Successful Immobilization of Lanthanides Doped TiO2 on Inert Foam for Repeatable Hydrogen Generation from Aqueous Ammonia
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Photocatalysts
2.2. Characterization Methods of Photocatalysts and Photocatalytic Tests
3. Results and Discussion
3.1. Structural and Textural Properties of Photocatalysts in Its Powder Form
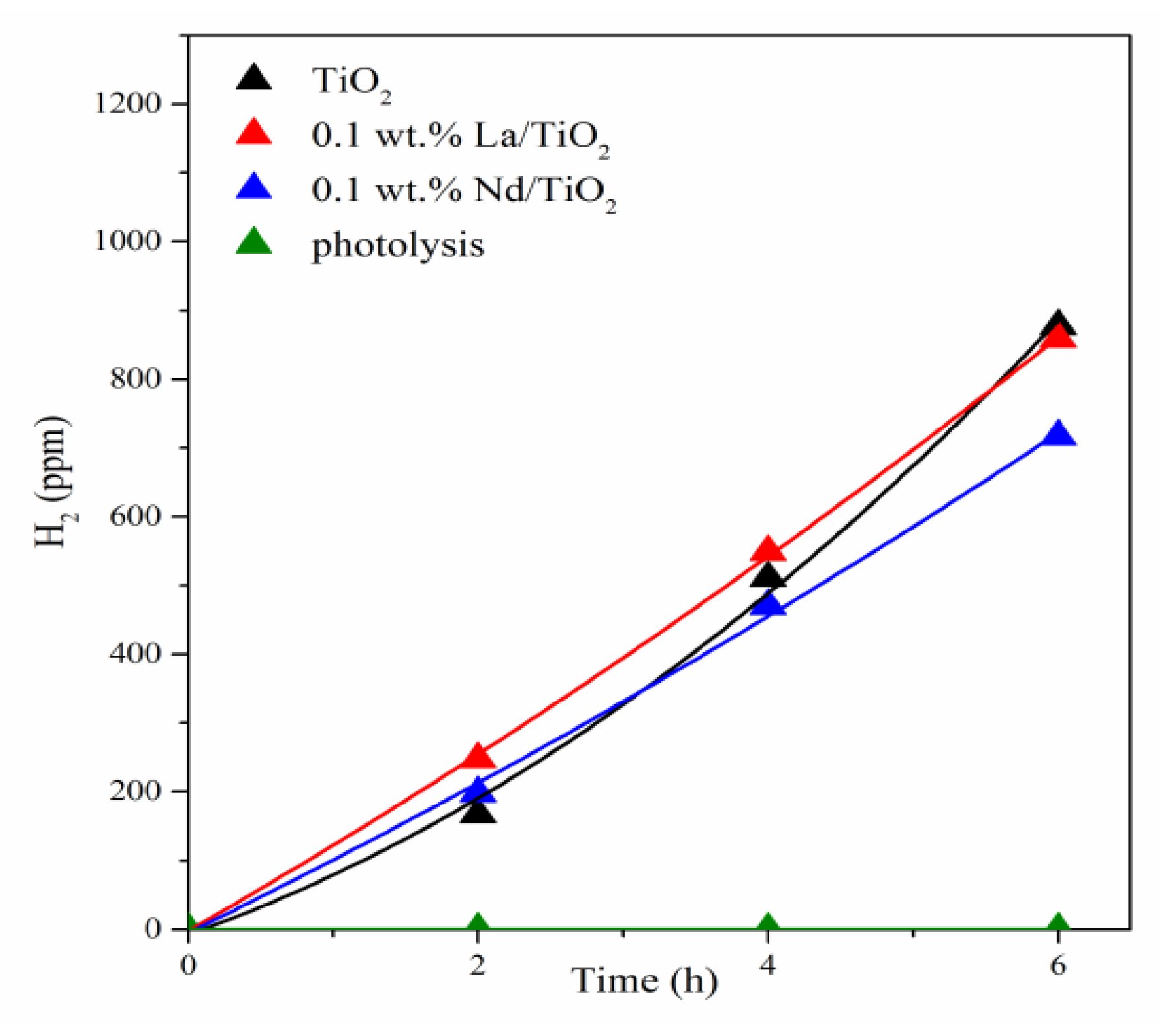
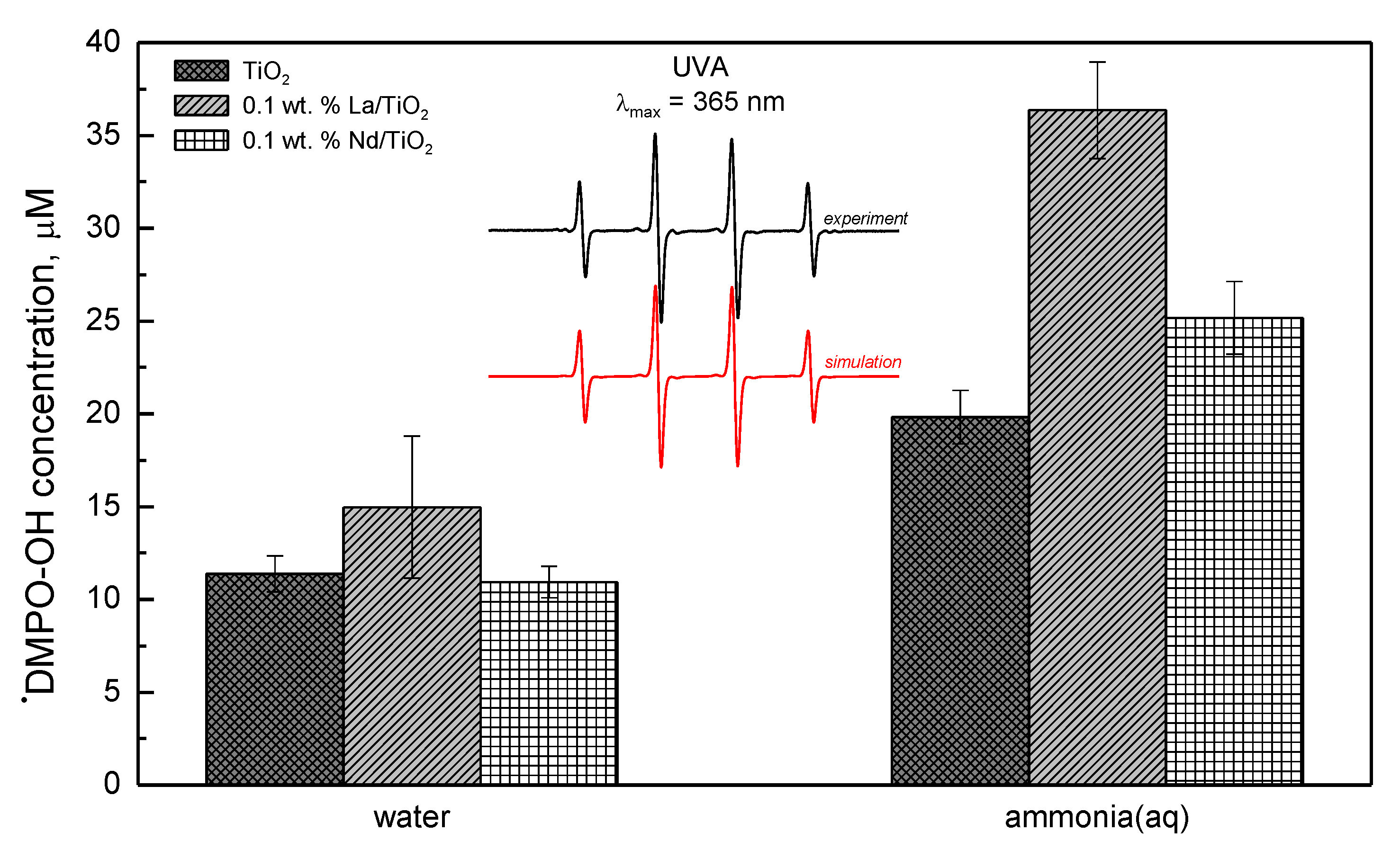
3.2. Photocatalytic Hydrogen Production from Ammonia
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Green, L. An ammonia energy vector for the hydrogen economy. Int. J. Hydrogen Energy 1982, 7, 355–359. [Google Scholar] [CrossRef]
- Altomare, M.; Chiarello, G.L.; Costa, A.; Guarino, M.; Selli, E. Photocatalytic abatement of ammonia in nitrogen-containing effluents. Chem. Eng. J. 2012, 191, 394–401. [Google Scholar] [CrossRef]
- Kominami, H.; Nishimune, H.; Ohta, Y.; Arakawa, Y.; Inaba, T. Photocatalytic hydrogen formation from ammonia and methyl amine in an aqueous suspension of metal-loaded titanium(IV) oxide particles. Appl. Catal. B 2012, 111, 297–302. [Google Scholar] [CrossRef]
- Shavisi, Y.; Sharifnia, S.; Hosseini, S.N.; Khadivi, M.A. Application of TiO2/perlite photocatalysis for degradation of ammonia in wastewater. J. Ind. Eng. Chem. 2014, 20, 278–283. [Google Scholar] [CrossRef]
- Obata, K.; Kishishita, K.; Okemoto, A.; Taniya, K.; Ichihashi, Y.; Nishiyama, S. Photocatalytic decomposition of NH3 over TiO2 catalysts doped with Fe. Appl. Catal. B 2014, 160, 200–203. [Google Scholar] [CrossRef]
- Malato, S.; Fernández-Ibáñez, P.; Maldonado, M.I.; Blanco, J.; Gernjak, W. Decontamination and disinfection of water by solar photocatalysis: Recent overview and trends. Catal. Today 2009, 147, 1–59. [Google Scholar] [CrossRef]
- Reli, M.; Edelmannova, M.; Sihor, M.; Praus, P.; Svoboda, L.; Mamulova Kutlakova, K.; Otoupalikova, H.; Capek, L.; Hospodkova, A.; Obalova, L. Photocatalytic H2 generation from aqueous ammonia solution using ZnO photocatalysts prepared by different methods. Int. J. Hydrogen Energy 2015, 40, 8530–8538. [Google Scholar] [CrossRef]
- Low, J.; Cheng, B.; Yu, J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: a review. Appl. Surf. Sci. 2017, 392, 658–686. [Google Scholar] [CrossRef]
- Al-Mamun, M.R.; Kader, S.; Islam, M.S.; Khan, M.Z.H. Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103248. [Google Scholar] [CrossRef]
- Yadav, H.M.; Kim, J.S. Solvothermal synthesis of anatase TiO2-graphene oxide nanocomposites and their photocatalytic performance. J. Alloys Compd. 2016, 688, 123–129. [Google Scholar] [CrossRef]
- Mazierski, P.; Mikolajczyk, A.; Bajorowicz, B.; Malankowska, A.; Zaleska-Medynska, A.; Nadolna, J. The role of lanthanides in TiO2-based photocatalysis: A review. Appl. Catal. B 2018, 233, 301–317. [Google Scholar] [CrossRef]
- Li, X.; Zhang, F.; Zhao, D. Highly efficient lanthanide upconverting nanomaterials: Progresses and challenges. Nano Today 2013, 8, 643–676. [Google Scholar] [CrossRef]
- Alenzi, N.; Liao, W.S.; Cremer, P.S.; Sanchez-Torres, V.; Wood, T.K.; Ehlig-Economides, C.; Cheng, Z. Photoelectrochemical hydrogen production from water/methanol decomposition using Ag/TiO2 nanocomposite thin films. Int. J. Hydrogen Energy 2010, 35, 11768–11775. [Google Scholar] [CrossRef]
- Wood, D.; Shaw, S.; Cawte, T.; Shanen, E.; Van Heyst, B. An overview of photocatalyst immobilization methods for air pollution remediation. Chem. Eng. J. 2019, 123490. [Google Scholar] [CrossRef]
- Rao, K.V.S.; Subrahmanyam, M.; Boule, P. Immobilized TiO2 photocatalyst during long-term use: decrease of its activity. Appl. Catal. B 2004, 49, 239–249. [Google Scholar] [CrossRef]
- Hegedűs, P.; Szabó-Bárdos, E.; Horváth, O.; Szabó, P.; Horváth, K. Investigation of a TiO2 photocatalyst immobilized with poly(vinyl alcohol). Catal. Today 2017, 284, 179–186. [Google Scholar] [CrossRef]
- Matějová, L.; Cajthaml, T.; Matěj, Z.; Benada, O.; Klusoň, P.; Šolcová, O. Super/subcritical fluid extractions for preparation of the crystalline titania. J. Supercrit. Fluids 2010, 52, 215–221. [Google Scholar] [CrossRef]
- Kočí, K.; Troppová, I.; Edelmannová, M.; Starostka, J.; Matějová, L.; Lang, J.; Reli, M.; Drobná, H.; Rokicińska, A.; Kuśtrowski, P. Photocatalytic decomposition of methanol over La/TiO2 materials. Environ. Sci. Pollut. Res. Int. 2018, 25, 34818–34825. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Edelmannová, M.; Troppová, I.; Drobná, H.; Rokicińska, A.; Kuśtrowski, P.; Dvoranová, D.; Čapek, L. Photocatalytic hydrogen production from methanol over Nd/TiO2. J. Photochem. Photobiol. A 2018, 366, 55–64. [Google Scholar] [CrossRef]
- Koci, K.; Troppova, I.; Reli, M.; Matejova, L.; Edelmannova, M.; Drobna, H.; Dubnova, L.; Rokicinska, A.; Kustrowski, P.; Capek, L. Nd/TiO2 Anatase-Brookite Photocatalysts for Photocatalytic Decomposition of Methanol. Front. Chem. 2018, 6, 1–11. [Google Scholar] [CrossRef]
- Reli, M.; Ambrozova, N.; Sihor, M.; Matejova, L.; Capek, L.; Obalova, L.; Matej, Z.; Kotarba, A.; Koci, K. Novel cerium doped titania catalysts for photocatalytic decomposition of ammonia. Appl. Catal. B 2015, 178, 108–116. [Google Scholar] [CrossRef]
- Dubnová, L.; Zvolská, M.; Edelmannová, M.; Matějová, L.; Reli, M.; Drobná, H.; Kuśtrowski, P.; Kočí, K.; Čapek, L. Photocatalytic decomposition of methanol-water solution over N-La/TiO2 photocatalysts. Appl. Surf. Sci. 2019, 469, 879–886. [Google Scholar] [CrossRef]
- Tahir, B.; Tahir, M.; Amin, N.A.S. Tailoring performance of La-modified TiO2 nanocatalyst for continuous photocatalytic CO2 reforming of CH4 to fuels in the presence of H2O. Energy Convers. Manage. 2018, 159, 284–298. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, S.; Li, J.; Wang, Y.; Jiang, G.; Zhao, Z.; Liu, B.; Gong, X.; Duan, A.; Liu, J.; et al. Photocatalytic reduction of CO2 with water vapor on surface La-modified TiO2 nanoparticles with enhanced CH4 selectivity. Appl. Catal. B 2015, 168, 125–131. [Google Scholar] [CrossRef]
- Choudhury, B.; Borah, B.; Choudhury, A. Ce–Nd codoping effect on the structural and optical properties of TiO2 nanoparticles. Mater. Sci. Eng. B 2013, 178, 239–247. [Google Scholar] [CrossRef]
- Ali, A.; Yassitepe, E.; Ruzybayev, I.; Ismat Shah, S.; Bhatti, A.S. Improvement of (004) texturing by slow growth of Nd doped TiO2 films. J. Appl. Phys. 2012, 112, 113505. [Google Scholar] [CrossRef]
- Yuzawa, H.; Mori, T.; Itoh, H.; Yoshida, H. Reaction Mechanism of Ammonia Decomposition to Nitrogen and Hydrogen over Metal Loaded Titanium Oxide Photocatalyst. J. Phys. Chem. C 2012, 116, 4126–4136. [Google Scholar] [CrossRef]
- Dvoranova, D.; Barbierikova, Z.; Brezova, V. Radical intermediates in photoinduced reactions on TiO2 (an EPR spin trapping study). Molecules 2014, 19, 17279–17304. [Google Scholar] [CrossRef]
- Csányi, L.J.; Nagy, L.; Galbács, Z.M.; Horváth, I. Alkali-Induced Generation of Superoxide and Hydroxyl Radicals from Aqueous Hydrogen Peroxide Solution. Z. Phys. Chem. 1983, 138, 107. [Google Scholar] [CrossRef]
- Mack, J.; Bolton, J.R. Photochemistry of nitrite and nitrate in aqueous solution: a review. J. Photochem. Photobiol. A 1999, 128, 1–13. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Recycling of carbon dioxide to renewable fuels by photocatalysis: Prospects and challenges. Renew. Sust. Energ. Rev. 2013, 25, 560–579. [Google Scholar] [CrossRef]
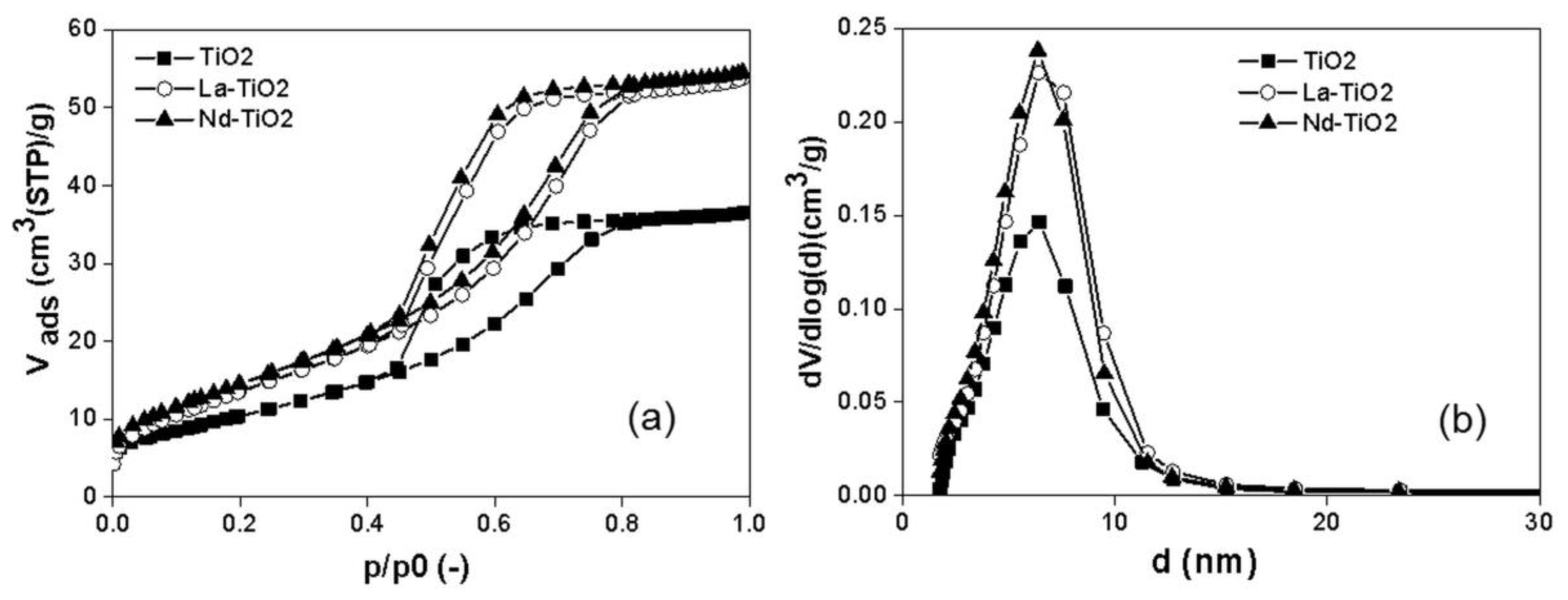
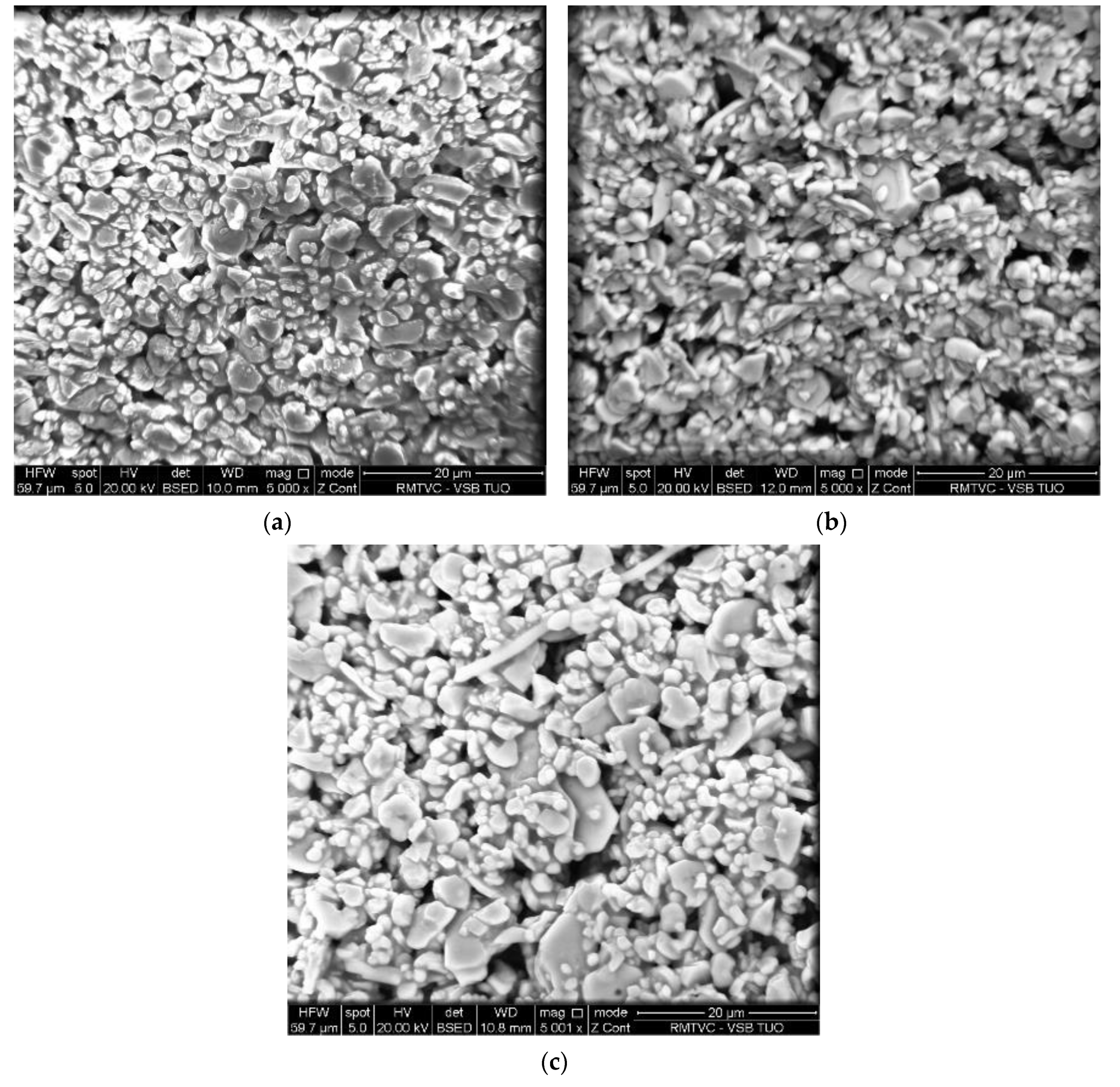
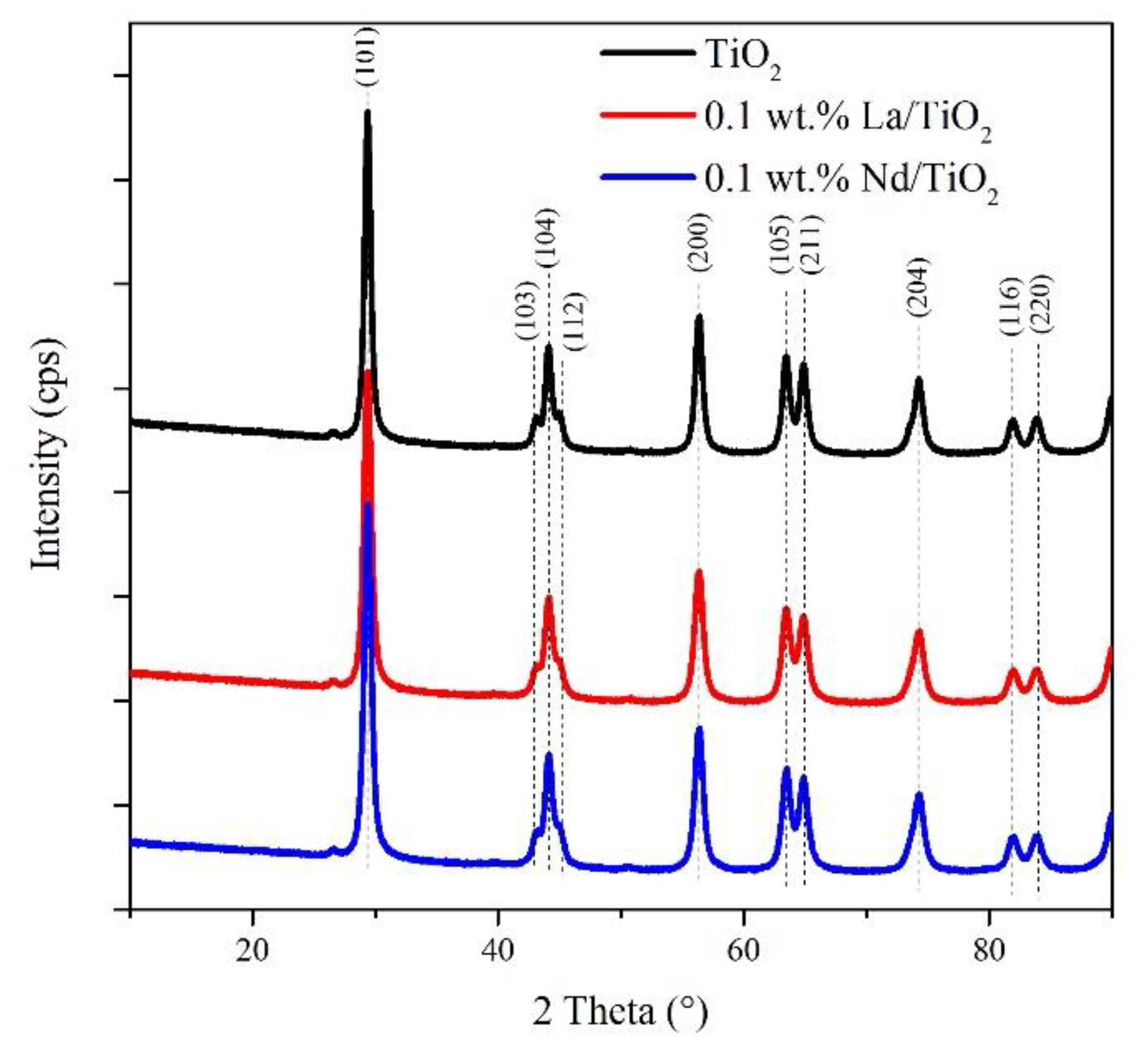
| Photocatalyst | SBET 1 | Vtot 2 | Amount of Metal La/Nd Determined by XRF | Indirect Band Gap | Lattice and Chemisorbed Oxygen or/and Hydroxyl Species |
|---|---|---|---|---|---|
| (cm3(STP)/g) | (wt.%) | (eV) | (at.%) | ||
| TiO2 | 39 | 37 | - | 3.0 | 63.4 |
| 0.1 wt.% La/TiO2 | 53 | 54 | 0.08 | 3.0 | 63.7 |
| 0.1 wt.% Nd/TiO2 | 56 | 55 | 0.10 | 3.0 | 63.6 |
| Photocatalyst | Crystallite Size (nm) | Lattice Parameters | Cell Volume (nm3) | |
|---|---|---|---|---|
| a(Å) | c(Å) | |||
| TiO2 | 14.0 | 3.7853 | 9.51301 | 13.630 |
| 0.1 wt.% La/TiO2 | 12.1 | 3.7844 | 9.50750 | 13.617 |
| 0.1 wt.% Nd/TiO2 | 8.8 | 3.7880 | 9.51650 | 13.655 |
| Photocatalyst | Kinetic Constant for Photocatalysts Immobilized on Foam (104 h−1) |
|---|---|
| TiO2 | 2.55 ± 0.15 |
| 0.1 wt.% La/TiO2 | 2.68 ± 0.02 |
| 0.1 wt.% Nd/TiO2 | 2.33 ± 0.04 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Edelmannová, M.; Reli, M.; Matějová, L.; Troppová, I.; Dubnová, L.; Čapek, L.; Dvoranová, D.; Kuśtrowski, P.; Kočí, K. Successful Immobilization of Lanthanides Doped TiO2 on Inert Foam for Repeatable Hydrogen Generation from Aqueous Ammonia. Materials 2020, 13, 1254. https://doi.org/10.3390/ma13051254
Edelmannová M, Reli M, Matějová L, Troppová I, Dubnová L, Čapek L, Dvoranová D, Kuśtrowski P, Kočí K. Successful Immobilization of Lanthanides Doped TiO2 on Inert Foam for Repeatable Hydrogen Generation from Aqueous Ammonia. Materials. 2020; 13(5):1254. https://doi.org/10.3390/ma13051254
Chicago/Turabian StyleEdelmannová, Miroslava, Martin Reli, Lenka Matějová, Ivana Troppová, Lada Dubnová, Libor Čapek, Dana Dvoranová, Piotr Kuśtrowski, and Kamila Kočí. 2020. "Successful Immobilization of Lanthanides Doped TiO2 on Inert Foam for Repeatable Hydrogen Generation from Aqueous Ammonia" Materials 13, no. 5: 1254. https://doi.org/10.3390/ma13051254
APA StyleEdelmannová, M., Reli, M., Matějová, L., Troppová, I., Dubnová, L., Čapek, L., Dvoranová, D., Kuśtrowski, P., & Kočí, K. (2020). Successful Immobilization of Lanthanides Doped TiO2 on Inert Foam for Repeatable Hydrogen Generation from Aqueous Ammonia. Materials, 13(5), 1254. https://doi.org/10.3390/ma13051254