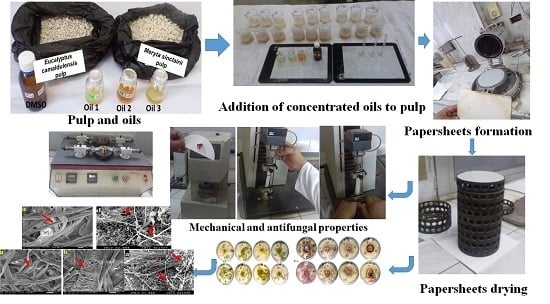
Impact of Three Natural Oily Extracts as Pulp Additives on the Mechanical, Optical, and Antifungal Properties of Paper Sheets Made from Eucalyptus camaldulensis and Meryta sinclairii Wood Branches
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Wood-Branch Shavings
2.2. Chemical Analysis of Wood Branch Shavings
2.2.1. Solubility in Cold and Hot Water
2.2.2. Alcohol-Benzene Extractive Determination (T204 cm-17)
2.2.3. Acid-Insoluble Lignin (T222 om-15)
2.2.4. Pentosans Determination (T223 cm-10)
2.2.5. Holocellulose Determination by Chlorination Method
2.2.6. Ash Content (T211 om-16)
2.3. Pulping Process
2.4. Extraction of Oily Extracts and GC/MS Analysis
2.5. Addition of Diluted Oily Extracts
2.6. Paper Sheet Formation
2.7. Testing of Mechanical Strength and Optical Properties
2.7.1. Tensile Index
2.7.2. Burst Index
2.7.3. Tear Index
2.7.4. Fold Number
2.7.5. Antifungal Evaluation In Vitro
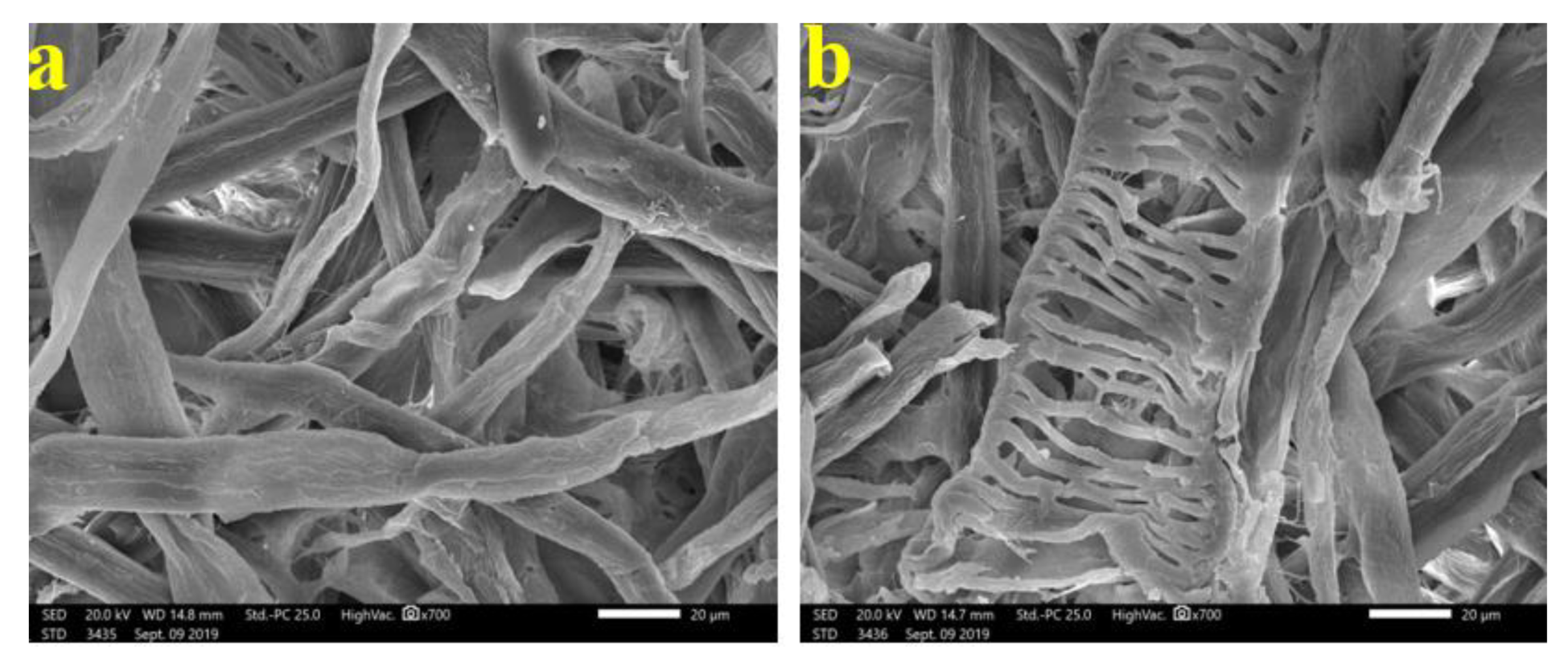
2.7.6. SEM Examination of Produced Paper Sheets
2.8. Statistical Analysis
3. Results and Discussion
3.1. Chemical and Physical Analysis of Wood Materials before Pulping and Unbleached Pulp
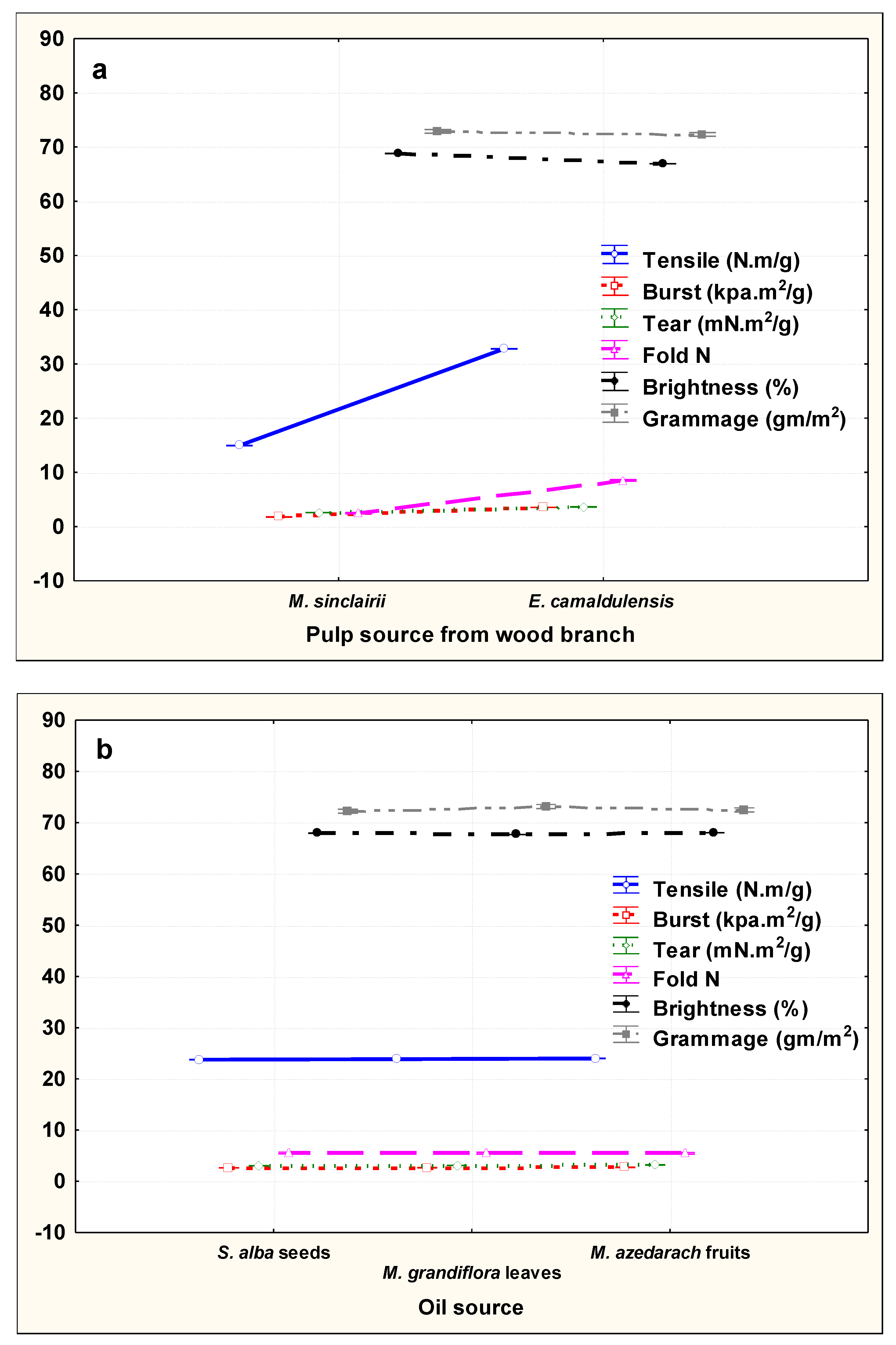
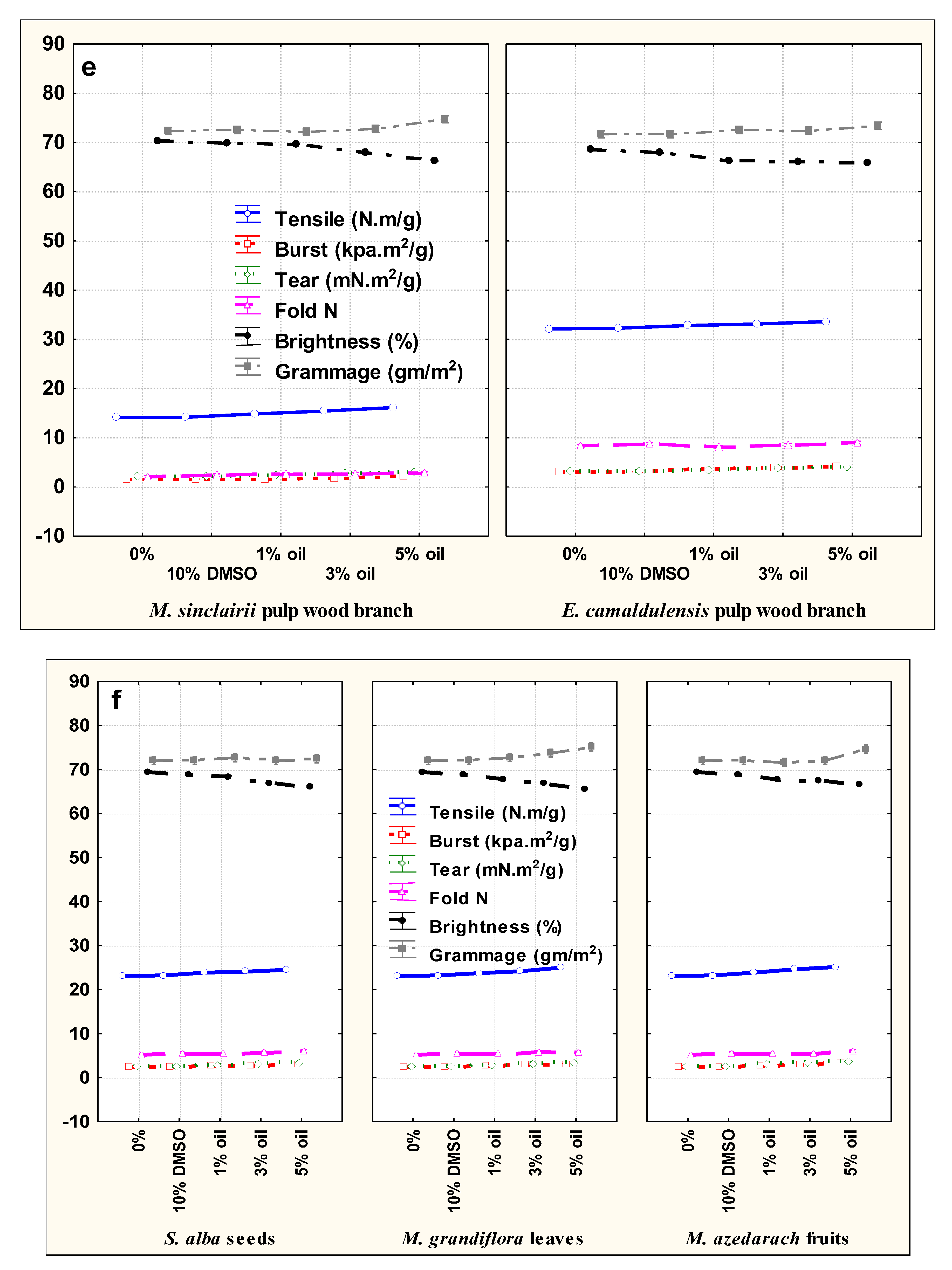
3.2. Paper Sheet Testing
3.3. Evaluation of Antifungal Activity of Paper Sheets
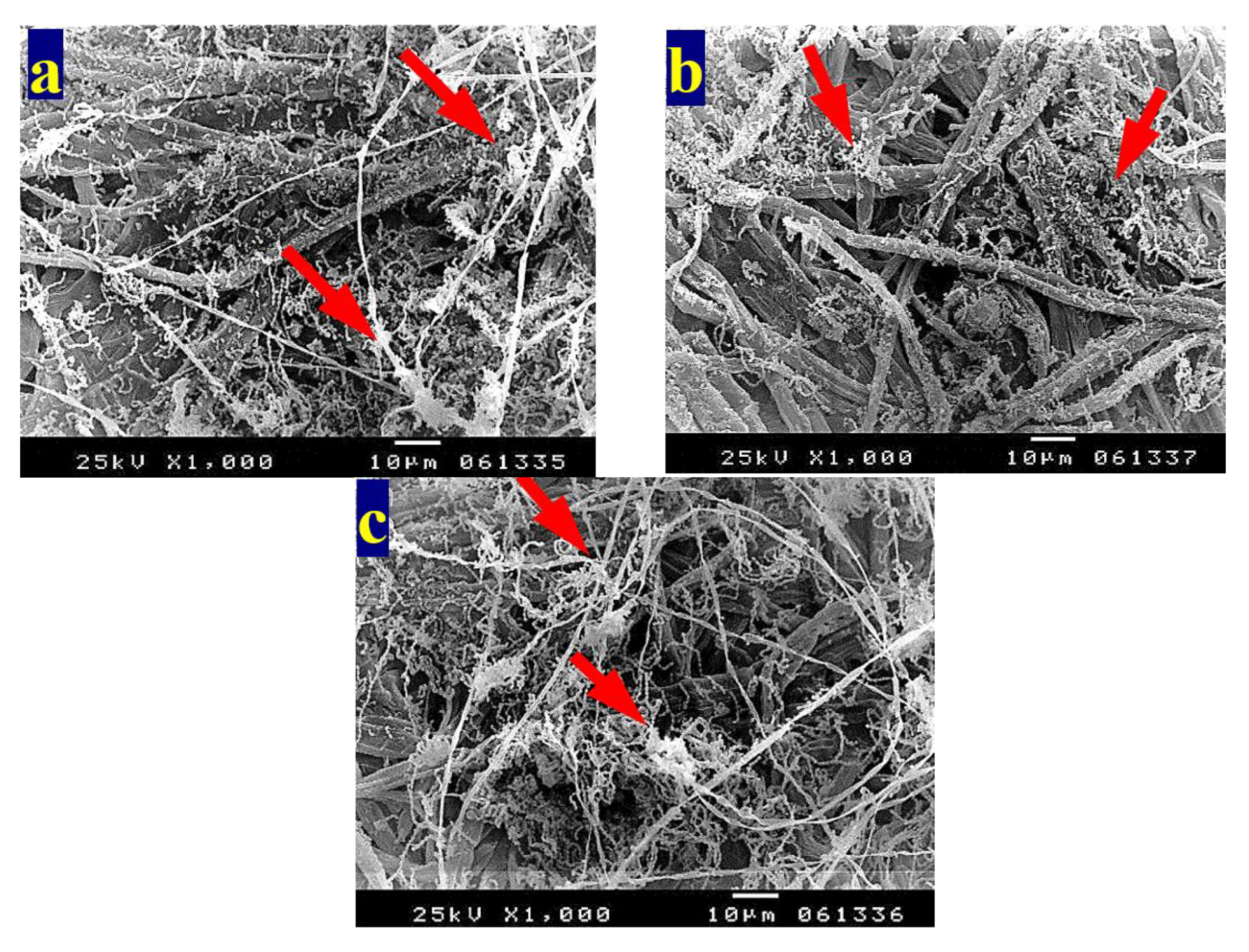
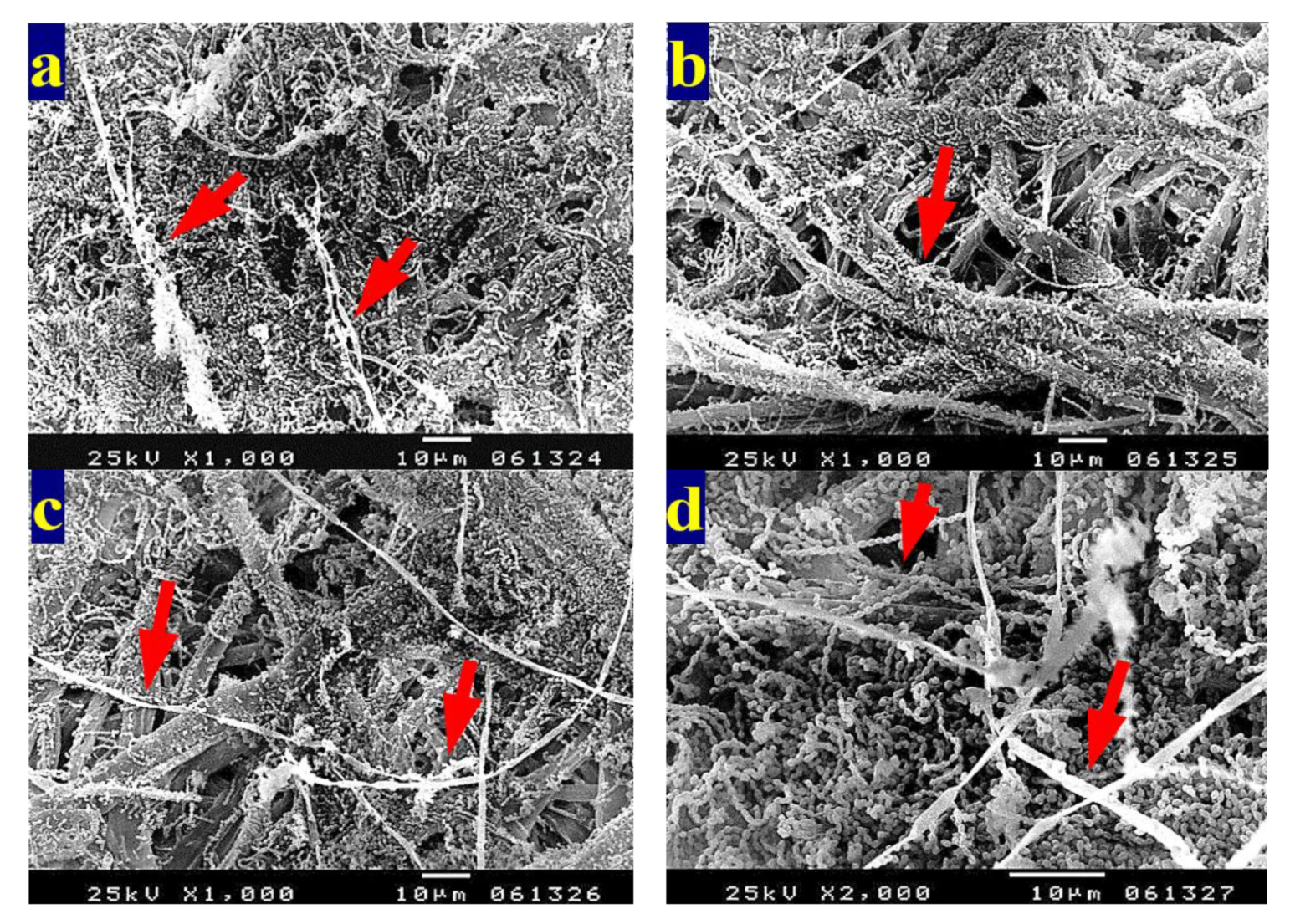
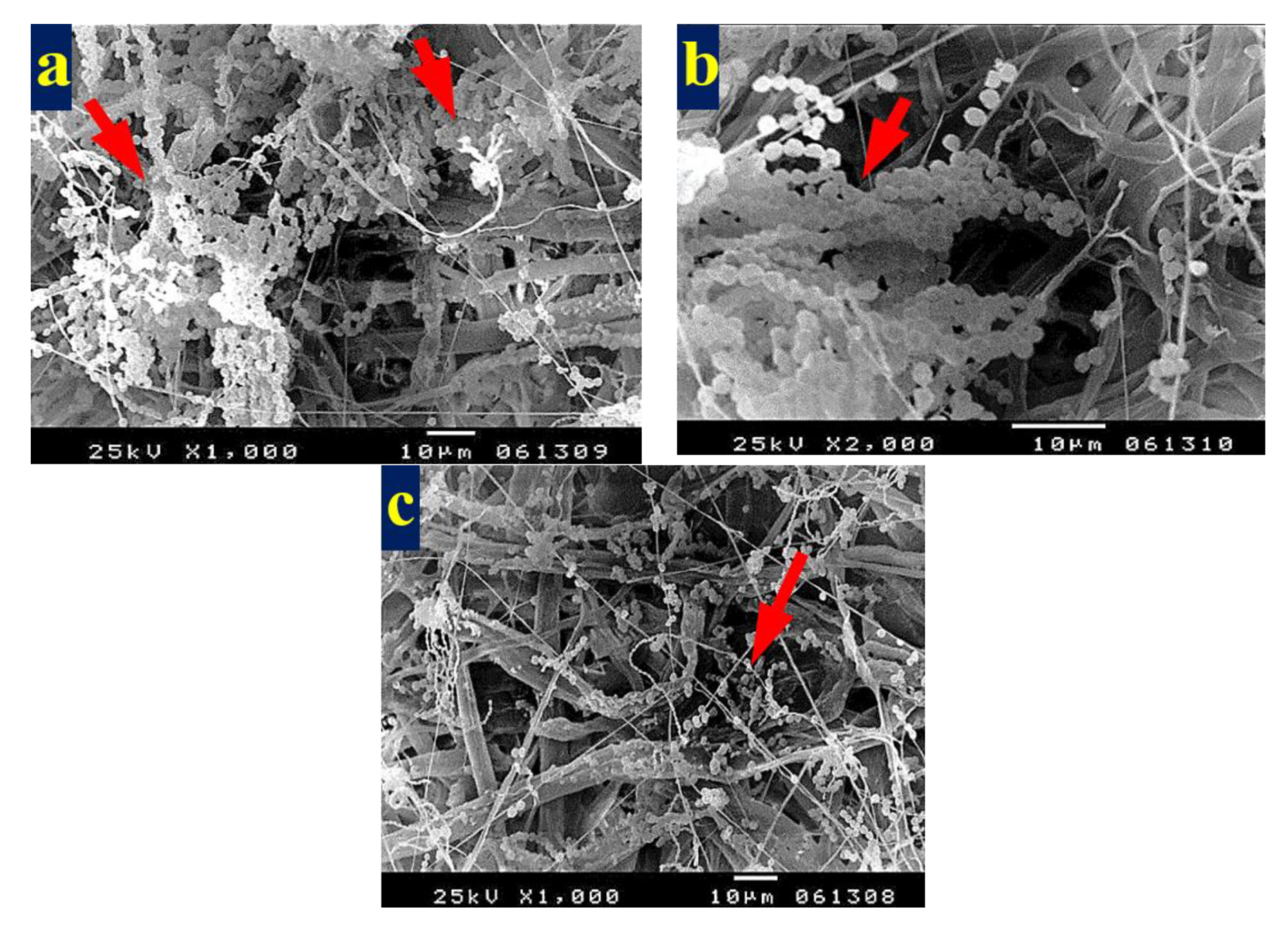
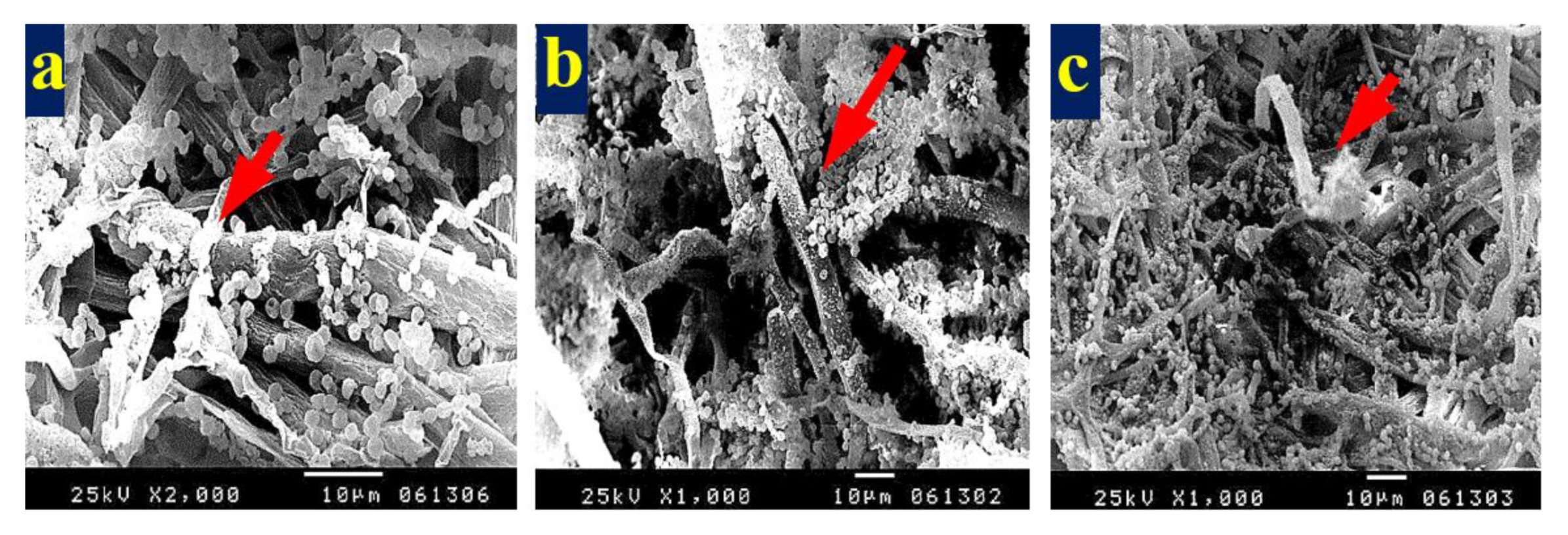
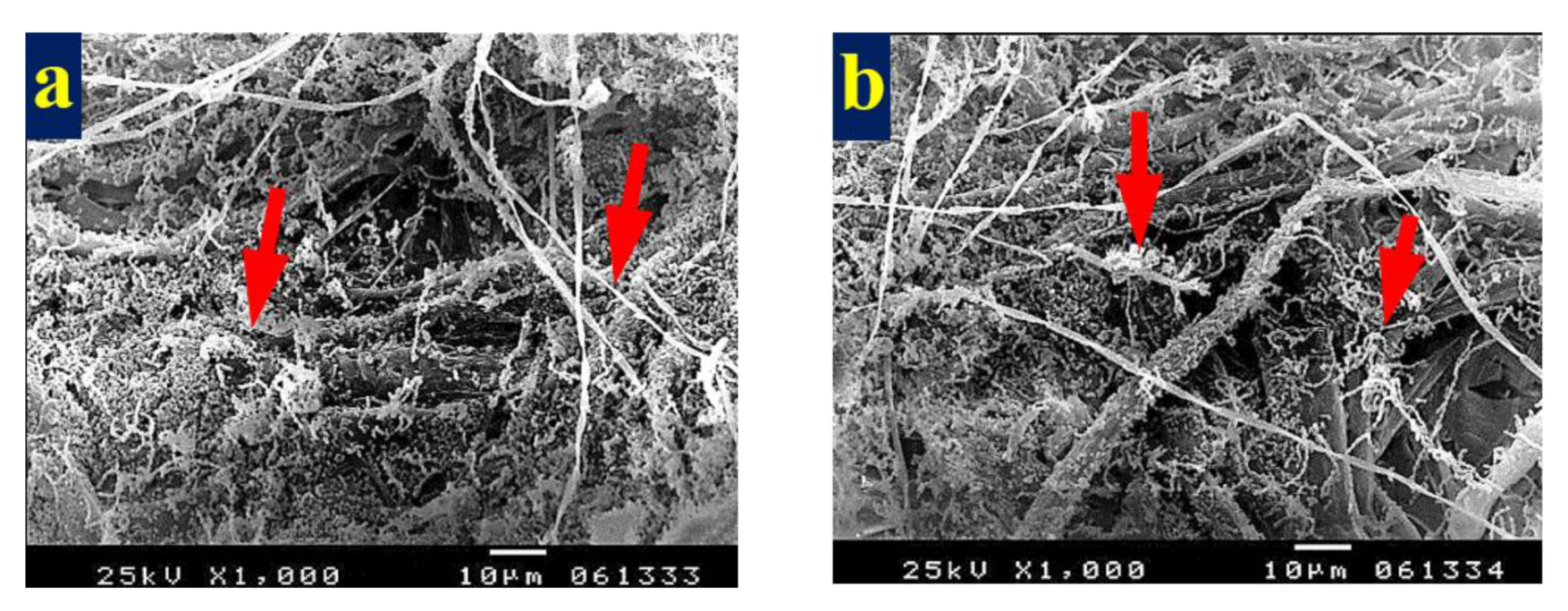
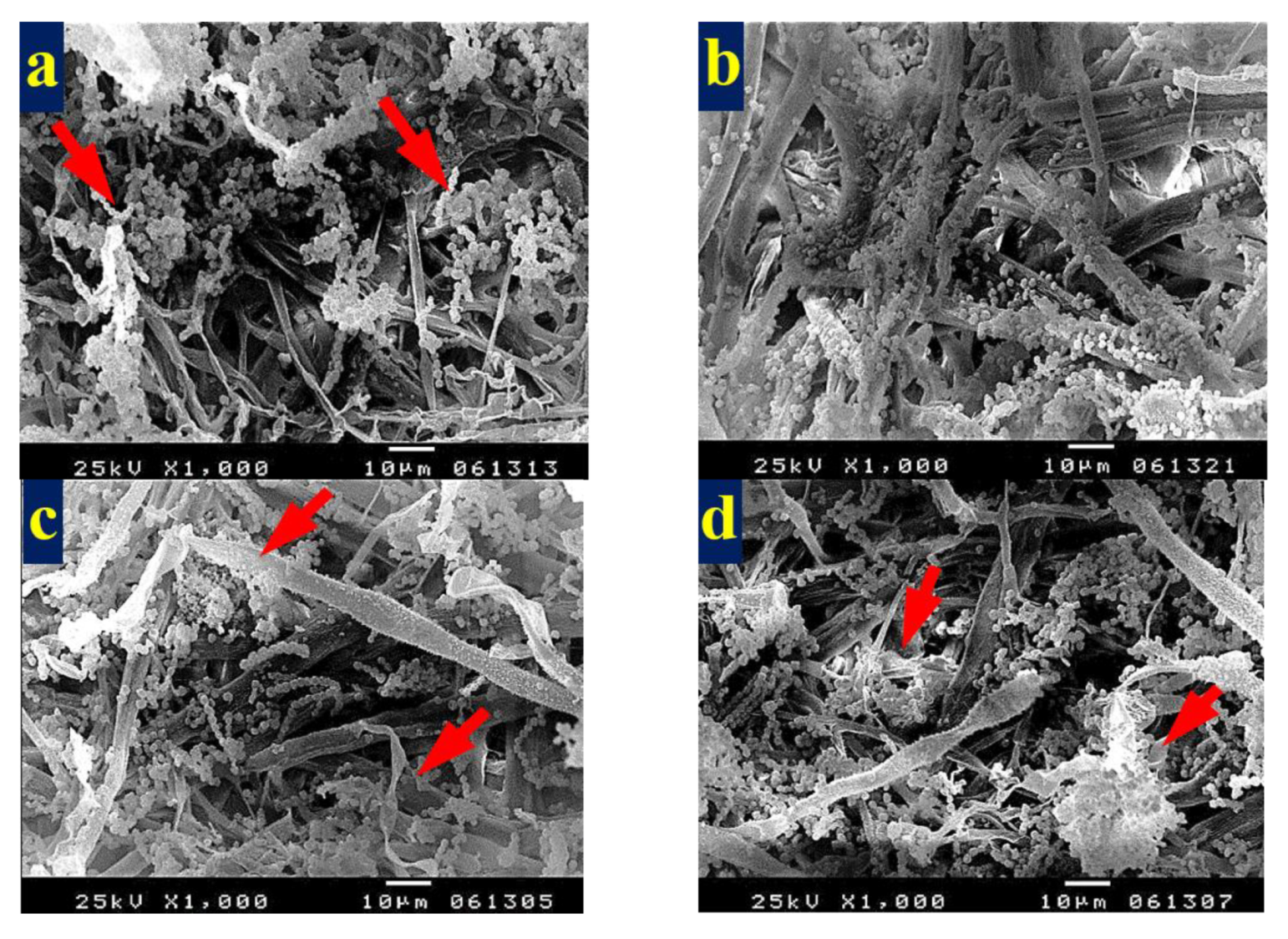
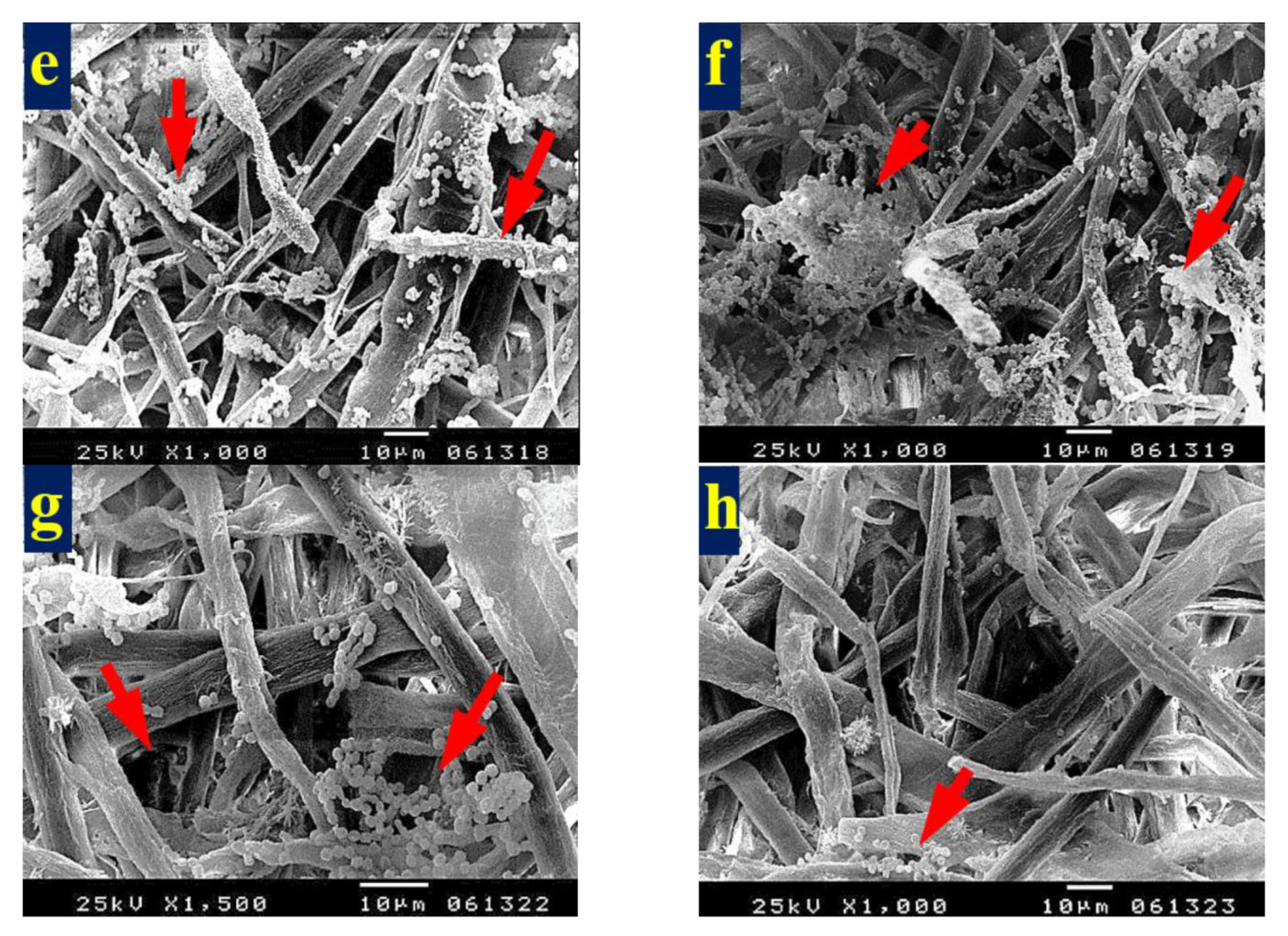
3.4. SEM Examination of Inoculated Paper Sheets
3.4.1. Fungal Infestation of Inoculated Paper Sheets
3.4.2. Inhibition of Fungal Growth of Inoculated Paper Sheets
3.5. Chemical Composition of the Oils
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hamzeh, Y.; Ashori, A.; Khorasani, Z.; Abdulkhani, A.; Abyaz, A. Pre-extraction of hemicelluloses from bagasse fibers: Effects of dry-strength additives on paper properties. Ind. Crop. Prod. 2013, 43, 365–371. [Google Scholar] [CrossRef]
- Hamzeh, Y.; Sabbaghi, S.; Ashori, A.; Abdulkhani, A.; Soltani, F. Improving wet and dry strength properties of recycled old corrugated carton (OCC) pulp using various polymers. Carbohyd. Polym. 2013, 94, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.; Singh, S.; Gangwar, A.; Mishra, O.P.; Chakrabarti, S.K.; Bhardwaj, N.K.; Varadhan, R. Blending of banana stem with wheat straw and bagasse to enhance physical strength properties of paper. IPPTA Q. J. Indian Pulp Pap. Tech. Assoc. 2013, 25, 121–126. [Google Scholar]
- Tajik, M.; Torshizi, H.J.; Resalati, H.; Hamzeh, Y. Effects of cationic starch in the presence of cellulose nanofibrils on structural, optical and strength properties of paper from soda bagasse pulp. Carbohyd. Polym. 2018, 194, 1–8. [Google Scholar] [CrossRef]
- Taha, A.S.; Salem, M.Z.M.; Abo Elgat, W.A.A.; Ali, H.M.; Hatamleh, A.A.; Abdel-Salam, E.M. Assessment of the impact of different treatments on technological and antifungal properties of produced Papyrus (Cyperus papyrus L.) sheets. Materials 2019, 12, 620. [Google Scholar] [CrossRef]
- Taha, A.S.; Abo Elgat, W.A.A.; Salem, M.Z.M.; Ali, H.M.; Fares, Y.G.E.; Elshikh, M.S. Impact of some plant source additives on enhancing the properties and antifungal activities of pulp made from Linen fibers. BioRes 2019, 14, 6025–6046. [Google Scholar]
- Abo Elgat, W.A.A.; Taha, A.S.; Böhm, M.; Vejmelková, E.; Mohamed, W.S.; Fares, Y.G.D.; Salem, M.Z.M. Evaluation of the Mechanical, Physical, and Anti-Fungal Properties of Flax Laboratory Papersheets with the Nanoparticles Treatment. Materials 2020, 13, 363. [Google Scholar] [CrossRef]
- Tripathi, S.; Nirmal Sharma, N.; Bhardwaj, N.K.; Varadhan, R. Improvement in Kraft Pulp Yield and Strength Properties of Paper by Black Liquor Pre-Treatment of Chips. Lignocellulose 2016, 5, 3–14. [Google Scholar]
- Hassan, R.R.A.; Mansour, M.M.A. A microscopic study of paper decayed by Trichoderma harzianum and Paecilomyces variotii. J. Polym. Environ. 2018, 26, 2698–2707. [Google Scholar] [CrossRef]
- İnan, Ö.; Özcan, M.M.; Al Juhaimi, F.Y. Antioxidant effect of mint, laurel and myrtle leaves essential oils on pomegranate kernel, poppy, grape and linseed oils. J. Clean. Prod. 2012, 27, 151–154. [Google Scholar] [CrossRef]
- Abdelsalam, N.R.; Salem, M.Z.M.; Ali, H.M.; Mackled, M.I.; EL-Hefny, M.; Elshikh, M.S.; Hatamleh, A.A. Morphological, biochemical, molecular, and oil toxicity properties of Taxodium trees from different locations. Ind. Crop. Prod. 2019, 139, 111515. [Google Scholar] [CrossRef]
- EL-Hefny, M.; Salem, M.Z.M.; Behiry, S.I.; Ali, H.M. The Potential Antibacterial and Antifungal Activities of Wood Treated with Withania somnifera Fruit Extract, and the Phenolic, Caffeine and Flavonoid Composition of the Extract According to HPLC. Processes 2020, 8, 113. [Google Scholar] [CrossRef]
- Mansour, M.M.A.; EL-Hefny, M.; Salem, M.Z.M.; Ali, H.M. The Biofungicide Activity of Some Plant Essential Oils for the Cleaner Production of Model Linen Fibers Similar to Those Used in Ancient Egyptian Mummification. Processes 2020, 8, 79. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Hamed, S.A.M.; Mansour, M.M.A. Assessment of efficacy and effectiveness of some extracted bio-chemicals as bio-fungicides on Wood. Drv. Ind. 2019, 70, 337–350. [Google Scholar] [CrossRef]
- Mohamed, A.A.; Behiry, S.I.; Younes, H.A.; Ashmawy, N.A.; Salem, M.Z.M.; Márquez-Molina, O.; Barbabosa-Pilego, A. Antibacterial activity of three essential oils and some monoterpenes against Ralstonia solanacearum phylotype II isolated from potato. Microb. Pathogen. 2019, 135, 103604. [Google Scholar] [CrossRef]
- Behiry, S.I.; EL-Hefny, M.; Salem, M.Z.M. Toxicity effects of Eriocephalus africanus L. leaf essential oil against some molecularly identified phytopathogenic bacterial strains. Nat. Prod. Res. 2019, 1–5. [Google Scholar] [CrossRef]
- Hamad, Y.K.; Abobakr, Y.; Salem, M.Z.M.; Ali, H.M.; Al-Sarar, A.S.; Al-Zabib, A.A. Activity of plant extracts/essential oils against some plant pathogenic fungi and mosquitoes: GC/MS analysis of bioactive compounds. BioRes 2019, 14, 4489–4511. [Google Scholar]
- Salem, M.Z.M.; Behiry, S.I.; EL-Hefny, M. Inhibition of Fusarium culmorum, Penicillium chrysogenum and Rhizoctonia solani by n-hexane extracts of three plant species as a wood-treated oil fungicide. J. Appl. Microbiol. 2019, 126, 1683–1699. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Vallejo, L.H.; Salem, A.Z.M.; Salem, M.Z.M.; Camacho, L.M.; Buendía, R.; Odongo, N.E. Effects of Schizochytrium microalgae and sunflower oil as sources of unsaturated fatty acids for the sustainable mitigation of ruminal biogases methane and carbon dioxide. J. Clean. Prod. 2017, 168, 1389–1397. [Google Scholar] [CrossRef]
- Lehn, D.N.; Esquerdo, V.M.; Júnior, M.A.D.; Dall’Agnol, W.; de Almeida Pinto, L.A. Microencapsulation of different oils rich in unsaturated fatty acids using dairy industry waste. J. Clean. Prod. 2018, 196, 665–673. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Zidan, Y.E.; Mansour, M.M.A.; El Hadidi, N.M.N.; Abo Elgat, W.A.A. Antifungal activities of two essential oils used in the treatment of three commercial woods deteriorated by five common mold fungi. Int. Biodeterior. Biodegrad. 2016, 106, 88–96. [Google Scholar] [CrossRef]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytochemisty 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Wilson, A.E.; Bergaentzle, M.; Bindler, F.; Marchioni, E.; Lintz, A.; Ennahar, S. In vitro efficacies of various isothiocyanates from cruciferous vegetables as antimicrobial agents against foodborne pathogens and spoilage bacteria. Food Control 2013, 30, 318–324. [Google Scholar] [CrossRef]
- Ildikó, S.-G.; Klára, K.A.; Marianna, T.-M.; Gnes, B.Á.; Zsuzsanna, M.-B.; B’alint, C. The effect of radio frequency heat treatment on nutritional and colloid-chemical properties of different white mustard (Sinapis alba L.) varieties. Innov. Food Sci. Emerg. Technol. 2006, 7, 74–79. [Google Scholar]
- Ali, A.; McKay, J.E. The chemical and physical characteristics and fatty acid composition of seed oils extracted from cruciferous species cultivated in Pakistan. Food Chem. 1982, 8, 225–231. [Google Scholar] [CrossRef]
- Al-Jasass, F.M.; Al-Jasser, M.S. Chemical composition and fatty acid content of some spices and herbs under Saudi Arabia conditions. Sci. World J. 2012, 2012, 859892. [Google Scholar] [CrossRef]
- Suhr, I.K.; Nielsen, V.P. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spolage fungi. J. Appl. Micrbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef]
- Valladares, G.; Garbin, L.; Defago, M.; Carpinella, C.; Palacios, S. Actividad antialimentaria e insecticida de un extracto de hojas senescentes de Melia azedarach (Meliaceae). Rev. Soc. Entomol. Argent. 2003, 62, 53–61. [Google Scholar]
- Farag, M.; Ahmed, M.H.M.; Yousef, H.; Abdel-Rahman, A.A.-H. Repellent and Insecticidal Activities of Melia azedarach L. against Cotton Leafworm, Spodoptera littoralis (Boisd.). Z. Naturforsch. C 2011, 66, 129–135. [Google Scholar] [CrossRef]
- Khan, I.H.; Javaid, A. Antifungal activity of Melia azedarach L. fruit extract against Sclerotium rolfsii, the cause of collar rot disease of chickpea. Mycopath 2013, 11, 9–13. [Google Scholar]
- Carpinella, M.C.; Herrero, G.G.; Alonso, R.; Palacios, S.M. Antifungal activity of Melia azedarach fruit extract. Fitoterapia 1999, 70, 296–298. [Google Scholar] [CrossRef]
- Abbas, M.K.; Ahmad, M.; Barkat, K.; Aslam, N. Antifungal, antioxidant and phytochemical screening of Melia azedarach flower extracts by using different solvents. J. Pharma. Res. Int. 2017, 20, 1–12. [Google Scholar] [CrossRef]
- Meziane, M.; Goumri, H. The antimicrobial effect of extracts of Melia azedarach on some pathogenic microorganisms. Int. J. Appl. Nat. Sci. 2014, 3, 173–180. [Google Scholar]
- Akacha, M.; Lahbib, K.; Remadi, M.D.; Boughanmi, N.G. Antibacterial, antifungal and anti-inflammatory activities of Melia azedarach ethanolic leaf extract. Bangladesh J. Pharmacol. 2016, 11, 666–674. [Google Scholar] [CrossRef]
- Farag, M.A.; El Din, R.S.; Fahmy, S. Headspace analysis of volatile compounds coupled to chemometrics in leaves from the Magnoliaceae family. Rec. Nat. Prod. 2015, 9, 153–158. [Google Scholar] [CrossRef]
- Guerra-Boone, L.; Alvarez-Román, R.; Salazar-Aranda, R.; Torres-Cirio, A.; Rivas-Galindo, V.M.; Waksman de Torres, N.; González, G.G.M.; Pérez-López, L.A. Chemical compositions and antimicrobial and antioxidant activities of the essential oils from Magnolia grandiflora, Chrysactinia mexicana, and Schinus molle found in northeast Mexico. Nat. Prod. Commun. 2013, 8, 135–138. [Google Scholar] [CrossRef]
- Jerusik, R. Fungi and paper manufacture. Fungal Biol. Rev. 2010, 24, 68–72. [Google Scholar] [CrossRef]
- Szczepanowska, H.; Lovett, C.M. A study of the removal and prevention of fungal stains on paper. J. Am. Inst. Conserv. 1992, 31, 147–160. [Google Scholar] [CrossRef]
- Alwaeli, M. The impact of product charges and EU directives on the level of packaging waste recycling in Poland. Resour. Conserv. Recycl. 2010, 54, 609–614. [Google Scholar] [CrossRef]
- Torres, C.E.; Lenon, G.; Craperi, D.; Wilting, R.; Blanco, Á. Enzymatic treatment for preventing biofilm formation in the paper industry. Appl. Microbiol. Biotechnol. 2011, 92, 95–103. [Google Scholar] [CrossRef]
- Guzińska, K.; Owczarek, M.; Dymel, M. Investigation in the microbiological purity of paper and board packaging intended for contact with food. Fibres Text. East. Eur. 2012, 20, 186–190. [Google Scholar]
- Vikele, L.; Laka, M.; Sable, I.; Rozenberga, L.; Grinfelds, U.; Zoldners, J.; Passas, R.; Mauret, E. Effect of chitosan on properties of paper for packaging. Cellul. Chem. Technol. 2017, 51, 67–73. [Google Scholar]
- Zemljič, L.F.; Valh, J.V.; Kreže, T. Preparation of antimicrobial paper sheets using chitosan. Cellul. Chem. Technol. 2017, 51, 75–81. [Google Scholar]
- Vellingiri, K.; Ramachandran, T.; Senthilkumar, M. Eco-friendly application of nano chitosan in antimicrobial coatings in the textile industry. Nanosci. Nanotechnol. 2013, 3, 75–89. [Google Scholar] [CrossRef]
- Lim, J.-K.; Melani, L.; Kim, H.-J. Effects of Ag/ZnO Composite Fillers on the Antifungal Activity of Paper. Palpu Chongi Gisul/J. Korea Technol. Assoc. Pulp Pap. Ind. 2017, 49, 134–142. [Google Scholar]
- Nasser, R.A.; Salem, M.Z.M.; Al-Mefarrej, H.A.; Aref, I.M. Measurement of some strength properties and chemical composition of seven hardwood Species grown in Northwest Egypt. J. Test. Eval. 2016, 44, 1629–1639. [Google Scholar] [CrossRef]
- Nasser, R.A.; AI-Meffarrei, H.A.; Abdel-Aal, M.A.; Hegazy, S.S. Chemical and Mechanical Properties of Melia Azedarach Mature Wood as Affected by Primary Treated Sewage-Effluent Irrigation. Am.-Eurasian J. Agric. Environ. Sci. 2010, 7, 697–704. [Google Scholar]
- ASTM D2395-84. Standard Test Method for Specific Gravity of Wood and Wood-Base Materials—Method A; ASTM International: West Conshohocken, PA, USA, 1989; Available online: www.astm.org (accessed on 15 August 1993).
- Mohamed, W.A.; Mansour, M.M.A.; Salem, M.Z.M. Lemna gibba and Eichhornia crassipes extracts: Clean alternatives for deacidification, antioxidation and fungicidal treatment of historical paper. J. Clean. Prod. 2019, 219, 846–855. [Google Scholar] [CrossRef]
- Okla, M.K.; Alamri, S.A.; Salem, M.Z.M.; Ali, H.M.; Behiry, S.I.; Nasser, R.A.; Alaraidh, I.A.; Al-Ghtani, S.M.; Soufan, W. Yield, phytochemical constituents, and antibacterial activity of essential oils from the leaves/twigs, branches, branch wood, and branch bark of Sour Orange (Citrus aurantium L.). Processes 2019, 7, 363. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Mansour, M.M.A.; Elansary, H.O. Evaluation of the effect of inner and outer bark extracts of Sugar Maple (Acer saccharum var. saccharum) in combination with citric acid against the growth of three common molds. J. Wood Chem. Technol. 2019, 39, 136–147. [Google Scholar] [CrossRef]
- TAPPI Test Methom; Tappi Press: Atlanta, GA, USA, 2018.
- Reinprecht, L.; Kizlink, J. Wood preservatives prepared from electrical and cooling wastes. Acta Fac. Ecol. Tu Zvolen 2017, 15, 71–76. [Google Scholar]
- SAS. User Guide: Statistics (Release 8.02); SAS Institute: Cary, NC, USA, 2001. [Google Scholar]
- Soliman, A.S.; Shehata, M.S.; Ahmad, F.; Abdel-Atty, M. Evaluation of paper pulp and paper making characteristics produced from different African woody trees grown in Egypt. Res. J. 2017, 11, 19–27. [Google Scholar]
- Vicentim, M.P.; de Almeida Faria, R.; Ferraz, A. High-yield kraft pulping of Eucalyptus grandis Hill ex Maiden biotreated by Ceriporiopsis subvermispora under two different culture conditions. Holzforsch. Int. J. Biol. Chem. Phys. Technol. Wood 2009, 63, 408–413. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Gutiérrez, A.; Ibarra, D.; Li, J.; Gellerstedt, G.; Santos, J.I.; Jiménez-Barbero, J.; Martínez, A.T.; del Río, J.C. Structural characterization of milled wood lignin from different eucalypt species. Holzforschung 2008, 62, 514–526. [Google Scholar] [CrossRef]
- Baeza, J.; Urizar, S.; Erismann, N.M.; Freer, J.; Schmidt, E.; Durán, N. Organosolv pulping-V: Formic acid delignification of Eucalyptus globulus and Eucalyptus Grandis. Bioresour. Technol. 1991, 37, 1–6. [Google Scholar] [CrossRef]
- Vennila, S.; Parthiban, K.T.; Seenivasan, R.; Saravanan, V.; Anbu, P.V.; Kanna, S.U.; Durairasu, P. Pulpwood characterization and screening short rotation Eucalyptus clones. Indian J. Ecol. 2011, 38, 84–90. [Google Scholar]
- Antova, G.A.; Angelova–Romova, M.I.; Petkova, Z.Y.; Teneva, O.T.; Marcheva, M.P. Lipid composition of mustard seed oils (Sinapis alba L.). Bulg. Chem. Commun. 2017, 49, 55–60. [Google Scholar]
- Mortuza, M.G. Tocopherol and sterol content of some rapeseed/mustard cultivars developed in Bangladesh. Pak. J. Biol. Sci. 2006, 9, 1812–1816. [Google Scholar]
- Vaidya, B.; Choe, E. Stability of tocopherols and lutein in oil extracted from roasted or unroasted mustard seeds during the oil oxidation in the dark. Food Sci. Biotechnol. 2011, 20, 193–199. [Google Scholar] [CrossRef]
- Singh, S.; Das, S.S.; Singh, G.; Perroti, M.; Schuff, C.; Catalán, C.A.N. Comparison of chemical composition, antioxidant and antimicrobial potentials of essential oils and oleoresins obtained from seeds of Brassica juncea and Sinapis Alba. MOJ Food Process. Technol. 2017, 4, 113–120. [Google Scholar]
- Huang, L.; Sun, H.; He, L.; Li, Z.; Yang, M. Fatty acids in leaves of Magnoliaceae. Acta Hortic. 2008, 769, 379–386. [Google Scholar] [CrossRef]
- Wang, Y.; Mu, R.; Wang, X.; Liu, S.; Fan, Z. Chemical composition of volatile constituents of Magnolia grandiflora. Chem. Nat. Compd. 2009, 45, 257–258. [Google Scholar] [CrossRef]
- McGaw, L.J.; Jäger, A.K.; van Staden, J. Antibacterial effects of fatty acids and related compounds from plants. S. Afr. J. Bot. 2002, 68, 417–423. [Google Scholar] [CrossRef]
- Seidel, V.; Taylor, P.W. In vitro activity of extracts and constituents of Pelargonium against rapidly growing mycobacteria. Int. J. Antimicrob. Agents 2004, 23, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, R.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar]
- Hadjiakhoondi, A.; Vatandoost, H.; Khanavi, M.; Sadeghipour-Roodsari, H.R.; Vosoughi, M.; Kazemi, M.; Abai, M.R. Fatty acid composition and toxicity of Melia azedarach L. fruits against malaria vector Anopheles stephensi. Iran. J. Pharm. Sci. 2006, 2, 97–102. [Google Scholar]









| Bleaching Conditions | E. camaldulensis | M. sinclairii |
|---|---|---|
| 1. Sodium hydroxide stage | ||
| Sodium hydroxide %, for 2 h at 75 °C | 2 | 2 |
| Initial brightness | 24 | 28 |
| 2. Hypochlorite stage | ||
| Hypochlorite %, for 3 h at 35 °C | 4 | 4 |
| Final brightness | 68.56 | 70.31 |
| Paper Sheet Properties | Standard Method | Model Machine Used |
|---|---|---|
| Tear index, mN·m2/g | T 414 om-12 | FRANK-PTI GMBH, Elmendorf tear tester, model 53984, serial no. 40551 |
| Tensile index, N m/g | T 404 wd-03 | Adamel Lhomargy, model 596420, DY-30, Saint-Baldoph, France |
| Burst index, kPa m2/g | T 403 om-15 | Tecnolab Company, model BS 20 E, serial no. 160.08, Laakirchen, Austria |
| Double fold number | T 511 om-13 | Folding endurance, KÖGEL LEIPZIG, DFP 6/60 9401 |
| Brightness, % | T525 om-17 | Color Touch Model ISO, model CTH-ISO, serial no. CTH A 2054, Technidyne Corporation, New Albany, IN, USA |
| Analysis | Value in Wood-Branch Shavings | |
|---|---|---|
| E. camaldulensis | M. sinclairii | |
| Solubility in cold water | 4.45% | 6.95% |
| Solubility in hot water | 10.2% | 11.3% |
| Benzene and alcohol extractives | 8.92% | 6.32% |
| Acid-insoluble lignin (Klason lignin) | 27% | 23% |
| Wood shaving ash | 0.9% | 1.1% |
| Pentosans | 14% | 16% |
| Holocellulose | 56% | 61% |
| Moisture content | 9.6% | 9.1% |
| Pulp Properties | Wood-Branch Pulp | |
|---|---|---|
| E. camaldulensis | M. sinclairii | |
| Pulp yield (%) | 48 | 37 |
| Initial Schopper-Riegler, SR0 | 27 | 19 |
| Kappa number | 24 | 18 |
| Wood Pulp | HeOE Additives | Conc. | Mechanical Properties | Optical Properties | Grammage g/m2 | |||
|---|---|---|---|---|---|---|---|---|
| Tensile Index | Tear Index | Burst Index | Double Fold | Brightness | ||||
| N·m/g | mN·m2/g | kPa·m2/g | No. | % | ||||
| E. camaldulensis | Without additive | 0% | 32.10 ± 0.17 * | 3.32 ± 0.005 | 3.08 ± 0.04 | 8.33 ± 0.57 | 68.56 ± 0.057 | 71.65 ± 0.49 |
| DMSO | 10% | 32.26 ± 0.15 | 3.34 ± 0.01 | 3.12 ± 0.01 | 8.66 ± 0.57 | 67.87 ± 0.005 | 72.31 ± 0.28 | |
| S. alba | 1% | 32.86 ± 0.02 | 3.72 ± 0.005 | 3.54 ± 0.01 | 8.00 | 66.35 ± 0.03 | 71.82 ± 0.28 | |
| 3% | 33.40 ± 0.17 | 3.86 ± 0.01 | 3.92 ± 0.06 | 8.33 ± 0.57 | 67.13 ± 0.05 | 73.31 ± 1.59 | ||
| 5% | 33.90 ± 0.05 | 4.11 ± 0.11 | 4.11 ± 0.01 | 9.00 | 67.63 ± 0.11 | 72.98 ± 0.28 | ||
| M. azedarach | 1% | 32.87 ± 0.005 | 3.44 ± 0.005 | 3.76 ± 0.005 | 8.33 ± 0.57 | 66.5 4 ± 0.005 | 72.48 ± 0.28 | |
| 3% | 32.98 ± 0.005 | 3.87 ± 0.005 | 3.87 ± 0.01 | 8.66 ± 0.57 | 66.17 ± 0.112 | 71.98 ± 0.76 | ||
| 5% | 33.14 ± 0.049 | 4.07 ± 0.06 | 3.97 ± 0.01 | 9.00 | 65.76 ± 0.057 | 72.15 ± 0.86 | ||
| M. grandiflora | 1% | 32.69 ± 0.16 | 3.44 ± 0.02 | 3.66 ± 0.005 | 8.00 | 65.66 ± 0.057 | 72.98 ± 1.52 | |
| 3% | 32.98 ± 0.01 | 3.81 ± 0.06 | 3.87 ± 0.02 | 8.66 ± 0.57 | 64.89 ± 0.011 | 74.97 ± 0.28 | ||
| 5% | 33.76 ± 0.005 | 4.06 ± 0.063 | 3.98 ± 0.01 | 9.00 | 64.19 ± 0.06 | 71.65 ± 0.49 | ||
| M. sinclairii | Without additive | 0% | 14.14 ± 0.16 | 2.23 ± 0.02 | 1.56 ± 0.005 | 2.00 | 70.31 ± 0.025 | 72.31 ± 0.28 |
| DMSO | 10% | 14.16 ± 0.057 | 2.26 ± 0.015 | 1.58 ± 0.02 | 2.33 ± 0.57 | 69.81 ± 0.106 | 72.48 ± 0.28 | |
| S. alba | 1% | 14.83 ± 0.11 | 2.55 ± 0.005 | 1.75 ± 0.005 | 2.66 ± 0.57 | 68.98 ± 0.01 | 70.99 ± 3.76 | |
| 3% | 15.80 ± 0.10 | 3.10 ± 0.10 | 1.96 ± 0.017 | 2.33 ± 0.57 | 67.65 ± 0.005 | 72.32 ± 0.28 | ||
| 5% | 16.36 ± 0.057 | 3.40 ± 0.17 | 2.56 ± 0.32 | 3.00 | 65.73 ± 0.05 | 75.96 ± 0.76 | ||
| M. azedarach | 1% | 14.83 ± 0.11 | 2.27 ± 0.011 | 1.61 ± 0.005 | 2.33 ± 0.57 | 70.23 ± 0.005 | 72.32 ± 0.28 | |
| 3% | 15.16 ± 0.057 | 2.56 ± 0.011 | 1.67 ± 0.015 | 2.66 ± 0.57 | 67.46 ± 0.21 | 71.49 ± 0.28 | ||
| 5% | 15.83 ± 0.057 | 2.94 ± 0.011 | 1.96 ± 0.011 | 3.00 | 66.60 ± 0.10 | 72.81 ± 0.28 | ||
| M. grandiflora | 1% | 14.80 ± 0.10 | 2.43 ± 0.01 | 1.65 ± 0.011 | 2.66 ± 0.57 | 69.92 ± 0.05 | 73.31 ± 2.91 | |
| 3% | 15.33 ± 0.11 | 2.87 ± 0.01 | 1.87 ± 0.01 | 3.00 | 68.73 ± 0.057 | 74.47 ± 1.03 | ||
| 5% | 16.20 ± 0.10 | 2.98 ± 0.01 | 2.20 ± 0.17 | 2.33 ± 0.57 | 66.60 ± 0.10 | 75.30 ± 1.43 | ||
| Minimum significant difference ** | 0.367 | 0.154 | 0.224 | 1.476 | 0.235 | 3.487 | ||
| WB Pulp | HeOE | Conc. | A. terreus | A. flavus | ||
|---|---|---|---|---|---|---|
| Inhibition Zone (mm) | Growth on Disc (mm) | Inhibition Zone (mm) | Growth on Disc (mm) | |||
| 14th day | 14th day | |||||
| E. camaldulensis | No additives | 0 | 0 | 5–7 | 0 | 10 |
| DMSO | 10% | 0 | 6–7 | 0 | 10 | |
| M. azedarach | 1% | 0 | 1–3 | 0 | 1–2 | |
| 3% | 3–7 | 0 | 0 | 0–2 | ||
| 5% | 5–7 | 0 | 1 | 0 | ||
| S. alba | 1% | 0 | 3–8 | 0 | 2–3 | |
| 3% | 2–5 | 0 | 0 | 0.5–2 | ||
| 5% | 3–7 | 0 | 0.5 | 0–1 | ||
| M. grandiflora | 1% | 0 | 8–9 | 0 | 8–9 | |
| 3% | 2–6 | 0 | 0 | 7–8 | ||
| 5% | 3–7 | 0 | 0 | 1–4 | ||
| M. sinclairii | No additives | 0 | 0 | 9–10 | 0 | 3–8 |
| DMSO | 10% | 0 | 9–10 | 0 | 4–8 | |
| M. azedarach | 1% | 0 | 9–10 | 0 | 3–4 | |
| 3% | 1–3 | 0 | 1 | 0 | ||
| 5% | 3–5 | 0 | 4 | 0 | ||
| S. alba | 1% | 0–2 | 0–3 | 0 | 0–1 | |
| 3% | 1–4 | 0 | 0 | 0–1 | ||
| 5% | 2–3 | 0 | 0 | 0–1 | ||
| M. grandiflora | 1% | 0 | 0.5–2 | 0 | 5–6 | |
| 3% | 0–2 | 0–0.5 | 0 | 3–9 | ||
| 5% | 0.5–2 | 0 | 0–2 | 0–1 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salem, M.Z.M.; Abo Elgat, W.A.A.; Taha, A.S.; Fares, Y.G.D.; Ali, H.M. Impact of Three Natural Oily Extracts as Pulp Additives on the Mechanical, Optical, and Antifungal Properties of Paper Sheets Made from Eucalyptus camaldulensis and Meryta sinclairii Wood Branches. Materials 2020, 13, 1292. https://doi.org/10.3390/ma13061292
Salem MZM, Abo Elgat WAA, Taha AS, Fares YGD, Ali HM. Impact of Three Natural Oily Extracts as Pulp Additives on the Mechanical, Optical, and Antifungal Properties of Paper Sheets Made from Eucalyptus camaldulensis and Meryta sinclairii Wood Branches. Materials. 2020; 13(6):1292. https://doi.org/10.3390/ma13061292
Chicago/Turabian StyleSalem, Mohamed Z. M., Wael A. A. Abo Elgat, Ayman S. Taha, Yahia G. D. Fares, and Hayssam M. Ali. 2020. "Impact of Three Natural Oily Extracts as Pulp Additives on the Mechanical, Optical, and Antifungal Properties of Paper Sheets Made from Eucalyptus camaldulensis and Meryta sinclairii Wood Branches" Materials 13, no. 6: 1292. https://doi.org/10.3390/ma13061292
APA StyleSalem, M. Z. M., Abo Elgat, W. A. A., Taha, A. S., Fares, Y. G. D., & Ali, H. M. (2020). Impact of Three Natural Oily Extracts as Pulp Additives on the Mechanical, Optical, and Antifungal Properties of Paper Sheets Made from Eucalyptus camaldulensis and Meryta sinclairii Wood Branches. Materials, 13(6), 1292. https://doi.org/10.3390/ma13061292