Modified Manganese Phosphate Conversion Coating on Low-Carbon Steel
Abstract
1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Preparation of the Standard Phosphating Bath
2.3. Preparation of Modified Phosphating Baths
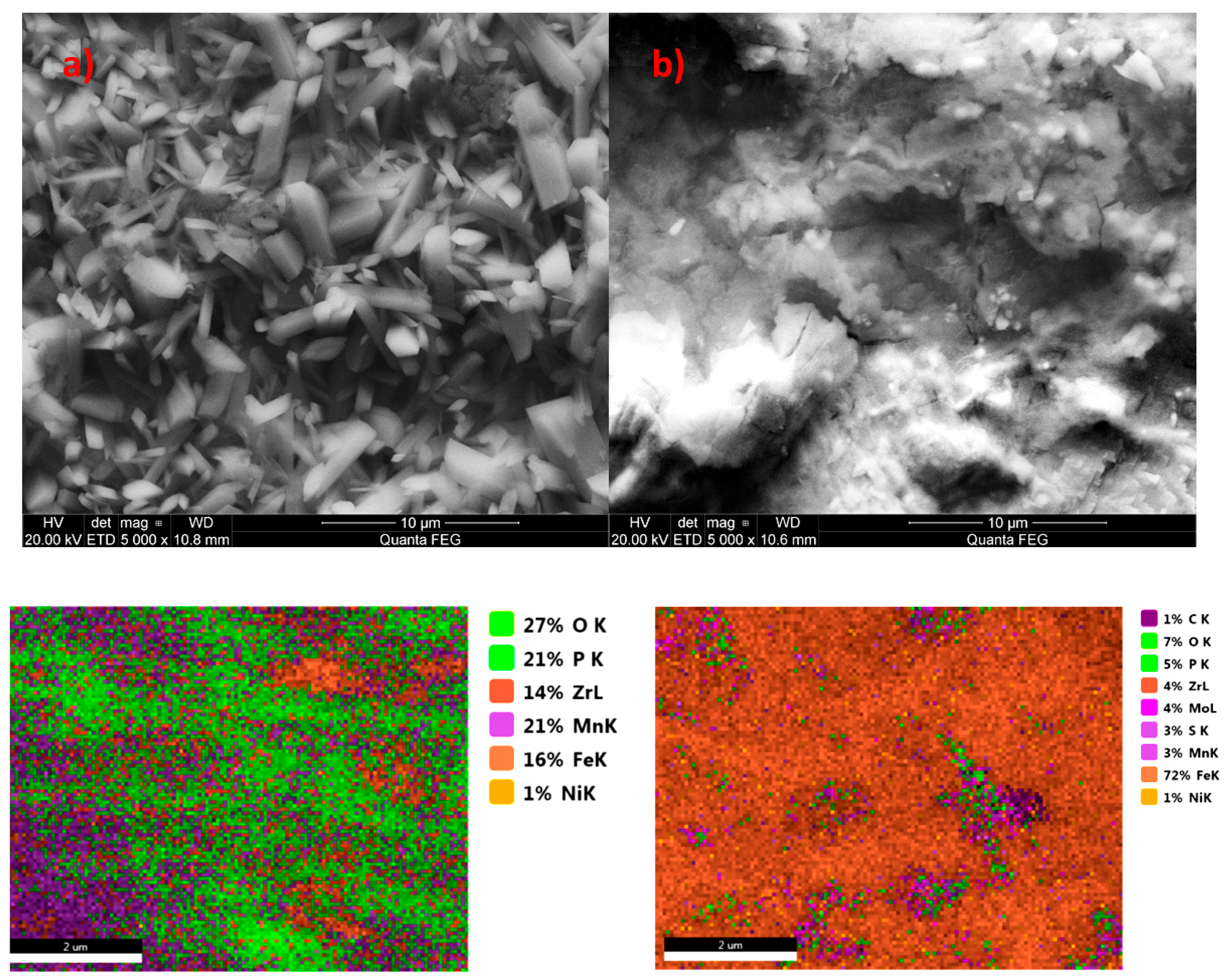
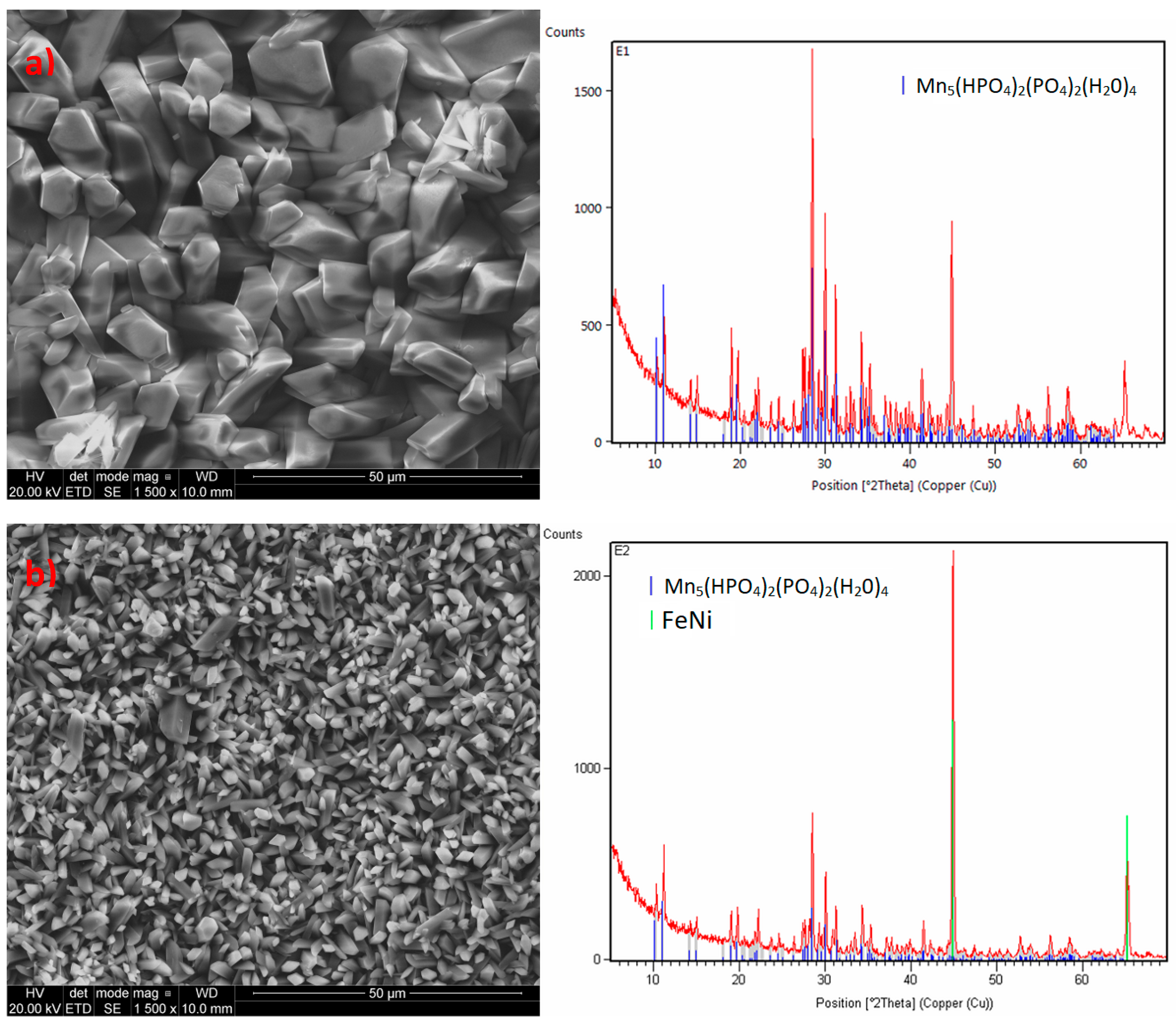
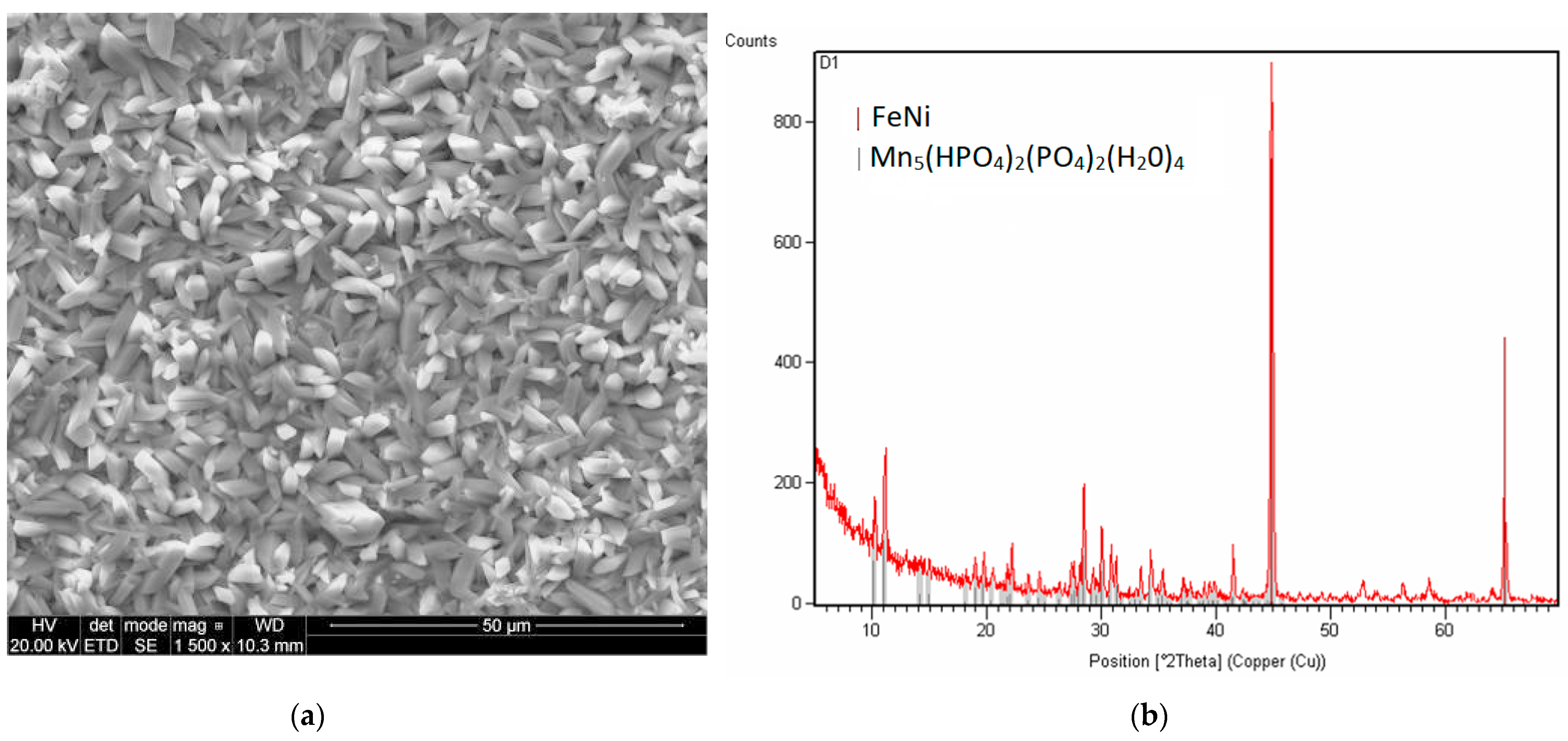
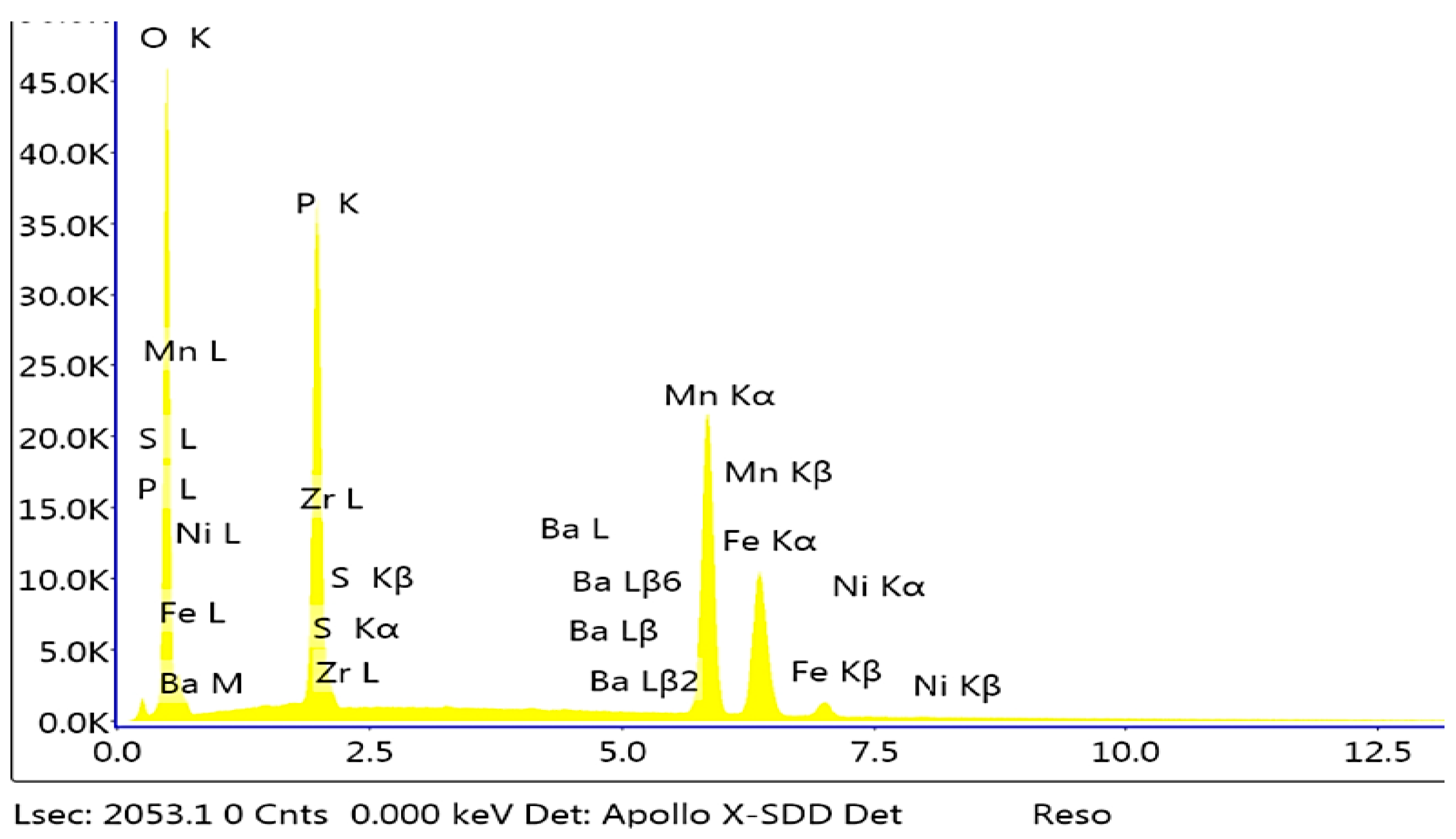
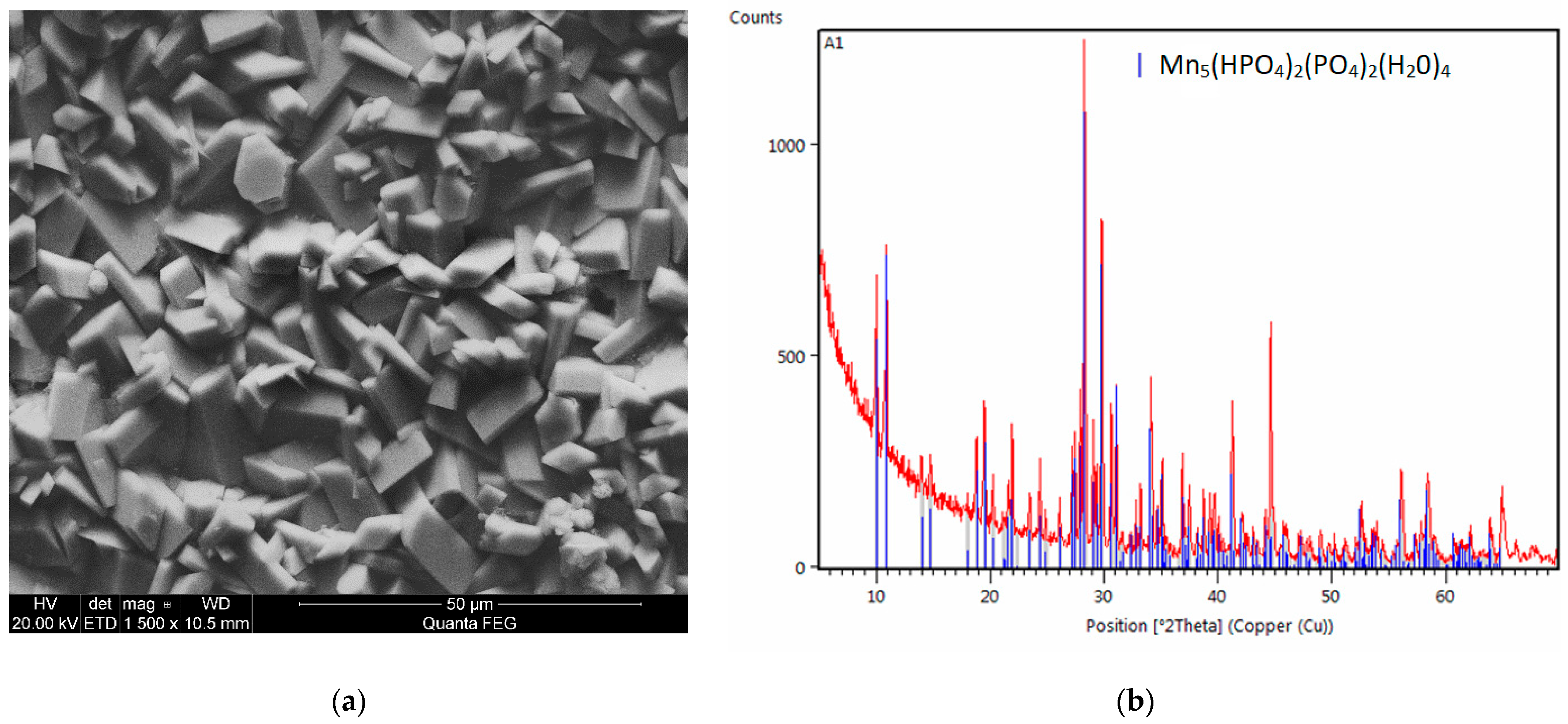
2.4. Microstructure of the Manganese Coating
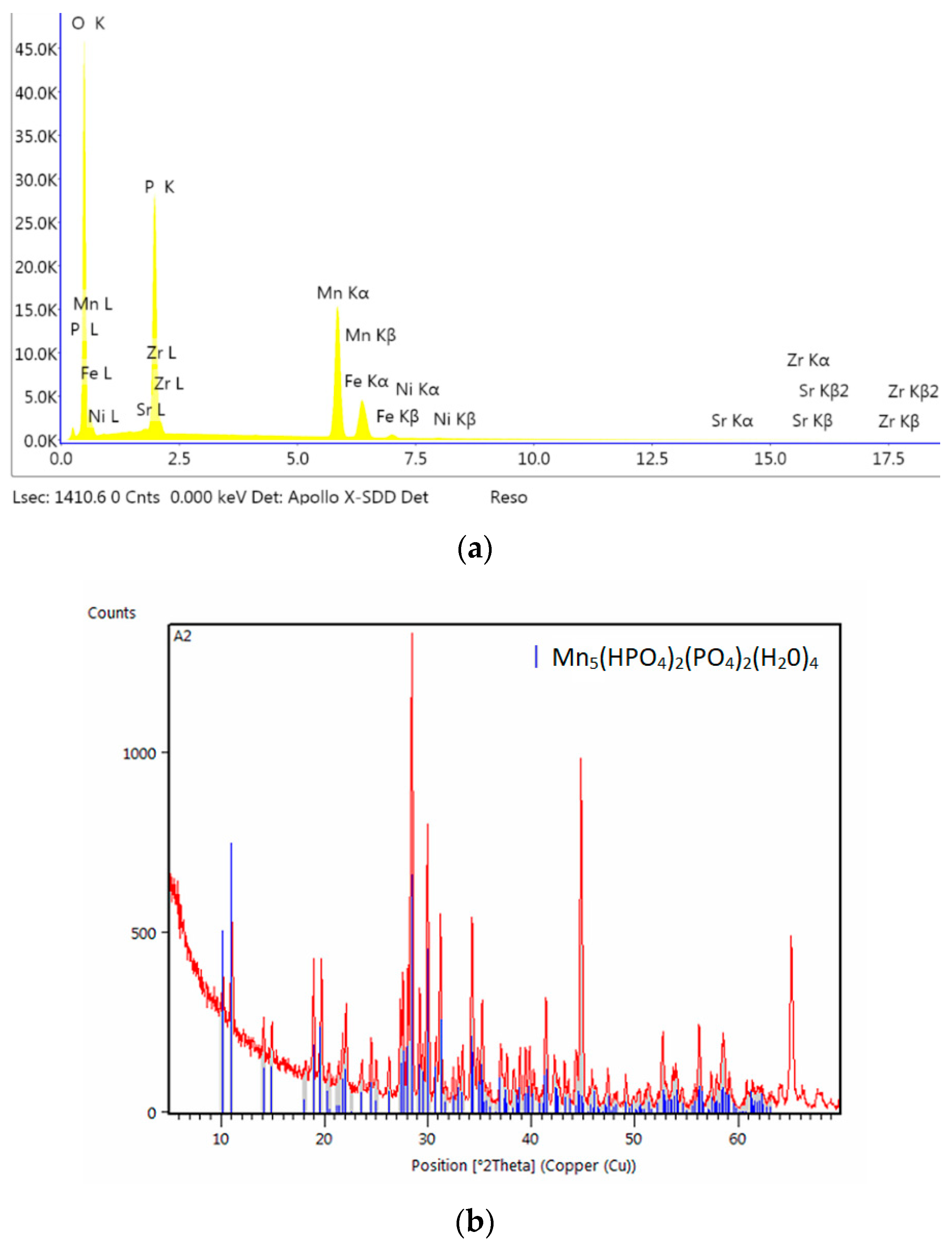
2.5. X-Ray Diffraction Analysis
2.6. Electrochemical Analysis
2.7. Integrity of the Coating
2.8. Coating Mass
3. Results and Discussion
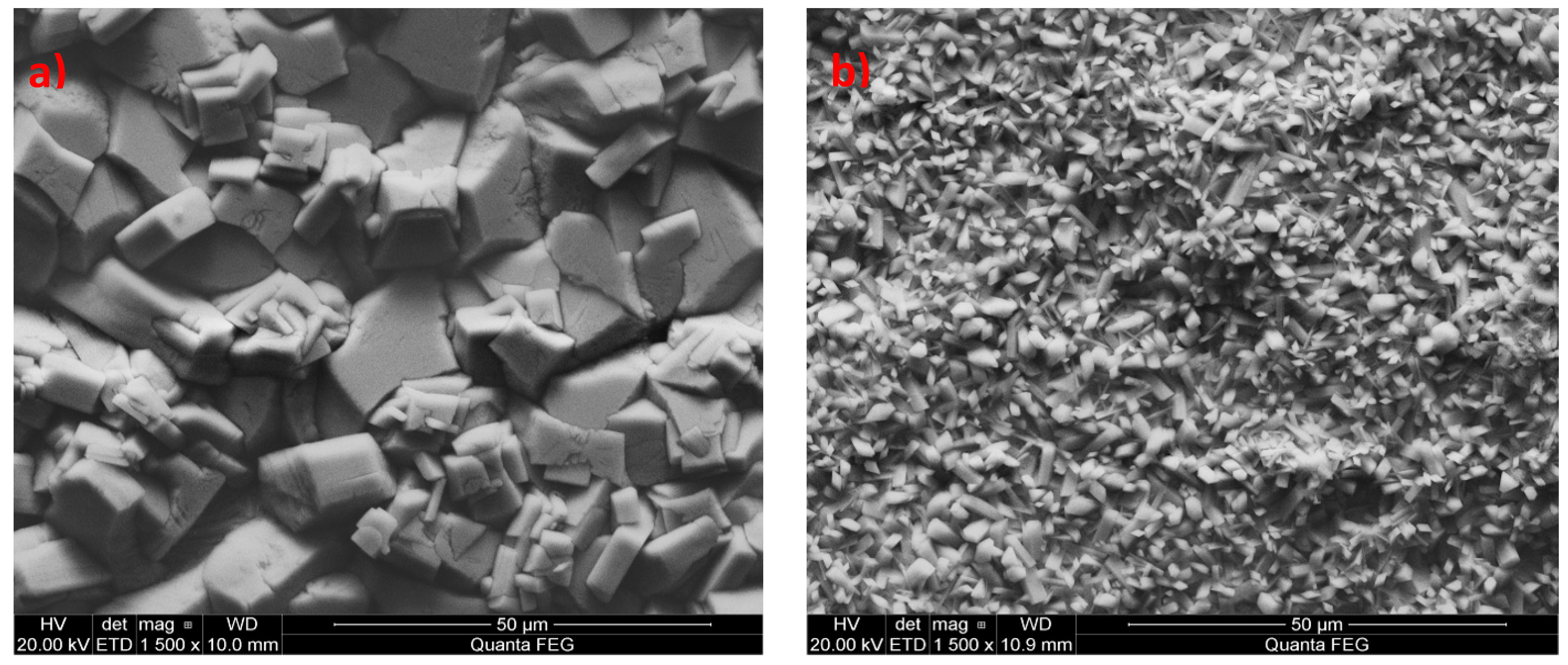
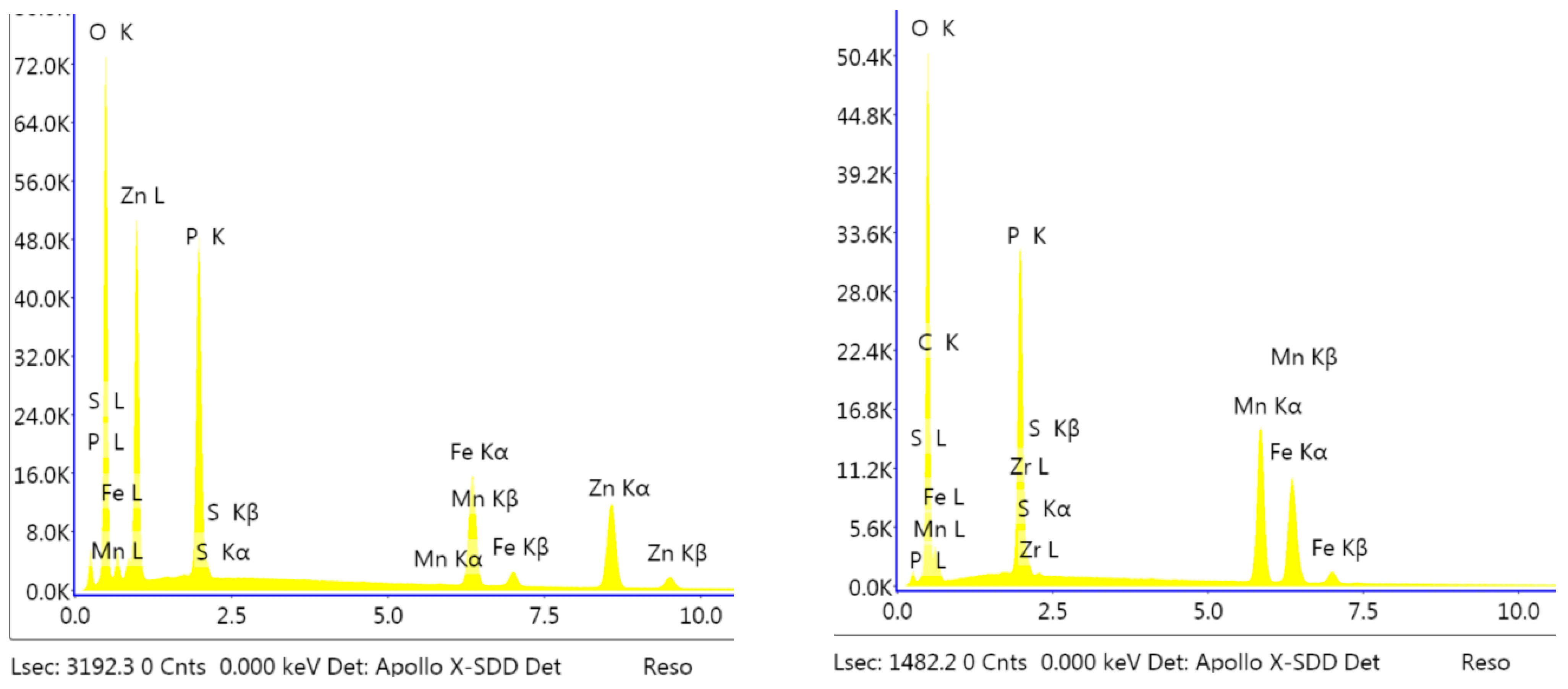
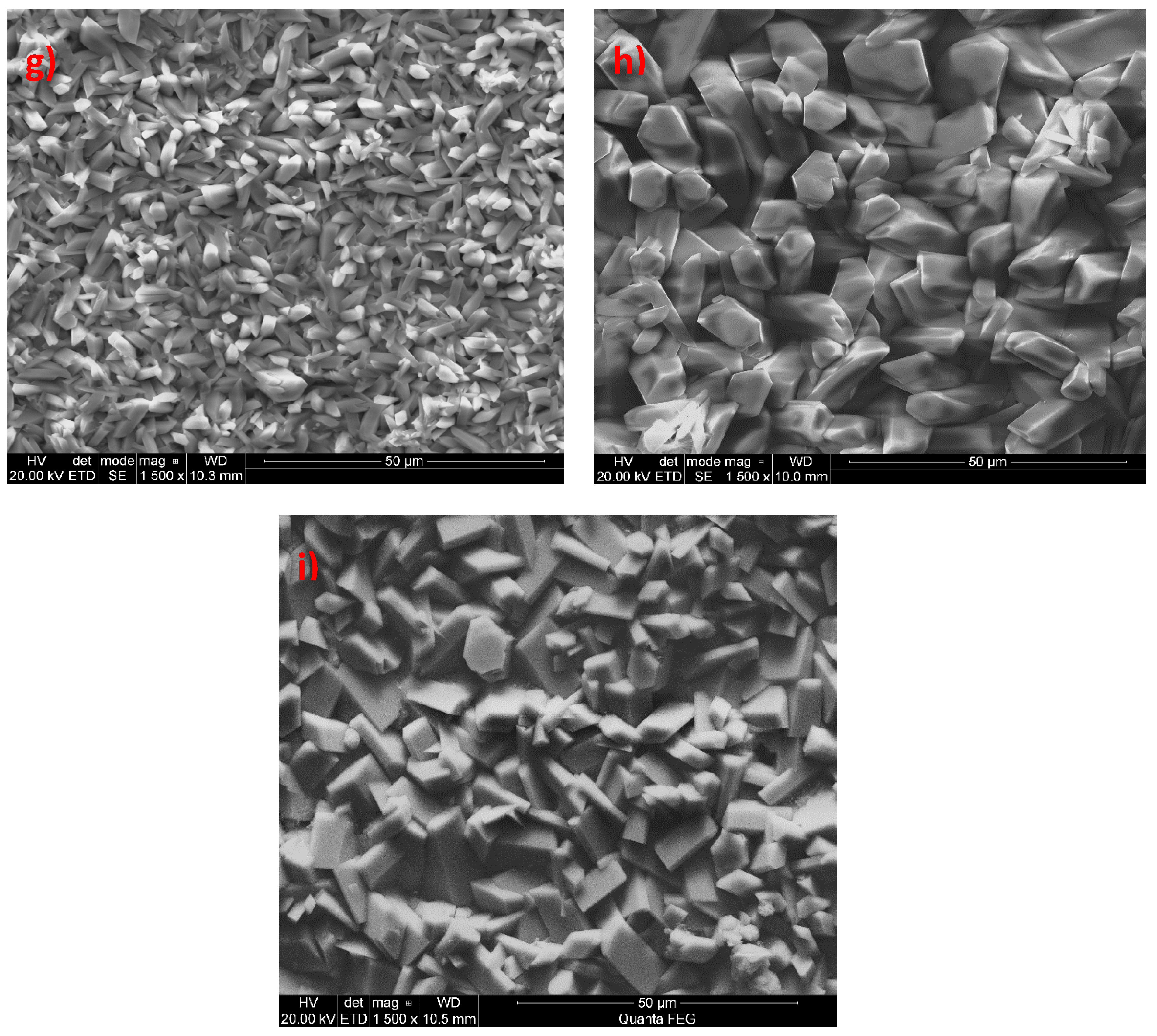
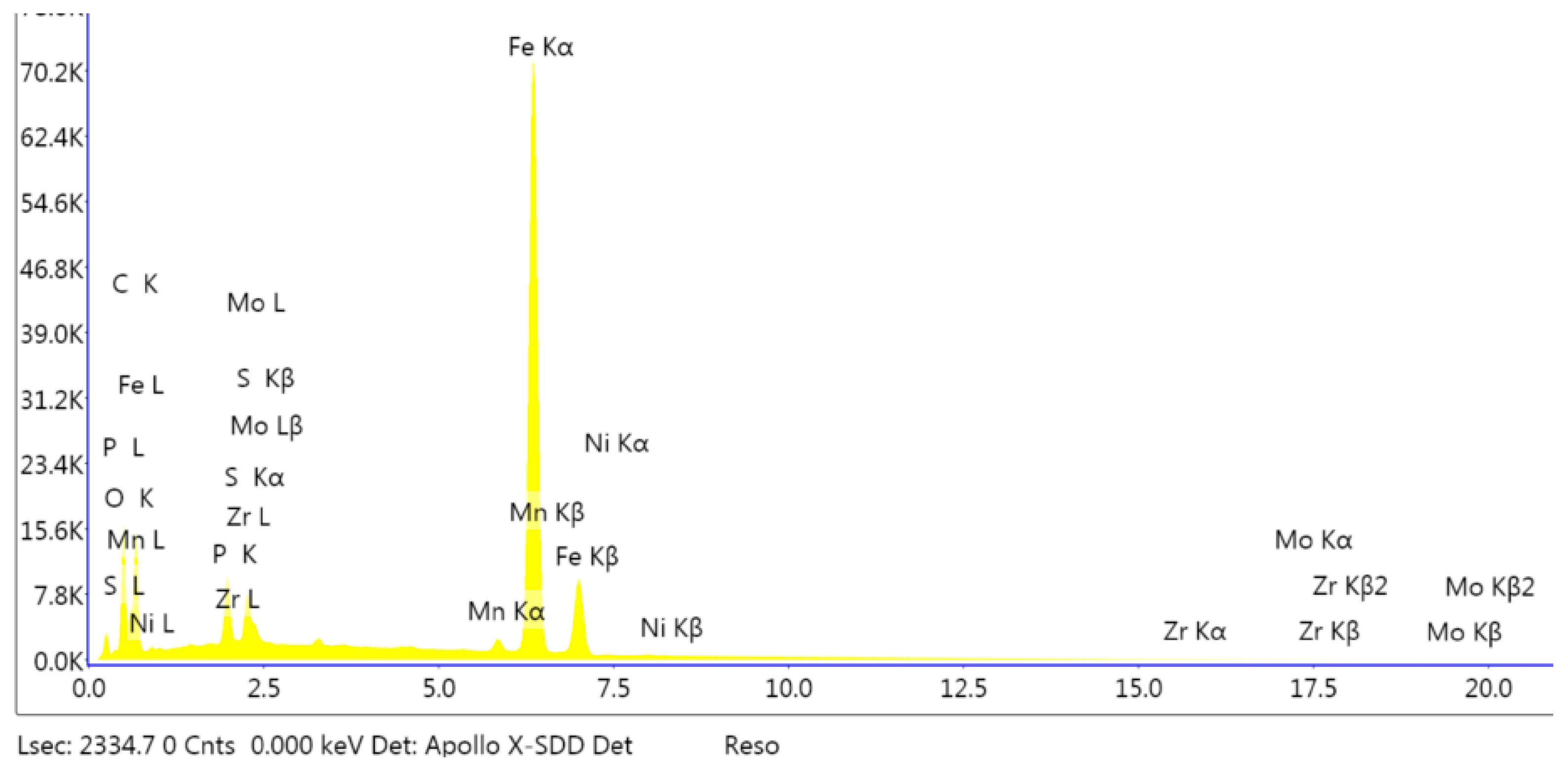
3.1. Analysis of the Morphology of the Samples
3.2. Impact of the Metal Additives on the Phosphating Bath
3.2.1. Impact of the Addition of Molybdenum
3.2.2. Impact of the Addition of Calcium
3.2.3. Impact of the Addition of Strontium
3.2.4. Impact of the Addition of Zinc
3.2.5. Impact of the Addition of Copper
3.2.6. Impact of the Addition of Cadmium
3.2.7. Impact of the Addition of Barium
3.2.8. Impact of the Addition of Cerium
3.3. Electrochemical Analyses
3.4. Integrity of the Coating
3.5. Coating Mass
- m1—mass in milligrams of the coated test sample;
- m2—mass in milligrams of the test sample after coating dissolution;
- A—area of the coated test sample in square centimeters (8 cm2).
4. Conclusions
- (1)
- The study found that the molybdenum compound had a harmful impact on the quality of the created phosphate coating.
- (2)
- Based on a comparison of the electrochemical results for the standard manganese sample, zinc sample, and modified manganese sample, it can be found that the addition of zinc to the manganese bath has a significant impact on corrosion resistance. The corrosion rate for the standard manganese sample is 7.58 CR/mpy, and for the manganese sample modified with zinc 0.37 CR/mpy.
- (3)
- The results of the integrity analysis indicate that the sample modified with molybdenum does not meet the criteria for integrity. The sample modified with the zinc compound has two colored spots on an area of 2 cm2, which means that this sample meets the integrity criterion. The sample modified with copper nitrate has six colored spots on an area of 2 cm2, which means that the sample does meet not the criterion for coating integrity. The remaining samples meet the coating integrity criterion.
- (4)
- The largest coating mass was recorded for the sample modified with barium (0.030 g/m2), and the smallest mass was observed in the sample modified with cerium (0.013 g/m2).
- (5)
- The chemical composition of the metallic substrate has a significant influence on the produced phosphate coating. The coating produced on chromium-molybdenum steel had worse visual quality and lower abrasion resistance.
- (6)
- Summarizing the results of SEM tests, electrochemical tests and XRD tests and measurements of integrity and coating mass, it was found that the best anti-corrosive properties were demonstrated by the phosphate manganese coating modified with zinc. The corrosion rate for the manganese phosphate coating is 7.58 CR/mpy, and for the coating modified with zinc 0.37 CR/mpy. Comparison of the mass of the manganese phosphate coating (0.010 g/m2) and the manganese coating modified with zinc (0.014 g/m2) indicated that the latter coating has the best protective properties.
Author Contributions
Funding
Conflicts of Interest
References and Notes
- Weng, D.; Jokiel, P.; Uebleis, A.; Boehni, H. Corrosion and protection characteristics of zinc and manganese phosphate coatings. Surf. Coat. Technol. 1996, 88, 147–156. [Google Scholar] [CrossRef]
- Chen, T.-T.; Ke, S.-T.; Liu, M.Y.; Hou, K.H. The study on Optimizing the Zinc Phosphate Conversion Coating Process and Its Corrosion Resistance. J. Chung Cheng Inst. Technol. 2006, 34, 1–11. [Google Scholar]
- Chen, X.-B.; Zhou, X.; Abbott, T.B.; Easton, M.A. Double-layered manganese phosphate conversion coating on magnesium alloy AZ91D: Insights into coating formation, growth corrosion resistance. Surf. Coat. Technol. 2013, 217, 147–155. [Google Scholar] [CrossRef]
- Nittel, K.-D.; Seifert, D.; Stickler, R. Method for Applying Manganese Phosphate Layers. U.S. Patent Appl. No.: 12/971,806, 14 April 2011. Filed: 17 December 2010. [Google Scholar]
- Boulos, M.S.; Montrose, D.C.; Michael, P. Moderate Temperature Manganese Phosphate Conversion Coating Composition and Process. U.S. Patent Appl. No.: 747,136, 17 March 1998. Filed: 12 November 1996. [Google Scholar]
- Wang, C.M.; Liau, H.C.; Tsai, W.T. Effects of temperature and applied potential on the microstructure and electrochemical behavior of manganese phosphate coating. Surf. Technol. 2006, 201, 2994–3001. [Google Scholar] [CrossRef]
- Westberg, H.J.; Nilsson, P.H.; Rosén, B.G.; Stenbom, B. Manganese Phosphating of Gears and Surface Roughness Consequence; Chalmers University of Technology, Department of Production Engineering, Volvo Technological Development: Gothenburg, Sweden, 2000. [Google Scholar]
- Duszczyk, J.; Siuzdak, K.; Klimczuk, T.; Strychalska-Nowak, J.; Zaleska-Medynska, A. Manganese Phosphatizing Coatings: The Effects of Preparation Conditions on Surface Properties. Materials 2018, 11, 2585. [Google Scholar] [CrossRef] [PubMed]
- Zang, L.; Chen, Y.; Ran, L.; Zheng, Y.; Li, K.; Ju, D. Comparative Study on the Tribological Properties of AISI52100 Steel Surface Modified by Graphite/MoS2 Composite Coating and Manganese Phosphate Coating. Mater. Sci. 2019, 25, 383–393. [Google Scholar]
- Noh, Y.-T.; Byoun, Y.-M.; Park, J.-K.; Seo, S.-K.; Hyun, S.-K.; Lee, C.-H. Effect of the Manganese Phosphate Solution with Additive Agent of Tartaric Acid. J. Nanosci. Nanotechnol. 2018, 18, 1152–1157. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Susac, D.; Li, R.; Wong, K.C.; Foster, T.; Mitchell, K.A.R. Some observations for effects of copper on zinc phosphate conversion coatings on aluminum surfaces. Surf. Coat. Technol. 2002, 155, 46–50. [Google Scholar] [CrossRef]
- Li, Q.; Xu, S.; Hu, J.; Zhang, S.; Zhong, X.; Yang, X. The effects to the structure and electrochemical behavior of zinc phosphate conversion coatings with ethanolamine on magnesium alloy AZ91D. Electrochim. Acta 2010, 55, 887–894. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Du, Y. Effect of sodium silicate pretreatment on phosphate layer: Morphology and corrosion resistance behavior. Mater. Corros. 2012, 63, 317–322. [Google Scholar] [CrossRef]
- Winiarski, J.; Szczygieł, B. Odporność na korozję powłok bezchromowych otrzymanych z kąpieli na bazie H2ZrF6 modyfikowanej dodatkiem 3-aminopropylotrietoksysilanu. Ochr. Przed Korozją 2012, 11, 541–543. [Google Scholar]
- Winiarski, J.; Szczygieł, B. Właściwości ochronne powłok otrzymanych z roztworów na bazie silanów oraz związków tytanu(IV). Ochrona przed Korozją 2011, 54, 446–448. [Google Scholar]
- Shibli, S.M.A.; Chacko, F. Development of nano TiO2-incorporated phosphate coatings on hot dip zinc surface for good paintability and corrosion resistance. Appl. Surf. Sci. 2011, 257, 3111–3117. [Google Scholar] [CrossRef]
- Yingsamphancharoen, T.; Srisuwan, N.; Rodchanarowan, A. The electrochemical investigation of the corrosion rates of welde pipe. ASTM A106 Grade B. Metals 2016, 6, 207. [Google Scholar] [CrossRef]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef]
- ASTM. Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements (G 102-89 (Reapproved)); ASTM: West Conshohocken, PA, USA, 1994. [Google Scholar]
- Polska Norma (Polish Norm) PN-81 H97016 Ochrona przed korozją. Powłoki fosforanowe.
- Norma EN ISO 3892:2001. Conversion Coatings on Metallic Materials—Determination of Coating Mass Per Unit Area—Gravimetric Methods; BSI: London, UK, 2001.
- ISO 683-1. Paper Version. Available online: https://www.iso.org/obp/ui/#iso:std:iso:683:-1:ed-3:v1:en (accessed on 19 March 2020).
- Polish Norm PN-EN 10277:2018-09. Wyroby Stalowe o Powierzchni Jasnej—Warunki Techniczne Dostawy; Paper Version; 2018.
- Polish Norm PN-EN 10025-2:2007. Wyroby Walcowe na Gorąco ze Stali Konstrukcyjnej. Część 2: Warunki Techniczne; Paper Version; 2007.
- Roland, W.-A.; Gottwald, K.H. Nickel-Free Phosphating Process. U.S. Patent 5,792,283, 11 August 1998. [Google Scholar]
- Roland, W.-A.; Gottwald, K.H. Nickel-Free Phosphating Process. U.S. Patent 6,197,126, 6 March 2001. [Google Scholar]
- Ungun, A. Corrosion inhibition behavior of robiniapseudoacacia Leaves extract as a Eco-friendly inhibitor on mild steel in acidic media. Met. Mater. Int. 2019. [Google Scholar] [CrossRef]




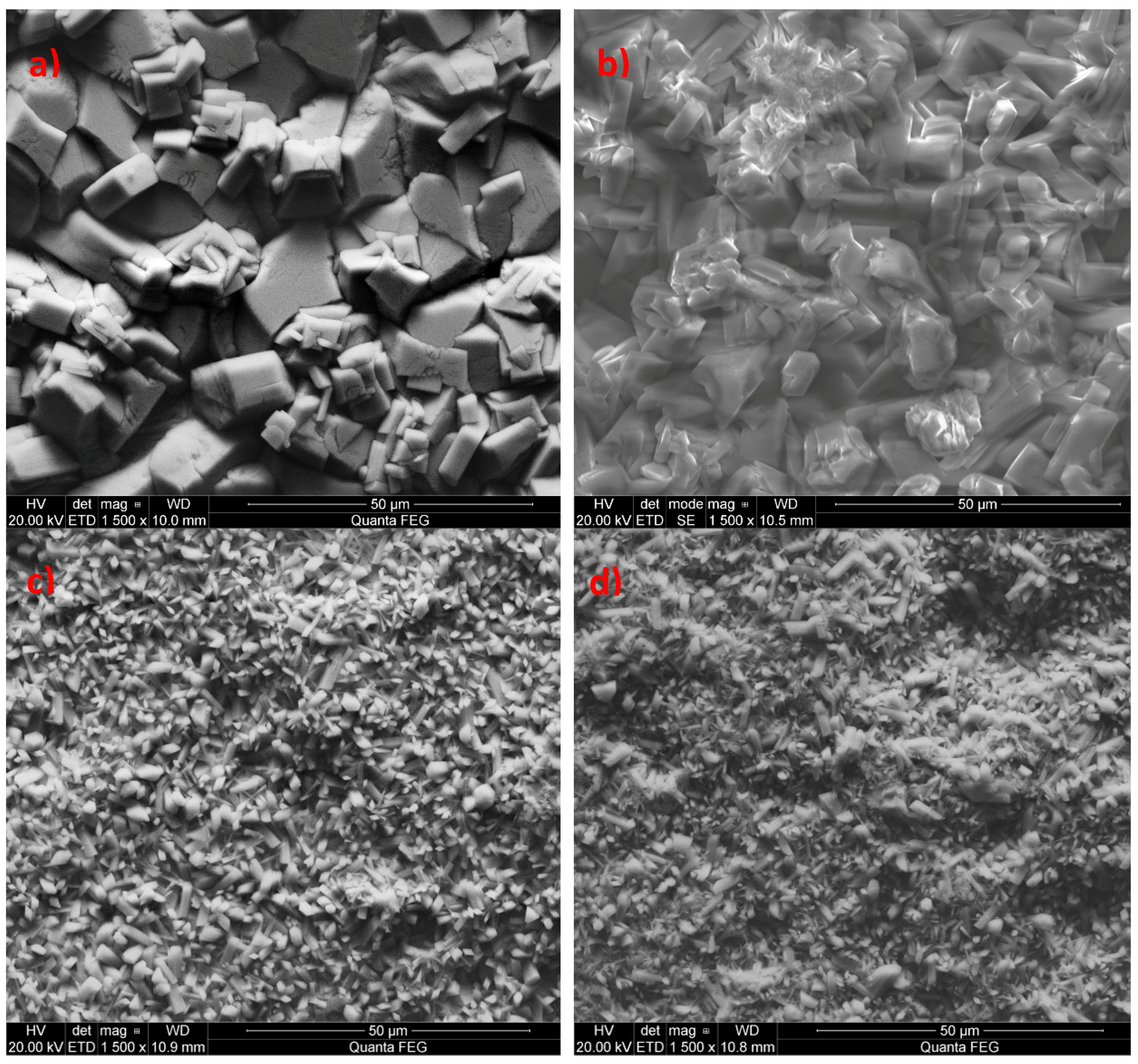
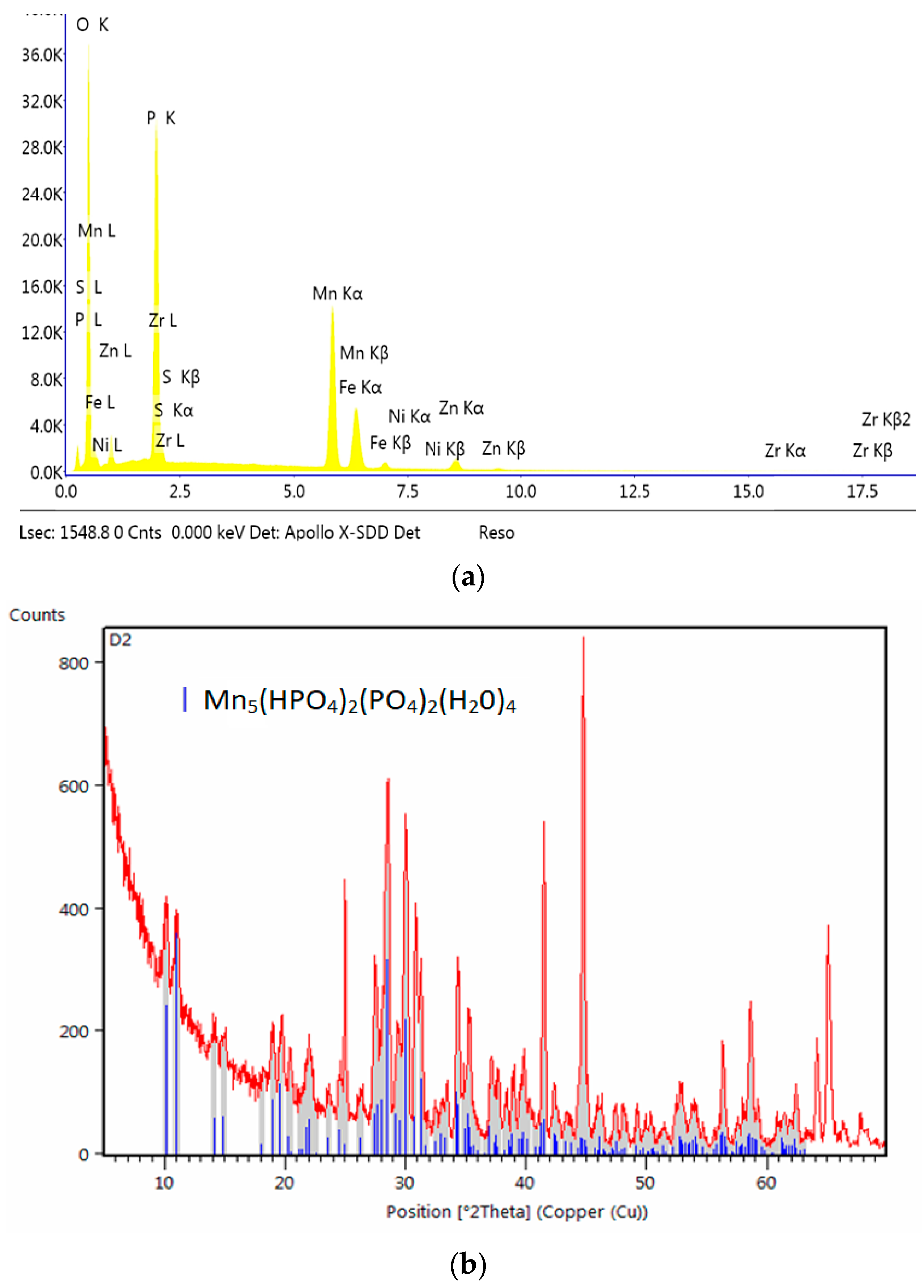
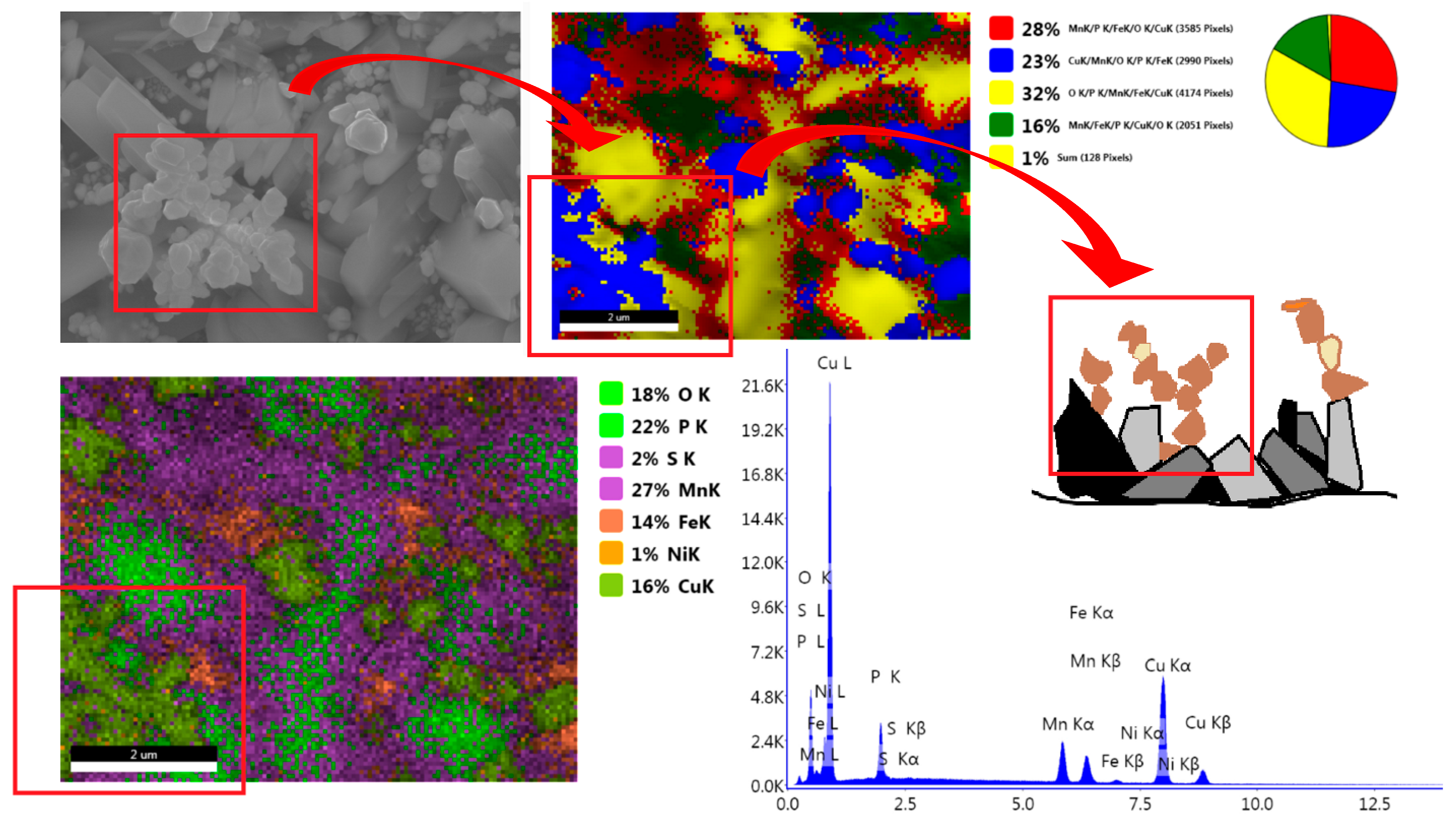
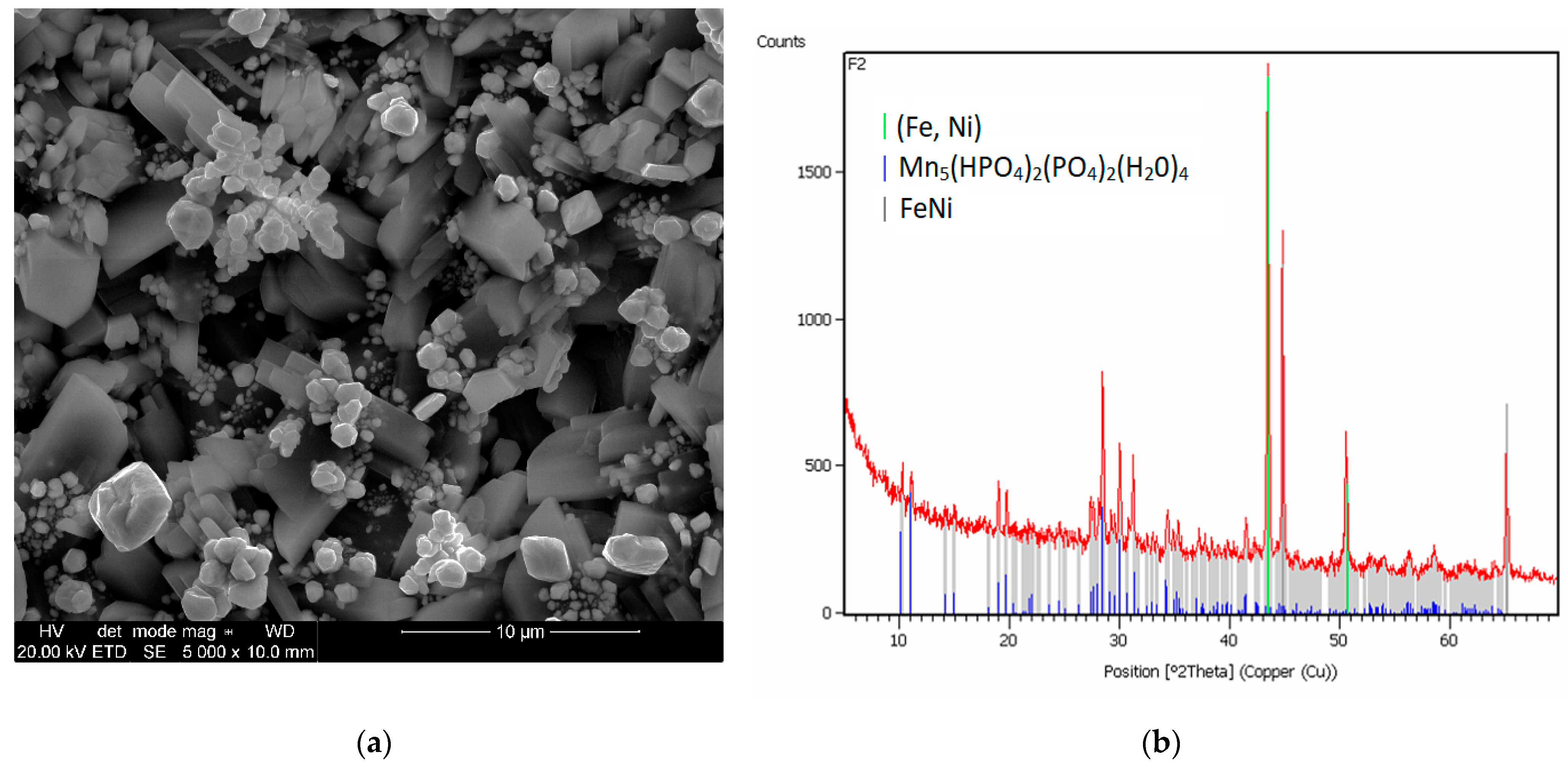



| Element | C | Si | Mn | P | S | Cr | Mo | Ni | Nb | Al | Cu |
| Weight Content (%) | 0.034 | 0.009 | 0.220 | 0.009 | 0.007 | 0.040 | 0.007 | 0.012 | 0.011 | 0.026 | 0.041 |
| Element | Co | B | Ti | W | Ca | Sn | Pb | Sb | Te | As | Fe |
| Weight Content (%) | 0.002 | <0.0001 | 0.010 | 0.008 | 0.001 | 0.001 | 0.006 | 0.008 | 0.001 | 0.001 | 99.545 |
| Stage No. | Process Type | Composition of Solution | Process Conditions |
|---|---|---|---|
| 1 | Degreasing | 10% (m/m) solution of NaOH 0.05% (m/m) sodium dodecyl sulfate (SLS) | mixing, temperature: 80–85 °C, 5 min. |
| 2 | Rinsing | Distilled water | mixing, room temperature, 3 min. |
| 3 | Etching | Solution of HCl 15% (m/m), 1–10 mL of 3% (m/m) H2O2, 0.05 g 1,2,3-benzotriazole | mixing, room temperature, 3 min. |
| 4 | Rinsing | Distilled water | mixing, room temperature, 1 min. |
| 5 | Activating | MnHPO4: 2 g Na4P2O7: 2 g H2O: 500 g | mixing, temperature: 40–45 °C, 4 min |
| 6 | Phosphating | H3PO4 (85%): 7.0 g Mn3(PO4)2: 15.0 g Mn(NO3)2: 6.0 g MnCO3: 0.5 g Ni(NO3)2: 0.3 g H2O: 531.5 g 1-methyl-3-nitroguanidine: 0.5 g | mixing, filtrated temperature: 95 °C, 15 min. |
| 7 | Rinsing | Distilled water | room temperature 3 min. |
| 8 | Passivation | ZrOCl2: 0.3 g Mn(NO3)2: 1.1 g NaNO3: 0.14 g HNO3: 0.14 g CH3OH: 0.14 g Na2CO3: 5.4 g H2O: 100 g | mixing, 25–30 °C, 2 min. |
| 9 | Conservation with oil | Solution of mineral oil solution of mineral, emulsifying with water oil (ZWEZ 4999) produced by ZWEZ. | mixing, 75 °C, 2 min. |
| Compound | Content (g) |
|---|---|
| H3PO4 (25% m/m solution) | 40.0 |
| Mn3(PO4)2 | 25.0 |
| Mn(NO3)2 | 10.0 |
| H2O | 25.0 |
| Ni(NO3)2 | 0.1 |
| 1-methyl-3-nitroguanidine (or nitroguanidine) | 1.0 |
| Ba-Ni-Mn Solution | Zn-Ni-Mn Solution | Cd-Mn Solution | Cd-Ni-Mn Solution | Mo-Ni-Mn Solution |
| H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g Ba(NO3)2: 1.0023 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g ZnO: 0.30 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Fe: 0.2 g H2O: 125 g CdO: 0.10 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g CdO: 0.10 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g Na2MoO4: 0.30 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. |
| Cu-Ni-Mn Solution | Ce-Ni-Mn Solution | Sr-Ni-Mn Solution | Ca-Ni-Mn Solution | Standard Bath Composition |
| H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g Cu(NO3)2: 0.2 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g Ce(NO3)2: 1.0 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g Sr(NO3)2: 2.5014 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g CaCO3: 0.10 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. | H3PO4: 5.2 g Mn3(PO4)2: 3.25 g Mn(NO3)2: 1.3 g MnCO3: 0.5 g Ni(NO3)2: 0.1 g Fe: 0.2 g H2O: 125 g 1-methyl-3-nitroguanidine: 0.3 g 95–98 °C, 15 min. |
| Modifying Substance | Average Crystal Size (µm) | Structure |
|---|---|---|
| Ba(NO3)2 | <5 | Large crystallites with oval edges |
| CaCO3 | <5 | Crystallites have the shape of sharp-tipped prisms |
| Ce(NO3)2 | <25 | Prismatic crystallites with distinct edges |
| Cu(NO3)2 | <10 | Spherical copper crystallites coated on manganese phosphate crystals |
| Na2MoO4 | N/A | Not uniform structure, made up of several superimposed layers |
| Sr(NO3)2 | <10 | Multilayer lamellar crystallites with a regular, prism-like structure |
| CdO − Ni(NO3)2 | <10 | Crystals in the form of sharp-tipped thick prisms |
| CdO + Ni(NO3)2 | 10–15 (1–5 for aggregates) | Crystals in the form of sharp-tipped thick prisms with oval shapes; in some points, there are aggregates of small needles and ball-shaped crystals |
| ZnO | <20 | Crystals have irregular shapes with large voids between them |
| Zn-reference | <10 | Crystals in the form of irregular polyhedra |
| Mn-reference | <5 | Crystals in the form of needles |
| Stal 42CrMoS4 + QT | |||||||
| C | Si | Mn | P | S | Cr | Mo | Ni |
| 0.38–0.45 | max 0.40 | 0.6–0.9 | max 0.025 | 0.02–0.04 | 0.9–1.2 | 0.15–0.30 | max 0.40 |
| Stal 235JR | |||||||
| max 0.17 | - | max 1.40 | max 0.035 | max 0.035 | - | - | - |
| Modyfying Substance | Corrosion Potential (Ecor/V) | Current Density (jcor/μAcm−2) | Corrosion Rate (CR/mpy) |
|---|---|---|---|
| Ba(NO3)2 | −0.344 | 1.88 | 0.87 |
| CaCO3 | −0.337 | 3.50 | 1.62 |
| Ce(NO3)2 | −0.343 | 4.84 | 2.24 |
| Cu(NO3)2 | −0.504 | 47.4 | 21.90 |
| Sr(NO3)2 | −0.410 | 4.72 | 2.18 |
| CdO − Ni(NO3)2 | −0.418 | 4.96 | 2.29 |
| CdO + Ni(NO3)2 | −0.341 | 1.30 | 0.60 |
| ZnO | −0.474 | 0.811 | 0.37 |
| Zn-reference | −0.365 | 0.251 | 0.11 |
| Mn-reference | −0.536 | 16.4 | 7.58 |
| Modifying Substance | Sample Mass before the Measurement (g) | Sample Mass after Etching (g) | Coating Mass (g/m2) |
|---|---|---|---|
| Ba(NO3)2 | 2.4879 | 2.4637 | 0.03025 |
| CaCO3 | 2.6173 | 2.6044 | 0.016125 |
| Ce(NO3)2 | 2.8642 | 2.8538 | 0.013 |
| Cu(NO3)2 | 2.9664 | 2.9446 | 0.02725 |
| Sr(NO3)2 | 2.4373 | 2.4288 | 0.010625 |
| CdO − Ni(NO3)2 | 2.1588 | 2.1503 | 0.010625 |
| CdO + Ni(NO3)2 | 2.5747 | 2.5641 | 0.01325 |
| ZnO | 2.4288 | 2.4169 | 0.014875 |
| Zn-reference | 2.2019 | 2.1953 | 0.00825 |
| Mn-reference | 2.5920 | 2.5834 | 0.01075 |
| Mn-reference with mineral oil | 2.7351 | 2.7220 | 0.016375 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duszczyk, J.; Siuzdak, K.; Klimczuk, T.; Strychalska-Nowak, J.; Zaleska-Medynska, A. Modified Manganese Phosphate Conversion Coating on Low-Carbon Steel. Materials 2020, 13, 1416. https://doi.org/10.3390/ma13061416
Duszczyk J, Siuzdak K, Klimczuk T, Strychalska-Nowak J, Zaleska-Medynska A. Modified Manganese Phosphate Conversion Coating on Low-Carbon Steel. Materials. 2020; 13(6):1416. https://doi.org/10.3390/ma13061416
Chicago/Turabian StyleDuszczyk, Jakub, Katarzyna Siuzdak, Tomasz Klimczuk, Judyta Strychalska-Nowak, and Adriana Zaleska-Medynska. 2020. "Modified Manganese Phosphate Conversion Coating on Low-Carbon Steel" Materials 13, no. 6: 1416. https://doi.org/10.3390/ma13061416
APA StyleDuszczyk, J., Siuzdak, K., Klimczuk, T., Strychalska-Nowak, J., & Zaleska-Medynska, A. (2020). Modified Manganese Phosphate Conversion Coating on Low-Carbon Steel. Materials, 13(6), 1416. https://doi.org/10.3390/ma13061416





