Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting
Abstract
1. Introduction
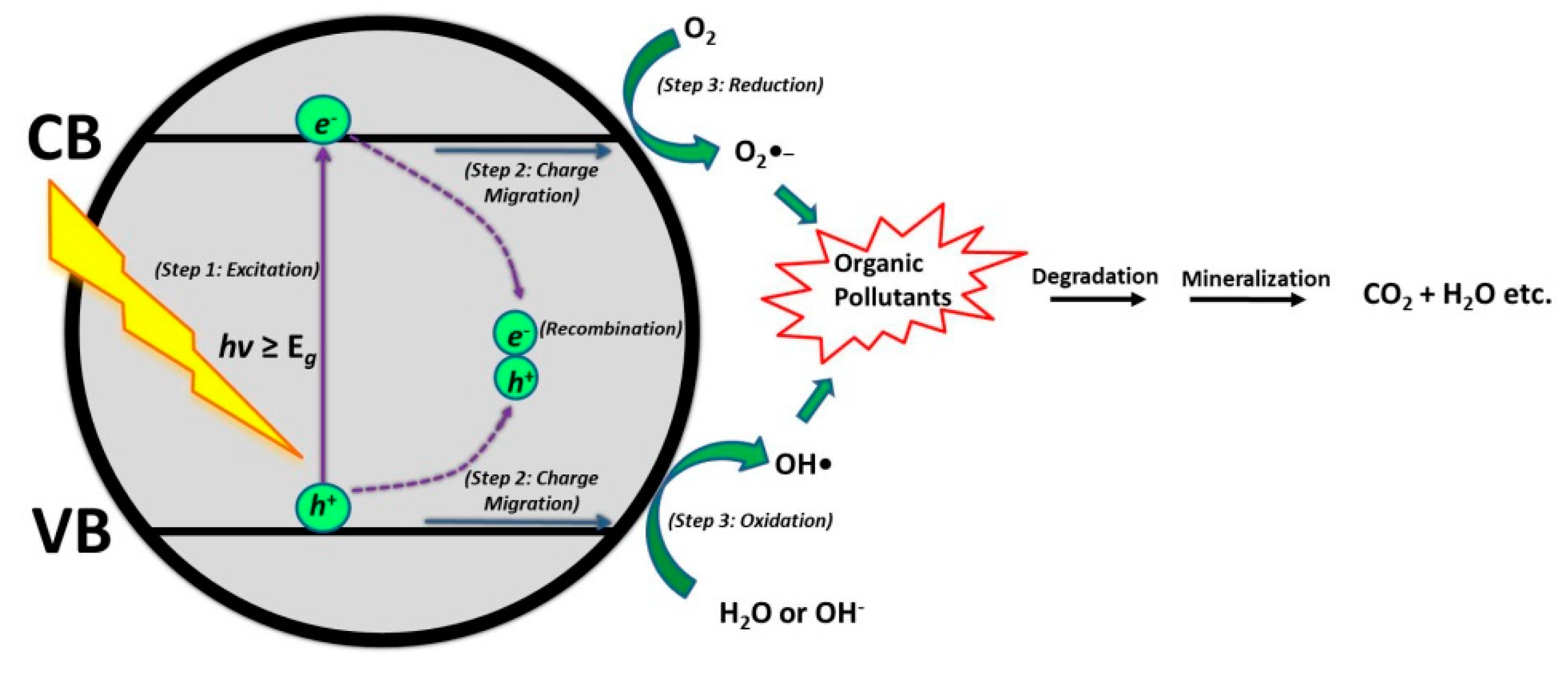
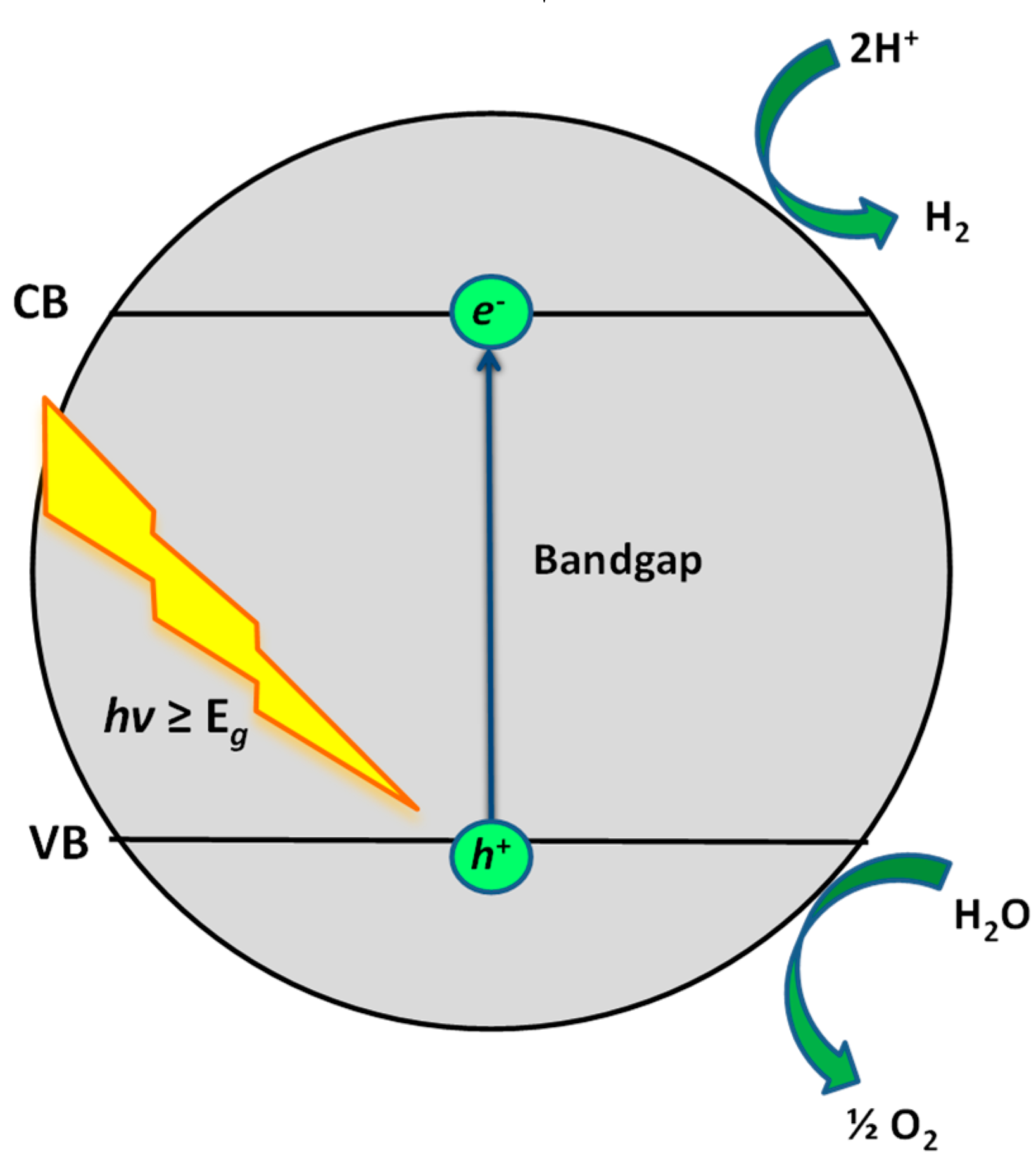
2. Photocatalytic Water Treatment
2.1. Coupling of TiO2 with Metal Oxides
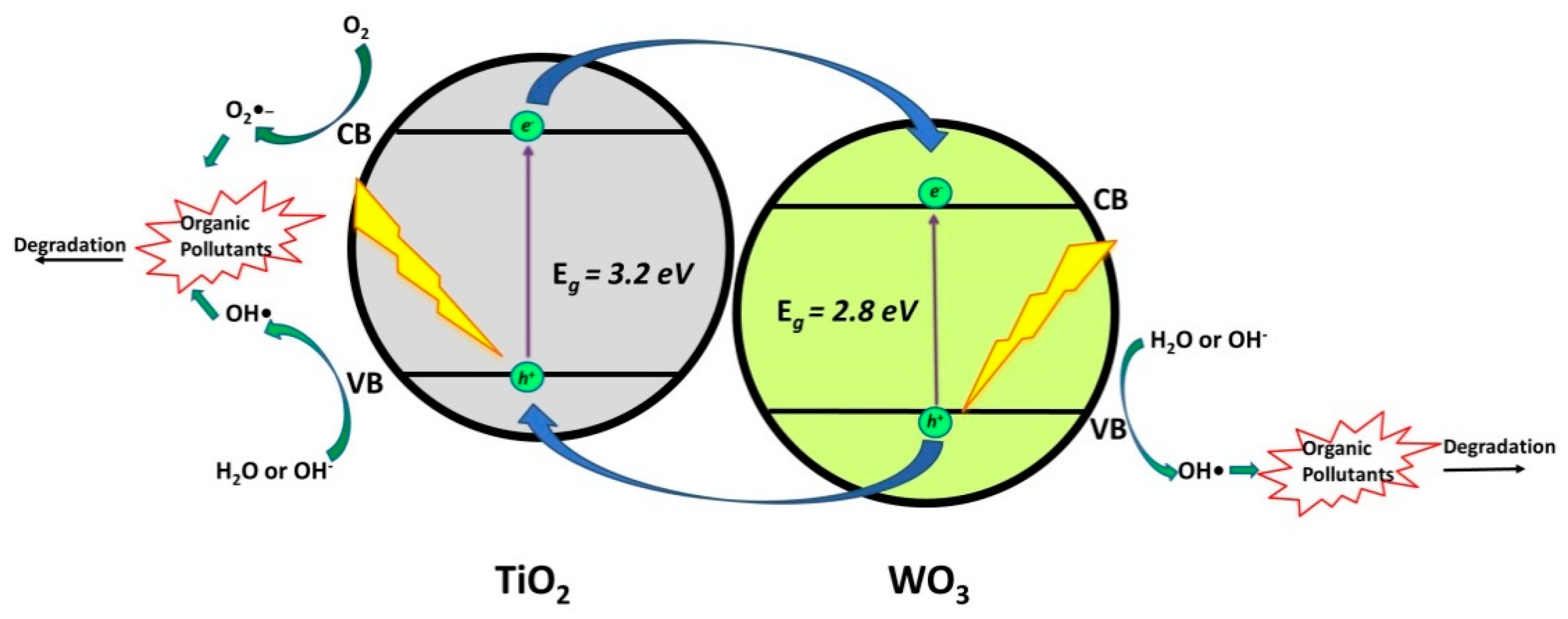
2.1.1. TiO2/WO3
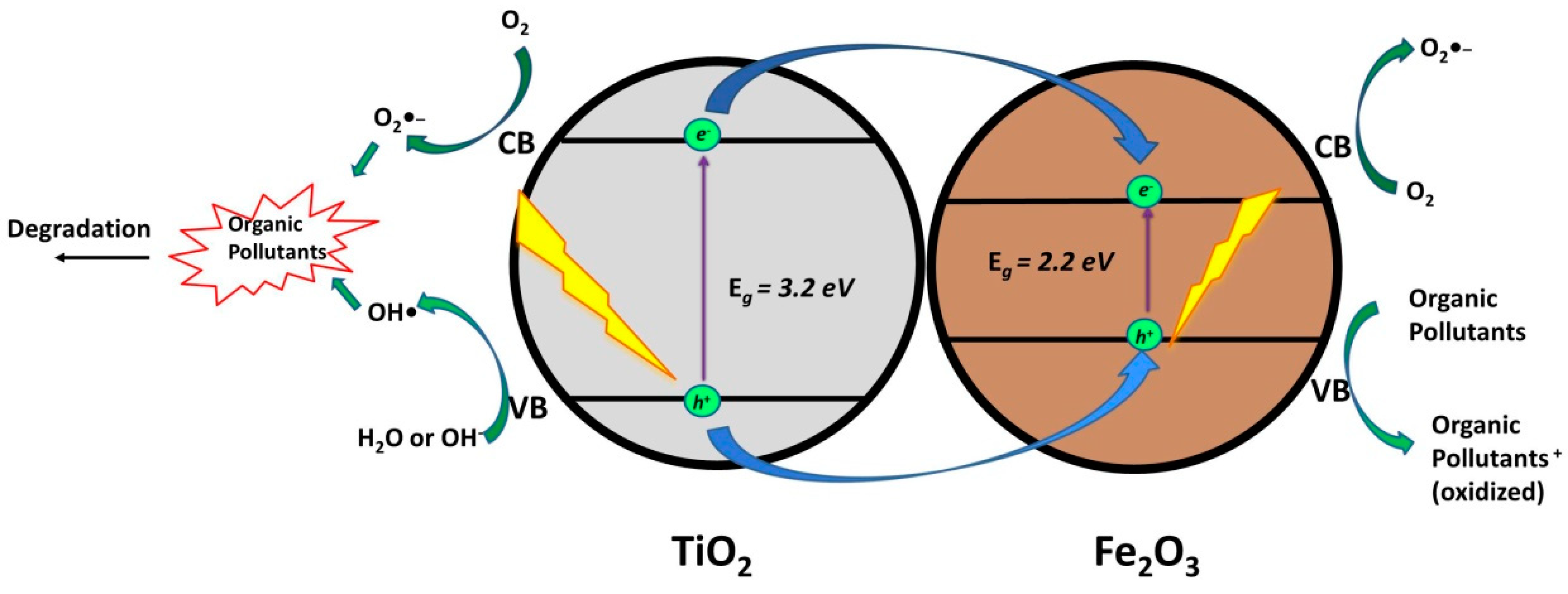
2.1.2. TiO2/Fe2O3
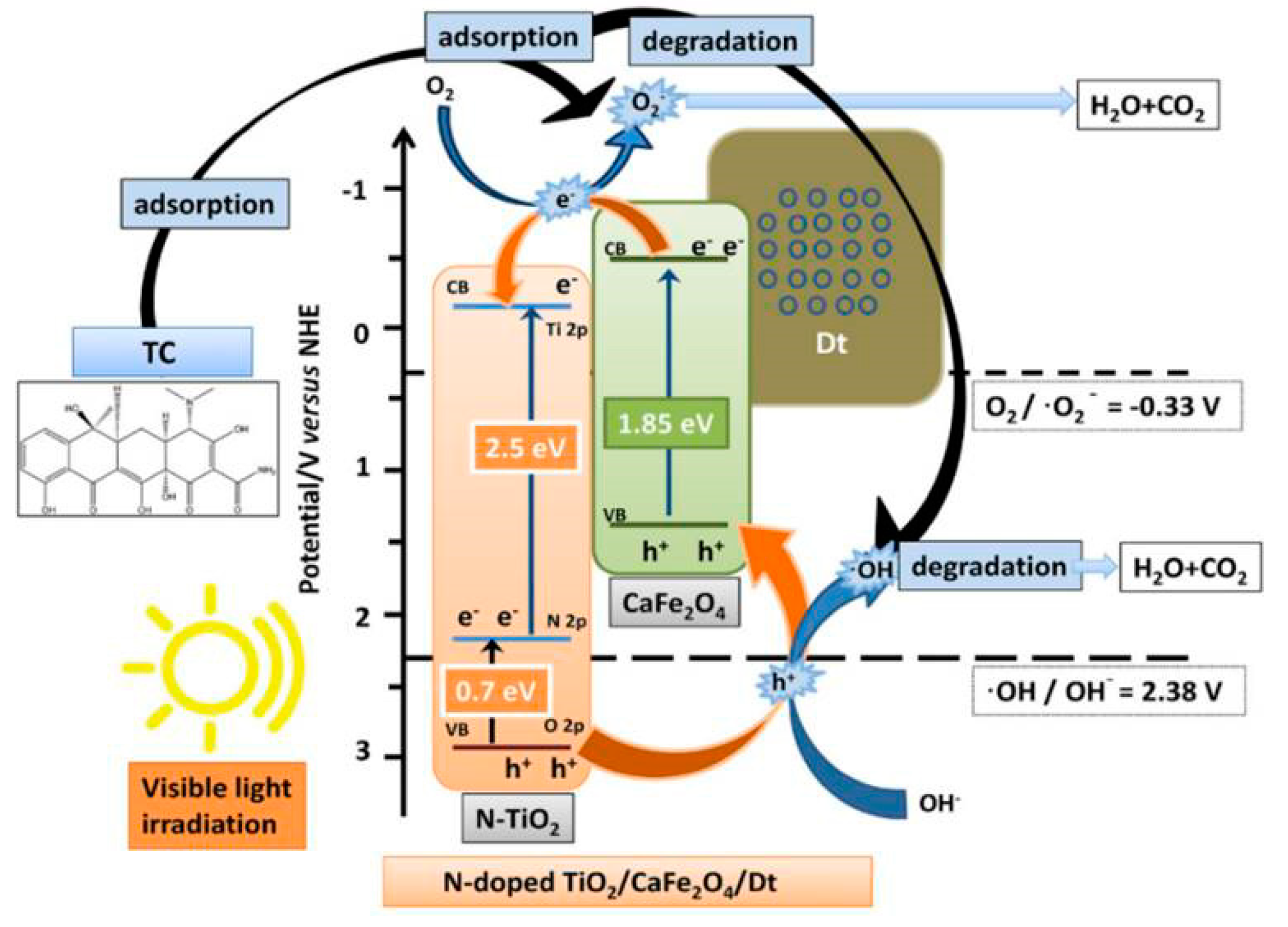
2.1.3. TiO2/Spinel Ferrite
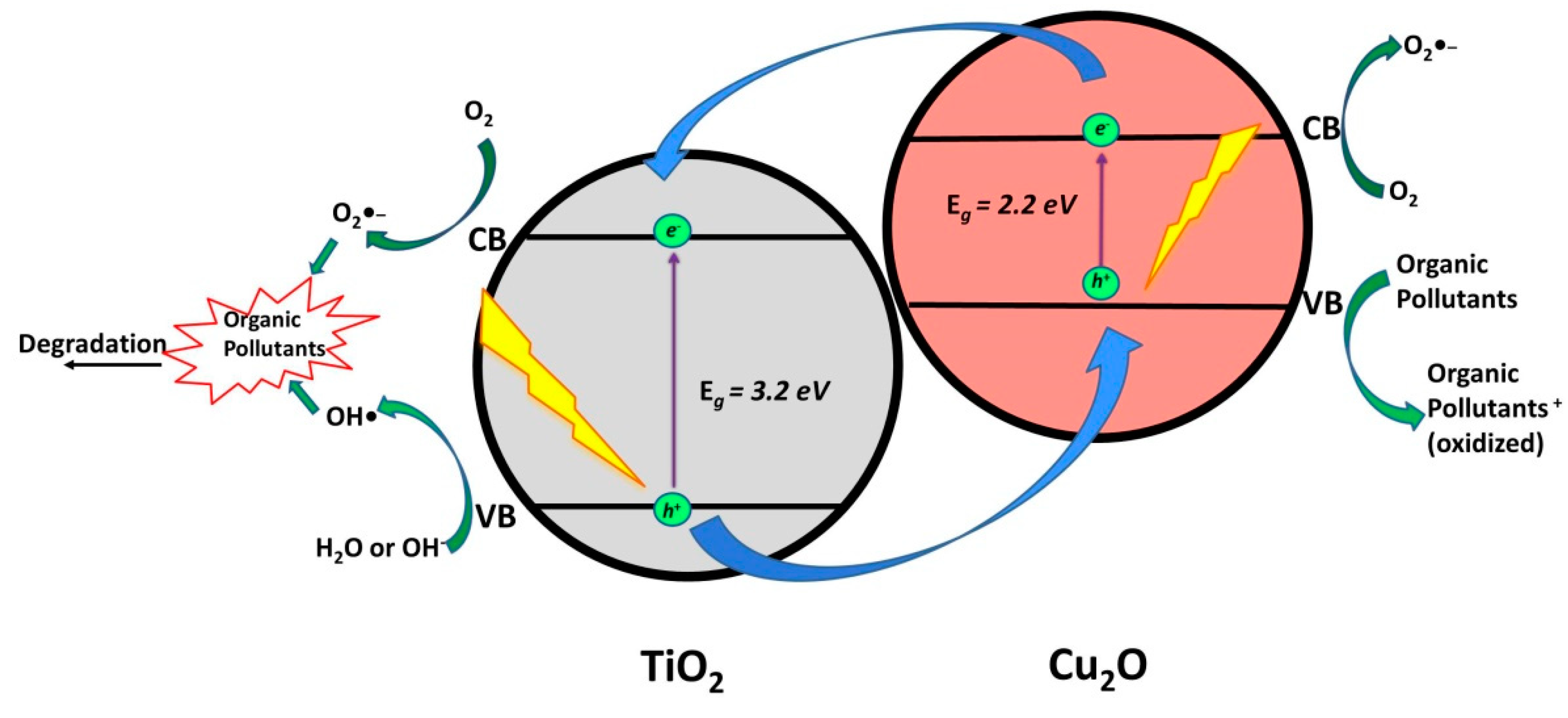
2.1.4. TiO2/Cu2O
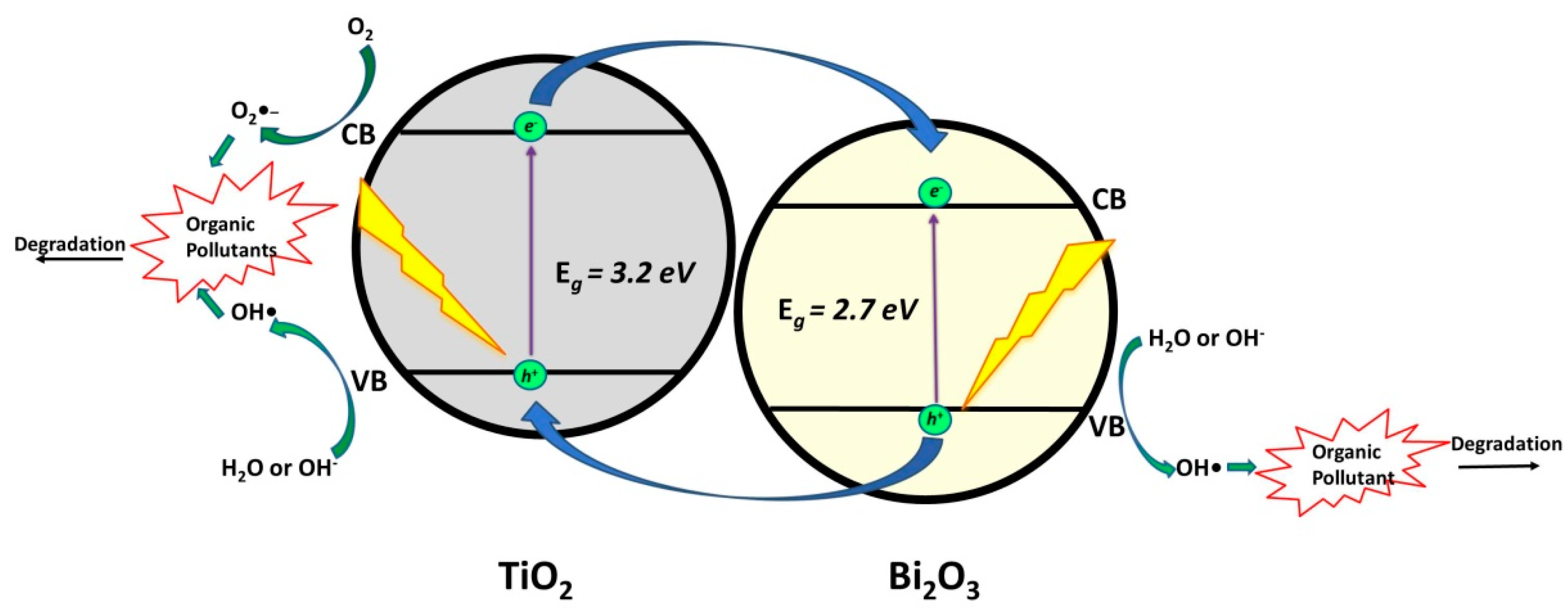
2.1.5. TiO2/Bi2O3
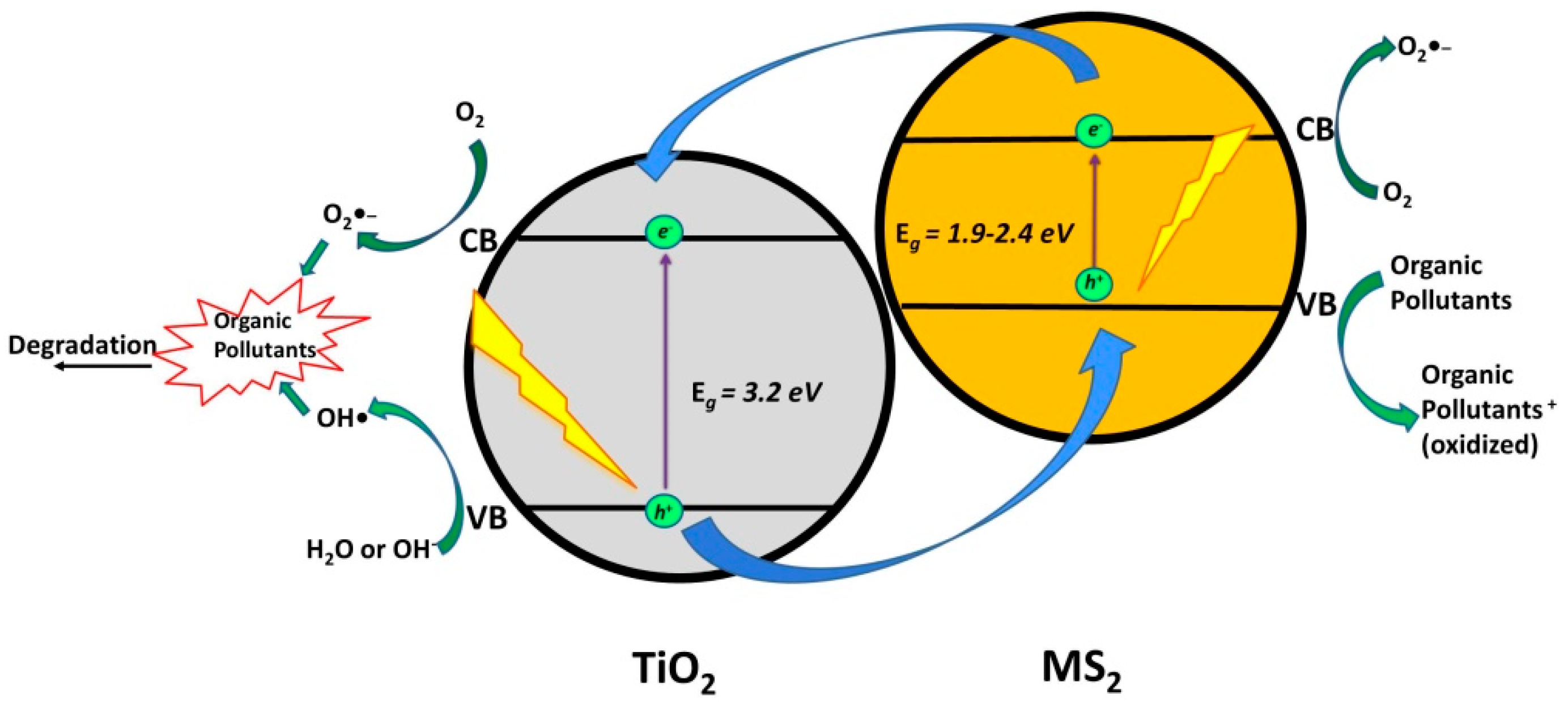
2.2. Coupling of TiO2 with Metal Sulfides
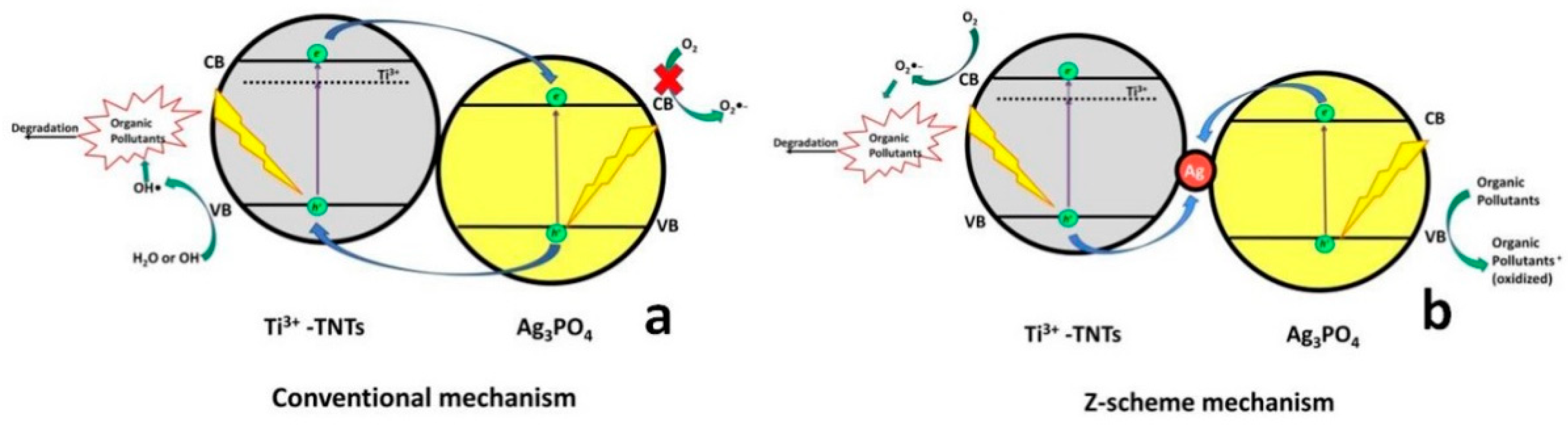
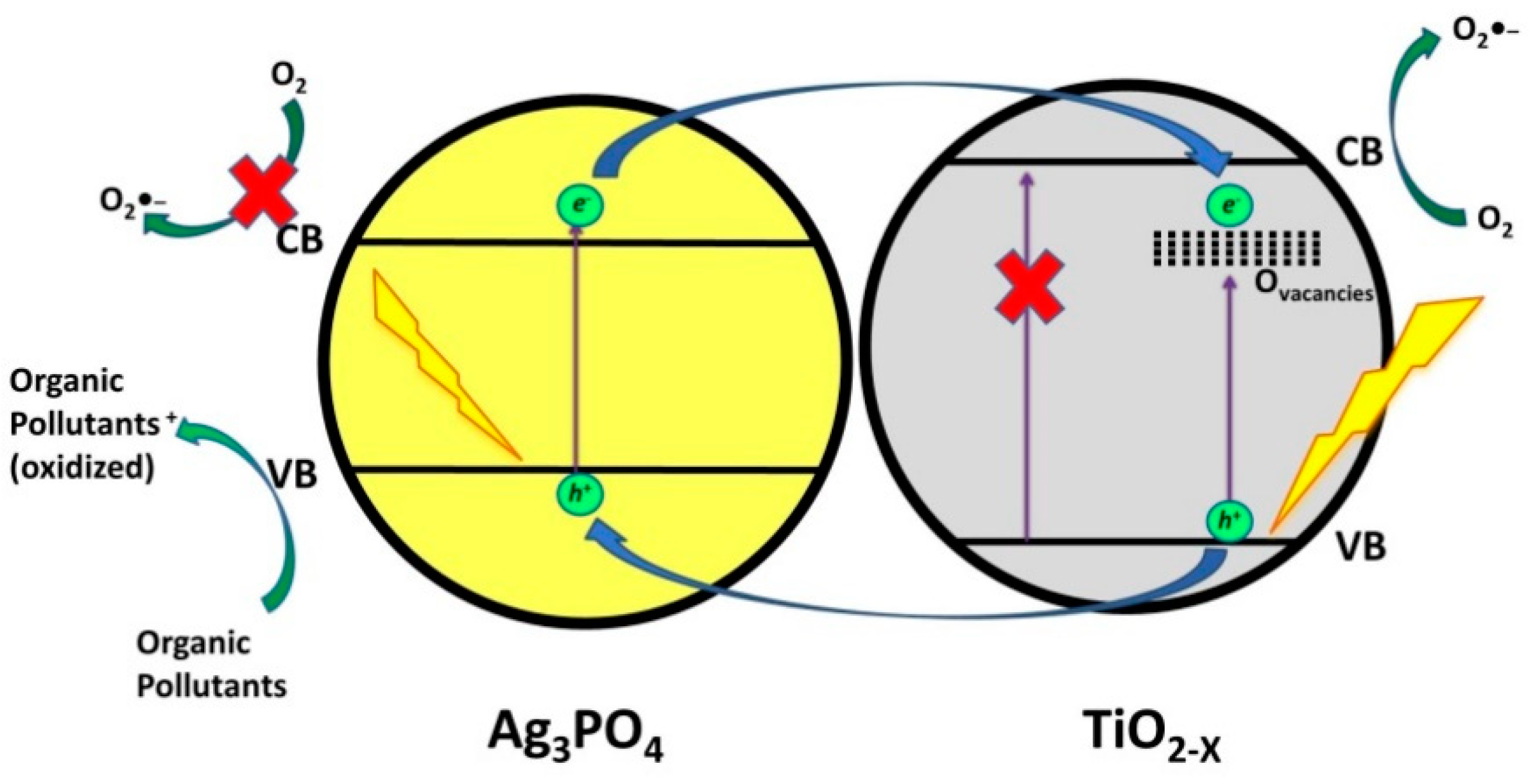
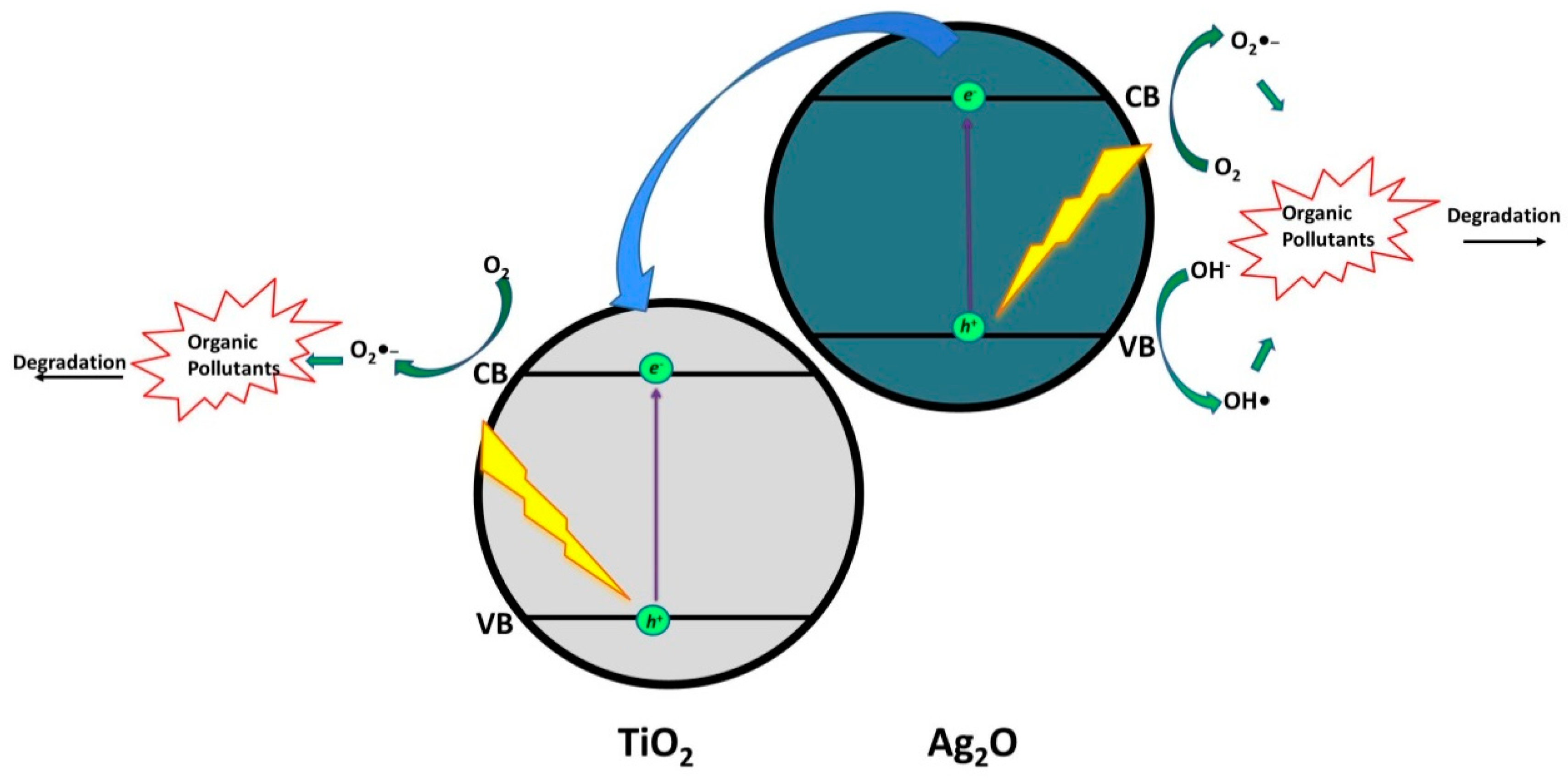
2.3. Coupling of TiO2 with Silver- Based Semiconductors
2.4. Coupling of TiO2 with Graphene and Graphene-Like Materials
2.4.1. TiO2/Graphene Composites
2.4.2. TiO2/Semiconductor/Graphene Composites
2.4.3. TiO2/g-C3N4
3. Photocatalytic Water Splitting
3.1. Carbon-Based/TiO2
3.1.1. TiO2/g-C3N4
3.1.2. TiO2-G/GO/rGO
3.1.3. TiO2/CNT
3.2. Transition-Metal Oxides/TiO2
3.3. Transition Metal Chalcogenides-TiO2
3.3.1. TiO2/CdS
3.3.2. TiO2/CuS
3.3.3. TiO2/MoS2
3.4. Multiple TiO2-Based Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council, of 23 October 2000. Off. J. Eur. Communities 2000, 327, 1–72. [Google Scholar]
- Water JPI. Available online: http://www.waterjpi.eu/mapping-agenda/strategic-re (accessed on 12 December 2019).
- European Commission. Energy Strategy. Available online: https://ec.europa.eu/energy/en/topics/energy-strategy (accessed on 12 December 2019).
- Martin, M. Alternative Energy Sources and Technologies; Springer: Basel, Switzerland, 2016. [Google Scholar]
- Hasan, M.M.; Allam, N.K. Unbiased spontaneous solar hydrogen production using stable TiO2-CuO composite nanofiber photocatalysts. RSC Adv. 2018, 8, 37219–37228. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.N.; Bard, A.J. Heterogeneous photocatalytic oxidation of cyanide ion in aqueous solutions at TiO2. J. Am. Chem. Soc. 1977, 99, 303–304. [Google Scholar] [CrossRef]
- Turchi, C.S.; Ollis, D.F. Photocatalytic degradation of organic water contaminants: Mechanisms involving hydroxyl radical attack. J. Catal. 1990, 122, 178–192. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Nolan, N.T.; Pillai, S.C.; Seery, M.K.; Falaras, P.; Kontos, A.G.; Dunlop, P.S.M.; Hamilton, J.W.J.; Byrne, J.A.; O’Shea, K.; et al. A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl. Catal. B Environ. 2012, 125, 331–349. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting—The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Jing, D.; Guo, L.; Zhao, L.; Zhang, X.; Liu, H.; Li, M.; Shen, S.; Liu, G.; Hu, X.; Zhang, X.; et al. Efficient solar hydrogen production by photocatalytic water splitting: From fundamental study to pilot demonstration. Int. J. Hydrogen Energy 2010, 35, 7087–7097. [Google Scholar] [CrossRef]
- Hafeez, H.Y.; Lakhera, S.K.; Bellamkonda, S.; Rao, G.R.; Shankar, M.V.; Bahnemann, D.W.; Neppolian, B. Construction of ternary hybrid layered reduced graphene oxide supported g-C3N4-TiO2 nanocomposite and its photocatalytic hydrogen production activity. Int. J. Hydrogen Energy 2018, 43, 3892–3904. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Pichat, P. Photocatalysis and Water Purification: From Fundamentals to Recent Applications; Wiley: Weinheim, Germany, 2013. [Google Scholar]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Qi, K.; Cheng, B.; Yu, J.; Ho, W. A review on TiO2 -based Z-scheme photocatalysts. Chin. J. Catal. 2017, 38, 1936–1955. [Google Scholar] [CrossRef]
- Calvete, M.J.F.; Piccirillo, G.; Vinagreiro, C.S.; Pereira, M.M. Hybrid materials for heterogeneous photocatalytic degradation of antibiotics. Coord. Chem. Rev. 2019, 395, 63–85. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic materials: Possibilities and challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef] [PubMed]
- Pasternak, S.; Paz, Y. On the similarity and dissimilarity between photocatalytic water splitting and photocatalytic degradation of pollutants. ChemPhysChem 2013, 14, 2059–2070. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Chen, Z.; Hu, J.; Li, S.; Wang, Z.; Liu, J.; Wang, X. Semiconductor heterojunction photocatalysts: Design, construction, and photocatalytic performances. Chem. Soc. Rev. 2014, 43, 5234–5244. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; Martin, D.J.; Guo, Z.X.; Tang, J. Visible-light driven heterojunction photocatalysts for water splitting-a critical review. Energy Environ. Sci. 2015, 8, 731–759. [Google Scholar] [CrossRef]
- Moniz, S.J.A.; Shevlin, S.A.; An, X.; Guo, Z.X.; Tang, J. Fe2O3-TiO2 nanocomposites for enhanced charge separation and photocatalytic activity. Chem. A Eur. J. 2014, 20, 15571–15579. [Google Scholar] [CrossRef]
- Macías-Tamez, R.; Villanueva-Rodríguez, M.; Ramos-Delgado, N.A.; Maya-Treviño, L.; Hernández-Ramírez, A. Comparative Study of the Photocatalytic Degradation of the Herbicide 2,4-D Using WO3/TiO2 and Fe2O3/TiO2 as Catalysts. Water Air Soil Pollut. 2017, 228, 379. [Google Scholar] [CrossRef]
- Luo, L.; Long, J.; Zhao, S.; Dai, J.; Ma, L.; Wang, H.; Xia, L.; Shu, L.; Jiang, F. Effective visible-light-driven photocatalytic degradation of 17α-ethynylestradiol by crosslinked CdS nano-rod/TiO2 (B) nano-belt composite. Process Saf. Environ. Prot. 2019, 130, 77–85. [Google Scholar] [CrossRef]
- Marschall, R. Semiconductor composites: Strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv. Funct. Mater. 2014, 24, 2421–2440. [Google Scholar] [CrossRef]
- Kaur, A.; Salunke, D.B.; Umar, A.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Visible light driven photocatalytic degradation of fluoroquinolone levofloxacin drug using Ag2 O/TiO2 quantum dots: A mechanistic study and degradation pathway. New J. Chem. 2017, 41, 12079–12090. [Google Scholar] [CrossRef]
- Gou, J.; Ma, Q.; Deng, X.; Cui, Y.; Zhang, H.; Cheng, X.; Li, X.; Xie, M.; Cheng, Q. Fabrication of Ag2O/TiO2-Zeolite composite and its enhanced solar light photocatalytic performance and mechanism for degradation of norfloxacin. Chem. Eng. J. 2017, 308, 818–826. [Google Scholar] [CrossRef]
- Dong, P.; Hou, G.; Xi, X.; Shao, R.; Dong, F. WO3-based photocatalysts: Morphology control, activity enhancement and multifunctional applications. Environ. Sci. Nano 2017, 4, 539–557. [Google Scholar] [CrossRef]
- Mugunthan, E.; Saidutta, M.B.; Jagadeeshbabu, P.E. Visible light assisted photocatalytic degradation of diclofenac using TiO2-WO3 mixed oxide catalysts. Environ. Nanotechnol. Monit. Manag. 2018, 10, 322–330. [Google Scholar]
- Cordero-García, A.; Turnes Palomino, G.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Maya-Teviño, L.; Hernández-Ramírez, A. Photocatalytic behaviour of WO3/TiO2-N for diclofenac degradation using simulated solar radiation as an activation source. Environ. Sci. Pollut. Res. 2017, 24, 4613–4624. [Google Scholar] [CrossRef]
- Cordero-García, A.; Guzmán-Mar, J.L.; Hinojosa-Reyes, L.; Ruiz-Ruiz, E.; Hernández-Ramírez, A. Effect of carbon doping on WO3/TiO2 coupled oxide and its photocatalytic activity on diclofenac degradation. Ceram. Int. 2016, 42, 9796–9803. [Google Scholar] [CrossRef]
- Pérez-Estrada, L.A.; Malato, S.; Gernjak, W.; Agüera, A.; Thurman, E.M.; Ferrer, I.; Fernández-Alba, A.R. Photo-fenton degradation of diclofenac: Identification of main intermediates and degradation pathway. Environ. Sci. Technol. 2005, 39, 8300–8306. [Google Scholar] [CrossRef]
- Salaeh, S.; Juretic Perisic, D.; Biosic, M.; Kusic, H.; Babic, S.; Lavrencic Stangar, U.; Dionysiou, D.D.; Loncaric Bozic, A. Diclofenac removal by simulated solar assisted photocatalysis using TiO2-based zeolite catalyst; mechanisms, pathways and environmental aspects. Chem. Eng. J. 2016, 304, 289–302. [Google Scholar] [CrossRef]
- Arce-Sarria, A.; Machuca-Martínez, F.; Bustillo-Lecompte, C.; Hernández-Ramírez, A.; Colina-Márquez, J. Degradation and loss of antibacterial activity of commercial amoxicillin with TiO2/WO3-assisted solar photocatalysis. Catalysts 2018, 8, 222. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Mirmasoomi, S.R.; Mehdipour Ghazi, M.; Galedari, M. Photocatalytic degradation of diazinon under visible light using TiO2/Fe2O3 nanocomposite synthesized by ultrasonic-assisted impregnation method. Sep. Purif. Technol. 2017, 175, 418–427. [Google Scholar] [CrossRef]
- Li, R.; Liu, J.; Jia, Y.; Zhen, Q. Photocatalytic degradation mechanism of oxytetracyclines using Fe2O3-TiO2 nanopowders. J. Nanosci. Nanotechnol. 2017, 17, 3010–3015. [Google Scholar] [CrossRef]
- Li, R.; Jia, Y.; Wu, J.; Zhen, Q. Photocatalytic degradation and pathway of oxytetracycline in aqueous solution by Fe2O3-TiO2 nanopowder. RSC Adv. 2015, 5, 40764–40771. [Google Scholar] [CrossRef]
- Lu, C.; Guan, W.; Zhang, G.; Ye, L.; Zhou, Y.; Zhang, X. TiO2 /Fe2O3/CNTs magnetic photocatalyst: A fast and convenient synthesis and visible-light-driven photocatalytic degradation of tetracycline. Micro Nano Lett. 2013, 8, 749–752. [Google Scholar] [CrossRef]
- Zheng, X.; Fu, W.; Kang, F.; Peng, H.; Wen, J. Enhanced photo-Fenton degradation of tetracycline using TiO2 -coated α-Fe2O3 core–shell heterojunction. J. Ind. Eng. Chem. 2018, 68, 14–23. [Google Scholar] [CrossRef]
- Casbeer, E.; Sharma, V.K.; Li, X.Z. Synthesis and photocatalytic activity of ferrites under visible light: A review. Sep. Purif. Technol. 2012, 87, 1–14. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; de la Peña O’Shea, V.A.; Keller, N. Ferrite Materials for Photoassisted Environmental and Solar Fuels Applications. Top. Curr. Chem. 2020, 378, 6. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, Q.; Wang, J.; Song, Y. The fabrication of magnetic recyclable nitrogen-doped titanium dioxide/calcium ferrite/diatomite heterojunction nanocomposite for improved visible-light-driven degradation of tetracycline. J. Chem. Technol. Biotechnol. 2019, 94, 2702–2712. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, K. Fabrication of magnetically recyclable Ce/N co-doped TiO2/NiFe2O4/diatomite ternary hybrid: Improved photocatalytic efficiency under visible light irradiation. J. Alloys Compd. 2017, 697, 161–173. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, Z. The fabrication of magnetic recyclable nitrogen modified titanium dioxide/strontium ferrite/diatomite heterojunction nanocomposite for enhanced visible-light-driven photodegradation of tetracycline. Int. J. Hydrogen Energy 2019, 44, 8261–8272. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Doong, R.A. Heterostructured ZnFe2O4/TiO2 nanocomposites with a highly recyclable visible-light-response for bisphenol a degradation. RSC Adv. 2017, 7, 50006–50016. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, Z.; Wang, B.; An, H.; Chen, Z.; Cui, H. Adsorption and photocatalytic degradation of tetracycline hydrochloride using a palygorskite-supported Cu2O-TiO2 composite. Appl. Clay Sci. 2016, 119, 311–320. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, X.; Dong, H.; Li, S.; Li, X.; Li, L. Efficient photocatalytic degradation of tetrabromodiphenyl ethers and simultaneous hydrogen production by TiO2-Cu2O composite films in N2 atmosphere: Influencing factors, kinetics and mechanism. J. Hazard. Mater. 2017, 340, 1–15. [Google Scholar] [CrossRef]
- Sud, D.; Syal, A. Investigations on the Phase Transformation, Optical Characteristics, and Photocatalytic Activity of Synthesized Heterostructured Nanoporous Bi2O3–TiO2. J. Chin. Chem. Soc. 2016, 63, 776–783. [Google Scholar] [CrossRef]
- Sood, S.; Mehta, S.K.; Sinha, A.S.K.; Kansal, S.K. Bi2O3/TiO2 heterostructures: Synthesis, characterization and their application in solar light mediated photocatalyzed degradation of an antibiotic, ofloxacin. Chem. Eng. J. 2016, 290, 45–52. [Google Scholar] [CrossRef]
- Kaur, A.; Umar, A.; Anderson, W.A.; Kansal, S.K. Facile synthesis of CdS/TiO2 nanocomposite and their catalytic activity for ofloxacin degradation under visible illumination. J. Photochem. Photobiol. A Chem. 2018, 360, 34–43. [Google Scholar] [CrossRef]
- Kušić, H.; Leszczynska, D. Altered toxicity of organic pollutants in water originated from simultaneous exposure to UV photolysis and CdSe/ZnS quantum dots. Chemosphere 2012, 89, 900–906. [Google Scholar] [CrossRef]
- Ning, X.; Li, J.; Yang, B.; Zhen, W.; Li, Z.; Tian, B.; Lu, G. Inhibition of photocorrosion of CdS via assembling with thin film TiO2 and removing formed oxygen by artificial gill for visible light overall water splitting. Appl. Catal. B Environ. 2017, 212, 129–139. [Google Scholar] [CrossRef]
- Pan, Y.X.; Zhou, T.; Han, J.; Hong, J.; Wang, Y.; Zhang, W.; Xu, R. CdS quantum dots and tungsten carbide supported on anatase-rutile composite TiO2 for highly efficient visible-light-driven photocatalytic H2 evolution from water. Catal. Sci. Technol. 2016, 6, 2206–2213. [Google Scholar] [CrossRef]
- Zhu, R.; Yang, R.; Hu, L.; Chen, B. Preparation of Z-Scheme system of CdS-RGO-BiVO4 and its activity for hydrogen production. Int. J. Hydrogen Energy 2019, 44, 25119–25128. [Google Scholar] [CrossRef]
- Kandi, D.; Behera, A.; Martha, S.; Naik, B.; Parida, K.M. Quantum confinement chemistry of CdS QDs plus hot electron of Au over TiO2 nanowire protruding to be encouraging photocatalyst towards nitrophenol conversion and ciprofloxacin degradation. J. Environ. Chem. Eng. 2019, 7, 102821. [Google Scholar] [CrossRef]
- Li, W.; Ding, H.; Ji, H.; Dai, W.; Guo, J.; Du, G. Photocatalytic degradation of tetracycline hydrochloride via a CdS-TiO2 heterostructure composite under visible light irradiation. Nanomaterials 2018, 8, 415. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, M.; Xin, Y.; Chai, C.; Chen, Q. Construction of immobilized CuS/TiO2 nanobelts heterojunction photocatalyst for photocatalytic degradation of enrofloxacin: Synthesis, characterization, influencing factors and mechanism insight. J. Chem. Technol. Biotechnol. 2019, 94, 2219–2228. [Google Scholar]
- Chen, Q.; Wu, S.; Xin, Y. Synthesis of Au-CuS-TiO2 nanobelts photocatalyst for efficient photocatalytic degradation of antibiotic oxytetracycline. Chem. Eng. J. 2016, 302, 377–387. [Google Scholar] [CrossRef]
- Irandost, M.; Akbarzadeh, R.; Pirsaheb, M.; Asadi, A.; Mohammadi, P.; Sillanpää, M. Fabrication of highly visible active N, S co-doped TiO2@MoS2 heterojunction with synergistic effect for photocatalytic degradation of diclofenac: Mechanisms, modeling and degradation pathway. J. Mol. Liq. 2019, 291, 111342. [Google Scholar] [CrossRef]
- Wu, L.; Shi, S.; Li, Q.; Zhang, X.; Cui, X. TiO2 nanoparticles modified with 2D MoSe2 for enhanced photocatalytic activity on hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 720–728. [Google Scholar] [CrossRef]
- Du, J.; Wang, H.; Yang, M.; Zhang, F.; Wu, H.; Cheng, X.; Yuan, S.; Zhang, B.; Li, K.; Wang, Y.; et al. Highly efficient hydrogen evolution catalysis based on MoS2/CdS/TiO2 porous composites. Int. J. Hydrogen Energy 2018, 43, 9307–9315. [Google Scholar] [CrossRef]
- Kumar, N.; Bhadwal, A.S.; Mizaikoff, B.; Singh, S.; Kranz, C. Electrochemical detection and photocatalytic performance of MoS2/TiO2 nanocomposite against pharmaceutical contaminant: Paracetamol. Sens. Bio-Sens. Res. 2019, 24, 100288. [Google Scholar] [CrossRef]
- Deng, L.; Liu, H.; Gao, X.; Su, X.; Zhu, Z. SnS2/TiO2 nanocomposites with enhanced visible light-driven photoreduction of aqueous Cr(VI). Ceram. Int. 2016, 42, 3808–3815. [Google Scholar] [CrossRef]
- Kovacic, M.; Kopcic, N.; Kusic, H.; Bozic, A.L. Solar driven degradation of 17β-estradiol using composite photocatalytic materials and artificial irradiation source: Influence of process and water matrix parameters. J. Photochem. Photobiol. A Chem. 2018, 361, 48–61. [Google Scholar] [CrossRef]
- Kovacic, M.; Papac, J.; Kusic, H.; Karamanis, P.; Loncaric Bozic, A. Degradation of polar and non-polar pharmaceutical pollutants in water by solar assisted photocatalysis using hydrothermal TiO2-SnS2. Chem. Eng. J. 2019, 382, 122826. [Google Scholar] [CrossRef]
- Yan, T.; Guan, W.; Li, W.; You, J. Ag3PO4 photocatalysts loaded on uniform SiO2 supports for efficient degradation of methyl orange under visible light irradiation. RSC Adv. 2014, 4, 37095–37099. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuart-Williams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z.; et al. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef]
- Ren, J.; Chai, Y.; Liu, Q.; Zhang, L.; Dai, W.L. Intercorrelated Ag3PO4 nanoparticles decorated with graphic carbon nitride: Enhanced stability and photocatalytic activities for water treatment. Appl. Surf. Sci. 2017, 403, 177–186. [Google Scholar] [CrossRef]
- Meng, S.; Ning, X.; Zhang, T.; Chen, S.F.; Fu, X. What is the transfer mechanism of photogenerated carriers for the nanocomposite photocatalyst Ag3PO4/g-C3N4, band-band transfer or a direct Z-scheme? Phys. Chem. Chem. Phys. 2015, 17, 11577–11585. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Lu, Y.; Qu, J.; Zhu, S.; Huo, M. A nano-composite comprised of Ti3+ -doped TiO2 nanotubes and Ag3PO4 quantum dots with enhanced photocatalytic activity under visible light. Mater. Lett. 2019, 240, 35–38. [Google Scholar] [CrossRef]
- Du, J.; Ma, S.; Yan, Y.; Li, K.; Zhao, F.; Zhou, J. Corn-silk-templated synthesis of TiO2 nanotube arrays with Ag3PO4 nanoparticles for efficient oxidation of organic pollutants and pathogenic bacteria under solar light. Colloids Surf. A Physicochem. Eng. Asp. 2019, 572, 237–249. [Google Scholar] [CrossRef]
- Liu, J.; Xie, F.; Li, R.; Li, T.; Jia, Z.; Wang, Y.; Wang, Y.; Zhang, X.; Fan, C. TiO2-x /Ag3PO4 photocatalyst: Oxygen vacancy dependent visible light photocatalytic performance and BPA degradative pathway. Mater. Sci. Semicond. Process. 2019, 97, 1–10. [Google Scholar] [CrossRef]
- Police, A.K.R.; Chennaiahgari, M.; Boddula, R.; Vattikuti, S.V.P.; Mandari, K.K.; Chan, B. Single-step hydrothermal synthesis of wrinkled graphene wrapped TiO2 nanotubes for photocatalytic hydrogen production and supercapacitor applications. Mater. Res. Bull. 2018, 98, 314–321. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, B.; Yang, Y.; Huang, E.; Ge, Z.; Zhang, T.; Zhang, S.; Li, L.; Guan, N.; Ma, Y.; et al. High activity of hot electrons from bulk 3D graphene materials for efficient photocatalytic hydrogen production. Nano Res. 2017, 10, 1662–1672. [Google Scholar] [CrossRef]
- Hao, X.; Li, M.; Zhang, L.; Wang, K.; Liu, C. Photocatalyst TiO2/WO3/GO nano-composite with high efficient photocatalytic performance for BPA degradation under visible light and solar light illumination. J. Ind. Eng. Chem. 2017, 55, 140–148. [Google Scholar] [CrossRef]
- Wang, G.; Chen, Q.; Xin, Y.; Liu, Y.; Zang, Z.; Hu, C.; Zhang, B. Construction of graphene-WO3/TiO2 nanotube array photoelectrodes and its enhanced performance for photocatalytic degradation of dimethyl phthalate. Electrochim. Acta 2016, 222, 1903–1913. [Google Scholar] [CrossRef]
- Bilgin Simsek, E.; Kilic, B.; Asgin, M.; Akan, A. Graphene oxide based heterojunction TiO2–ZnO catalysts with outstanding photocatalytic performance for bisphenol-A, ibuprofen and flurbiprofen. J. Ind. Eng. Chem. 2018, 59, 115–126. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Hou, Y.; Li, L. Hierarchical structured ZnFe2O4@RGO@TiO2 composite as powerful visible light catalyst for degradation of fulvic acid. J. Nanopart. Res. 2017, 19, 178. [Google Scholar] [CrossRef]
- Luo, L.j.; Li, J.; Dai, J.; Xia, L.; Barrow, C.J.; Wang, H.; Jegatheesan, J.; Yang, M. Bisphenol A removal on TiO2–MoS2–reduced graphene oxide composite by adsorption and photocatalysis. Process Saf. Environ. Prot. 2017, 112, 274–279. [Google Scholar] [CrossRef]
- Wang, W.; Han, Q.; Zhu, Z.; Zhang, L.; Zhong, S.; Liu, B. Enhanced photocatalytic degradation performance of organic contaminants by heterojunction photocatalyst BiVO4/TiO2/RGO and its compatibility on four different tetracycline antibiotics. Adv. Powder Technol. 2019, 30, 1882–1896. [Google Scholar] [CrossRef]
- Alcudia-Ramos, M.A.; Fuentez-Torres, M.O.; Ortiz-Chi, F.; Espinosa-González, C.G.; Hernández-Como, N.; García-Zaleta, D.S.; Kesarla, M.K.; Torres-Torres, J.G.; Collins-Martínez, V.; Godavarthi, S. Fabrication of g-C3N4/TiO2 heterojunction composite for enhanced photocatalytic hydrogen production. Ceram. Int. 2020, 46, 38–45. [Google Scholar] [CrossRef]
- Liu, C.; Wu, P.; Wu, J.; Hou, J.; Bai, H.; Liu, Z. Effective protect of oxygen vacancies in carbon layer coated black TiO2-x/CNNS hetero-junction photocatalyst. Chem. Eng. J. 2019, 359, 58–68. [Google Scholar] [CrossRef]
- Yang, Y.; Li, X.; Lu, C.; Huang, W. G-C3N4 Nanosheets Coupled with TiO2 Nanosheets as 2D/2D Heterojunction Photocatalysts Toward High Photocatalytic Activity for Hydrogen Production. Catal. Lett. 2019, 140, 2930–2939. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, X.; Wang, C.; Xu, J.; Du, X.; Li, L. Ti3+ defect mediated g-C3N4 /TiO2 Z-scheme system for enhanced photocatalytic redox performance. Appl. Surf. Sci. 2018, 448, 288–296. [Google Scholar] [CrossRef]
- Wang, J.; Wang, G.; Wang, X.; Wu, Y.; Su, Y.; Tang, H. 3D/2D direct Z-scheme heterojunctions of hierarchical TiO2 microflowers/g-C3N4 nanosheets with enhanced charge carrier separation for photocatalytic H2 evolution. Carbon 2019, 149, 618–626. [Google Scholar] [CrossRef]
- Pan, C.; Jia, J.; Hu, X.; Fan, J.; Liu, E. In situ construction of g-C3N4 /TiO2 heterojunction films with enhanced photocatalytic activity over magnetic-driven rotating frame. Appl. Surf. Sci. 2018, 430, 283–292. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.W.; Ma, D.; Fan, Z.; Lu, L.; Niu, C. In situ synthesis of C-doped TiO2@g-C3N4 core-shell hollow nanospheres with enhanced visible-light photocatalytic activity for H2 evolution. Chem. Eng. J. 2017, 322, 435–444. [Google Scholar] [CrossRef]
- Ma, J.; Zhou, W.; Tan, X.; Yu, T. Potassium ions intercalated into g-C3N4-modified TiO2 nanobelts for the enhancement of photocatalytic hydrogen evolution activity under visible-light irradiation. Nanotechnology 2018, 29, 215706. [Google Scholar] [CrossRef]
- Pan, J.; You, M.; Chi, C.; Dong, Z.; Wang, B.; Zhu, M.; Zhao, W.; Song, C.; Zheng, Y.; Li, C. The two dimension carbon quantum dots modified porous g-C3N4/TiO2 nano-heterojunctions for visible light hydrogen production enhancement. Int. J. Hydrogen Energy 2018, 43, 6586–6593. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Corredor, J.; Rivero, M.J.; Rangel, C.M.; Gloaguen, F.; Ortiz, I. Comprehensive review and future perspectives on the photocatalytic hydrogen production. J. Chem. Technol. Biotechnol. 2019, 94, 3049–3063. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4 -based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Huang, H.; He, M.; Yang, X.; Tian, Z.; Hu, J.; Wen, B. One-pot hydrothermal synthesis of TiO2/RCN heterojunction photocatalyst for production of hydrogen and rhodamine B degradation. Appl. Surf. Sci. 2019, 493, 202–211. [Google Scholar] [CrossRef]
- Yang, Z.; Yan, J.; Lian, J.; Xu, H.; She, X.; Li, H. g-C3N4/TiO2 Nanocomposites for Degradation of Ciprofloxacin under Visible Light Irradiation. ChemistrySelect 2016, 1, 5679–5685. [Google Scholar] [CrossRef]
- Li, G.; Nie, X.; Gao, Y.; An, T. Can environmental pharmaceuticals be photocatalytically degraded and completely mineralized in water using g-C3N4/TiO2 under visible light irradiation?-Implications of persistent toxic intermediates. Appl. Catal. B Environ. 2016, 180, 726–732. [Google Scholar] [CrossRef]
- Yu, S.; Wang, Y.; Sun, F.; Wang, R.; Zhou, Y. Novel mpg-C3N4/TiO2 nanocomposite photocatalytic membrane reactor for sulfamethoxazole photodegradation. Chem. Eng. J. 2018, 337, 183–192. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Shen, H.; Gu, Y.; Xu, T.; Zhu, Z.; Wang, G.; Chen, W. Solar-driven efficient degradation of emerging contaminants by g-C3N4-shielding polyester fiber/TiO2 composites. Appl. Catal. B Environ. 2019, 258, 117960. [Google Scholar] [CrossRef]
- Xie, X.; Chen, C.; Wang, X.; Li, J.; Naraginti, S. Efficient detoxification of triclosan by a S-Ag/TiO2@g-C3N4 hybrid photocatalyst: Process optimization and bio-toxicity assessment. RSC Adv. 2019, 9, 20439–20449. [Google Scholar] [CrossRef]
- Song, J.; Wu, X.; Zhang, M.; Liu, C.; Yu, J.; Sun, G.; Si, Y.; Ding, B. Highly flexible, core-shell heterostructured, and visible-light-driven titania-based nanofibrous membranes for antibiotic removal and E. coil inactivation. Chem. Eng. J. 2020, 379, 122269. [Google Scholar] [CrossRef]
- Yang, L.; Bai, X.; Shi, J.; Du, X.; Xu, L.; Jin, P. Quasi-full-visible-light absorption by D35-TiO2/g-C3N4 for synergistic persulfate activation towards efficient photodegradation of micropollutants. Appl. Catal. B Environ. 2019, 256, 117759. [Google Scholar] [CrossRef]
- Su, Y.; Chen, P.; Wang, F.; Zhang, Q.; Chen, T.; Wang, Y.; Yao, K.; Lv, W.; Liu, G. Decoration of TiO2/g-C3N4 Z-scheme by carbon dots as a novel photocatalyst with improved visible-light photocatalytic performance for the degradation of enrofloxacin. RSC Adv. 2017, 7, 34096–34103. [Google Scholar] [CrossRef]
- Wang, F.; Chen, P.; Feng, Y.; Xie, Z.; Liu, Y.; Su, Y.; Zhang, Q.; Wang, Y.; Yao, K.; Lv, W.; et al. Facile synthesis of N-doped carbon dots/g-C3N4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of indomethacin. Appl. Catal. B Environ. 2017, 207, 103–113. [Google Scholar] [CrossRef]
- Nie, Y.C.; Yu, F.; Wang, L.C.; Xing, Q.J.; Liu, X.; Pei, Y.; Zou, J.P.; Dai, W.L.; Li, Y.; Suib, S.L. Photocatalytic degradation of organic pollutants coupled with simultaneous photocatalytic H2 evolution over graphene quantum dots/Mn-N-TiO2/g-C3N4 composite catalysts: Performance and mechanism. Appl. Catal. B Environ. 2018, 227, 312–321. [Google Scholar] [CrossRef]
- Jo, W.K.; Adinaveen, T.; Vijaya, J.J.; Sagaya Selvam, N.C. Synthesis of MoS2 nanosheet supported Z-scheme TiO2/g-C3N4 photocatalysts for the enhanced photocatalytic degradation of organic water pollutants. RSC Adv. 2016, 6, 10487–10497. [Google Scholar]
- Tahir, M.B.; Sagir, M.; Shahzad, K. Removal of acetylsalicylate and methyl-theobromine from aqueous environment using nano-photocatalyst WO3-TiO2 @g-C3N4 composite. J. Hazard. Mater. 2019, 363, 205–213. [Google Scholar] [CrossRef]
- Schubert, J.S.; Popovic, J.; Haselmann, G.M.; Nandan, S.P.; Wang, J.; Giesriegl, A.; Cherevan, A.S.; Eder, D. Immobilization of Co, Mn, Ni and Fe oxide co-catalysts on TiO2 for photocatalytic water splitting reactions. J. Mater. Chem. A 2019, 7, 18568–18579. [Google Scholar] [CrossRef]
- Patil, S.B.; Basavarajappa, P.S.; Ganganagappa, N.; Jyothi, M.S.; Raghu, A.V.; Reddy, K.R. Recent advances in non-metals-doped TiO2 nanostructured photocatalysts for visible-light driven hydrogen production, CO2 reduction and air purification. Int. J. Hydrogen Energy 2019, 44, 13022–13039. [Google Scholar] [CrossRef]
- Yang, Q.; Dong, L.; Su, R.; Hu, B.; Wang, Z.; Jin, Y.; Wang, Y.; Besenbacher, F.; Dong, M. Nanostructured heterogeneous photo-catalysts for hydrogen production and water splitting: A comprehensive insight. Appl. Mater. Today 2019, 17, 159–182. [Google Scholar] [CrossRef]
- Bhagya, T.C.; Krishnan, A.; Arunima Rajan, S.; Ameen Sha, M.; Sreelekshmy, B.R.; Jineesh, P.; Shibli, S.M.A. Exploration and evaluation of proton source-assisted photocatalyst for hydrogen generation. Photochem. Photobiol. Sci. 2019, 18, 1716–1726. [Google Scholar] [CrossRef]
- Hernández-Majalca, B.C.; Meléndez-Zaragoza, M.J.; Salinas-Gutiérrez, J.M.; López-Ortiz, A.; Collins-Martínez, V. Visible-light photo-assisted synthesis of GO-TiO2 composites for the photocatalytic hydrogen production. Int. J. Hydrogen Energy 2019, 44, 12381–12389. [Google Scholar] [CrossRef]
- Wang, D.; Saleh, N.B.; Sun, W.; Park, C.M.; Shen, C.; Aich, N.; Peijnenburg, W.J.G.M.; Zhang, W.; Jin, Y.; Su, C. Next-Generation Multifunctional Carbon–Metal Nanohybrids for Energy and Environmental Applications. Environ. Sci. Technol. 2019, 53, 7265–7287. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Reddy, N.L.; Kumari, M.M.; Cheralathan, K.K.; Ravi, P.; Sathish, M.; Neppolian, B.; Reddy, K.R.; Shetti, N.P.; Prathap, P.; et al. Sustainable hydrogen production for the greener environment by quantum dots-based efficient photocatalysts: A review. J. Environ. Manag. 2019, 248, 109246. [Google Scholar] [CrossRef]
- Yi, L.; Lan, F.; Li, J.; Zhao, C. Efficient Noble-Metal-Free Co-NG/TiO2 Photocatalyst for H2 Evolution: Synergistic Effect between Single-Atom Co and N-Doped Graphene for Enhanced Photocatalytic Activity. ACS Sustain. Chem. Eng. 2018, 6, 12766–12775. [Google Scholar] [CrossRef]
- Rabin, N.N.; Ohmagari, H.; Islam, M.S.; Karim, M.R.; Hayami, S. A procession on photocatalyst for solar fuel production and waste treatment. J. Incl. Phenom. Macrocycl. Chem. 2019, 94, 263–281. [Google Scholar] [CrossRef]
- Lv, T.; Wang, H.; Hong, W.; Wang, P.; Jia, L. In situ self-assembly synthesis of sandwich-like TiO2/reduced graphene oxide/LaFeO3 Z-scheme ternary heterostructure towards enhanced photocatalytic hydrogen production. Mol. Catal. 2019, 475, 110497. [Google Scholar] [CrossRef]
- Iwase, A.; Ng, Y.H.; Ishiguro, Y.; Kudo, A.; Amal, R. Reduced Graphene Oxide as a Solid-State Electron Mediator in Z-Scheme Photocatalytic Water Splitting under Visible Light. J. Am. Chem. Soc. 2011, 133, 11054–11057. [Google Scholar] [CrossRef]
- Samal, A.; Das, D.P. Transfiguring UV light active “metal oxides” to visible light active photocatayst by reduced graphene oxide hypostatization. Catal. Today 2018, 300, 124–135. [Google Scholar] [CrossRef]
- Ida, S.; Wilson, P.; Neppolian, B.; Sathish, M.; Karthik, P.; Ravi, P. Ultrasonically aided selective stabilization of pyrrolic type nitrogen by one pot nitrogen doped and hydrothermally reduced Graphene oxide/Titania nanocomposite (N-TiO2/N-RGO) for H2 production. Ultrason. Sonochem. 2019, 57, 62–72. [Google Scholar] [CrossRef]
- Olowoyo, J.O.; Kumar, M.; Jain, S.L.; Babalola, J.O.; Vorontsov, A.V.; Kumar, U. Insights into Reinforced Photocatalytic Activity of the CNT-TiO2 Nanocomposite for CO2 Reduction and Water Splitting. J. Phys. Chem. C 2019, 123, 367–378. [Google Scholar] [CrossRef]
- Umer, M.; Tahir, M.; Azam, M.U.; Tahir, B.; Jaffar, M.M.; Alias, H. Montmorillonite dispersed single wall carbon nanotubes (SWCNTs)/TiO2 heterojunction composite for enhanced dynamic photocatalytic H2 production under visible light. Appl. Clay Sci. 2019, 174, 110–119. [Google Scholar] [CrossRef]
- Bellamkonda, S.; Thangavel, N.; Hafeez, H.Y.; Neppolian, B.; Ranga Rao, G. Highly active and stable multi-walled carbon nanotubes-graphene-TiO2 nanohybrid: An efficient non-noble metal photocatalyst for water splitting. Catal. Today 2019, 321, 120–127. [Google Scholar] [CrossRef]
- Haque, F.; Daeneke, T.; Kalantar-zadeh, K.; Ou, J.Z. Two-Dimensional Transition Metal Oxide and Chalcogenide-Based Photocatalysts. Nano-Micro Lett. 2018, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Camposeco, R.; Castillo, S.; Rodriguez-González, V.; Hinojosa-Reyes, M.; Medina-Álvares, M.I.; Mejía-Centeno, I. Promotional effect of Rh nanoparticles on WO3/TiO2 titanate nanotube photocatalysts for boosted hydrogen production. J. Photochem. Photobiol. A Chem. 2018, 353, 114–121. [Google Scholar] [CrossRef]
- Ren, X.; Gao, P.; Kong, X.; Jiang, R.; Yang, P.; Chen, Y.; Chi, Q.; Li, B. NiO/Ni/TiO2 nanocables with Schottky/p-n heterojunctions and the improved photocatalytic performance in water splitting under visible light. J. Colloid Interface Sci. 2018, 530, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Madhumitha, A.; Preethi, V.; Kanmani, S. Photocatalytic hydrogen production using TiO2 coated iron-oxide core shell particles. Int. J. Hydrogen Energy 2018, 43, 3946–3956. [Google Scholar] [CrossRef]
- Xie, M.Y.; Su, K.Y.; Peng, X.Y.; Wu, R.J.; Chavali, M.; Chang, W.C. Hydrogen production by photocatalytic water-splitting on Pt-doped TiO2–ZnO under visible light. J. Taiwan Inst. Chem. Eng. 2017, 70, 161–167. [Google Scholar] [CrossRef]
- Chen, Q.; Tong, R.; Chen, X.; Xue, Y.; Xie, Z.; Kuang, Q.; Zheng, L. Ultrafine ZnO quantum dot-modified TiO2 composite photocatalysts: The role of the quantum size effect in heterojunction-enhanced photocatalytic hydrogen evolution. Catal. Sci. Technol. 2018, 8, 1296–1303. [Google Scholar] [CrossRef]
- Liu, J.; Ke, J.; Li, Y.; Liu, B.; Wang, L.; Xiao, H.; Wang, S. Co3O4 quantum dots/TiO2 nanobelt hybrids for highly efficient photocatalytic overall water splitting. Appl. Catal. B Environ. 2018, 236, 396–403. [Google Scholar] [CrossRef]
- Zhang, Q.; Hai, Z.; Jian, A.; Xu, H.; Xue, C.; Sang, S. Synthesis of p-Co3O4/n-TiO2 nanoparticles for overall water splitting under visible light irradiation. Nanomaterials 2016, 6, 138. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Gao, H.; Hu, J.; Mao, J.; Liang, C.; Zhang, P.; Shao, G. 3D CuO network supported TiO2 nanosheets with applications for energy storage and water splitting. Sci. Adv. Mater. 2016, 8, 1256–1262. [Google Scholar] [CrossRef]
- Wei, T.; Zhu, Y.-N.; An, X.; Liu, L.-M.; Cao, X.; Liu, H.; Qu, J. Defect Modulation of Z-Scheme TiO2 /Cu2O Photocatalysts for Durable Water Splitting. ACS Catal. 2019, 9, 8346–8354. [Google Scholar] [CrossRef]
- Rao, V.N.; Reddy, N.L.; Kumari, M.M.; Ravi, P.; Sathish, M.; Neppolian, B.; Shankar, M.V. Synthesis of titania wrapped cadmium sulfide nanorods for photocatalytic hydrogen generation. Mater. Res. Bull. 2018, 103, 122–132. [Google Scholar] [CrossRef]
- Du, J.; Wang, H.; Yang, M.; Li, K.; Zhao, L.; Zhao, G.; Li, S.; Gu, X.; Zhou, Y.; Wang, L.; et al. Pyramid-like CdS nanoparticles grown on porous TiO2 monolith: An advanced photocatalyst for H2 production. Electrochim. Acta 2017, 250, 99–107. [Google Scholar] [CrossRef]
- Peng, R.; Wu, C.M.; Baltrusaitis, J.; Dimitrijevic, N.M.; Rajh, T.; Koodali, R.T. Solar hydrogen generation over CdS incorporated in Ti-MCM-48 mesoporous materials under visible light illumination. Int. J. Hydrogen Energy 2016, 41, 4106–4119. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, C.F. Recent advances in the TiO2/CdS nanocomposite used for photocatalytic hydrogen production and quantum-dot-sensitized solar cells. Renew. Sustain. Energy Rev. 2016, 54, 1048–1059. [Google Scholar] [CrossRef]
- Qi, L.; Yu, J.; Jaroniec, M. Preparation and enhanced visible-light photocatalytic H2- production activity of CdS-sensitized Pt/TiO2 nanosheets with exposed (001) facets. Phys. Chem. Chem. Phys. 2011, 13, 8915–8923. [Google Scholar] [CrossRef]
- Li, C.; Yuan, J.; Han, B.; Jiang, L.; Shangguan, W. TiO2 nanotubes incorporated with CdS for photocatalytic hydrogen production from splitting water under visible light irradiation. Int. J. Hydrogen Energy 2010, 35, 7073–7079. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Yan, W.; Wu, Y.P.; Wang, Z.H. Synthesis of TiO2 nanotubes coupled with CdS nanoparticles and production of hydrogen by photocatalytic water decomposition. Mater. Lett. 2008, 62, 3846–3848. [Google Scholar] [CrossRef]
- Chandra, M.; Bhunia, K.; Pradhan, D. Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting. Inorg. Chem. 2018, 57, 4524–4533. [Google Scholar] [CrossRef]
- Dang, H.; Cheng, Z.; Yang, W.; Chen, W.; Huang, W.; Li, B.; Shi, Z.; Qiu, Y.; Dong, X.; Fan, H. Room-temperature synthesis of CuxS (x = 1 or 2) co-modified TiO2 nanocomposite and its highly efficient photocatalytic H2 production activity. J. Alloys Compd. 2017, 709, 422–430. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, J.; Sun, H.; Ng, Y.H.; Zhang, K.Q.; Lai, Y. MoS2 Quantum Dots@TiO2 Nanotube Arrays: An Extended-Spectrum-Driven Photocatalyst for Solar Hydrogen Evolution. ChemSusChem 2018, 11, 1708–1721. [Google Scholar] [CrossRef] [PubMed]
- Ng, B.J.; Putri, L.K.; Tan, L.L.; Pasbakhsh, P.; Chai, S.P. All-solid-state Z-scheme photocatalyst with carbon nanotubes as an electron mediator for hydrogen evolution under simulated solar light. Chem. Eng. J. 2017, 316, 41–49. [Google Scholar] [CrossRef]
- Yang, C.; Qin, J.; Rajendran, S.; Zhang, X.; Liu, R. WS2 and C-TiO2 Nanorods Acting as Effective Charge Separators on g-C3N4 to Boost Visible-Light Activated Hydrogen Production from Seawater. ChemSusChem 2018, 11, 4077–4085. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Xu, C.; Jin, C.; Fan, J.; Hu, X. Carbon quantum dots bridged TiO2 and Cd0.5Zn0.5S film as solid-state Z-scheme photocatalyst with enhanced H2 evolution activity. J. Taiwan Inst. Chem. Eng. 2019, 97, 316–325. [Google Scholar] [CrossRef]
- Zou, Y.; Shi, J.W.; Ma, D.; Fan, Z.; Niu, C.; Wang, L. Fabrication of g-C3N4/Au/C-TiO2 Hollow Structures as Visible-Light-Driven Z-Scheme Photocatalysts with Enhanced Photocatalytic H2 Evolution. ChemCatChem 2017, 9, 3752–3761. [Google Scholar] [CrossRef]
- Yang, G.; Ding, H.; Chen, D.; Feng, J.; Hao, Q.; Zhu, Y. Construction of urchin-like ZnIn2S4-Au-TiO2 heterostructure with enhanced activity for photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2018, 234, 260–267. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Y.; Dai, C.; Liang, Q.; Yang, P.; Yang, H.; Yan, J. Constructing ZnO/ZnCr2O4@TiO2 -NTA Nanocomposite for Photovoltaic Conversion and Photocatalytic Hydrogen Evolution. J. Electron. Mater. 2019, 48, 1724–1729. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, J.; Dai, K.; Liang, C. Construction of flourinated-TiO2 nanosheets with exposed {001} facets/CdSe-DETA nanojunction for enhancing visible-light-driven photocatalytic H2 evolution. Ceram. Int. 2020, 46, 866–876. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lim, S.K.; Park, H.; Hoffmann, M.R.; Kim, S. Trilayer CdS/carbon nanofiber (CNF) mat/Pt-TiO2 composite structures for solar hydrogen production: Effects of CNF mat thickness. Appl. Catal. B Environ. 2016, 196, 216–222. [Google Scholar] [CrossRef]
- Dai, K.; Lv, J.; Zhang, J.; Zhu, G.; Geng, L.; Liang, C. Efficient Visible-Light-Driven Splitting of Water into Hydrogen over Surface-Fluorinated Anatase TiO2 Nanosheets with Exposed {001} Facets/Layered CdS-Diethylenetriamine Nanobelts. ACS Sustain. Chem. Eng. 2018, 6, 12817–12826. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, Z.; Cui, X.; Liu, H.; Tai, C.; Song, Y. Fabrication of Hollow Mesoporous CdS@TiO2@Au Microspheres with High Photocatalytic Activity for Hydrogen Evolution from Water under Visible Light. ACS Sustain. Chem. Eng. 2018, 6, 13766–13777. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, M.; Liu, J.; Deng, Z.; Li, Y.; Su, B.L. Synergistic promotion of solar-driven H2 generation by three-dimensionally ordered macroporous structured TiO2-Au-CdS ternary photocatalyst. Appl. Catal. B Environ. 2016, 184, 182–190. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, J.; Liu, C.; Gong, Y.; Wang, R.; He, B.; Wang, H. Rational Design and Fabrication of Noble-metal-free NixP Cocatalyst Embedded 3D N-TiO2/g-C3N4 Heterojunctions with Enhanced Photocatalytic Hydrogen Evolution. ChemCatChem 2018, 10, 3069–3077. [Google Scholar] [CrossRef]
- Sampath, S.; Sellappa, K. Visible-light-driven photocatalysts for hydrogen production by water splitting. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 42, 719–729. [Google Scholar] [CrossRef]
- Azam, M.U.; Tahir, M.; Umer, M.; Tahir, B.; Shehzad, N.; Siraj, M. In-situ synthesis of TiO2/La2O2CO3/rGO composite under acidic/basic treatment with La3+/Ti3+ as mediators for boosting photocatalytic H2 evolution. Int. J. Hydrogen Energy. 2019, 44, 23669–23688. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, M.; Xiong, J.; Yan, Y.; Li, W.; Cheng, G. Electrostatically assembled construction of ternary TiO2-Cu@C hybrid with enhanced solar-to-hydrogen evolution employing amorphous carbon dots as electronic mediator. Chem. Eng. J. 2019, 375, 121902. [Google Scholar] [CrossRef]
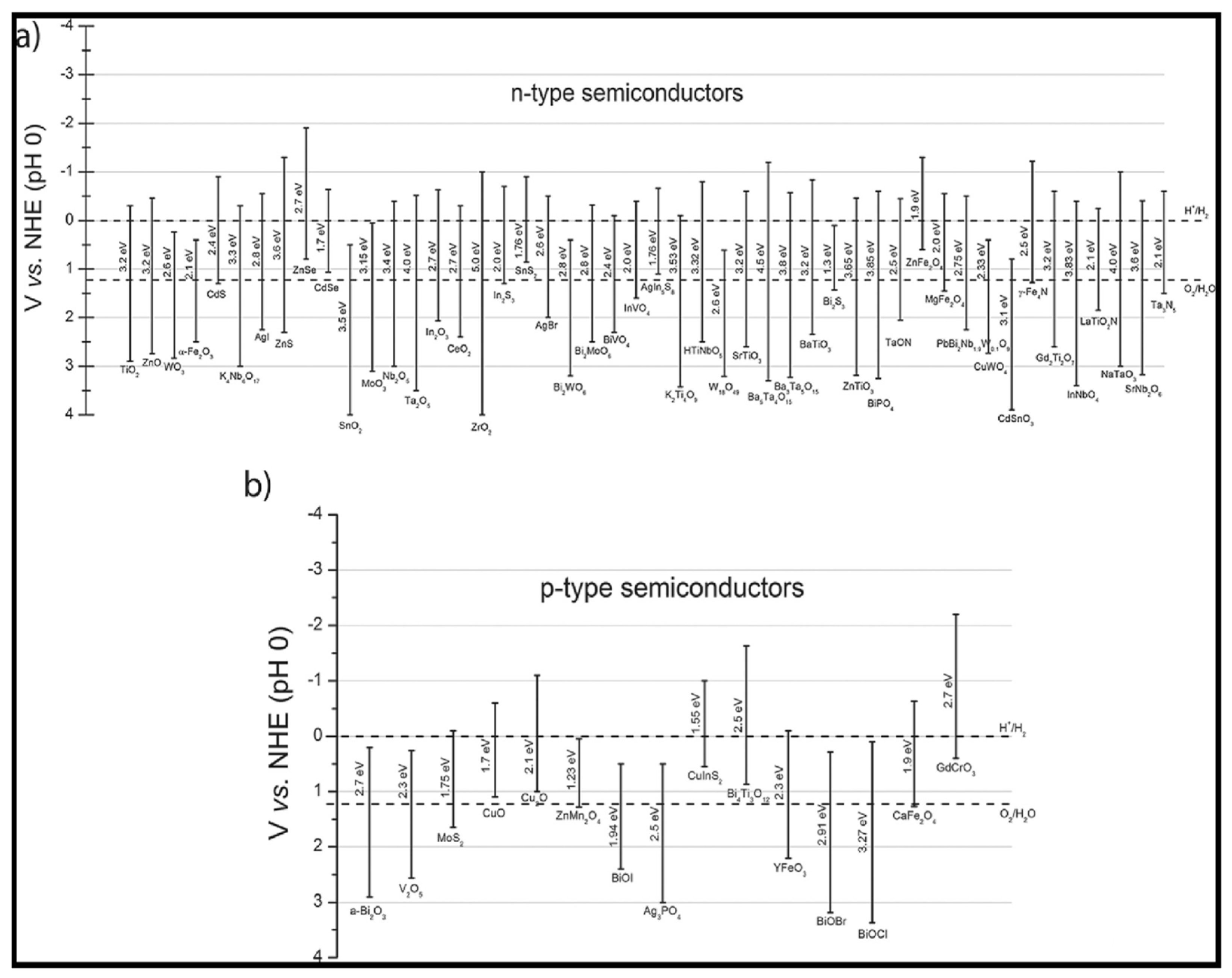
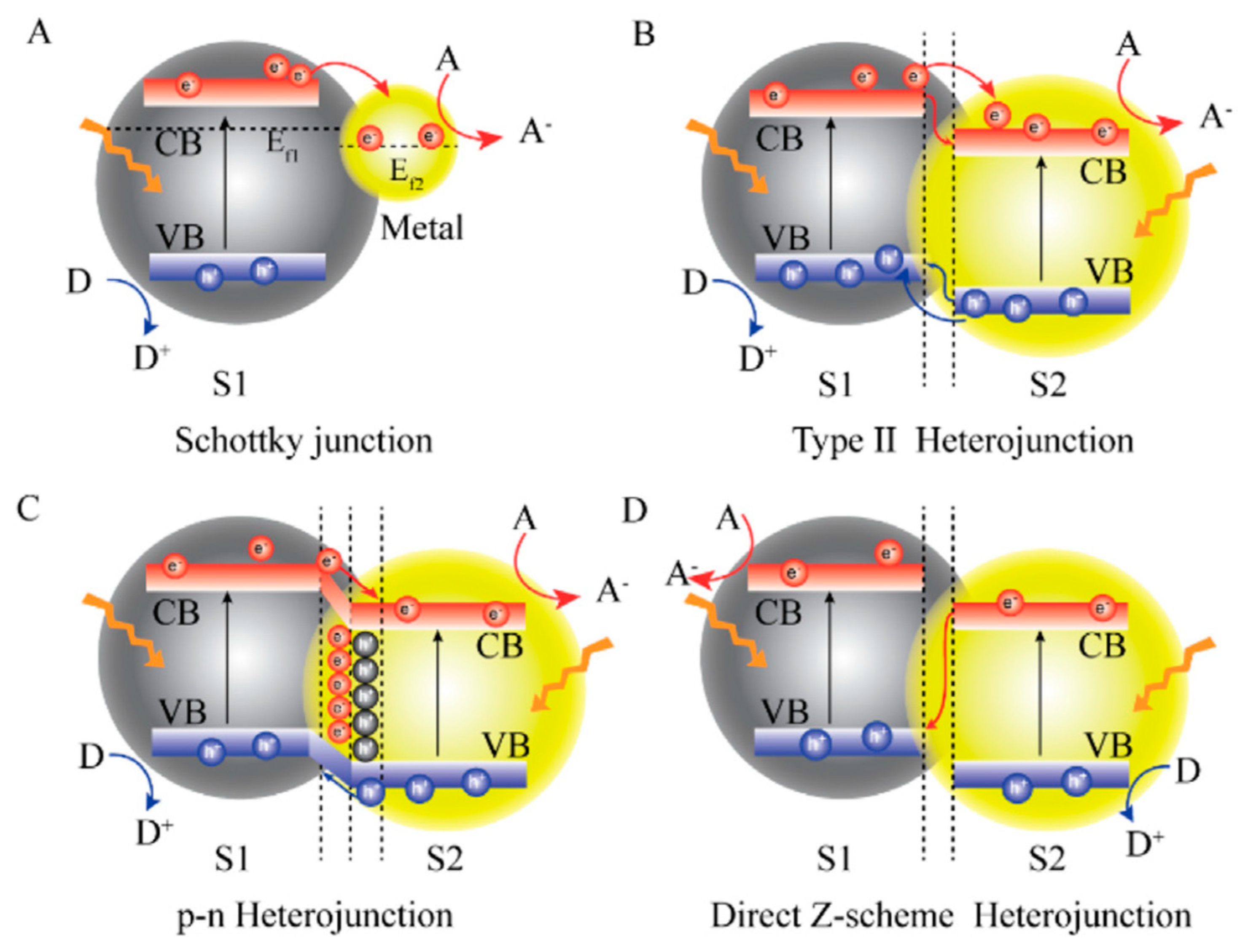
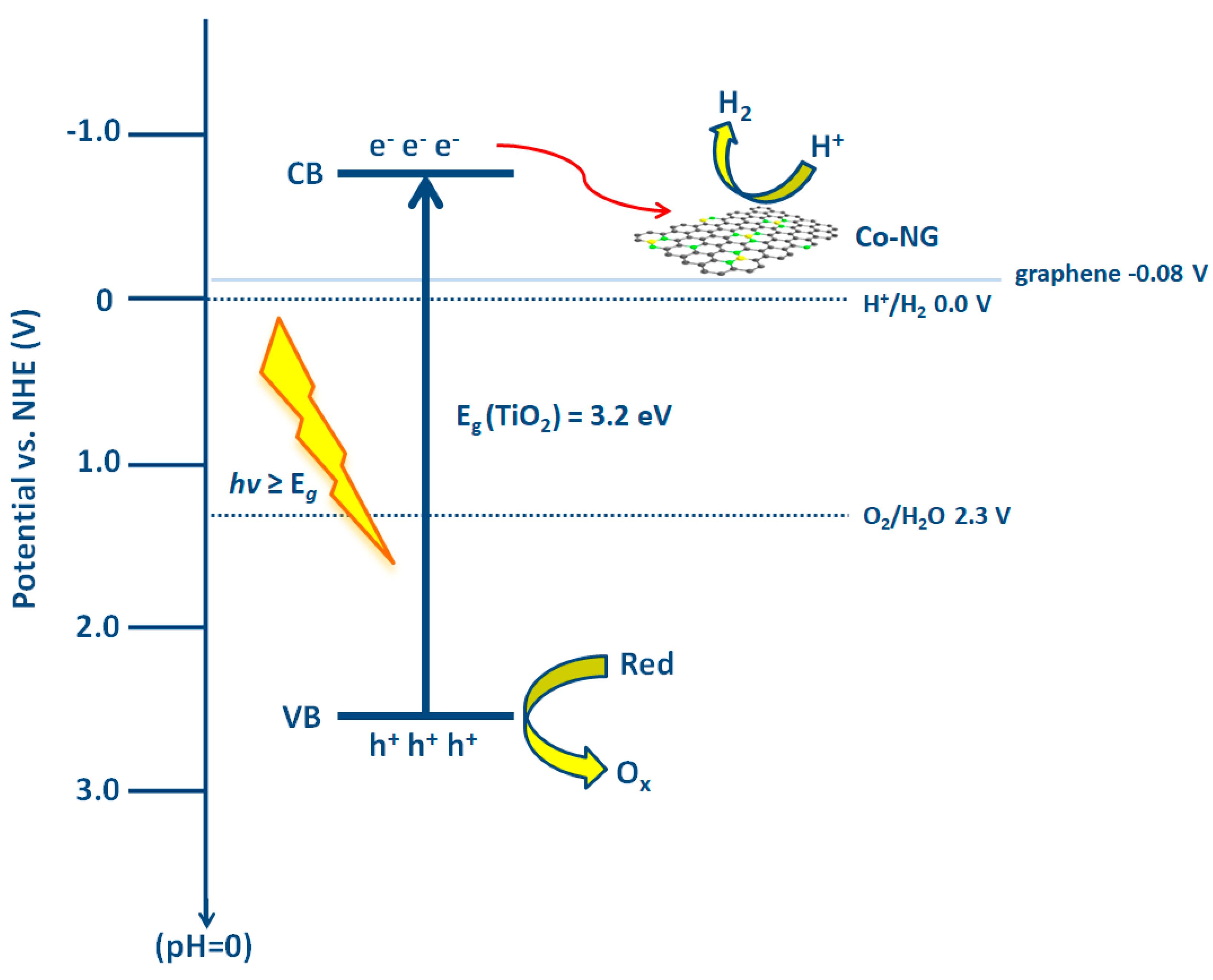
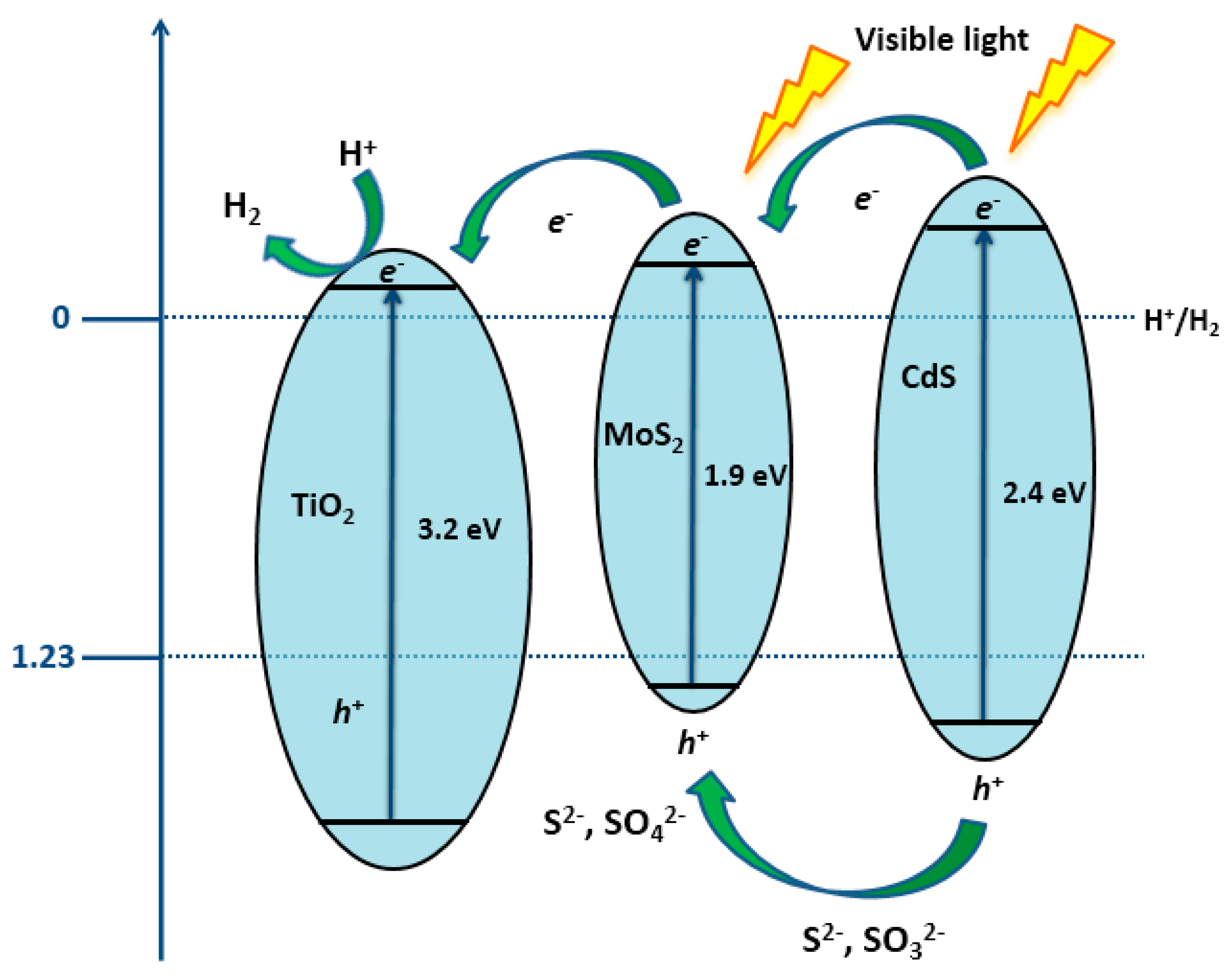
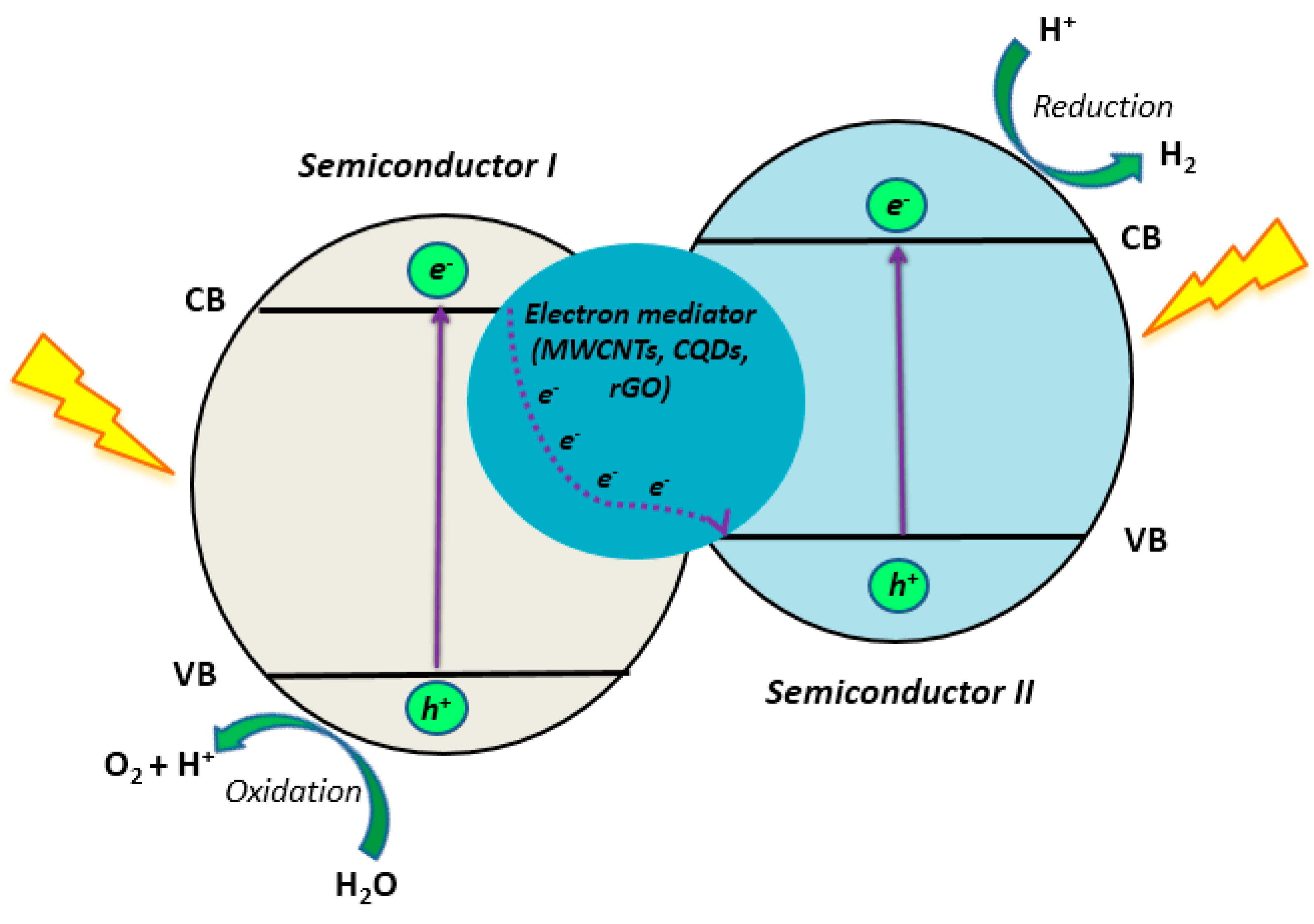
| Catalyst | Target Pollutant | Initial Concentration/Working Volume ((mg L−1) /mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| TiO2 - WO3 (0.5 g/L ) | 2,4-dichlorophenoxy acetic acid | 50 (in 250 mL) | Light Source: natural sunlight 11AM-4PM; pH = 4 | 120 min | 94.6 (TOC = 88.6) | [24] |
| TiO2 - WO3 (0.6 g/L) | Diclofenac | 25 (in 100 mL) | Light Source: 400 W Metal Halide Lamp; pH = 5 | 240 min | TOC = 91 | [30] |
| TiO2 - WO3 (0.1 g/L) | Amoxicillin | 100 (in 25,000 mL) | CPC Reactor with accumulated energy 550,000 J/m2 | NA | 64.4 (@ 550 kJ/m2) | [35] |
| (WO3/TiO2-C) (1.0 g/L) | Diclofenac | 10 (in 300 mL) | Light Source: 1500 W Xenon Lamp with filter(λ > 290 nm) ; pH = 7 | NA | 100 (@ 250 kJ/m2) (TOC = 82.4 @ 400 kJ/m2) | [32] |
| (WO3/TiO2-N) (1.0 g/L) | Diclofenac | 10 | Light Source: 1500 W Xenon Lamp with ID65 solar filter; pH = 6.5 | NA | 100 (@ 250 kJ/m2) (TOC = 100 @400 kJ/m2) | [31] |
| Catalyst | Target Pollutant | Initial Concentration/Working Volume ((mg L−1) /mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| TiO2/Fe2O3 (0.1 g/L ) | Diazinon | 10 (in 300 mL) | Light Source: 60 W Philips Visible lamp; pH = natural | 45 min | 95.07 | [37] |
| TiO2/Fe2O3 (10 mg) | 2,4-dichlorophenoxy acetic acid | 50 (in 100 mL) | Light Source: 300 W Xenon Lamp; pH = natural | 120 min | 100 (TOC = 100 @ 150 min.) | [23] |
| TiO2/Fe2O3 (0.5 g/L ) | 2,4-dichlorophenoxy acetic acid | 50 (in 250 mL) | Light Source: natural sunlight 11AM-4PM; pH = 4 | 120 min | 96.8 (TOC =90 @ 240 min.) | [24] |
| TiO2/Fe2O3 (70 mg) | Oxytetracycline Hydrochloride | 60 (in 70 mL) | Light Source: 300 W Iodine Tungsten Lamp; pH = 5.5 | 300 min | 75.6 | [38] |
| TiO2/Fe2O3 (1.0 g/L) | Oxytetracycline | 60 | Light Source: 300 W Iodine Tungsten Lamp; pH = 5.5 | 300 min | ~80 | [39] |
| TiO2/Fe2O3/CNT (100 mg) | Tetracycline | 20 (in 100mL) | Light Source: 300 W Xenon Lamp; pH = natural | 90 min | 89.41 | [40] |
| TiO2-coated α-Fe2O3 core-shell (100 mg) | Tetracycline Hydrochloride | 50 (in 200 mL) | Light Source: 300 W Xenon Lamp (λ > 420 nm) ; pH = 5. 45 Oxidant = 120 µL (30% H2O2) | 90 min | 100 | [41] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1) /mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | reference |
|---|---|---|---|---|---|---|
| N-TiO2/ CaFe2O4 /diatomite (2.0 g/L) | Tetracycline | 10 (in 200 mL) | Light Source: 150 W Xenon Lamp with UV light filter | 150 min | 91.7 (TOC =~80 @ 2h) | [44] |
| N-TiO2/ SrFe2O4 /diatomite (2.0 g/L) | Tetracycline | 10 (in 200 mL) | Light Source: 150 W Xenon Lamp with UV light filter | 150 min | 92 (TOC = ~80 @ 2h) | [46] |
| Ce/N co-doped TiO2 / NiFe2O4 diatomite (0.5 g/L) | Tetracycline | 20 (in 200 mL) | Light Source: 150 W Xenon Lamp with UV light filter | 180 min | 98.2 (TOC = ~95) | [45] |
| ZnFe2O4 / TiO2 (1.0 g/L) | Bisphenol A | 10 (in 200 mL) | Light Source: 300 W Xenon Lamp pH= 7 | 30 min | 100 | [47] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1) /mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| Cu2O-TiO2 supported palygorskite (1.0 g/L) | Tetracycline Hydrochloride | 30 (in 50mL) | Light Source: 500 Xe Lamp; pH = 8.7 | 240 min | 88.81 | [48] |
| TiO2-Cu2O film | Tetrabromodiphenyl Ethers | 5 (in 100 mL) | Light Source: 300 W Xenon Lamp; pH = natural solvent CH3OH:H2O (50:50 v/v) | 150 min | 90 | [49] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1) /mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| Bi2O3–TiO2 (50 mg) | Quinalphos | 25 (in 50 mL) | Light Source: Visible light with 1.56µmol/m2/s; pH = 8 | 100 min | 92 | [50] |
| Bi2O3–TiO2 (0.5 g/L) | Ofloxacin | 25 | Light Source: 70.3 K lux; pH = 7 | 120 min | 92 | [51] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| CdS –TiO2 (50 mg) | Tetracycline Hydrochloride | 50 (in 50mL) | Light Source: 500 W Xenon Lamp with filter (λ > 400 nm); pH = natural | 480 min | 87.0 | [58] |
| Au-CdS/TiO2 nanowire (20 mg) | Ciprofloxacin | 20 | Average solar light intensity = 100, 000 | 60 min | 99 | [57] |
| CdS/TiO2 (450 mg) | Ofloxacin | 10 (in 100mL) | Light Source: 85 W Oreva bulb with 4150 lumens (λ = 450-650 nm); pH = natural | 180 min | 86 | [52] |
| CdS nano-rod/TiO2 nano-belt ( 0.50 g/L) | 17α-ethynylestradiol | 3 (in 10 mL) | Light Source: 500 W Xenon Lamp with filter (λ > 420 nm); pH = natural | 120 min | 92 | [25] |
| CuS/TiO2 nanobelts | Enrofloxacin | 5 (in 35 mL) | Light Source: 35 W Xenon Lamp; pH = natural | 120 min | 85.5 (TOC = 27.7) | [59] |
| Au-CuS-TiO2 nanobelts | Oxytetracycline | 5 ( in 35 mL) | Light Source: 35 W Xenon Lamp; pH = natural | 60 min | 96 (TOC = 68) | [60] |
| MoS2 /TiO2 (25 mg/L) | Acetaminophen | 302 | Light Source: Sunlight; pH = natural | 25 min | 40 | [64] |
| N,S co-doped TiO2 @MoS2 (0.98g/L) | Diclofenac | 0.15 ( in 100 mL) | Light Source: 60 W LED lamp; pH = 5.5 | 150 min | 98 | [61] |
| TiO2/SnS2 films | 17β-estradiol | 1.36 (in 90 mL) | Light Source: 450 W Xenon Arc Lamp | 90 min | 51.0 | [66] |
| TiO2/SnS2 films | Diclofenac | 31.8 ( in 90mL) | Light Source: 450 W Xenon Arc Lamp; pH = 4 | 60 min | 76.21 | [67] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| Ti3+ -doped TiO2 nanotubes/ Ag3PO4 quantum dots (0.5 g/L) | Tetracycline | 10 (NA) | Light Source: 400 W Xenon Lamp; pH = natural | 8 min | 90 | [72] |
| TiO2 nanotube/ Ag3PO4 nanoparticles (40 mg) | Ciprofloxacin | 10 (in 40 mL) | Light Source: 300 W Xenon Lamp | 60 min | 85.3 | [73] |
| TiO2-x / Ag3PO4 (100 mg) | Bisphenol A | 10 (in 100 mL) | Light Source: 500 W Xenon Lamp with filter (λ = 420 nm); pH = natural | 16 min | 95 | [74] |
| Ag2O/ TiO2 quantum dots (0.25 g/L) | Levofloxacin | 10 (in 100 mL) | Light Source: 85 W Oreva CFL (4150 lumens) (λ = 380–700 nm) pH=4 | 90 min | 81 | [27] |
| Ag2O /TiO2 –zeolite (50 mg) | Norfloxacin | 5 (in 100 mL) | Light Source: 35 W Xenon Lamp | 60 min | 98.7 (TOC = 83.1) | [28] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| TiO2/ WO3/GO (2 mg) | Bisphenol A | 20 ( in 50 mL) | Light Source: sunlight Ph = 7 | 7 h | 93.2 | [77] |
| Graphene-WO3 /TiO2 nanotube (photoelectrodes ) | Dimethyl Phthalate | 10 (in 40 mL) | Light Source: 150W Xe lamps | 120 min | 75.9 | [78] |
| TiO2 /ZnO/GO (0.5 g/L) | Bisphenol A | 10 (in 50 mL) | Light Source: 18 UV lamps (λ =365 nm) ;Visible metal halide lamps(λ = 400–800 nm) pH = 6 | 120 min. (UV) 180 min. (Vis) | 99.7 (UV) 94.9 (Vis) | [79] |
| TiO2 /ZnO/GO (0.5 g/L) | Ibuprofen | 10 (in 50 mL) | Light Source: 18 UV lamps (λ = 365 nm) ;Visible metal halide lamps(λ = 400–800 nm) pH = 6 | 120 min. (UV) 180 min. (Vis) | 98.5 (UV) 79.6 (Vis) | [79] |
| TiO2 /ZnO/GO (0.5 g/L) | Flurbiprofen | 10 ( in 50 mL) | Light Source: 18 UV lamps (λ=365 nm) ;Visible metal halide lamps(λ= 400–800 nm) pH= 6 | 120 min. (UV) 180 min. (Vis) | 98.1(UV) 82.2 (Vis) | [79] |
| ZnFe2O4/rGO/TiO2 (0.1 g) | Fulvic Acid | 20 (in 50 mL) | Light Source: 300 W (λ=420 nm); Vol H2O2 = 0.8 mL, pH= 7 | 180 min | 95.4% | [80] |
| TiO2 /MoS2 /rGO (0.5 g/L) | Bisphenol A | 10 (in 50 mL) | Light Source: 20 W (λ = 254 nm); | 300 min | 62.4 | [81] |
| TiO2/BiVO4/rGO | Tetracycline | 10 µg/L (NA) | Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 96.2 | [82] |
| TiO2/BiVO4/rGO | Chlorotetracycline | 10 µg/L (NA) | Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 97.5 | [82] |
| TiO2/BiVO4/rGO | Oxytetracycline | 10 µg/L (NA) | Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 98.7 | [82] |
| TiO2/BiVO4/rGO | Doxycycline | 10 µg/L (NA) | Light Source: 1000 W Xe Lamp (λ = 420 nm) with filter | 120 min | 99.6 | [82] |
| Catalyst | Target Pollutant | Initial Concentration/ Working Volume ((mg L−1)/mL) | Experimental Conditions | Reaction Time | Removal Extent (%) | Reference |
|---|---|---|---|---|---|---|
| g-C3N4/TiO2 (30 mg) | Ciprofloxacin | 10 (in 80 mL) | Light Source: 300 W Xe Lamp with filter (λ > 400 nm) pH = natural | 180 min | 88.1 | [96] |
| g-C3N4/TiO2 (30 mg) | Acyclovir | 10 (in 100 mL) | Light Source: 300 W Xe Lamp with filter (λ > 420 nm) pH = natural | 90 min | 100 | [97] |
| mpg-C3N4/TiO2 (membrane) | Sulfamethoxazole | 10 (in 50 mL) | Light Source: 300 W Xe Lamp pH = natural Flow rate = 13 mL/min. Membrane flux = 918 L /m2 h | 1800 min | 69 | [98] |
| TiO2@g-C3N4 core-shell (100 mg) | Tetracycline | 20 (in 100 mL) | Light Source: Xenon Lamp with full spectrum pH = natural | 9 min | (2.2 mg/min.) | [99] |
| g-C3N4 –shielding polyester/ TiO2 (130 mg) | sulfaquinoxaline | 2 × 10−5 mol/L (30 mL) | Light Source: Q-Sun Xe-1 test, pH = 7 | 90 min | 97 | [101] |
| g-C3N4 –shielding polyester/ TiO2 (130 mg) | thiamethoxam | 2 × 10−5 mol/L (30 mL) | Light Source: Q-Sun Xe-1 test, pH = 7 | 180 min | ~95 | [101] |
| g-C3N4/TiO2/kaolinite (200 mg) | Ciprofloxacin | 10 (in 100 mL) | Light Source: Ave. light intensity =90 mW/cm2 ; Xe Lamp with filter (λ > 400 nm), pH = natural | 240 min | 92 | [100] |
| S-Ag/ TiO2 @ g-C3N4 (0.20 g/L) | Triclosan | 10 (in 100 mL) | Light Source: 250 W Xe Lamp with filter (λ > 420 nm), pH = 7.8 | 60 min | 92.3 (Detoxification Efficiency= 64.3± 3.9) | [102] |
| Co-TiO2 @g-C3N4 (5 mg ; 2 × 2 cm2 membranes) | Tetracycline Hydrochloride | 20 (in 10 mL) | Light Source: 300 W Xe Lamp with filter (λ > 420 nm), pH = 7 | 60 min. | 90.8 | [103] |
| D35-TiO2/g-C3N4 (0.5g/L) | Bisphenol A | 10 (in 100 mL) | Light Source: 300 W Metal Halide pH = 7, Oxidant = 2mM Persulfate | 15 min | 100 (TOC= 50) | [104] |
| C dots decorated g-C3N4/ TiO2 (1.0 g/L) | Enrofloxacin | 4 ( in 50 mL) | Light Source: 350 W Xe Lamp with filter (λ > 420 nm) pH = natural | 60 min | 91.6 | [105] |
| graphene quantum dots/ Mn-N-TiO2 /g-C3N4 (45 mg) | Ciprofloxacin | 10 (in 80 mL) | Light Source: 300 W Xe Lamp (320 ≤λ ≤ 780 nm), pH = 7 | 120 min | 89 | [107] |
| graphene quantum dots/ Mn-N-TiO2 /g-C3N4 (45 mg) | Diethyl Phthalate | 10 (in 80mL) | Light Source: 300 W Xe Lamp (320 ≤ λ ≤ 780 nm), pH = 7 | 120 min | 70.4 | [107] |
| MoS2 supported TiO2/g-C3N4 (30 mg) | Atrazine | 10 (in 100 mL) | Light Source: 500 W Xe Lamp (λ > 420 nm), pH = 7 | 300 min | 86.5 | [108] |
| WO3–TiO2 @g-C3N4 | Acetylsalicylate | 10 (in 100 mL) | Light Source: 500 W Metal Halide pH = natural | 90 min | 98 | [109] |
| WO3–TiO2 @g-C3N4 | Methyl-theobromine | 10 (in 100 mL) | Light Source: 500 W Metal Halide pH = natural | 90 min | 97 | [109] |
| Photocatalyst | Light Source | HER | Reference |
|---|---|---|---|
| CdS/Pt/TiO2 film | 300 W Xe lamp | 3.074 µmol/h/g | [54] |
| CdS/Pt/TiO2 nanosheets | 350 W Xe arc lamp | 265 µmol/h | [141] |
| CdS/Pt/TiO2 nanotubes | 300 W Xe lamp | 402 µmol/h | [142] |
| CdS/TiO2 nanotubes | 350 W Xe lamp | 2585 µL/h/g | [143] |
| CdS-Ti-MCM-48-21-25 | 300 W Xe lamp | 2726 µmol/h | [139] |
| Photocatalyst | Reaction Conditions | HER | Reference |
|---|---|---|---|
| CdSQDs/WC/TiO2 | Photocatalyst dispersed in 20 vol.% lactic acid as an electron donor under visible light. | 624.9 µmol/h/ | [55] |
| ZnO/ZnCr2O4@TiO2-NTA | Photocatalyst dispersed in aqueous methanol solution under simulated solar light. | 1680 µmol/cm2 | [152] |
| F-TiO2/CdSe-DETA | Photocatalyst was dispersed in a mixed solution of Na2S and Na2SO3 as a sacrificial agents under visible light with the use of Pt as a cocatalyst. | 12381 µmol/h/g | [153] |
| g-C3N4/TiO2/RGO | 5 mg of the g-C3N4-TiO2/RGO nano-composite was dispersed in 50 mL glycerol-water solution under UV-vis light. | 19610 µmol/h/g | [13] |
| CDS/CNF/Pt-TiO2 | Photocatalyst was dispersed in a mixed solution of Na2S and Na2SO3 as a sacrificial agents under visible light. | 16.34 µmol for 3 h | [154] |
| F-TiO2/CdS-DETA, Pt as a cocatalyst | 50 mg of the photocatalyst was dispersed in 100 mL of mixed aqueous solution containing 0.35 mg/L Na2S and 0.25 mg/L Na2SO3 with the use of Pt as a cocatalyst. | 5342.86 µmol/h/g | [155] |
| CdS@TiO2@Au | 20 mg of the photocatalyst was dispersed in 40 ml of aqueous solution containing 0.1 M Na2S and 0.1 M Na2SO3 as the sacrificial agents under visible light. | 1720 µmol/h/g | [156] |
| TiO2-Au-CdS | 0.1 g of the sample was immersed in an aqueous solution containing 0.1 M Na2S and 0.1 M Na2SO3 as the sacrificial agents under visible light. | 1810 µmol/h/g | [157] |
| N-TiO2/g-C3N4@NixP | 50 mg photocatalyst was suspended in a 100 mL solution containing 10 vol.% triethanolamine (TEOA) under 300 W Xe lamp irradiation. | 5438 µmol/h/g | [158] |
| TiO2/CdS/CNT | 0.1 g of photocatalyst was dispersed in solution containing 70 mL of distilled water or seawater and 30 mL of sacrificial agent. The photocatalyst were irradiated using three visible-light sunlamps of each 100 W and UV lamp of 8 W. | 3502 µmol/h from pure water and 1373 µmol/h from seawater | [159] |
| WS2/ C-TiO2/ g-C3N4 | 50 mg of photocatalyst was added in to 80 mL TEOA aqueous solution with the use of Pt as a cocatalyst. | 17726 for DI water and 29978 µmol/g for seawater | [148] |
| TiO2/La2O2CO3/rGO | 0.05 g of powder catalyst was dispersed in 80 Ml ethylene-glycol water (5/95, v/v) solution under UV-vis light. | 583 µmol/h | [160] |
| TiO2-Cu@C | Photocatalyst was dispersed in methanol aqueous solution under UV-vis light. | 3911 µmol/g/h | [161] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perović, K.; dela Rosa, F.M.; Kovačić, M.; Kušić, H.; Štangar, U.L.; Fresno, F.; Dionysiou, D.D.; Loncaric Bozic, A. Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials 2020, 13, 1338. https://doi.org/10.3390/ma13061338
Perović K, dela Rosa FM, Kovačić M, Kušić H, Štangar UL, Fresno F, Dionysiou DD, Loncaric Bozic A. Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials. 2020; 13(6):1338. https://doi.org/10.3390/ma13061338
Chicago/Turabian StylePerović, Klara, Francis M. dela Rosa, Marin Kovačić, Hrvoje Kušić, Urška Lavrenčič Štangar, Fernando Fresno, Dionysios D. Dionysiou, and Ana Loncaric Bozic. 2020. "Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting" Materials 13, no. 6: 1338. https://doi.org/10.3390/ma13061338
APA StylePerović, K., dela Rosa, F. M., Kovačić, M., Kušić, H., Štangar, U. L., Fresno, F., Dionysiou, D. D., & Loncaric Bozic, A. (2020). Recent Achievements in Development of TiO2-Based Composite Photocatalytic Materials for Solar Driven Water Purification and Water Splitting. Materials, 13(6), 1338. https://doi.org/10.3390/ma13061338







_Dionysiou.jpg)

