The Influence of Plasma-Assisted Production and Milling Processes of DLC Flakes on Their Size, Composition and Chemical Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Diamond-Like Carbon Flakes Deposition Process
2.2. Milling Process
2.3. Surface Characterisation
3. Results and Discussion
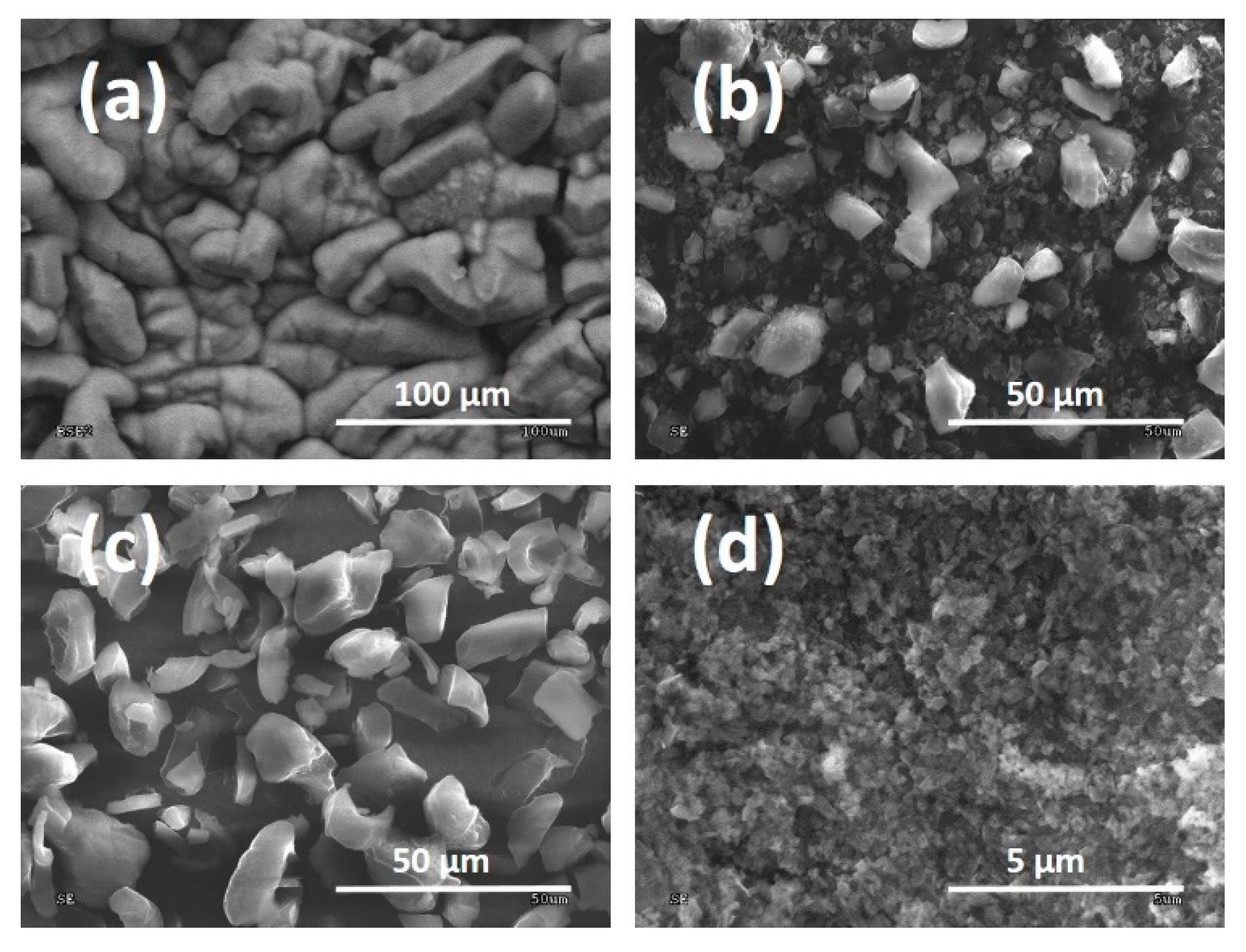
3.1. DLC Flakes Produced Using the MW/RF PACVD Method
3.2. DLC Flake Milling
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salvo-Comino, C.; Rassas, I.; Minot, S.; Bessueille, F.; Arab, M.; Chevallier, V.; Rodriguez-Mendez, M.L.; Errachid, A.; Jaffrezic-Renault, N. Voltammetric Sensor Based on Molecularly Imprinted Chitosan-Carbon Nanotubes Decorated with Gold Nanoparticles Nanocomposite Deposited on Boron-Doped Diamond Electrodes for Catechol Detection. Materials 2020, 13, 688. [Google Scholar] [CrossRef]
- Lu, X.; Liang, B.; Sheng, X.; Yuan, T.; Qu, J. Enhanced thermal conductivity of polyurethane/wood powder composite phase change materials via incorporating low loading of graphene oxide nanosheets for solar thermal energy storage. Sol. Energy Mater. Sol. Cells 2020, 208, 110391. [Google Scholar] [CrossRef]
- Kume, A.; Mochalin, V.N. Sonication-assisted hydrolysis of ozone oxidized detonation nanodiamond. Diam. Relat. Mater. 2020, 103, 107705. [Google Scholar] [CrossRef]
- Shah, K.A.; Tali, B.A. Synthesis of carbon nanotubes by catalytic chemical vapour deposition: A review on carbon sources, catalysts and substrates. Mater. Sci. Semicond. Process. 2016, 41, 67–82. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Edgington, R.; Spillane, K.M.; Papageorgiou, G.; Wray, W.; Ishiwata, H.; Labarca, M.; Leal-Ortiz, S.; Reid, G.; Webb, M.; Foord, J.; et al. Functionalisation of Detonation Nanodiamond for Monodispersed, Soluble DNA-Nanodiamond Conjugates Using Mixed Silane Bead-Assisted Sonication Disintegration. Sci. Rep. 2018, 8, 728. [Google Scholar] [CrossRef] [PubMed]
- Kaczorowski, W.; Szymanski, W.; Batory, D.; Niedzieslki, P. Tribological Properties and Characterization of Diamond like Carbon Coatings Deposited by MW/RF and RF Plasma-Enhanced CVD Method on Poly (ether-ether-ketone). Plasma Process. Polym. 2014, 11, 878–887. [Google Scholar] [CrossRef]
- García, J.A.; Rivero, P.J.; Barba, E.; Fernández, I.; Santiago, J.A.; Palacio, J.F.; Fuente, G.G.; Rodríguez, R.J. A Comparative Study in the Tribological Behavior of DLC Coatings Deposited by HiPIMS Technology with Positive Pulses. Metals 2020, 10, 174. [Google Scholar] [CrossRef]
- Choudhury, D.; Morita, T.; Sawae, Y.; Lackner, J.M.; Towler, M.; Krupka, I. A novel functional layered diamond like carbon coating for orthopedics applications. Daim. Relat. Mater. 2016, 61, 56–69. [Google Scholar] [CrossRef]
- Liao, T.T.; Zhang, T.F.; Li, S.S.; Deng, Q.Y.; Wu, B.J.; Zhang, Y.Z.; Zhou, Y.J.; Guo, Y.B.; Leng, Y.X.; Huang, N. Biological responses of diamond-like carbon (DLC) films with different structures in biomedical application. Mater. Sci. Eng. C 2016, 69, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Bouabibsa, I.; Lamri, S.; Sanchette, F. Structure, Mechanical and Tribological Properties of Me-Doped Diamond-Like Carbon (DLC) (Me = Al, Ti, or Nb) Hydrogenated Amorphous Carbon Coatings. Coatings 2018, 8, 370. [Google Scholar] [CrossRef]
- Batory, D.; Jedrzejczak, A.; Kaczorowski, W.; Kolodziejczyk, L.; Burnat, B. The effect of Si incorporation on the corrosion resistance of a-C:H:SiOx coatings. Daim. Relat. Mater. 2016, 67, 1–7. [Google Scholar] [CrossRef]
- Mazare, A.; Anghel, A.; Surdu-Bob, C.; Totea, G.; Demetrescu, I.; Ionita, D. Silver doped diamond-like carbon antibacterial and corrosion resistance coatings on titanium. Thin Solid Films 2018, 657, 16–23. [Google Scholar] [CrossRef]
- Ohana, T.; Nakamura, T.; Tanaka, A. Tribological properties of polymer composites with diamond-like carbon flakes. Daim. Relat. Mater. 2010, 19, 894–898. [Google Scholar] [CrossRef]
- Solarska, K.; Gajewska, A.; Kaczorowski, W.; Bartosz, G.; Mitura, K. Effect of nanodiamond powders on the viability and production of reactive oxygen and nitrogen species by human endothelial cells. Daim. Relat. Mater. 2012, 21, 107–113. [Google Scholar] [CrossRef]
- Grill, A. Diamond-like carbon: State of the art. Daim. Relat. Mater. 1999, 8, 428–434. [Google Scholar] [CrossRef]
- Grill, A. Plasma-deposited diamondlike carbon and related materials. IBM J. Res. Dev. 1999, 43, 147–162. [Google Scholar] [CrossRef]
- Batory, M.; Batory, D.; Grabarczyk, J.; Kaczorowski, W.; Kupcewicz, B.; Mitura, K.; Nasti, T.H.; Yusuf, N.; Niedzielski, P. Biological properties of carbon powders synthesized using CVD and detonation methods. J. Nanosci. Nanotechnol. 2012, 12, 9037–9046. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Ding, J.; Xu, Y.; Chen, G.; Liu, J.; Zhao, L.; Huang, N.; He, Z.; Li, Y.; et al. Diamond-like carbon structure-doped carbon dots: A new class of self-quenching-resistant solid-state fluorescence materials toward light-emitting diodes. Carbon 2019, 149, 342–349. [Google Scholar] [CrossRef]
- Lin, H.C.; Yu, J.F.; Shiue, S.T.; Lin, H.Y. Characteristics of carbon coatings on optical fibers prepared by radio-frequency plasma enhanced chemical vapor deposition with different H2/C2H2 ratios. Thin Solid Films 2010, 518, 7492–7496. [Google Scholar] [CrossRef]
- Bakowicz-Mitura, K.; Bartosz, G.; Mitura, S. Influence of diamond powder particles on human gene expression. Surf. Coat. Technol. 2007, 201, 6131–6135. [Google Scholar] [CrossRef]
- Kazimierczak, T.; Kaczorowski, W.; Niedzieslki, P. CVD carbon powders modified by ball milling. Mater. Sci.-Poland 2015, 33, 521–528. [Google Scholar] [CrossRef][Green Version]
- Kaczorowski, W.; Niedzieslki, P. Morphology and Growth Process of Carbon Films Prepared by Microwave / Radio Frequency Plasma Assisted CVD. Adv. Eng. Mater. 2008, 19, 651–656. [Google Scholar] [CrossRef]
- Vesel, A.; Zaplotnik, R.; Primc, G.; Mozetič, M. Synthesis of Vertically Oriented Graphene Sheets or Carbon Nanowalls—Review and Challenges. Materials 2019, 12, 2968. [Google Scholar] [CrossRef]
- Yamamoto, S.; Kawana, A.; Masuda, C. Tribological behavior of diamond-like carbon produced by rf-PCVD based on energetic evaluation. Surf. Coat. Technol. 2013, 236, 457–464. [Google Scholar] [CrossRef]
- Kaczorowski, W.; Batory, D.; Szymanski, W.; Niedzielski, P. Evaluation of the surface properties of PEEK substrate after two-step plasma modification: Etching and deposition of DLC coatings. Surf. Coat. Technol. 2015, 265, 92–98. [Google Scholar] [CrossRef]
- Itoh, M.; Suda, Y.; Bratescu, M.; Sakai, Y.; Suzuki, K. Amorphous carbon nitride film preparation by plasma-assisted pulsed laser deposition method. Appl. Phys. 2004, 79, 1575–1578. [Google Scholar] [CrossRef]
- Choi, J.; Ishii, K.; Kato, T.; Kawaguchi, M.; Lee, W. Structural and mechanical properties of DLC films prepared by bipolar PBII&D. Daim. Relat. Mater. 2011, 20, 845–848. [Google Scholar]

| RF Power [W] | MW Power [W] | Gas Flow [sccm] | Pressure [Pa] | Time of Deposition [min] | ||||
|---|---|---|---|---|---|---|---|---|
| CH4 | H2 | Ar | O2 | N2 | ||||
| 500 | 500 | 180 | - | - | - | - | 100–130 | 120–200 |
| 20 | - | - | - | 120 | ||||
| - | 20 | - | - | 120 | ||||
| - | - | 20 | - | 120 | ||||
| - | - | - | 20 | 120 | ||||
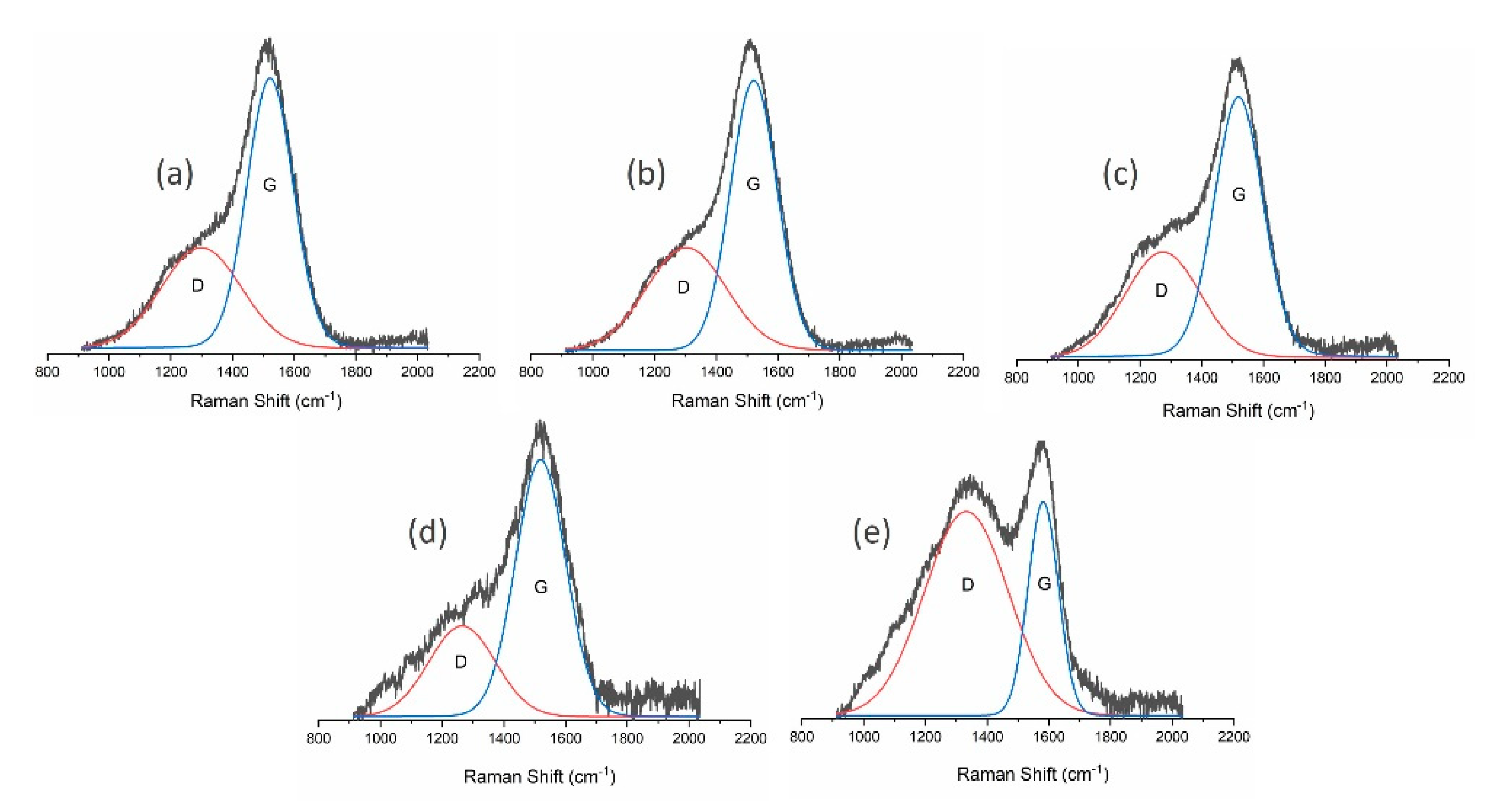
| Sample | ID/IG | G Position (cm−1) | D Position (cm−1) | FWHM of G Peak (cm−1) |
|---|---|---|---|---|
| CH4 | 0.63 | 1521 | 1299 | 177 |
| CH4/H2 | 0.65 | 1521 | 1303 | 177 |
| CH4/Ar | 0.60 | 1519 | 1274 | 186 |
| CH4/O2 | 0.46 | 1519 | 1264 | 192 |
| CH4/N2 | 2.64 | 1582 | 1333 | 115 |
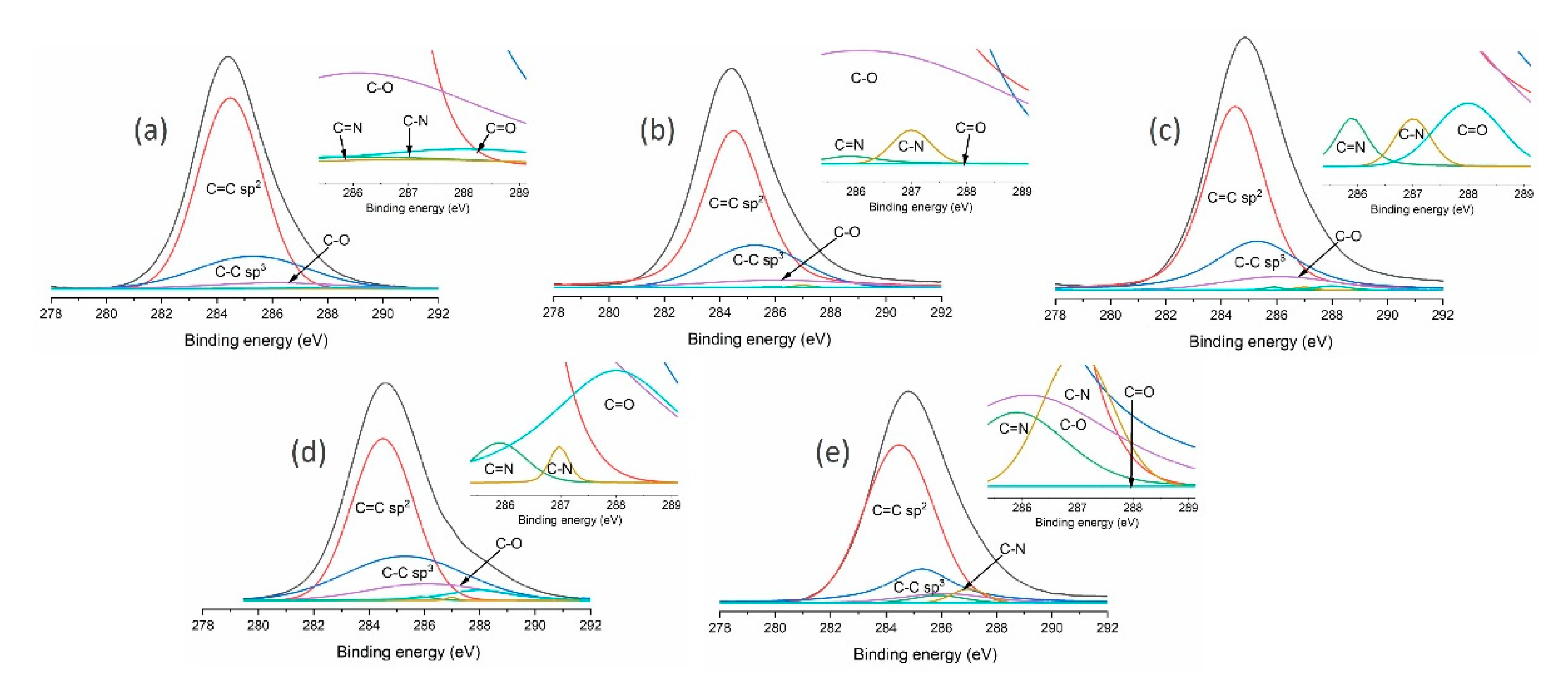
| Sample | Structural Composition | Chemical Composition | sp3/ (sp3+sp2) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C=C sp2 (284.5 eV) | C-C sp3 (285.3 eV) | C=N (285.9 eV) | C-O (286.1 eV) | C-N (287 eV) | C=O (288 eV) | C (%) | 0 (%) | Fe (%) | N (%) | ||
| CH4 | 68.1 | 24.3 | 0.4 | 5.8 | 0.4 | 1.0 | 92.8 | 6.3 | 0.3 | 0.6 | 0.26 |
| CH4/H2 | 68.9 | 24.0 | 0.1 | 6.6 | 0.3 | 0.1 | 92.6 | 6.9 | 0.2 | 0.3 | 0.25 |
| CH4/Ar | 64.6 | 27.1 | 0.3 | 7.3 | 0.3 | 0.4 | 91.5 | 7.9 | 0.2 | 0.4 | 0.29 |
| CH4/O2 | 54.6 | 28.8 | 0.6 | 10.7 | 0.2 | 5.1 | 82.1 | 16.9 | 0.3 | 0.7 | 0.35 |
| CH4/N2 | 67.7 | 20.5 | 2.7 | 6.0 | 3.0 | 0.1 | 87.8 | 6.1 | 0.1 | 6.0 | 0.23 |
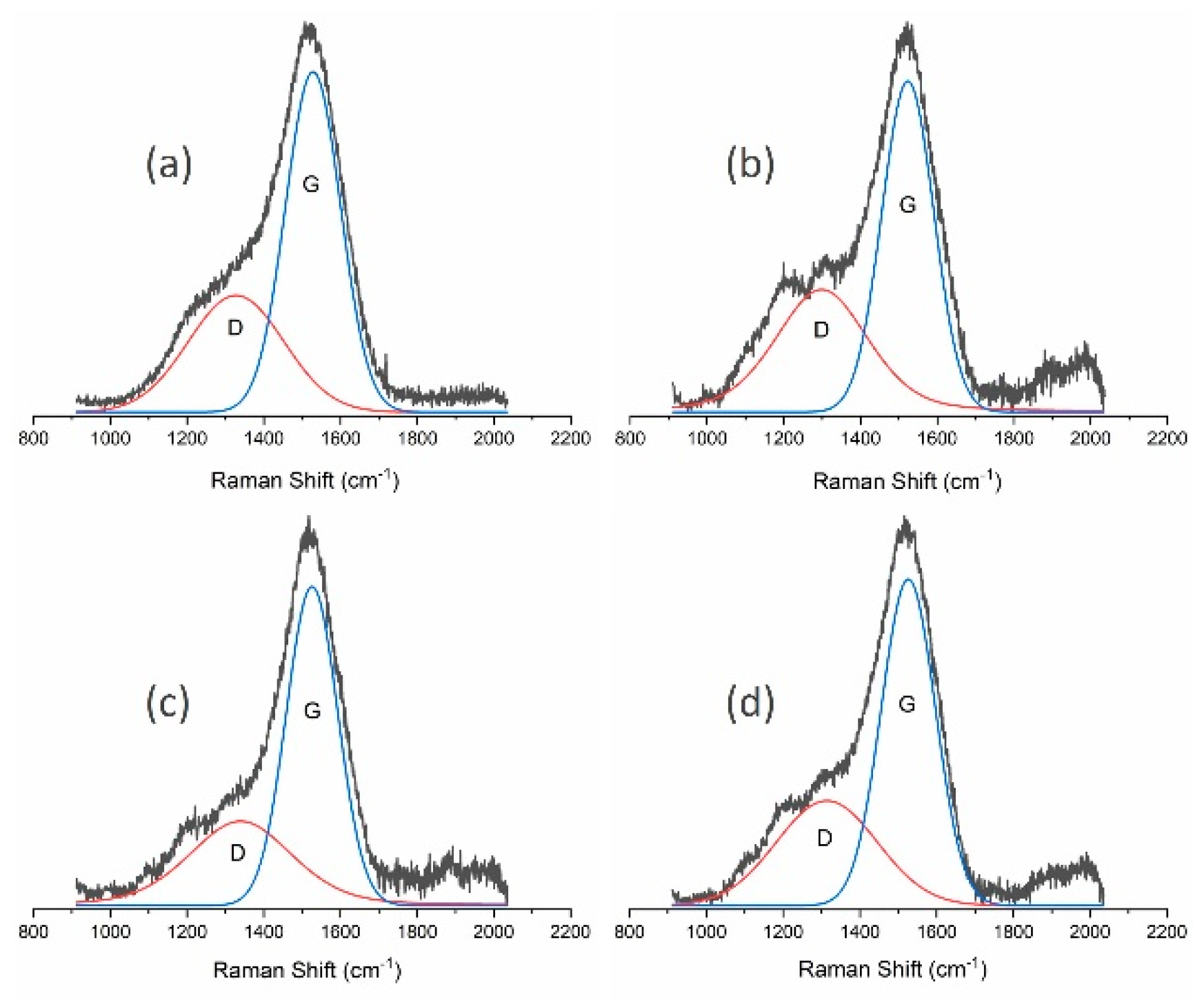
| Sample | ID/IG | G Position (cm−1) | D Position (cm−1) | FWHM of G Peak (cm−1) |
|---|---|---|---|---|
| MW/RF PACVD | 0.60 | 1528 | 1327 | 169 |
| MW/RF PACVD after dry milling | 0.75 | 1523 | 1299 | 159 |
| MW/RF PACVD after milling with water | 0.61 | 1526 | 1340 | 158 |
| MW/RF PACVD after milling with methanol | 0.60 | 1526 | 1314 | 166 |
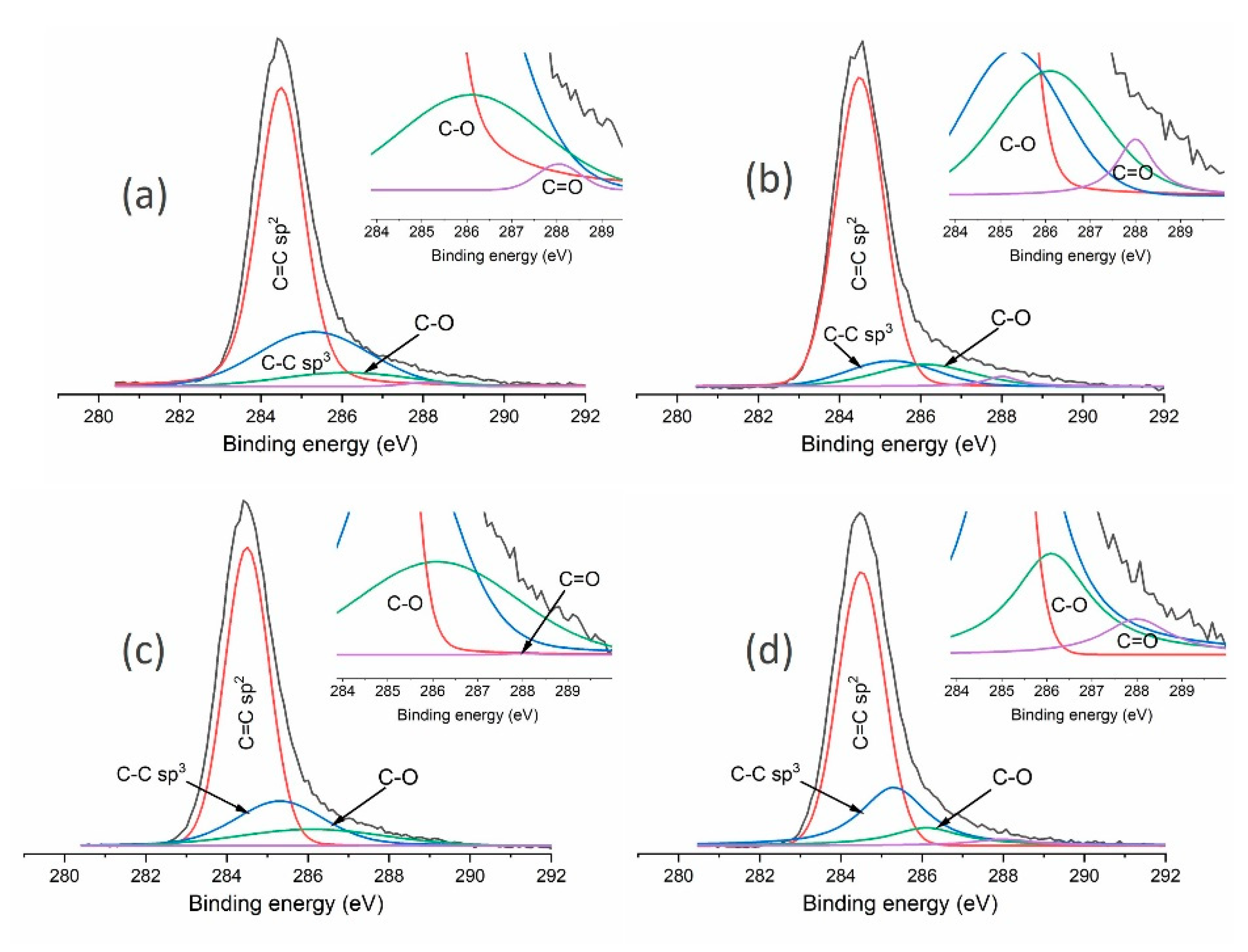
| Sample | Structural Composition | Chemical Composition | sp3/(sp3 + sp2) | |||||
|---|---|---|---|---|---|---|---|---|
| C=C sp2 (284.5 eV) | C-C sp3 (285.3 eV) | C-O (286.1 eV) | C=O (288 eV) | C (%) | 0 (%) | Zr (%) | ||
| MW/RF PACVD | 66.1 | 25.9 | 7.5 | 0.6 | 90.5 | 9.5 | - | 0.28 |
| MW/RF PACVD after dry milling | 75.2 | 11.0 | 11.3 | 2.5 | 85.7 | 13.8 | 0.5 | 0.13 |
| MW/RF PACVD after milling with water | 66.8 | 21.6 | 11.6 | - | 88.3 | 11.6 | 0.1 | 0.24 |
| MW/RF PACVD after milling with methanol | 62.1 | 24.2 | 11.1 | 2.6 | 87.4 | 12.8 | 0.8 | 0.28 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaźmierczak, T.; Niedzielski, P.; Kaczorowski, W. The Influence of Plasma-Assisted Production and Milling Processes of DLC Flakes on Their Size, Composition and Chemical Structure. Materials 2020, 13, 1209. https://doi.org/10.3390/ma13051209
Kaźmierczak T, Niedzielski P, Kaczorowski W. The Influence of Plasma-Assisted Production and Milling Processes of DLC Flakes on Their Size, Composition and Chemical Structure. Materials. 2020; 13(5):1209. https://doi.org/10.3390/ma13051209
Chicago/Turabian StyleKaźmierczak, Tomasz, Piotr Niedzielski, and Witold Kaczorowski. 2020. "The Influence of Plasma-Assisted Production and Milling Processes of DLC Flakes on Their Size, Composition and Chemical Structure" Materials 13, no. 5: 1209. https://doi.org/10.3390/ma13051209
APA StyleKaźmierczak, T., Niedzielski, P., & Kaczorowski, W. (2020). The Influence of Plasma-Assisted Production and Milling Processes of DLC Flakes on Their Size, Composition and Chemical Structure. Materials, 13(5), 1209. https://doi.org/10.3390/ma13051209