Production of Gamma Alumina Using Plasma-Treated Aluminum and Water Reaction Byproducts
Abstract
1. Introduction
2. Experimental Details
2.1. Synthesis Procedure
2.2. Characterization
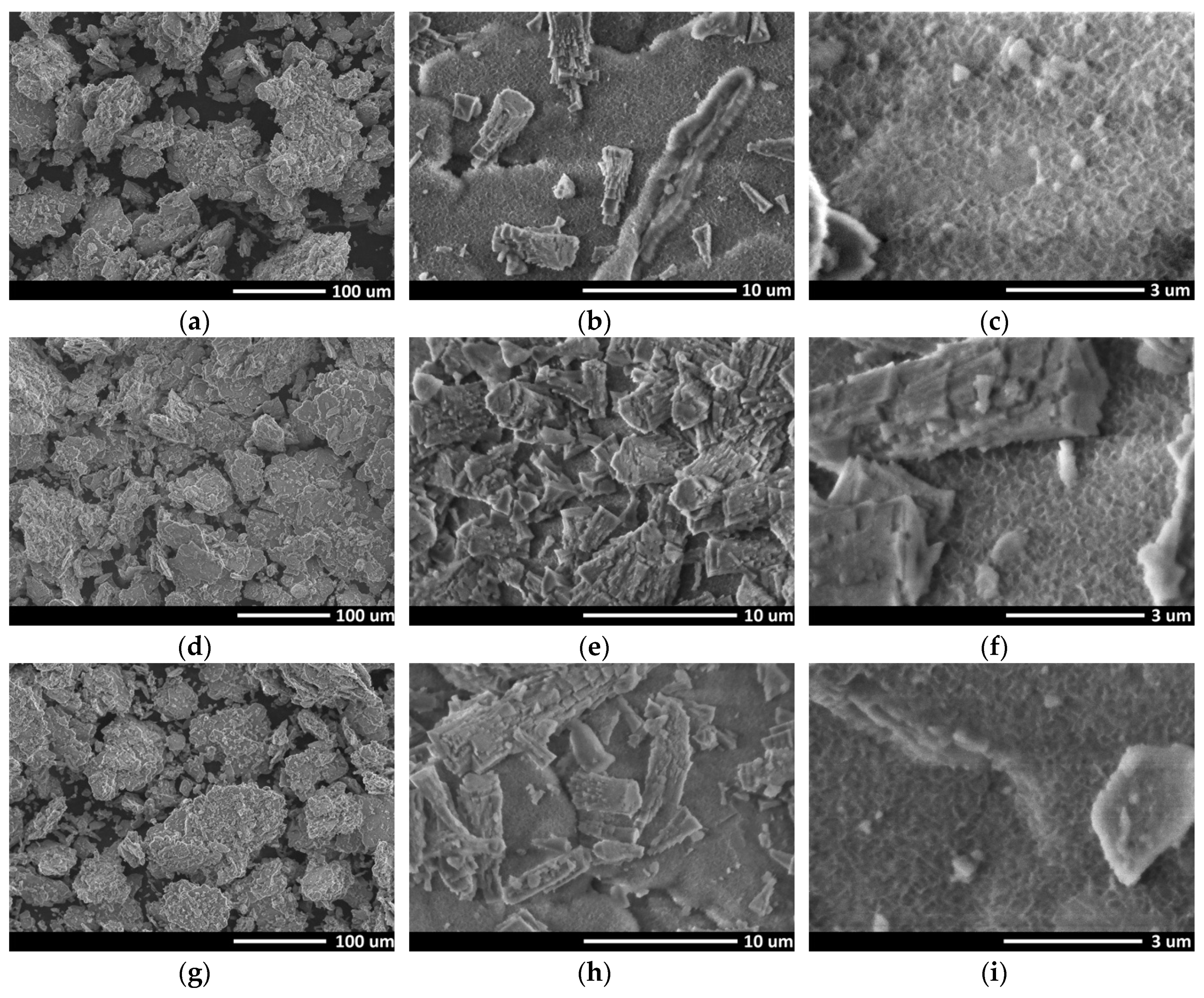
3. Results and Discussion
4. Conclusions
5. Patents
Author Contributions
Funding
Conflicts of Interest
References
- Busca, G. The surface of transitional aluminas: A critical review. Catal. Today 2014, 226, 2–13. [Google Scholar] [CrossRef]
- Hong, Y.; Wang, H.; Ibrahim, A.-R.; Su, Y.; Li, J.; Xu, J.; Hu, X. Preparation of large pore volume γ-alumina and its performance as catalyst support in phenol hydroxylation. Microporous Mesoporous Mater. 2016, 231, 1–8. [Google Scholar]
- Samain, L.; Seo, D.-K.; Javier Garcia-Garcia, F.; Jaworski, A.; Edén, M.; Häussermann, U.; Ladd, D.M. Structural analysis of highly porous γ-Al2O3. J. Solid State Chem. 2014, 217, 1–8. [Google Scholar] [CrossRef]
- Chauruka, S.R.; Roberts, K.J.; Stitt, H.; Hassanpour, A.; Brydson, R.; Ghadiri, M. Effect of mill type on the size reduction and phase transformation of gamma alumina. Chem. Eng. Sci. 2015, 134, 774–783. [Google Scholar] [CrossRef]
- Pakharukova, V.P.; Yatsenko, D.A.; Gerasimov, E.Y.; Shalygin, A.S.; Martyanov, O.N.; Tsybulya, S.V. Coherent 3D nanostructure of γ-Al2O3: Simulation of whole X-ray powder diffraction pattern. J. Solid State Chem. 2017, 246, 284–292. [Google Scholar] [CrossRef]
- Baghalha, M.; Mohammadi, M.; Ghorbanpour, A. Coke deposition mechanism on the pores of a commercial Pt-Re/γ-Al2O3 naphtha reforming catalyst. Fuel Process. Technol. 2010, 91, 714–722. [Google Scholar] [CrossRef]
- Bose, S.; Das, C. Preparation, characterization, and activity of γ-alumina-supported molybdenum/cobalt catalyst for the removal of elemental sulfur. Appl. Catal. A Gen. 2016, 512, 15–26. [Google Scholar] [CrossRef]
- Hartmann, S.; Sachse, A.; Galarneau, A. Challenges and strategies in the synthesis of mesoporous alumina powders and hierarchical alumina monoliths. Materials 2012, 5, 336–349. [Google Scholar] [CrossRef]
- Navas, M.; Sánchez, M.; Casella, M.L.; Aracil, J.; Ruggera, J.F.; Martínez, M. Biodiesel production optimization using γAl2O3 based catalysts. Energy 2014, 73, 661–669. [Google Scholar]
- Matori, K.; Wah, L.; Hashim, M.; Ismail, I.; Zaid, M. Phase transformations of α-alumina made from waste aluminum via a precipitation technique. Int. J. Mol. Sci. 2012, 13, 16812–16821. [Google Scholar] [CrossRef]
- Bilir, G.; Liguori, J. Laser diode induced white light emission of γ-Al2O3 nano-powders. J. Lumin. 2014, 153, 350–355. [Google Scholar] [CrossRef]
- Asencios, Y.J.O.; Sun-Kou, M.R. Synthesis of high-surface-area γ-Al2O3 from aluminum scrap and its use for the adsorption of metals: Pb(II), Cd(II) and Zn(II). Appl. Surf. Sci. 2012, 258, 10002–10011. [Google Scholar] [CrossRef]
- Murafa, N.; Cordero, T.; Pascual-Cosp, J.; Balek, V.; Pérez-Maqueda, L.A.; Martín-Ruiz, M.M.; Subrt, J. High surface area α-alumina preparation by using urban waste. Ceram. Int. 2008, 35, 2111–2117. [Google Scholar]
- Han, Q.; Setchi, R.; Evans, S.L. Synthesis and characterisation of advanced ball-milled Al-Al2O3 nanocomposites for selective laser melting. Powder Technol. 2016, 297, 183–192. [Google Scholar] [CrossRef]
- Kwak, J.H.; Hu, J.; Mei, D.; Yi, C.-W.; Kim, D.H.; Peden, C.H.F.; Allard, L.F.; Szanyi, J. Coordinatively unsaturated Al3+ centers as binding sites for active catalyst phases of platinum on-Al2O3. Science 2009, 325, 1670–1673. [Google Scholar] [CrossRef] [PubMed]
- Alphonse, P.; Courty, M. Structure and thermal behavior of nanocrystalline boehmite. Thermochim. Acta 2005, 425, 75–89. [Google Scholar] [CrossRef]
- Chen, J.; Cai, W.; Xia, T.; Hu, Y.; Zhang, G. Synthesis of nanorod-like mesoporous γ-Al2O3 with enhanced affinity towards Congo red removal: Effects of anions and structure-directing agents. Cryst. Eng. Comm 2011, 14, 972–977. [Google Scholar]
- Li, G.; Liu, Y.; Liu, C. Solvothermal synthesis of gamma aluminas and their structural evolution. Microporous Mesoporous Mater. 2013, 167, 137–145. [Google Scholar] [CrossRef]
- Bora, B.; Aomoa, N.; Bordoloi, R.K.; Srivastava, D.N.; Bhuyan, H.; Das, A.K.; Kakati, M. Free-flowing, transparent γ-alumina nanoparticles synthesized by a supersonic thermal plasma expansion process. Curr. Appl. Phys. 2012, 12, 880–884. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Q.; Huang, Y. Preparation and adsorption properties of the biomimetic gama-alumina. Mater. Lett. 2015, 157, 67–69. [Google Scholar] [CrossRef]
- Tanna, J.A.; Chaudhary, R.G.; Gandhare, N.V.; Juneja, H.D. Alumina nanoparticles: A new and reusable catalyst for synthesis of dihydropyrimidinones derivatives. Adv. Mater. Lett. 2016, 7, 933–938. [Google Scholar] [CrossRef]
- Song, X.; He, X.; Qu, P.; Yang, H.; Qiu, G. Synthesis of γ-Al2O3 nanoparticles by chemical precipitation method. J. Cent. South Univ. Technol. 2007, 12, 536–541. [Google Scholar] [CrossRef]
- Reza, A.; Rezaei, M.; Yaripour, F. Nanocrystalline gamma-alumina: A highly active catalyst for dimethyl ether synthesis. Powder Technol. 2010, 199, 176–179. [Google Scholar]
- Xu, N.; Liu, Z.; Dong, Y.; Hong, T.; Dang, L.; Li, W. Controllable synthesis of mesoporous alumina with large surface area for high and fast fluoride removal. Ceram. Int. 2016, 42, 15253–15260. [Google Scholar] [CrossRef]
- Márquez-Alvarez, C.; Žilková, N.; Pérez-Pariente, J.; Čejka, J. Synthesis, characterization and catalytic applications of organized mesoporous aluminas. Catal. Rev. 2008, 50, 222–286. [Google Scholar] [CrossRef]
- Baburao, N.S.; Umarji, A.M. Synthesis of γ-alumina by solution combustion method using mixed fuel approach (urea + glycine fuel). Int. J. Res. Eng. Technol. 2013, 2, 434–438. [Google Scholar]
- Huang, B.; Bartholomew, C.H.; Smith, S.J.; Woodfield, B.F. Facile solvent-deficient synthesis of mesoporous γ-alumina with controlled pore structures. Microporous Mesoporous Mater. 2013, 165, 70–78. [Google Scholar] [CrossRef]
- Nordahl, C.S.; Messing, G.L. Sintering of α-Al2O3-seeded nanocrystalline γ-Al2O3 powders. J. Eur. Ceram. Soc. 2002, 22, 415–422. [Google Scholar] [CrossRef]
- Petrovic, J.; Thomas, G. Reaction of aluminum with water to produce hydrogen. In White Paper for U.S. Department of Energy; United States Department of Energy: Washington, DC, USA, 2008; pp. 1–26. [Google Scholar]
- Urbonavicius, M.; Varnagiris, S.; Milcius, D. Generation of hydrogen through the reaction between plasma-modified aluminum and water. Energy Technol. 2017, 5, 2300–2308. [Google Scholar] [CrossRef]
- Ziegler, F.J. SRIM/TRIM. Available online: http://www.srim.org (accessed on 1 February 2019).
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Halawy, S.A.; Mohamed, M.A.; Abdelkader, A. Effect of precursor on the performance of alumina for the dehydration of methanol to dimethyl ether. Appl. Catal. B Environ. 2012, 127, 307–315. [Google Scholar] [CrossRef]
- Hosseini, Z.; Taghizadeh, M.; Yaripour, F. Synthesis of nanocrystalline γ-Al2O3 by sol-gel and precipitation methods for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 128–134. [Google Scholar] [CrossRef]
- Aboonasr Shiraz, M.H.; Rezaei, M.; Meshkani, F. Microemulsion synthesis method for preparation of mesoporous nanocrystalline γ-Al2O3 powders as catalyst carrier for nickel catalyst in dry reforming reaction. Int. J. Hydrogen Energy 2016, 41, 6353–6361. [Google Scholar] [CrossRef]
- Rozita, Y.; Brydson, R.; Scott, A.J. An investigation of commercial gamma-Al2O3 nanoparticles. J. Phys. Conf. Ser. 2010, 241, 012096. [Google Scholar] [CrossRef]
- Hosseinia, S.; Nikoub, M. Synthesis and characterization of nano-sized γ-Al2O3 catalyst for production of dimethyl ether via indirect method. In Proceedings of the 4th International Conference on Nanostructures (ICNS4), Kish Island, Hormozgãn, Iran, 12–14 March 2012; pp. 1032–1034. [Google Scholar]
- León, J.N.D.d.; Petranovskii, V.; Reyes, J.A.d.l.; Alonso-Nuñez, G.; Zepeda, T.A.; Fuentes, S.; García-Fierro, J.L. One dimensional (1D) γ-alumina nanorod linked networks: Synthesis, characterization and application. Appl. Catal. A Gen. 2014, 472, 1–10. [Google Scholar] [CrossRef]
- Wu, W.; Wan, Z.; Chen, W.; Zhu, M.; Zhang, D. Synthesis of mesoporous alumina with tunable structural properties. Microporous Mesoporous Mater. 2015, 217, 12–20. [Google Scholar] [CrossRef]
- Yi, J.H.; Sun, Y.Y.; Gao, J.F.; Xu, C.Y. Synthesis of crystalline γ-Al2O3 with high purity. Trans. Nonferrous Met. Soc. China 2009, 19, 1237–1242. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Y.; Zuhra, Z.; Butler, I.S. Synthesis of γ-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–218. [Google Scholar] [CrossRef]
- Yan, F.; Jiang, J.; Liu, N.; Gao, Y.; Meng, Y.; Li, K.; Chen, X. Green synthesis of mesoporous Γ-Al2O3 from coal fly ash with simultaneous on-site utilization of CO2. J. Hazard. Mater. 2018, 359, 535–543. [Google Scholar] [CrossRef]
- Segal, F.M.; Correa, M.F.; Bacani, R.; Castanheira, B.; Politi, M.J.; Brochsztain, S.; Triboni, E.R. A novel synthesis route of mesoporous? -alumina from polyoxohydroxide aluminum. Mater. Res. 2018, 21, 36–38. [Google Scholar] [CrossRef]
- khazaei, A.; Nazari, S.; Karimi, G.; Ghaderi, E.; Mansouri Moradian, K.; Bagherpor, Z. Synthesis and characterization of γ-alumina porous nanoparticles from sodium aluminate liquor with two different surfactants. Int. J. Nanosci. Nanotechnol. 2016, 12, 207–214. [Google Scholar]
- Liu, D.; Zhu, H.; Zhao, J.; Pan, L.; Dai, P.; Gu, X.; Li, L.; Liu, Y.; Zhao, X. Synthesis of mesoporous γ-Al2O3 with spongy structure: In-situ conversion of metal-organic frameworks and improved performance as catalyst support in hydrodesulfurization. Materials 2018, 11, 1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, K.; Zhang, L.; Wu, H.; Guo, J. Synthesis of mesoporous Γ-Al2O3 by using cellulose nanofiber as template for hydrodesulfurization of dibenzothiophene. Fuel 2019, 253, 431–440. [Google Scholar] [CrossRef]
- Siahpoosh, S.M.; Salahi, E.; Hessari, A.; Mobasherpour, I. Facile synthesis of Γ-alumina nanoparticles via the sol-gel method in presence of various solvents. Sigma J. Eng. Nat. Sci. 2017, 35, 441–456. [Google Scholar]
- Keshavarz, A.R.; Rezaei, M.; Yaripour, F. Preparation of nanocrystalline γ-Al2O3 catalyst using different procedures for methanol dehydration to dimethyl ether. J. Nat. Gas Chem. 2011, 20, 334–338. [Google Scholar] [CrossRef]
- Huang, B.; Bartholomew, C.H.; Woodfield, B.F. Facile structure-controlled synthesis of mesoporous γ-alumina: Effects of alcohols in precursor formation and calcination. Microporous Mesoporous Mater. 2013, 177, 37–46. [Google Scholar] [CrossRef]





| Samples | O, at.% | Al, at.% | C, at.% |
|---|---|---|---|
| Initial byproduct | 75.71 | 22.52 | 1.77 |
| Boehmite | 67.26 | 29.77 | 2.97 |
| Gamma alumina | 64.82 | 31.53 | 3.66 |
| Method | Precursor | BET Surface Area (m2/g) | Pore Characteristics | Ref. | |
|---|---|---|---|---|---|
| Volume (cm3/g) | Diameter (nm) | ||||
| Lime-sinter | Fly ash | 196.1–404.3 | 0.23–0.82 | 2.77–15.22 | [41] |
| Precipitation | Sodium aliuminate | 269.9 | 0.57 (mL/g) | - | [39] |
| Calcination | Polyoxohydroxide aluminum | 72–282 | 0,13–0,24 | 3,5–10,3 | [42] |
| Precipitation | AlCl3 × 6 H2O | 112.9 | - | 4.13 | [40] |
| Sol-gel | Aluminate liquor | 138.8–201.1 | 0.16–0.24 | 4.7–7.8 | [43] |
| Two-step pyrolysis | Al-based metal organic | 240–350 | 0.52–0.63 | 7.2–8.6 | [44] |
| Evaporation-induced self-assembly | Aluminum isopropoxide | 234–285 | 0.23–0.49 | 3.8–6.7 | [45] |
| Sol-gel | Aluminum isopropoxide | 306–351 | 0.226–1.29 | 5.34–6.13 | [46] |
| Solvent-deficient | Tri-sec-butoxide aluminum | 192–375 | 0.44–0.80 | 7.4–16.2 | [47] |
| Sol-gel | Aluminum isopropoxide | 180–260 | 0.8–1.4 | 7-37 | [48] |
| Commercial | Unknown | 220–280 | 0.8–1.2 | - | Alfa Aesar by Thermo Fisher Scientific |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbonavicius, M.; Varnagiris, S.; Pranevicius, L.; Milcius, D. Production of Gamma Alumina Using Plasma-Treated Aluminum and Water Reaction Byproducts. Materials 2020, 13, 1300. https://doi.org/10.3390/ma13061300
Urbonavicius M, Varnagiris S, Pranevicius L, Milcius D. Production of Gamma Alumina Using Plasma-Treated Aluminum and Water Reaction Byproducts. Materials. 2020; 13(6):1300. https://doi.org/10.3390/ma13061300
Chicago/Turabian StyleUrbonavicius, Marius, Sarunas Varnagiris, Liudas Pranevicius, and Darius Milcius. 2020. "Production of Gamma Alumina Using Plasma-Treated Aluminum and Water Reaction Byproducts" Materials 13, no. 6: 1300. https://doi.org/10.3390/ma13061300
APA StyleUrbonavicius, M., Varnagiris, S., Pranevicius, L., & Milcius, D. (2020). Production of Gamma Alumina Using Plasma-Treated Aluminum and Water Reaction Byproducts. Materials, 13(6), 1300. https://doi.org/10.3390/ma13061300





