1. Introduction
Bismuth vanadate is one of the most promising candidates for visible light photoelectrochemical water splitting [
1]. It is characterized by a moderated energy band gap [
2] and high absorption coefficient [
3] allowing material to be efficiently excited by visible light. The appropriate flat band potential makes BiVO
4 a good candidate as a photoanode for efficient water oxidation [
4]. It was estimated that BiVO
4 illuminated by AM1.5 solar light should generate 7.5 mA per cm
2 [
5]. The theoretical efficiency of water photooxidation has not been achieved yet. This is related to the adverse phenomena such as bulk e
−/h
+ recombination due to the poor hole mobility and recombination on the surface states. There are many literature reports about BiVO
4 modifications that lead to the enhancement of its performance [
6]. The most common ways consists of Mo and W doping [
7] and heterostructures formation [
8]. Up to now, the highest photocurrent densities were achieved for bulk (i) W-doped BiVO
4/V
2O
5 heterojunction (6.6 mA cm
−2) and (ii) electrode material that was prepared on a scaffold of polystyrene spheres scaffold and consisted of WO
3 and molybdenum doped BiVO
4 nanoparticles (5.8 mA cm
−2), both covered by a FeOOH/NiOOH dual layer catalyst [
9,
10]. However, the theoretical value of the photocurrent should be possible to achieve for “bare” BiVO
4. Thus, it is still worthwhile to focus on the optimization of BiVO
4 synthesis and layer preparation. In the literature, one can find different techniques to obtain bismuth vanadate films acting as photoelectrodes, e.g., electrostatic spray pyrolysis [
11], deposition of inverse opals using polystyrene spheres as a template [
12], simple dip-coating method [
13], polymer-assisted spin coating [
14], and most promising pulsed laser deposition technique (PLD) [
15]. The PLD is a common method of photoactive material deposition [
16]. The sputtering conditions during PLD strongly affect the properties of the resulting layer [
17]. It was shown that pulsed laser deposited BiVO
4 films on FTO substrates obtained at 230 °C can generate high photocurrents equal to about 3 mA cm
−2, but only in the hole-scavenger containing electrolyte [
18]. Other authors showed that the high temperature (500 °C) in the deposition chamber makes the growth of BiVO
4 crystallites anisotropic [
19]. The specific orientation and (001) facet exposition facilitates the photoexcited charge carriers transport through the layer and enhance the efficiency of photocurrent generation. A similar effect of BiVO
4 anisotropic growth was observed for the films deposited at 550 °C on the yttria-stabilized zirconia (YSZ), however the activity of the electrodes in water photooxidation reaction was unsatisfactory [
20]. Since the ambient gas pressure strongly affects the expansion dynamic of plasma plume, one may expect different properties of films deposited under different pressures [
21]. Indeed, it was reported that the PLD technique can be applied in order to form type-II heterojunction (BiVO
4/Bi
4V
2O
11) on specifically oriented substrates using stoichiometric BiVO
4 as a target and the composition of a resulting film can be tuned by changing oxygen pressure [
22]. The presence of oxygen in the deposition chamber is crucial to obtain metal oxides films using PLD. The deviations from stoichiometry were observed, e.g., for ZnO [
23]. Authors showed that Zn:O ratio was close to one only in the case of the deposition performed in oxygen atmosphere, and the oxygen depletion was observed for process performed in Ar and in vacuum. Oxygen deficiency was also reported for SiO
2 films deposited in vacuum [
24]. Thus, in order to avoid this effect, all depositions of BiVO
4 layers were carried in oxygen atmosphere. Some authors claimed that the excess of Bi in the target is necessary to get stoichiometric BiVO
4 films on YSZ substrates [
25]. The PLD technique carried out by sequential ablation was also utilized in order to prepare BiVO
4/WO
3/SnO
2 heterojunction, however deposition parameters were not thoroughly investigated [
26].
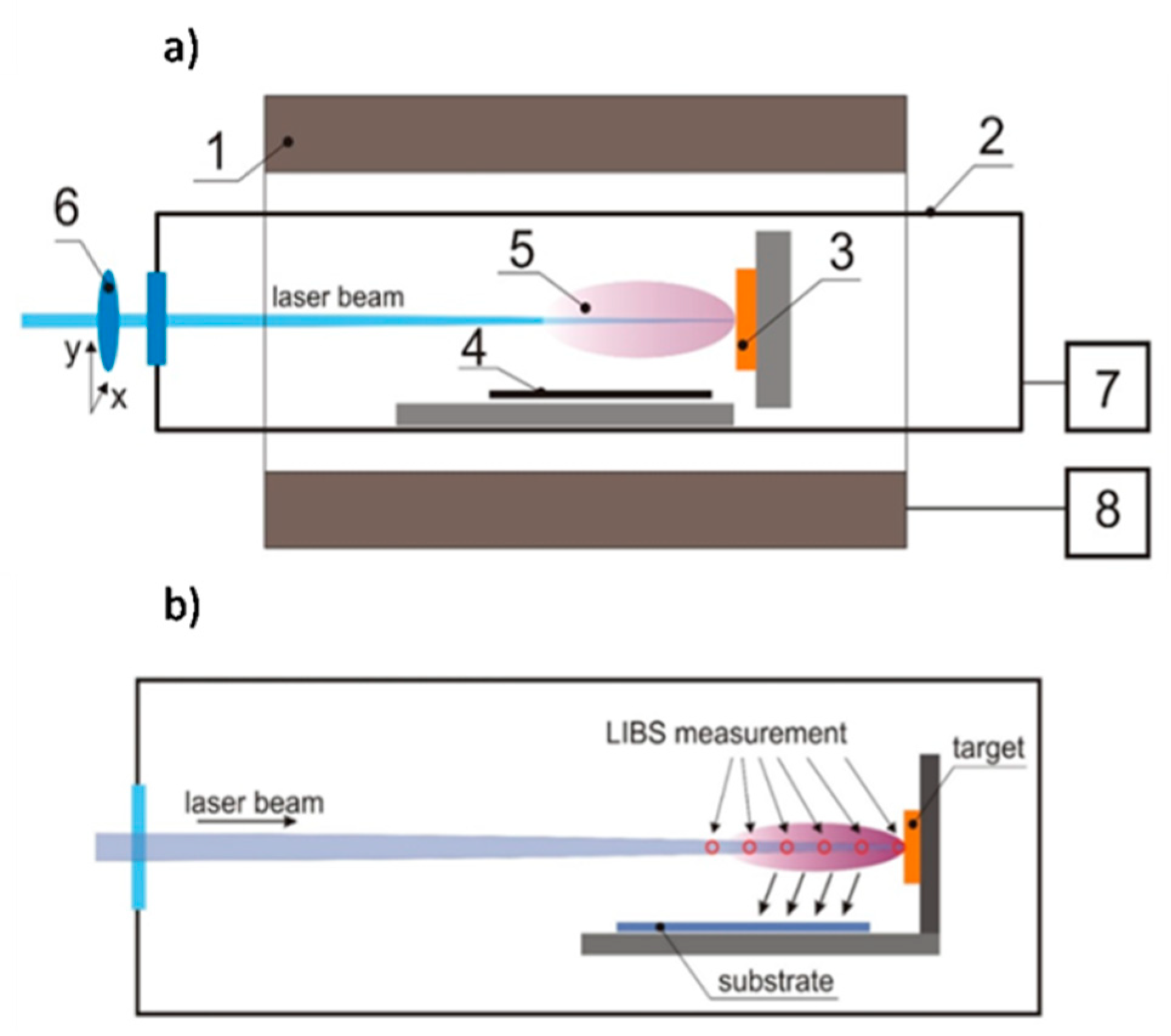
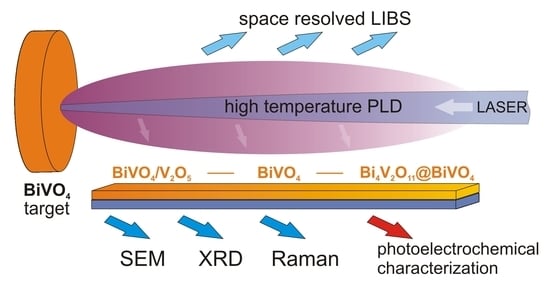
This work is focused on the influence of oxygen pressure in the chamber during pulsed laser deposition on the composition, morphology, and photoelectrochemical properties of bismuth vanadate. Bismuth vanadate layers were deposited on the commercially available FTO substrates from BiVO4 pellet (equimolar amounts of bismuth and vanadium). The substrate was placed in the chamber parallel to the laser beam. Such geometry is not chosen in order to obtain the highest possible photoelectrochemical activity of the deposited layer. The proposed geometry allows the spatial distribution of elements deposited on the substrate to be investigated. The results obtained from such geometry of the system give new insight into the mechanism of bismuth vanadate ablation from stoichiometric BiVO4 targets. The resulting films were characterized using XRD, Raman spectroscopy, energy dispersive X-ray spectroscopy, and scanning electron microscopy. The photoelectrochemical measurements were performed in an aqueous electrolyte under AM1.5 simulated solar illumination in order to investigate the influence of deposition parameters on the efficiency of photoelectrochemical water oxidation.
3. Results and Discussion
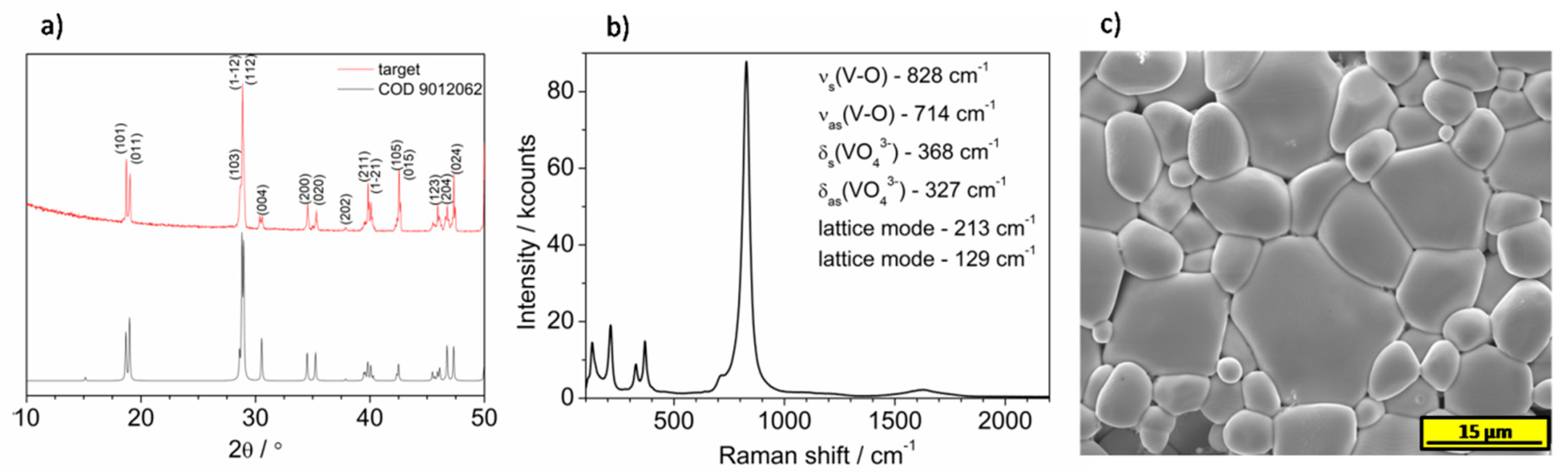
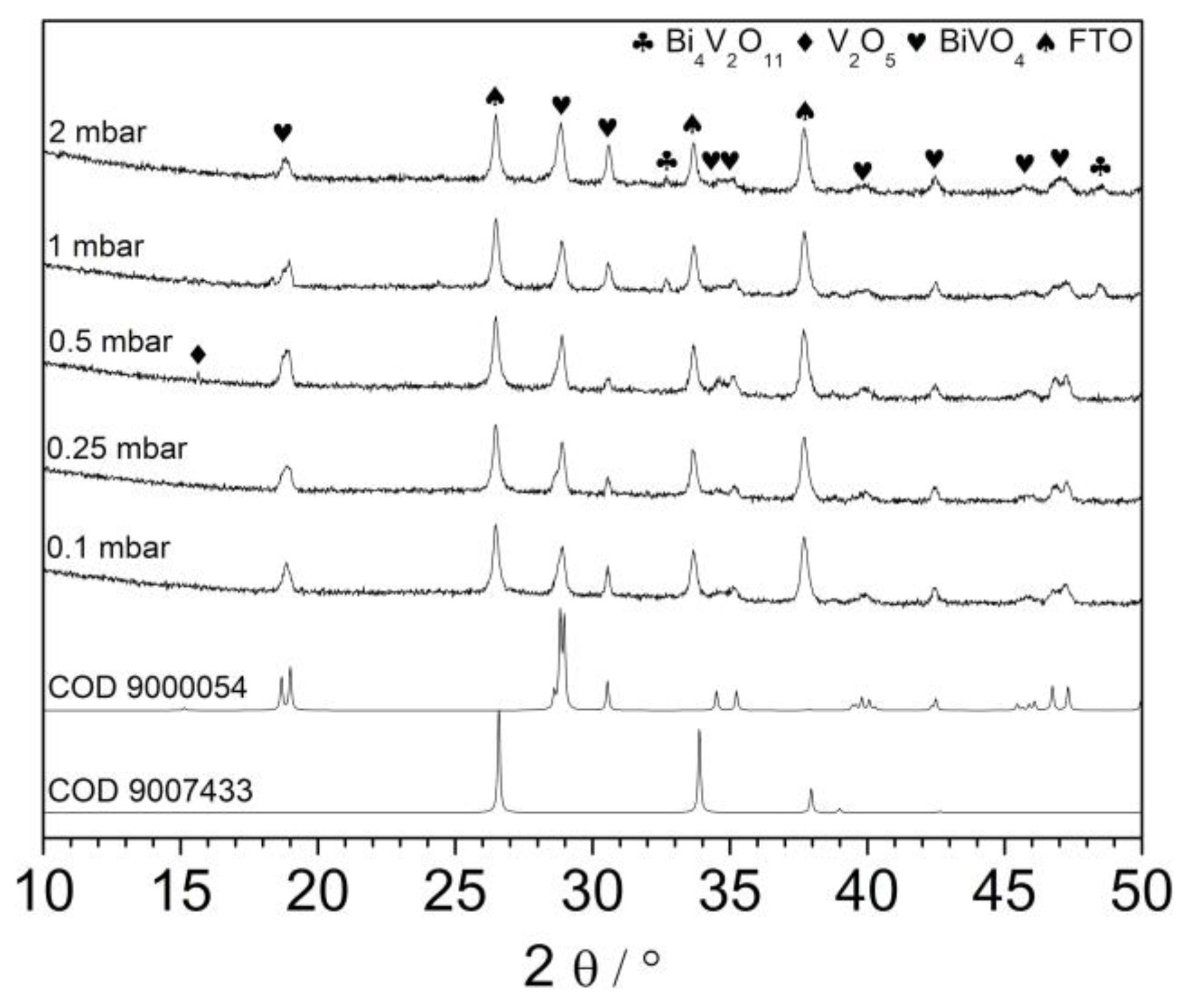
The prepared films were characterized using an XRD technique. As it is shown in
Figure 3, all patterns exhibits typical reflection characteristic for monoclinic bismuth vanadate. Recently, it was reported that deposition temperature significantly affects the orientation of BiVO
4 crystallites growing on the FTO substrates [
19]. Authors showed that oxygen pressure does not change significantly the crystal structure of resulting layers. Indeed, the presented diffractograms differ rather slightly. Differences may result i.a. from the different thicknesses of the layers and the relative intensity of peaks coming from the FTO substrate. Patterns consist of all the reflexes characteristic for the monoclinic BiVO
4 phase. However, in the case of the sample deposited under 0.5 mbar, reflex at about 15.5° was detected (V). It could be related to the (002) plane of BiVO
4 suggesting anisotropic growth of the crystallites, but the intensity of the peak recorded at 30.5° coming from (004) plane was not enhanced. Thus, it is very likely that it is coming from V
2O
5 (020) plane [
27]. The exemplary XRD pattern recorded in a small-angle scattering mode is shown in
Figure S3 for the samples deposited at 0.1, 0.5, and 2 mbar. The patterns are characterized by a much lower intensity of reflections from the FTO substrate as expected. Generally, the same reflexes have been recorded as in the case of conventional XRD analysis. However, the reflection coming from V
2O
5 (010) plane at 20.4° was clearly detected for the sample deposited under 0.5 mbar. It confirms that the V
2O
5 phase was present rather on the top of the thin film. Interestingly, the peak from a plane (200) (15.5°) was not detected, in contrast to the conventional XRD measurements. It may suggest a very specific arrangement of V
2O
5 crystallites. Additionally, the XRD patterns of samples deposited under 1 and 2 mbar consisted of peaks at 32.5° and 48.5° suggesting the coexistence of the Bi
4V
2O
11 (bismuth vanadate with Bi:V ratio 2:1), see
Figure 3. Thus, the oxygen pressure in the deposition chamber affects the composition of resulting films. Such a conclusion was already formulated previously. However, the Bi
4V
2O
11 phase crystallized at low O
2 pressure (unlike in this work), while the application of higher pressure in the deposition chamber led to the formation of pure BiVO
4 [
22]. The differences may result from (i) different substrates and (ii) different geometry of the deposition system. The crystallite size of the films was estimated on the basis of broadening of the XRD reflections. The crystallite size in (101) direction estimated from (101) reflection observed at 18.8° was between 30 and 40 nm. The largest crystallites were observed in the sample obtained at 0.1 mbar, whereas the crystallite size values in the other films were similar (0.25–2 mbar). On the other hand, the reflection at 28.8° corresponding to the (103) and (112) planes was narrower, the crystallite sizes were between 55 and 65 nm. Additionally, in this case, low oxygen partial pressure lead to the formation of larger crystallites.
Samples have been investigated using the Raman spectroscopy technique. The films showed inhomogeneity and spectra vary depending on the measurement spot distance from the edge of the sample. Thus, the spectra were measured every 500 μm from the edge that was the closest to the target during sputtering. Only in the case of the sample deposited under 0.1 mbar pressure, the film is homogenous, and exemplary spectra are presented in
Figure 4a. However, besides bands coming from BiVO
4, which are characterized by enhanced intensity due to the Raman resonance effect, the new low intensity bands are seen in the Raman spectra at 145, 284, 304, 404, 481, 528, and 995 cm
−1. All these bands confirm the presence of V
2O
5. Interestingly, V
2O
5 was not registered on this sample using the XRD measurement, suggesting only short-range ordering in V
2O
5 or too small of an amount of it on the sample. Thus, the proposed method of deposition using PLD at certain O
2 pressure leads to the formation of BiVO
4/V
2O
5 heterojunction on the whole considered surface. The Raman spectra of the film deposited at higher oxygen pressure (0.25 mbar) are presented in
Figure 4b. It can be concluded that V
2O
5 was not evenly distributed throughout the film. The highest intensity of the band coming from V
2O
5 (995 cm
−1) was detected 1000–2500 μm from the edge. In the further part of the layer, V
2O
5 does not occur and only bands originating from BiVO
4 were detected. Samples obtained at pressure 0.5 mbar exhibited a similar distribution of V
2O
5 on the surface, see
Figure 4c. The spectra recorded on the spots between 3000 and 6000 μm from the sample edge were characteristic for the pure BiVO
4 phase. Additionally, the spectrum measured at the opposite site of the layer exhibited a new feature (7000–8000 μm). The bands of BiVO
4 merged and a new band at about 870 cm
−1 appeared. In the case of the sample deposited under 1 mbar the effect of peaks merging was enhanced and visible over a larger area of the sample (5000–8000 μm), while maintaining the presence of V
2O
5 on the opposite site of the layer (1000–3000 μm), see
Figure 4d. Notably, two phenomena do not coexist on any of the same spots of the sample and there is a “buffer layer” containing only BiVO
4 (3000–5000 μm). In the case of the sample deposited at 2 mbar, the V
2O
5 was not detected, see
Figure 4e. The spectra collected closest to the edge of the sample (0–4000 μm) consisted of bands characteristic only of BiVO
4. However, spectra change with distance from the sample edge and new bands at 247 and 567 cm
−1 appear. Additionally, the two bands characteristic of V–O stretching vibration (800–900 cm
−1 range) were clearly separated. Thus, depending on the pressure of oxygen in the deposition chamber, the composition of resulting films varied. For the low oxygen pressure, the effect of V
2O
5 formation was enhanced. The V
2O
5 peaks appeared closer to the sample edge that was closest to the target during deposition. In the case of higher pressures, the V
2O
5 disappeared and the opposite side of the sample contains a new phase that can be described as Bi-rich bismuth vanadate—Bi
4V
2O
11. The results obtained suggest that ablation performed using UV laser leads to the generation of plasma plume where Bi and V atoms/ions can be separated. The oxygen pressure may strongly affect V atoms/ions in plasma plume, but heavier Bi atoms/ions were not significantly slowed down due to interactions with oxygen molecules in the deposition chamber. As a result, depending on the oxygen pressure, the V-rich phase (V
2O
5) can be formed on the one side of the sample, while the Bi-rich phase (Bi
4V
2O
11) on the opposite. The effect of nonstoichiometric plasma plume propagation due to the differences of cations masses has been already reported for pulsed laser deposition of LaAlO
3 and LaGaO
3 [
28,
29]. It was also reported on the basis on Langmuir probe measurements that lighter Cu
+ ions are more efficiently slowed down by collisions with oxygen molecules than heavier Ba
+ and Y
+ ions during deposition of YBa
2Cu
3O
7 [
30]. EDX (energy-dispersive X-ray spectroscopy) scans were performed in order to study the Bi:V atomic ratio along the layer. The results for layers obtained under 0.1, 0.5, and 2 mbar are presented in
Figure 4f. The spectra were measured point by point, approximately every 350 μm. The spread of results was relatively large, mainly due to irregularly occurring V
2O
5 crystallites, however the general relationship was clearly seen. The Bi:V ratio was close to one and more or less constant for films deposited at low pressures suggesting presence of BiVO
4 phase. The Bi:V ratio lower than one confirmed the presence of the V
2O
5 phase. Higher pressure in the deposition chamber results in a compositional gradient across the layer and the Bi concentration increased (in comparison with V concentration) from the edge that was the closest to the target during deposition. EDX analysis confirmed the presence of Bi excess and formation of Bi
4V
2O
11 for the films deposited under 2 mbar. The Bi:V ratio was not equal to 2, because two phases are present (BiVO
4 and Bi
4V
2O
11). The results obtained support the hypothesis that Bi and V atoms and ions in plasma plume can be separated due to the interactions or reaction with O
2 gas, what affects the spatial distribution of elements on the deposited films.
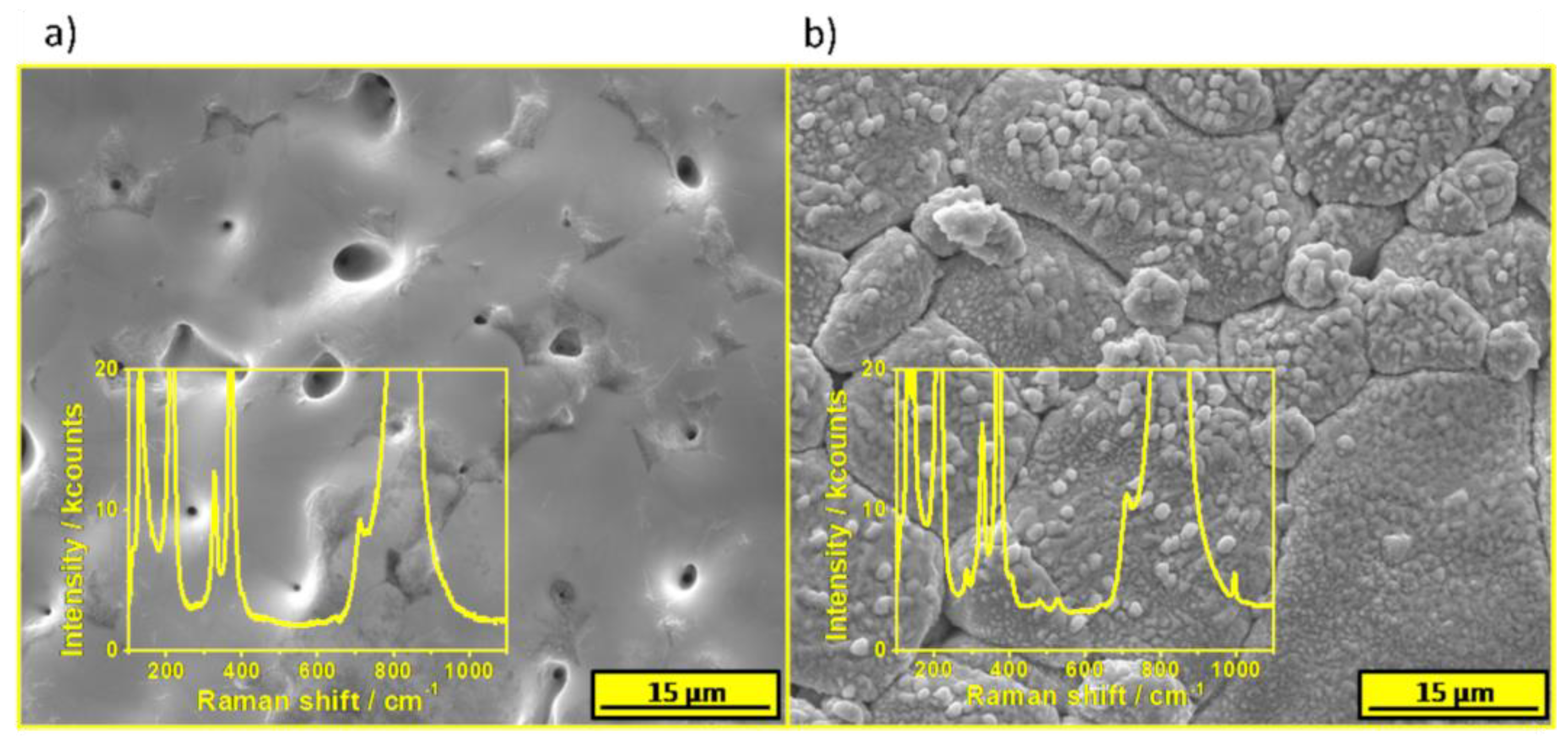
The morphology and composition of the spent target after deposition was investigated. The SEM micrograph of a target’s area exposed to laser pulses is presented in
Figure 5a. Generally, the area is rather flat and BiVO
4 particles cannot be distinguished, however some pinholes were formed. Raman spectrum of the area is characteristic of BiVO
4 and V and Bi rich phases were not detected, see the inset of
Figure 5a. It suggests that the ablation causes a formation of a plasma with a rather stoichiometric (Bi:V) composition. The pressure did not affect the ablation thresholds of V and Bi independently. The area of a target that was uncovered during deposition but not exposed to the laser pulses was also investigated. The SEM image is presented in
Figure 5b. The surface was covered by a new phase, which was not observed before the PLD procedure (compared with
Figure 1c). According to the Raman spectroscopy measurements, small particles contained the V
2O
5 phase as is shown in the inset of
Figure 5b, however the main signal comes from the underlying BiVO
4. Thus, vanadium was significantly affected by the oxygen gas in the chamber and it led to redeposition only of V
2O
5 on the target. Bi-rich phases were not detected there. Thus, it is very likely that the Bi/V separation occurs after ablation, during interactions with oxygen molecules with ions characterized by different masses.
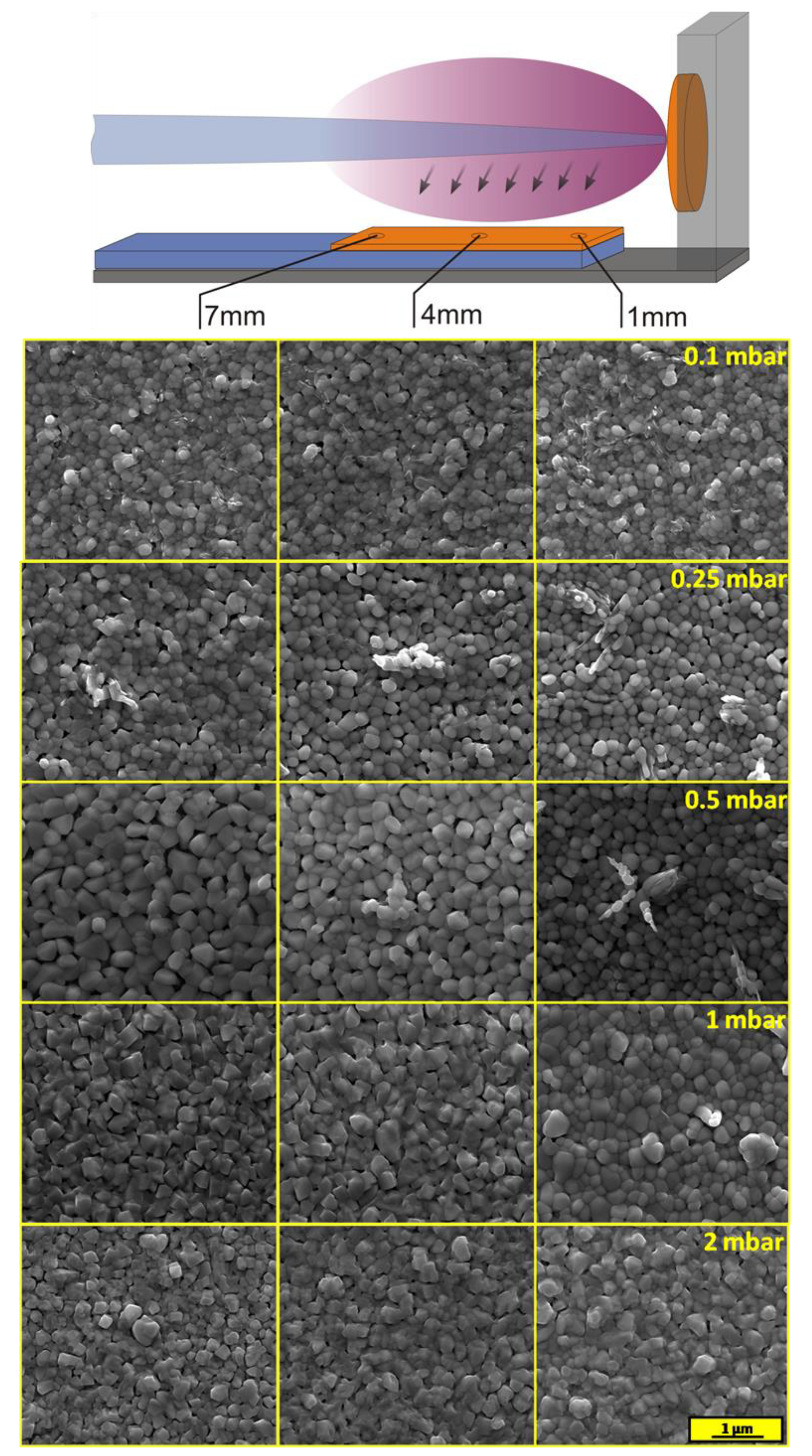
The morphology of layers was investigated using scanning electron microscopy. SEM images were recorded at three different spots for each sample, as is shown in
Figure 6. The sample deposited at the lowest pressure of O
2 was quite homogenous, which was consistent with the Raman spectroscopy measurements. Films were built by round-shaped grains with different diameters from about 100 to 200 nm. Inclusion of another phase, probably V
2O
5, was also visible. The higher pressure during deposition affected the amount of the V
2O
5 phase on the film surface. Layers deposited under 0.5 mbar were built by clearly bigger particles of BiVO
4. An additional phase of V
2O
5 on the top of BiVO
4 was observed in a form of protruding longitudinal crystallites (confirmed by EDX analysis, see
Figure S4), only near the edge of the sample that was the closest to the target. The cross-sectional micrograph of area rich in specific structures of the sample deposited under 0.5 mbar is presented in
Figure S5. Although the V
2O
5 phase was also present on the surface of the samples deposited under 0.1 and 0.25 mbar (which was confirmed by Raman spectroscopy), the XRD technique enabled detection of it only in the case of the sample deposited under 0.5 mbar on which crystallites were formed. The effect of Bi to the V ratio on the morphology was visible on SEM images of the samples deposited under 1 and 2 mbar. The higher concentration of bismuth made the crystallite edges sharper, while a lower Bi concentration area was characterized by rather round-shape grains. It should be noticed that the separated phase of Bi
4V
2O
11 could not be distinguished on the SEM micrographs. The SEM image of the larger area of the sample deposited under 2 mbar was added to the
Supplementary Information in order to prove that there are no separated crystallites of Bi
4V
2O
11, see
Figure S6. It is very likely that the bulk Bi
4V
2O
11@BiVO
4 heterojunction was formed, as opposed to BiVO
4/V
2O
5. It has been already shown previously that BiVO
4 and Bi
4V
2O
11 tend to form structures in which the components are indistinguishable (by SEM technique) due to the formation of nanosized interfacial contact [
31]. Additionally, AFM measurements were performed In order to characterize the topography of the deposited films. The results are presented in
Figure S7. The root mean square roughness of the sample deposited under 0.1 mbar was the lowest (32 nm), while the rest of the samples were characterized by rms equal to 38 nm.
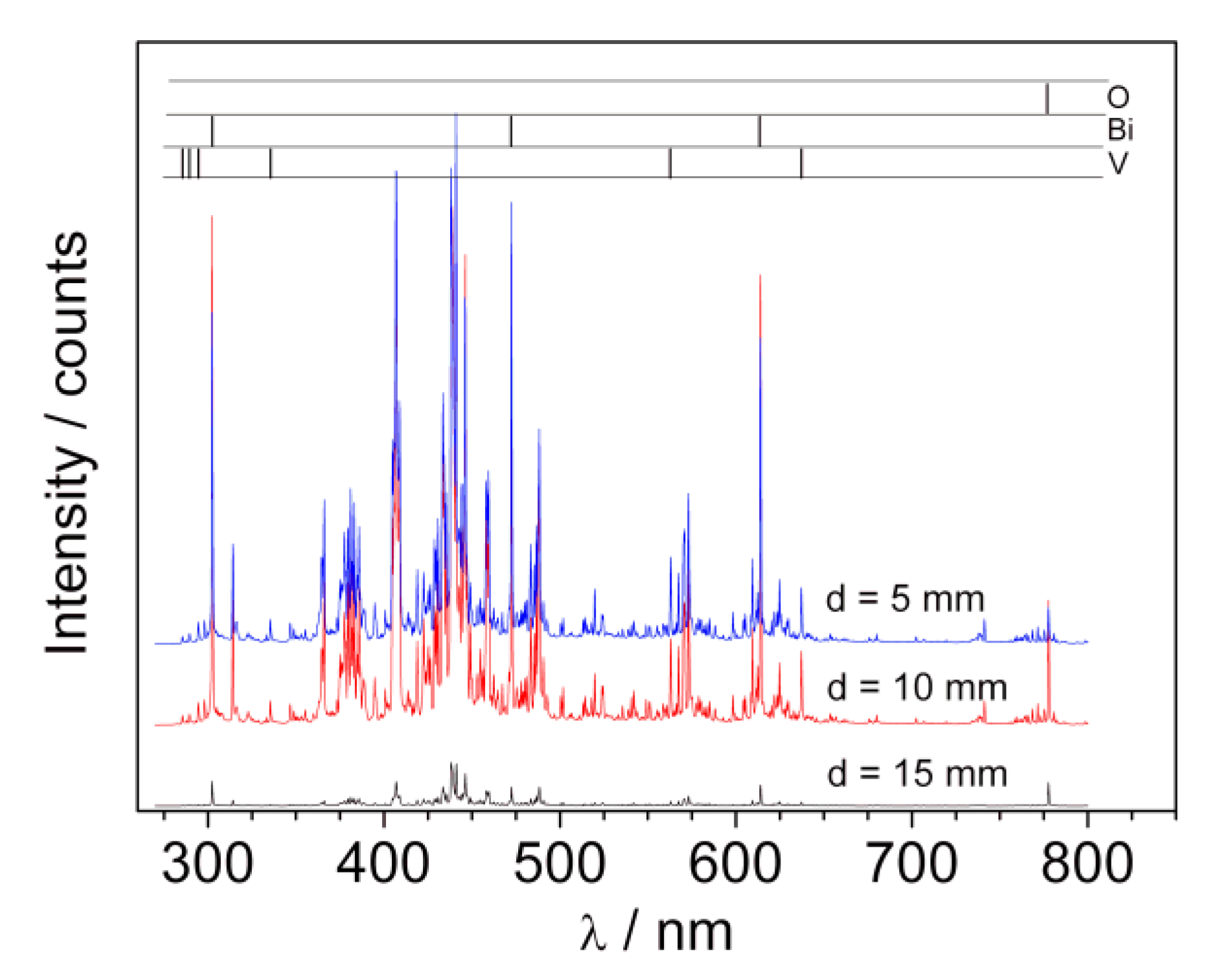
The plasma plume formed during deposition was also characterized using LIBS (laser-induced breakdown spectroscopy). The investigated radiation spectra contained clear atomic lines corresponding to oxygen, bismuth, and vanadium as well as continuous molecular spectra, which identification would require higher spectral resolution. Most probably these lines may correspond to oxygen and ozone molecules as well as bismuth and vanadium oxides or dimmers. The LIBS spectra recorded at different distances from the target during BiVO
4 deposition (p = 2 mbar) are presented in
Figure 7. The analyzed lines are marked in the upper part of the picture.
In the case of the signal coming from oxygen, there is a high intensity triplet O(777)—777.194, 777.417, and 777.539 nm (here seen as one line around 777 nm). The radiation results from optical transition 3s5S02→3p5P1 2 3. The energy of the higher transition level is around 10.74 eV and was not detected here. The bismuth lines observed in the spectra correspond to the transition from high energy levels 7s2,4P, 8s2P, and 6d2D (with energies in the range of 4–6 eV) to metastable 6p32D03/2, 5/2 and 6p3 2P01/2, 3/2. Lines corresponding to optical resonance transition (to ground electronic state 6p3 4S03/2) were not observed, which can be explained by full absorption in plasma volume. A radiation from vanadium atoms contains a large amount of spectral lines, which constitute the main part of the observed spectra. Energies of excited levels (3d34s4p 4D0, 3d44p 4P, and 3d44p 4F) for observed lines were of the order 4 ÷ 5.3 eV. The observed transitions end in the ground electronic state (3d34s2 4F) or metastable levels (3d44s 6D and 3d34s2 4P). It should be underlined that in contrast to the bismuth spectra, in the case of vanadium the electrons fell to metastable as well as ground states. This means that the resonance lines of vanadium were reabsorbed in the plasma less than those of bismuth. It may point to the fact that there were significantly fewer atoms of vanadium than bismuth in the investigated plasma. Since Bi and V ablate from the target in a stoichiometric way, the results obtained indicate that propagation of vanadium in the deposition chamber filled with oxygen was therefore difficult.
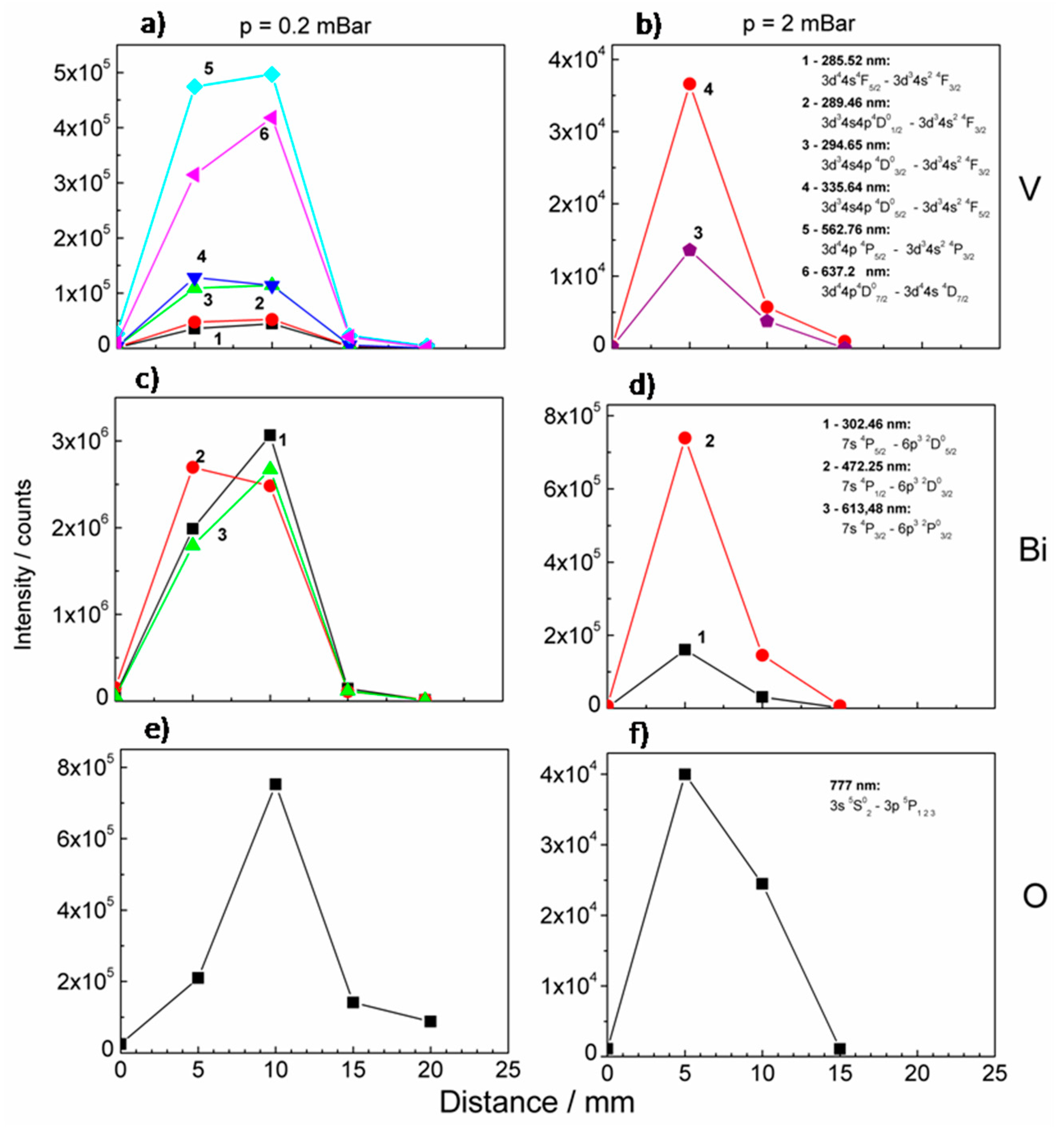
The intensities of the selected lines were plotted versus the distance from the BiVO
4 target and the resulting graphs are presented in
Figure 8. Measurements were performed under 0.2 and 2 mbar oxygen pressure. The dependence of intensities on a distance for low oxygen pressure was very similar for all observed vanadium lines, i.e., the intensities grew for a distance L < 10 mm and suddenly dropped later (
Figure 8a). Similar dependences were observed in the case of bismuth (
Figure 8c). As oxygen pressure grows the dependence changes. The maximum of intensities was shifted to lower L values, they grew for L < 5 mm and fell rapidly later, see
Figure 8b,d. The dependences differed significantly in the case of oxygen both in the case of lower and higher oxygen pressure, see
Figure 8e,f. Oxygen lines may originate from the oxygen ablated from the target. However, it is very likely that signals at about 777 nm originate from oxygen (ambient gas) species excited during interaction with plasma components.
During the laser ablation in the presence of ambient gas, the ablated species were decelerated and attenuated due to collision with gas molecules. These processes resulted in plasma confinement and generation of shock waves at the plasma propagation front, where chemical reactions of ablated materials with oxygen molecules were intensified. Higher ambient gas pressure results in the compression of plasma and shifting of the plasma front into the proximity of target. It was observed in the experiment as a change of spectral lines intensities versus the distance from a target measured for two different pressure values (
Figure 8). For a higher pressure the maximum intensity of excited oxygen shifted towards the target, which means that the zone of intensive collision excitation also shift. The decrease of intensity beyond the maximum indicates a collision recombination. The attenuation of the emission with an increase of distance from the target may result from non-radiative processes (including quenching and energy transfer processes) and adiabatic expansion of the plasma. However the kinetic energy of all species was still sufficient to reach the substrate and form thin film. In our experiment, we observed the presence of the V
2O
5 phase, in a form of irregularly occurring crystallites, that for higher oxygen pressure was shifted towards the target. This was in an agreement with the spectroscopic observation of plasma properties. It should be also noted that the increase of ambient gas pressure resulted in the decrease of plasma plume species velocity, which can influence the morphology of the deposited film. For a higher pressure and distances from the target, where relaxation processes in plasma could be observed, the thin film was more homogenous, which was confirmed by SEM images (see
Figure S6). The excess of vanadium in one part of the sample caused its deficiency in the area of the covered substrate, which was farther from the target. The effect of this phenomenon was a higher Bi:V ratio and possibility of Bi
4V
2O
11 crystallization and formation of the BiVO
4/Bi
4V
2O
11 system.
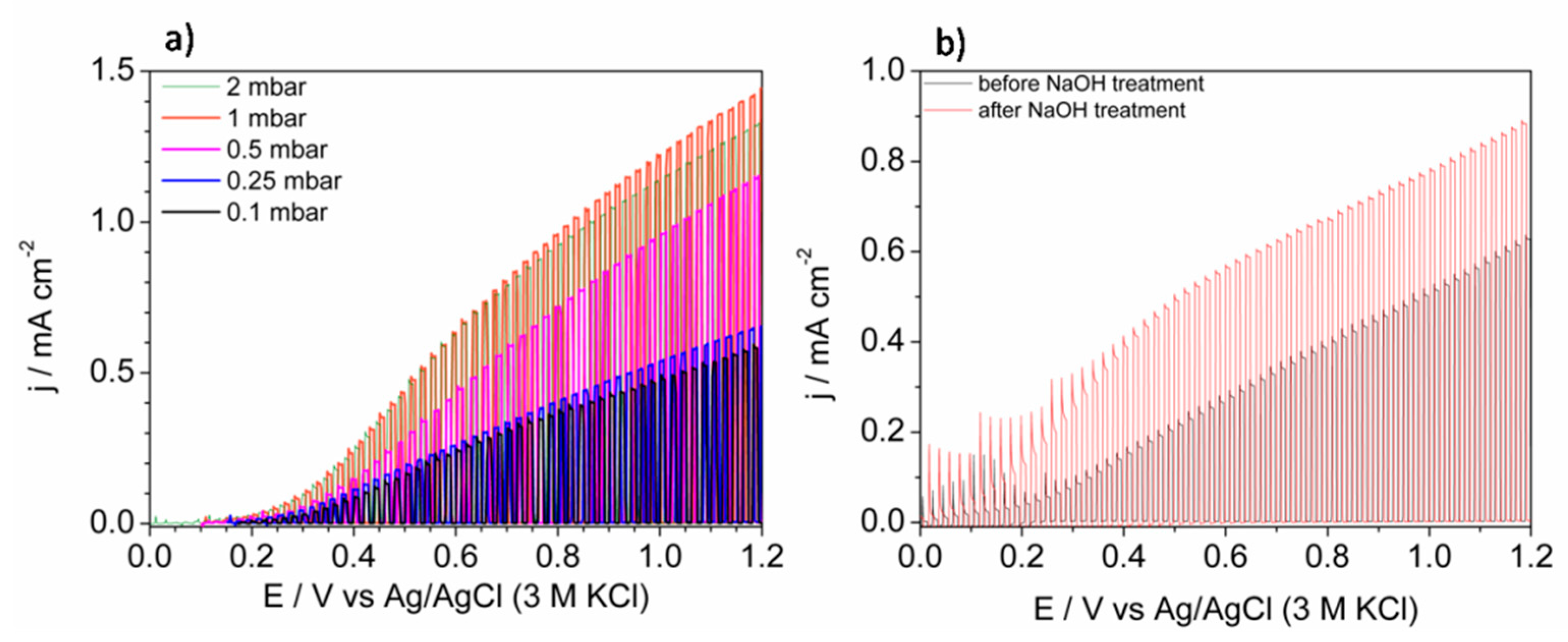
The most promising application of thin layers of BiVO
4 is photoelectrochemical water splitting. Thus, the obtained films were tested as photoanodes for water photooxidation under simulated solar light exposure. As it is shown in
Figure 9a, the shape of linear sweep voltammograms was similar for each sample and the presence of heterojunctions (BiVO
4/V
2O
5 and Bi
4V
2O
11@BiVO
4) did not clearly affect it. The curves were characteristic of n-type semiconductor electrodes under illumination [
32]. The lowest photoactivity was observed for samples deposited under 0.1 mbar. Generally, the formation of BiVO
4/V
2O
5 heterojunction can enhance the efficiency of photocurrent generation for both, layered and bulk types of junctions [
10]. However, the geometry of the system significantly affects its photoactivity [
33] due to the relative positions of conduction bands [
34]. The photoexcited electrons can be trapped due to the potential barrier if the V
2O
5 is mainly on the top of the electrode, as it is in the present work. Thus, FTO/BiVO
4 photoanode deposited under 0.1 mbar was immersed in 1 M NaOH in order to facilitate selective dissolution of V
2O
5. This is a procedure commonly used during some methods of BiVO
4 synthesis that uses the excess of the V-containing precursor [
35]. The comparison of the LSV (linear sweep voltammetry) curves recorded before and after soaking in NaOH solution is presented in
Figure 9b. Since the enhancement of photocurrent was achieved, the presented results confirmed the negative influence of V
2O
5 on the surface on BiVO
4. If BiVO
4 and V
2O
5 did not form a heterojunction, an increase in photocurrent in comparison with BiVO
4 would be expected due to the narrower energy band gap of vanadium pentaoxide. In the case of the sample deposited under 0.5 mbar, most of the sample surface consists of bare BiVO
4. The enhancement of the photocurrent, in comparison with samples deposited under lower pressures, results from the lack of adverse BiVO
4/V
2O
5 junction presence on a larger area of the film. The highest photocurrent of water oxidation was observed for the sample deposited under 1 mbar, however the system was very complex due to the presence of three phases: BiVO
4, V
2O
5, and Bi
4V
2O
11. A very similar result was achieved for the sample that consisted of the Bi
4V
2O
11@BiVO
4 heterojunction (deposited under 2 mbar). Notably, pristine FTO/BiVO
4 photoanodes (prepared under 0.1 mbar with removed V
2O
5) generated a lower photocurrent than photoanodes consisting of the BiVO
4@Bi
4V
2O
11 heterojunction. It has been already reported that such a system positively affects the photoelectroactivity in comparison to bare BiVO
4 [
31,
36]. The geometry of the system should be taken into account, as it was discussed for the BiVO
4/V
2O
5 photoanode. However, the crystallites of Bi
4V
2O
11 cannot be simply distinguished on the SEM images, thus it is very likely that bulk heterojunction was obtained. Since the pulsed laser deposited films of Bi
4V
2O
11 acts as a rather poor photoanode i.a. due to the low absorption coefficient [
37], in the case of the formation of BiVO
4 and Bi
4V
2O
11 separated phases, the enhancement would not be expected. Thus, the formation of heterojunction is the main reason of photocurrent improvement. The formation of heterojunction leads to the inhibition of bulk electron/hole recombination [
38]. Generally, the presence of type-II heterojunction enhances bulk e
−/h
+ separation efficiency due to the internal electric field generated on the phases interface, e.g., BiVO
4/Bi
4V
2O
11 [
36]. The enhancement of photoactivity in the case of the Bi
4V
2O
11-containing photoanode may be also related to improved charge transfer resistance through the photoactive film in comparison with pristine BiVO
4 as it was reported previously [
22]. It is not expected that improvement is related to better light absorption because the energy band gaps of BiVO
4 and Bi
4V
2O
11 are comparable [
39]. Since the morphology, thickness, and roughness of the samples were comparable, we claimed that differences in the photocurrent of water oxidation were related mainly to the composition of the samples. The recorded photocurrent seems to be lower in comparison with other pulsed laser deposited BiVO
4-based photoanodes [
22,
40,
41]. However it should be noticed that in the case of the presented results the photocurrent was related to water photooxidation and oxygen evolution, not oxidation of the consumable hole scavenger. On the other hand, results were comparable with the photocurrent of water oxidation that was achieved for unmodified BiVO
4 films with underlying hole-blocking layer sputtered using the PLD method [
42].