Surgery—Metallic Materials—Part 4: Cobalt-chromium-molybdenum Casting Alloy (ISO 5332-4:2014); Deutsches Institut für Normung e.V., Beuth Verlag GmbH: Berlin, Germany, 2015. [Google Scholar]
- Goldberg, J.R.; Gilbert, J.L.; Jacobs, J.J.; Bauer, T.W.; Paprosky, W.; Leurgans, S. A Multicenter retrieval study of the taper interfaces of modular hip prostheses. Clin. Orthop. Relat. Res. 2002, 401, 149–161. [Google Scholar] [CrossRef]
- Hood, R.W.; Wright, T.M.; Burstein, A.H. Retrieval analysis of total knee prostheses: A method and its application to 48 total condylar prostheses. J. Biomed. Mater. Res. 1983, 17, 829–842. [Google Scholar] [CrossRef]
- Arnholt, C.M.; MacDonald, D.W.; Malkani, A.L.; Klein, G.R.; Rimnac, C.M.; Kurtz, S.M.; Kocagoz, S.B.; Gilbert, J.L. Corrosion damage and wear mechanisms in long-term retrieved cocr femoral components for total knee arthroplasty. J. Arthroplast. 2016, 31, 2900–2906. [Google Scholar] [CrossRef] [Green Version]
- Kretzer, J.P.; Reinders, J.; Sonntag, R.; Hagmann, S.; Streit, M.; Jeager, S.; Moradi, B. Wear in total knee arthroplasty—Just a question of polyethylene? Int. Orthop. 2014, 38, 335–340. [Google Scholar] [CrossRef] [Green Version]
- Luetzner, J.; Krummenauer, F.; Lengel, A.M.; Ziegler, J.; Witzleb, W.-C. Serum metal ion exposure after total knee arthroplasty. Clin. Orthop. Relat. Res. 2007, 461, 136–142. [Google Scholar] [CrossRef]
- Lons, A.; Putman, S.; Pasquier, G.; Migaud, H.; Drumez, E.; Girard, J. Metallic ion release after knee prosthesis implantation: A prospective study. Int. Orthop. 2017, 41, 2503–2508. [Google Scholar] [CrossRef]
- Oğurel, T.; Serbest, S.; Oğurel, R.; Tiftikçi, U.; Ölmez, Y. Blood chromium-cobalt levels in patients after total knee arthroplasty and their effect on the retinal nerve fiber layer and macular ganglion cell complex. Retina 2019. [Google Scholar] [CrossRef]
- Arnholt, C.M.; White, J.B.; Lowell, J.A.; Perkins, M.R.; Mihalko, W.M.; Kurtz, S.M. Postmortem retrieval analysis of metallosis and periprosthetic tissue metal concentrations in total knee arthroplasty. J. Arthroplast. 2020, 35, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Lord, J.K.; Langton, D.J.; Nargol, A.V.F.; Joyce, T.J. Volumetric wear assessment of failed metal-on-metal hip resurfacing prostheses. Wear 2011, 272, 79–87. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Roques, A.; Taylor, A.; Kwon, Y.-M.; McLardy-Smith, P.; Gill, H.S.; Walter, W.; Tuke, M.; Murray, D. The in vivo linear and volumetric wear of hip resurfacing implants revised for pseudotumor. JBJS 2011, 93, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Gascoyne, T.C.; Turgeon, T.R.; Burnell, C.D. Retrieval analysis of large-head modular metal-on-metal hip replacements of a single design. J. Arthroplast. 2018, 33, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Klasan, A.; Meine, E.; Fuchs-Winkelmann, S.; Efe, T.; Boettner, F.; Heyse, T.J. Are serum metal ion levels a concern at mid-term followup of revision knee arthroplasty with a metal-on-metal hinge design? Clin. Orthop. Relat. Res. 2019, 477, 2007–2014. [Google Scholar] [CrossRef] [PubMed]
- Friesenbichler, J.; Sadoghi, P.; Maurer-Ertl, W.; Szkandera, J.; Glehr, M.; Ogris, K.; Wolf, M.; Weger, C.; Leithner, A. Serum metal ion concentrations in paediatric patients following total knee arthroplasty using megaprostheses. BioMed Res. Int. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Favetti, F.; Mazzotta, G.; Papalia, M.; Panegrossi, G.; Casella, F.; Falez, F. Contamination of revision procedures in patients with adverse tissues reaction to metal on metal implant. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 86–93. [Google Scholar]
- Wyles, C.C.; Van Demark, R.E.; Sierra, R.J.; Trousdale, R.T. High rate of infection after aseptic revision of failed metal-on-metal total hip arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 509–516. [Google Scholar] [CrossRef] [Green Version]
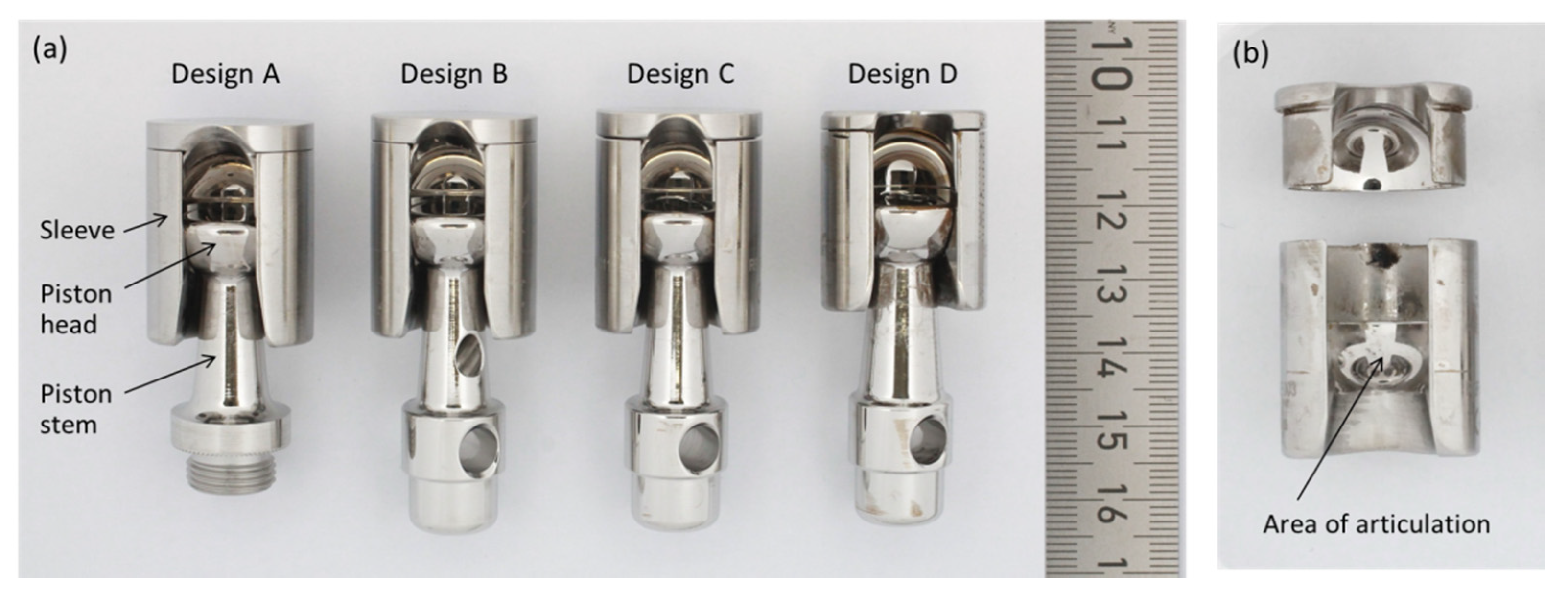
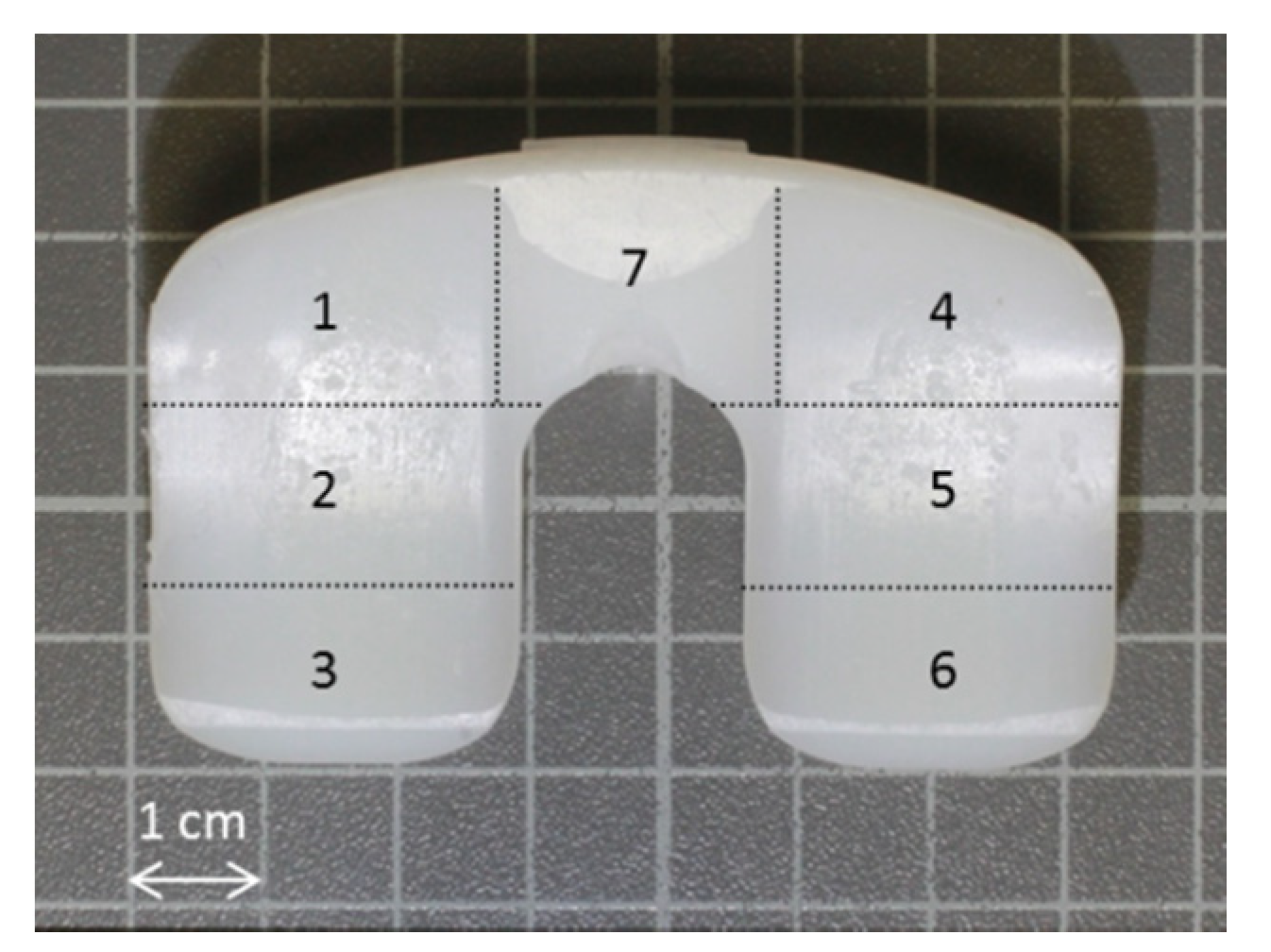
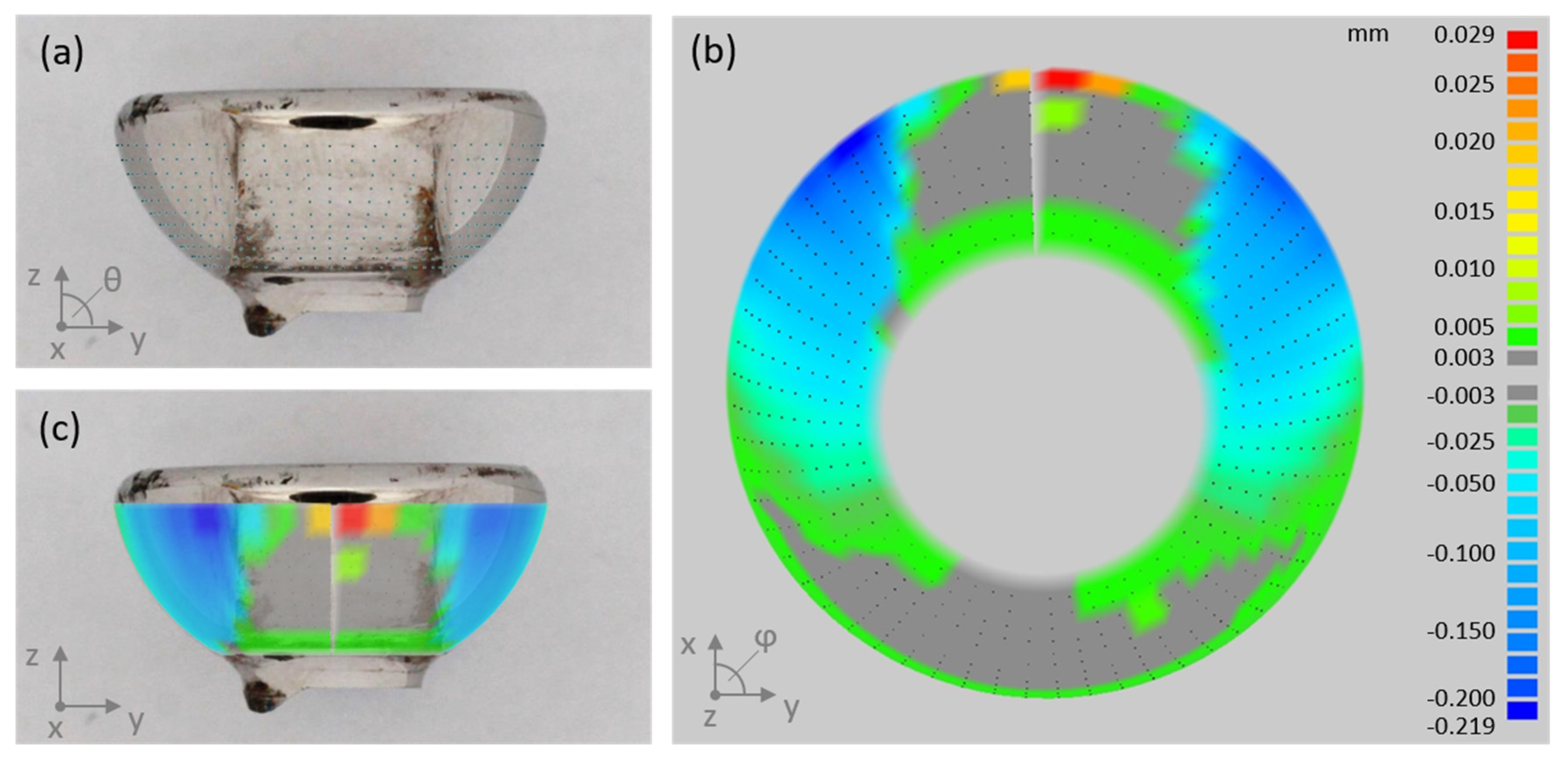
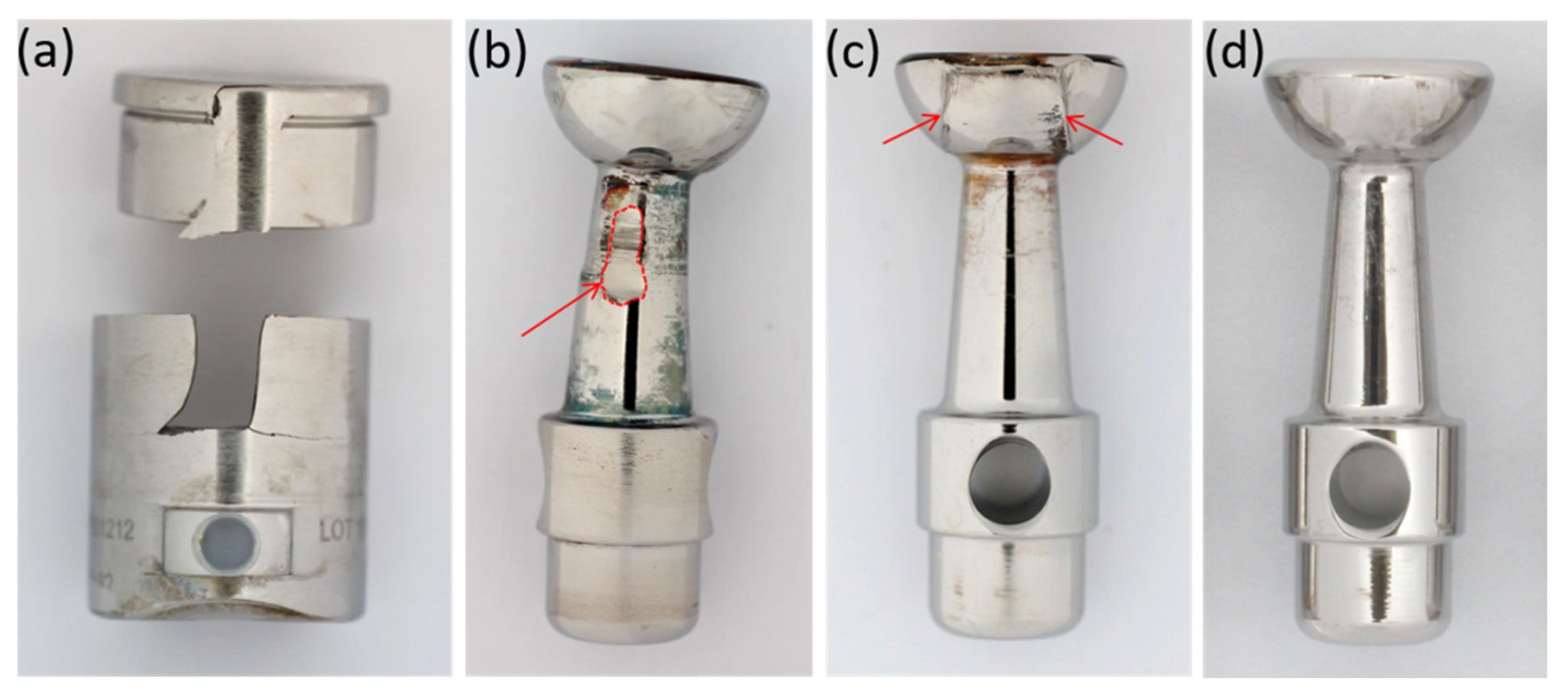

| Score | Sleeve (Each Quadrant) | Piston Head | Piston Stem |
|---|---|---|---|
| 0 | No damage | No damage | No damage |
| 1 | Scratches and/or polishing < 10% of the surface | Scratches visible | Scratches visible |
| 2 | Polishing 10–50% of the surface | Planar abrasion noticeable | Planar abrasion < 10% of the surface |
| 3 | Polishing > 50% of the surface | Planar abrasion with distinct edge on the surface | Planar abrasion > 10% of the surface |
| Number of Cases | Time in Vivo (months) | Total Wear Volume (mm3) | Wear Rate (mm3/year) | |
|---|---|---|---|---|
| n | mean (SD/range) | mean (SD/range) | mean (SD/range) | |
| Septic revision | 11 | 10.9 (10.1/0.4–33.2) | 3.5 (3.2/0.8–10.0) | 8.2 (10.7/0.6–39.1) |
| Aseptic revision | 9 | 34.3 (30.0/1.4–70.2) | 13.2 (9.2/0.5–30.0) | 6.7 (6.1/0.6–20.6) |
| p | − | <0.05 | <0.05 | 0.7 |
| All cases | 20 | 19.4 (18/0.4–70.2) | 7.9 (8.1/0.5–30.0) | 7.8 (8.6/0.6–39.1) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bormann, T.; Jäger, S.; Kretzer, J.P.; Nebel, L.; Clarius, L.; Omlor, G.; Bitsch, R.; Lehner, B. Retrieval Analysis of Modern Knee Tumor Megaendoprosthesis Shows Considerable Volumetric Metal Wear Generated at the Rotating Hinge. Materials 2020, 13, 1519. https://doi.org/10.3390/ma13071519
Bormann T, Jäger S, Kretzer JP, Nebel L, Clarius L, Omlor G, Bitsch R, Lehner B. Retrieval Analysis of Modern Knee Tumor Megaendoprosthesis Shows Considerable Volumetric Metal Wear Generated at the Rotating Hinge. Materials. 2020; 13(7):1519. https://doi.org/10.3390/ma13071519
Chicago/Turabian StyleBormann, Therese, Sebastian Jäger, J. Philippe Kretzer, Laura Nebel, Lucas Clarius, Georg Omlor, Rudi Bitsch, and Burkhard Lehner. 2020. "Retrieval Analysis of Modern Knee Tumor Megaendoprosthesis Shows Considerable Volumetric Metal Wear Generated at the Rotating Hinge" Materials 13, no. 7: 1519. https://doi.org/10.3390/ma13071519
APA StyleBormann, T., Jäger, S., Kretzer, J. P., Nebel, L., Clarius, L., Omlor, G., Bitsch, R., & Lehner, B. (2020). Retrieval Analysis of Modern Knee Tumor Megaendoprosthesis Shows Considerable Volumetric Metal Wear Generated at the Rotating Hinge. Materials, 13(7), 1519. https://doi.org/10.3390/ma13071519




