A Solar-Driven Flexible Electrochromic Supercapacitor
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of the Photoanode and Counter Electrode
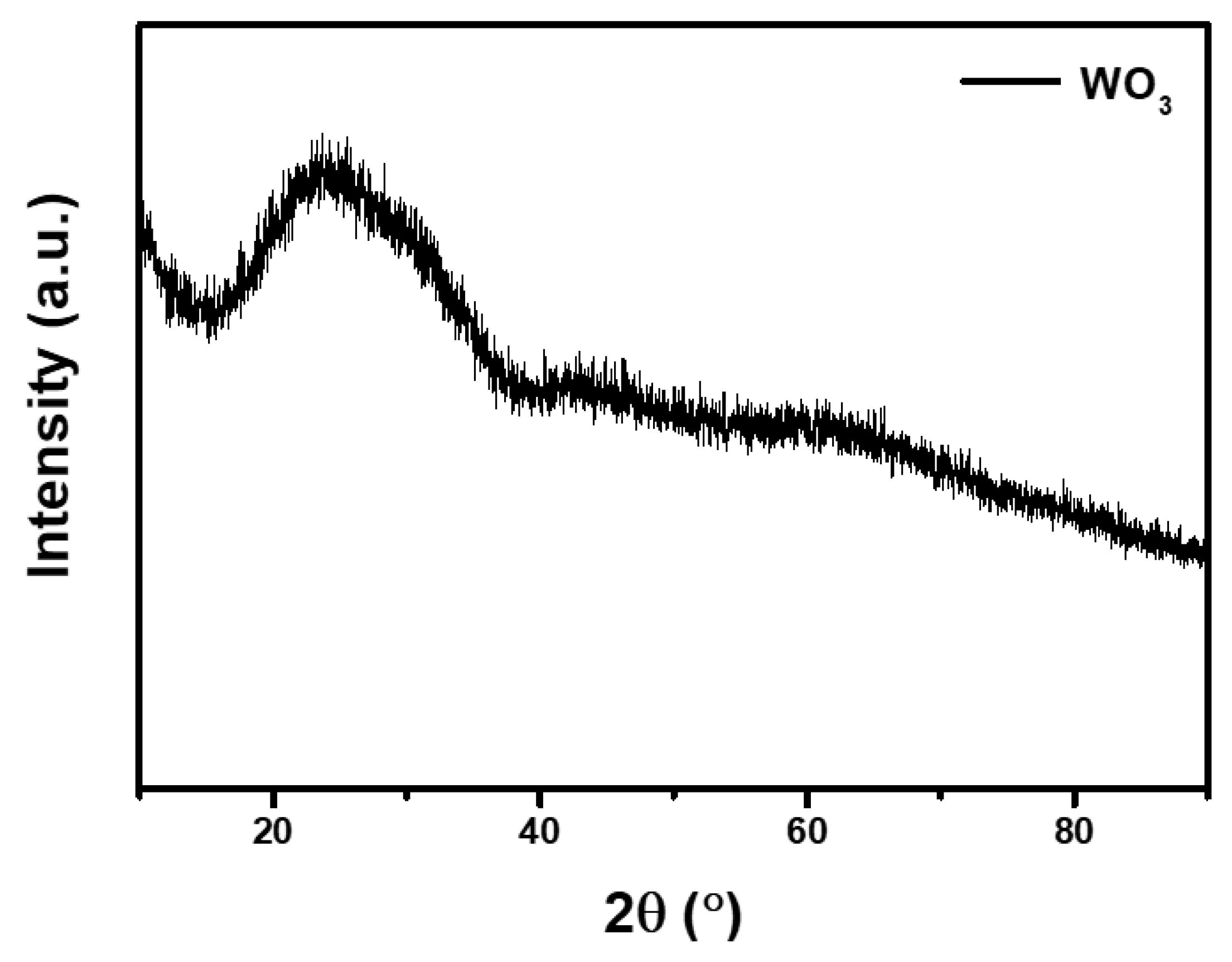
2.3. Preparation of WO3 Thin Film
2.4. Fabrication of the Electrolytes
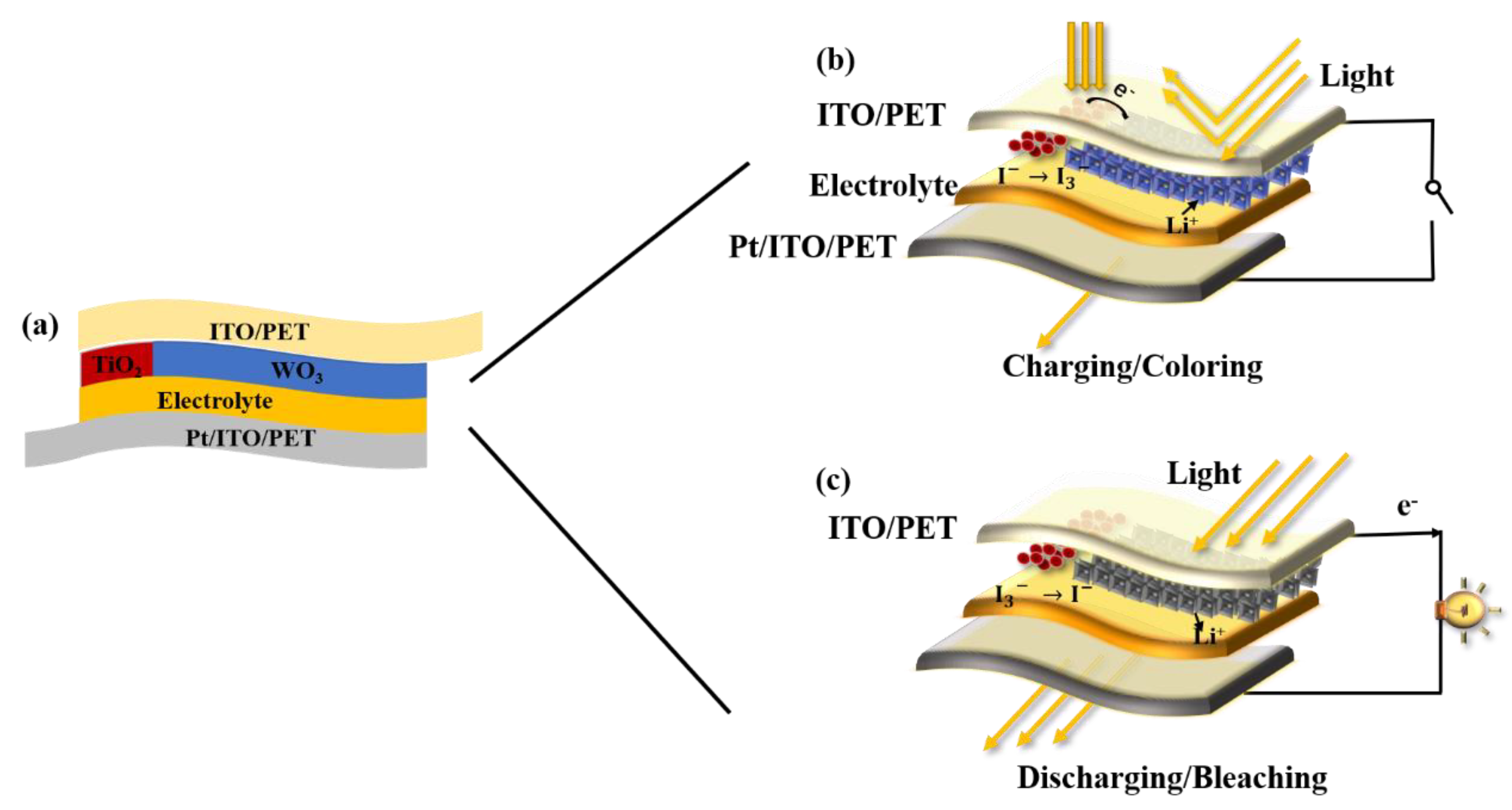
2.5. Fabrication of the PECD
2.6. Characterization
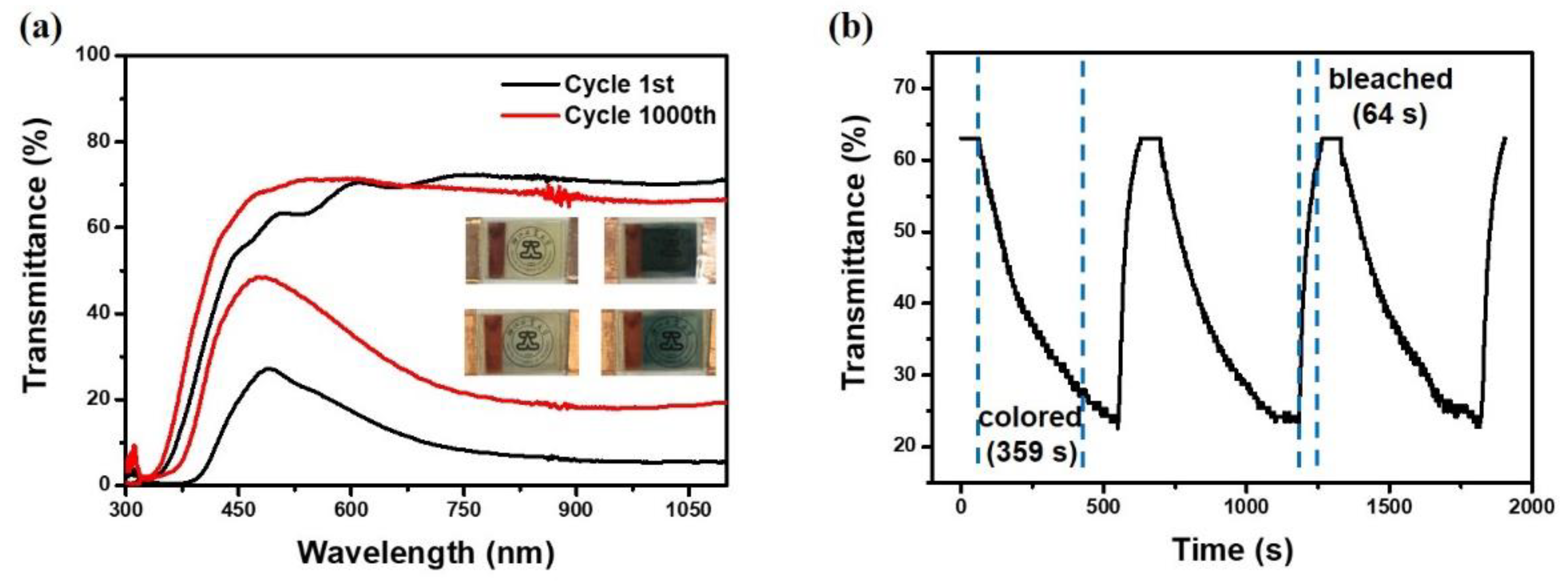
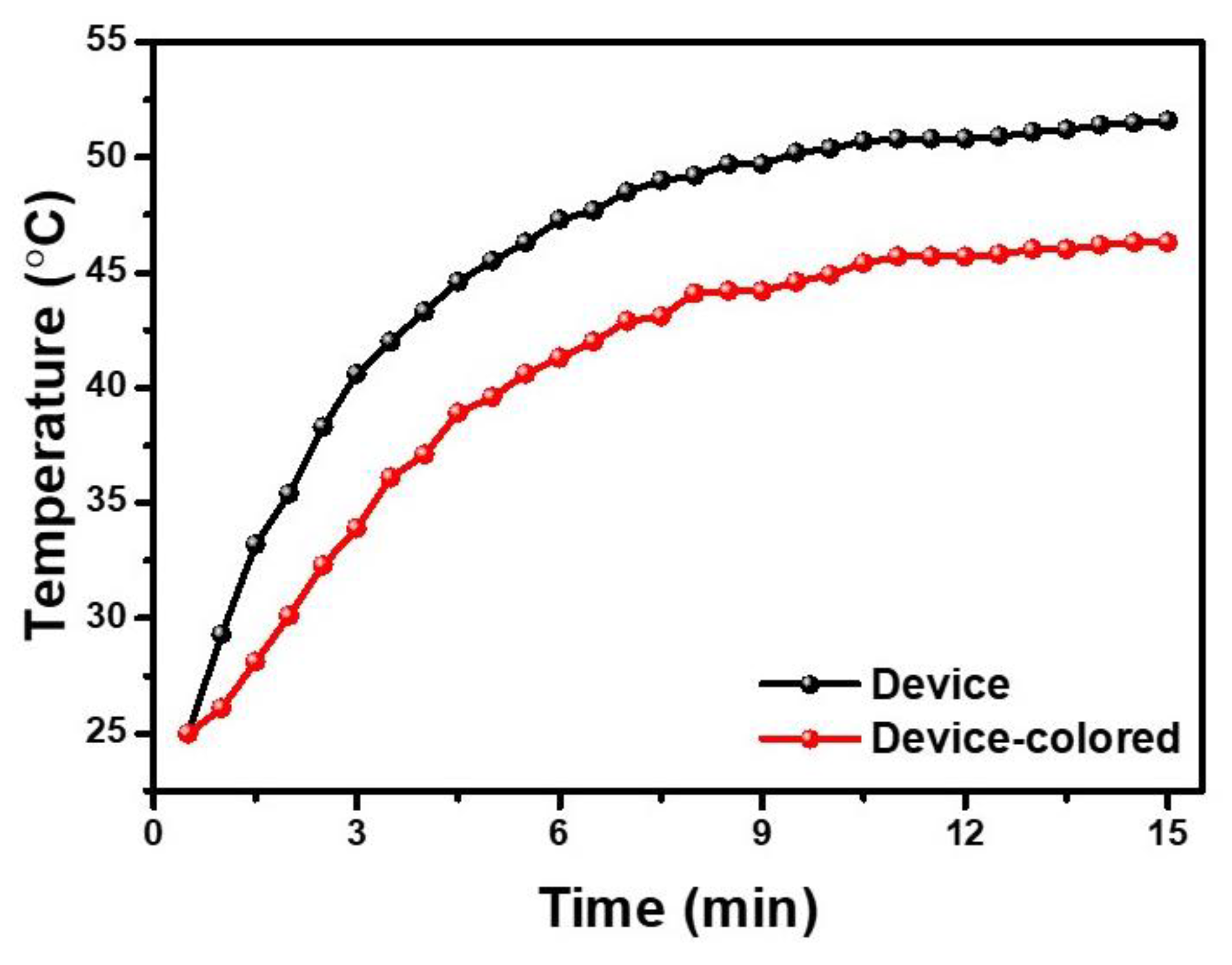
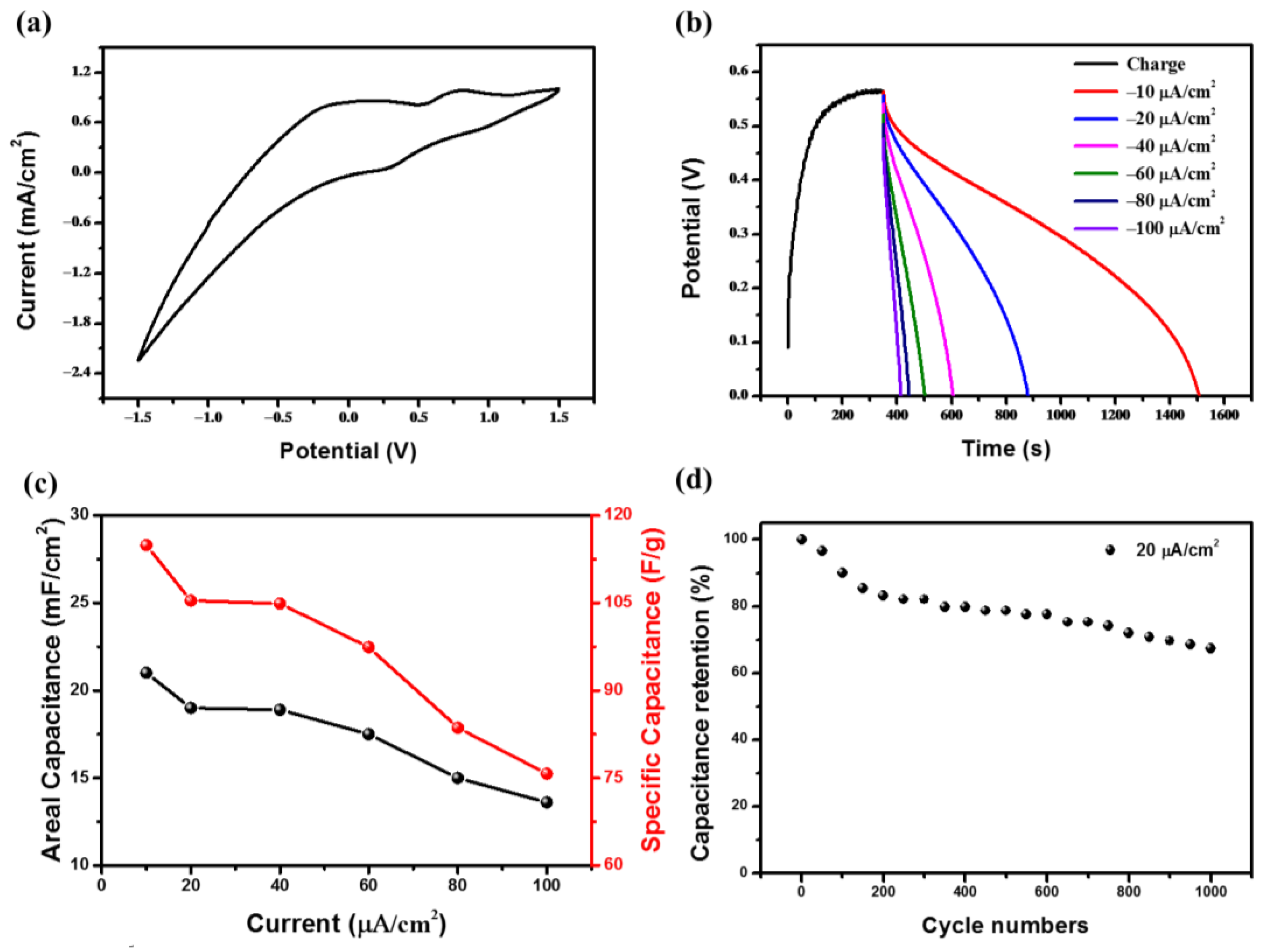
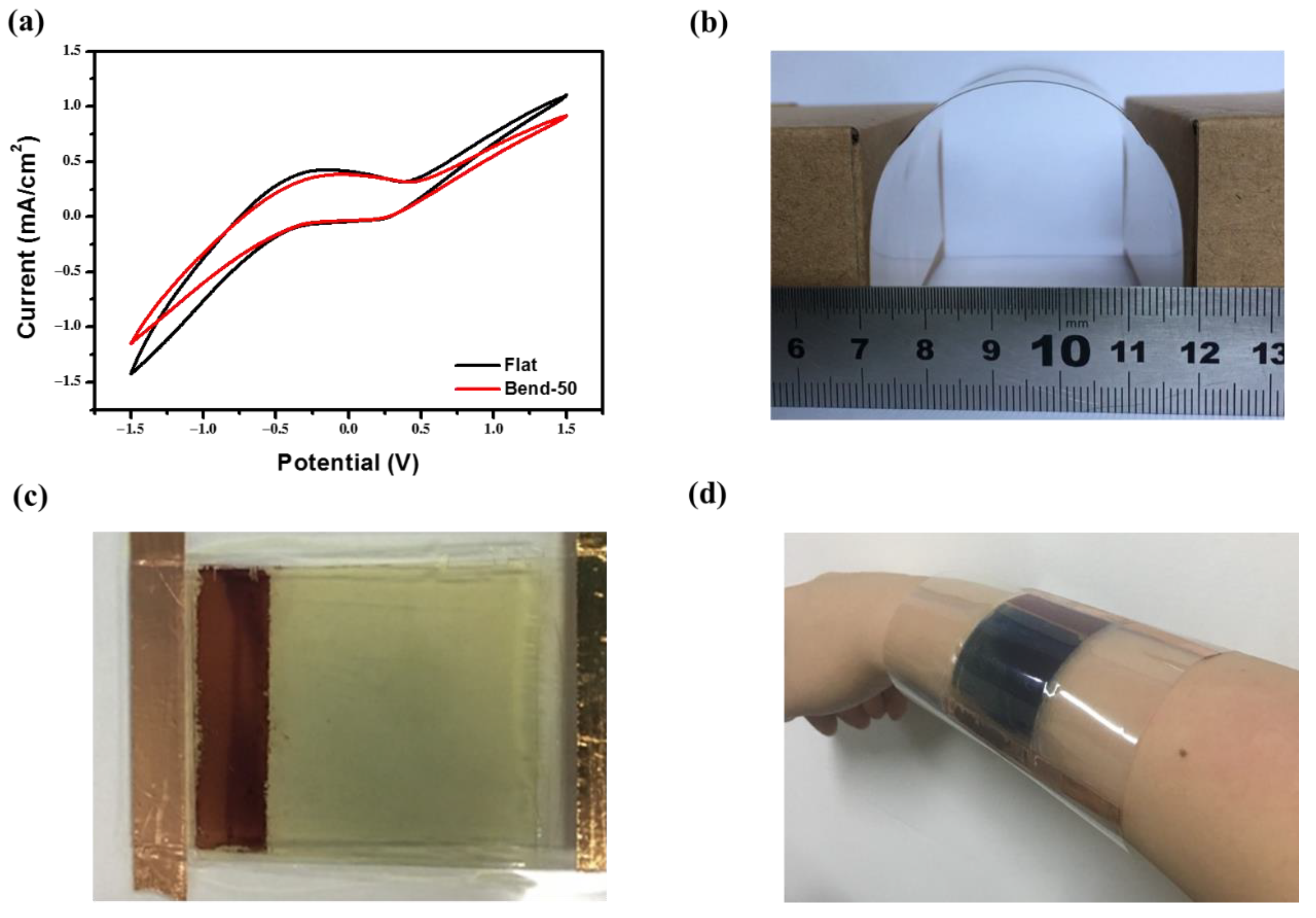
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Andric, I.; Pina, A.; Ferrao, P.; Lacarriere, B.; Le Corre, O. The impact of renovation measures on building environmental performance: An emergy approach. J. Clean. Prod. 2017, 162, 776–790. [Google Scholar] [CrossRef]
- O’Grady, M.; Lechowska, A.A.; Harte, A.M. Application of infrared thermography technique to the thermal assessment of multiple thermal bridges and windows. Energy Build. 2018, 168, 347–362. [Google Scholar] [CrossRef]
- Lin, S.; Wang, H.Y.; Zhang, X.N.; Wang, D.; Zu, D.; Song, J.A.; Liu, Z.L.; Huang, Y.; Huang, K.; Tao, N.A.; et al. Direct spray-coating of highly robust and transparent Ag nanowires for energy saving windows. Nano Energy 2019, 62, 111–116. [Google Scholar] [CrossRef]
- Krebs, F.C. Careful design of donor–acceptor polymer molecules with reversible redox properties gives access to polymer electrochromic displays with switchable absorption in the full visible range of the optical spectrum. Nat. Mater. 2008, 7, 766–767. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.H.; Sun, P.; Mai, W.J. Electrochromic energy storage devices. Mater. Today 2016, 19, 394–402. [Google Scholar] [CrossRef]
- Madasamy, K.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.C. Viologen-based electrochromic materials and devices. J. Mater. Chem. C 2019, 7, 4622–4637. [Google Scholar] [CrossRef]
- Zhao, Q.; Fang, Y.S.; Qiao, K.; Wei, W.; Yao, Y.J.; Gao, Y.F. Printing of WO3/ITO nanocomposite electrochromic smart windows. Sol. Energy Mater. Sol. Cells 2019, 194, 95–102. [Google Scholar] [CrossRef]
- Xie, S.J.; Chen, Y.B.; Bi, Z.J.; Jia, S.S.; Guo, X.X.; Gao, X.D.; Li, X.M. Energy storage smart window with transparent-to-dark electrochromic behavior and improved pseudocapacitive performance. Chem. Eng. J. 2019, 370, 1459–1466. [Google Scholar] [CrossRef]
- Zhai, Y.L.; Li, Y.; Zhang, H.; Yu, D.B.; Zhu, Z.J.; Sun, J.Z.; Dong, S.H. Self-rechargeable-battery-driven device for simultaneous electrochromic windows, ROS biosensing, and energy storage. ACS Appl. Mater. Interfaces 2019, 11, 28072–28077. [Google Scholar] [CrossRef]
- Zhang, S.H.; Chen, S.; Yang, F.; Hu, F.; Zhao, Y.H.; Yan, B.; Jiang, H.; Cao, Y. A facile preparation of SiO2/PEDOT core/shell nanoparticle composite film for electrochromic device. J. Mater. Sci. Mater. Electron. 2019, 30, 3994–4005. [Google Scholar] [CrossRef]
- Kateb, M.; Safarian, S.; Kolahdouz, M.; Fathipour, M.; Ahamdi, V. ZnO-PEDOT core-shell nanowires: An ultrafast, high contrast and transparent electrochromic display. Sol. Energy Mater. Sol. Cells 2016, 145, 200–205. [Google Scholar] [CrossRef]
- Qiu, M.J.; Sun, P.; Zhang, B.; Yu, J.H.; Fu, Y.; Yu, X.; Zhao, C.X.; Mai, W.J. Reliable information encryption and digital display applications based on multistate smart windows. Adv. Opt. Mater. 2018, 6, 1800338. [Google Scholar] [CrossRef]
- Kim, J.; Ong, G.K.; Wang, Y.; LeBlanc, G.; Williams, T.E.; Mattox, T.M.; Helms, B.A.; Milliron, D.J. Nanocomposite Architecture for Rapid, Spectrally-Selective Electrochromic Modulation of Solar Transmittance. Nano Lett. 2015, 15, 5574–5579. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.Z.; Balendhran, S.; Field, M.R.; McCulloch, D.G.; Zoolfakar, A.S.; Rani, R.A.; Zhuiykov, S.; O’Mullane, A.P.; Kalantar-zadeh, K. The anodized crystalline WO3 nanoporous network with enhanced electrochromic properties. Nanoscale 2012, 4, 5980–5988. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.L.; Liu, F.Q.; Li, T.; Xu, T. Fast and low voltage-driven solid-state electrochromics using 3-D conductive FTO nanobead electrodes. J. Mater. Chem. C 2014, 2, 618–621. [Google Scholar] [CrossRef]
- Li, M.; Wei, Y.X.; Zheng, J.M.; Zhu, D.; Xu, C.Y. Highly contrasted and stable electrochromic device based on well-matched viologen and triphenylamine. Org. Electron. 2014, 15, 428–434. [Google Scholar] [CrossRef]
- Eh, A.L.-S.; Tan, A.W.M.; Cheng, X.; Magdassi, S.; Lee, P.S. Recent advances in flexible electrochromic devices: Prerequisites, challenges, and prospects. Energy Technol. 2018, 6, 33–45. [Google Scholar] [CrossRef]
- Huang, J.H.; Hsu, M.H.; Hsiao, Y.S.; Chen, P.L.; Yu, P.C.; Chu, C.W. Performance of chromophore-type electrochromic devices employing indium tin oxide nanorod optical amplification. Sol. Energy Mater. Sol. Cells 2012, 98, 191–197. [Google Scholar] [CrossRef]
- Wang, X.; Liu, B.; Tang, J.; Dai, G.; Dong, B.; Cao, L.; Gao, R.; Su, G. Preparation of Ni(OH)2/TiO2 porous film with novel structure and electrochromic property. Sol. Energy Mater. Sol. Cells 2019, 191, 108–116. [Google Scholar] [CrossRef]
- Lee, K.-C.; Chang-Jian, C.-W.; Cho, E.-C.; Huang, J.-H.; Lin, W.-T.; Ho, B.-C.; Chou, J.-A.; Hsiao, Y.-S. Surface modification of Ni(OH)2 nanosheets with PEDOT:PSS for supercapacitor and bendable electrochromic applications. Sol. Energy Mater. Sol. Cells 2019, 195, 1–11. [Google Scholar] [CrossRef]
- Deng, B.; Hsu, P.-C.; Chen, G.; Chandrashekar, B.N.; Liao, L.; Ayitimuda, Z.; Wu, J.; Guo, Y.; Lin, L.; Zhou, Y.; et al. Roll-to-roll encapsulation of metal nanowires between graphene and plastic substrate for high-performance flexible transparent electrodes. Nano Lett. 2015, 15, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Li, X.; Ou, Y.; Xu, Q.; Zhang, X.M.; Xu, J.; Weng, J. Ultra-large optical modulation of a size-tunable flexible electrochromic honeycomb mesoporous tungsten oxide film. Inorg. Chem. Front. 2019, 6, 680–686. [Google Scholar] [CrossRef]
- Cai, G.; Darmawan, P.; Cui, M.; Wang, J.; Chen, J.; Magdassi, S.; Lee, P.S. Highly stable transparent conductive silver grid/PEDOT:PSS electrodes for integrated bifunctional flexible electrochromic supercapacitors. Adv. Energy Mater. 2016, 6, 1501882. [Google Scholar] [CrossRef]
- Wang, J.L.; Lu, Y.R.; Li, H.H.; Liu, J.W.; Yu, S.H. Large area co-assembly of nanowires for flexible transparent smart windows. J. Am. Chem. Soc. 2017, 139, 9921–9926. [Google Scholar] [CrossRef] [PubMed]
- Che, B.; Zhou, D.; Li, H.; He, C.; Liu, E.; Lu, X. A highly bendable transparent electrode for organic electrochromic devices. Org. Electron. 2019, 66, 86–93. [Google Scholar] [CrossRef]
- Lehtimaki, S.; Suominen, M.; Damlin, P.; Tuukkanen, S.; Kvarnstrom, C.; Lupo, D. Preparation of supercapacitors on flexible substrates with electrodeposited PEDOT/graphene composites. ACS Appl. Mater. Interfaces 2015, 7, 22137–22147. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Tharion, J.; Murugan, S.; Kumar, A. ITO-free solution-processed flexible electrochromic devices based on PEDOT:PSS as transparent conducting electrode. ACS Appl. Mater. Interfaces 2017, 9, 19427–19435. [Google Scholar] [CrossRef]
- Bi, Z.; Li, X.; He, X.; Chen, Y.; Xu, X.; Gao, X. Integrated electrochromism and energy storage applications based on tungsten trioxide monohydrate nanosheets by novel one-step low temperature synthesis. Sol. Energy Mater. Sol. Cells 2018, 183, 59–65. [Google Scholar] [CrossRef]
- Bogati, S.; Georg, A.; Graf, W. Photoelectrochromic devices based on sputtered WO3 and TiO2 films. Sol. Energy Mater. Sol. Cells 2017, 163, 170–177. [Google Scholar] [CrossRef]
- Dokouzis, A.; Theodosiou, K.; Leftheriotis, G. Assessment of the long-term performance of partly covered photoelectrochromic devices under insolation and in storage. Sol. Energy Mater. Sol. Cells 2018, 182, 281–293. [Google Scholar] [CrossRef]
- Azam, A.; Kim, J.; Park, J.; Novak, T.G.; Tiwari, A.P.; Song, S.H.; Kim, B.; Jeon, S. Two-dimensional WO3 nanosheets chemically converted from layered WS2 for high-performance electrochromic devices. Nano Lett. 2018, 18, 5646–5651. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Sun, P.; Chai, Z.; Huang, L.; Cai, X.; Tan, S.; Song, J.; Mai, W. Large-scale fabrication of pseudocapacitive glass windows that combine electrochromism and energy storage. Angew. Chem. Int. Ed. 2014, 53, 11935–11939. [Google Scholar] [CrossRef] [PubMed]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Development of photoelectrochromic devices for dynamic solar control in buildings. Sol. Energy Mater. Sol. Cells 2010, 94, 2304–2313. [Google Scholar] [CrossRef]
- Xia, X.; Ku, Z.; Zhou, D.; Zhong, Y.; Zhang, Y.; Wang, Y.; Huang, M.J.; Tu, J.; Fan, H.J. Perovskite solar cell powered electrochromic batteries for smart windows. Mater. Horiz. 2016, 3, 588–595. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. “Partly covered” photoelectrochromic devices with enhanced coloration speed and efficiency. Sol. Energy Mater. Sol. Cells 2012, 96, 86–92. [Google Scholar] [CrossRef]
- Leftheriotis, G.; Syrrokostas, G.; Yianoulis, P. Photocoloration efficiency and stability of photoelectrochromic devices. Solid State Ion. 2013, 231, 30–36. [Google Scholar] [CrossRef]
- Xu, Z.; Li, W.; Huang, J.; Guo, X.; Liu, Q.; Yu, R.; Miao, W.; Zhou, B.; Guoa, W.; Liu, X. Flexible, controllable and angle-independent photoelectrochromic display enabled by smart sunlight management. Nano Energy 2019, 63, 103830. [Google Scholar] [CrossRef]
- Tong, Z.Q.; Tian, Y.L.; Zhang, H.M.; Li, X.G.; Ji, J.Y.; Qu, H.Y.; Li, N.; Zhao, J.P.; Li, Y. Recent advances in multifunctional electrochromic energy storage devices and photoelectrochromic devices. Sci. Chin. Chem. 2017, 60, 13–37. [Google Scholar] [CrossRef]
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef]
- Deepa, M.; Sharma, R.; Basu, A.; Agnihotry, S.A. Effect of oxalic acid dihydrate on optical and electrochemical properties of sol-gel derived amorphous electrochromic WO3 films. Electrochim. Acta 2005, 50, 3545–3555. [Google Scholar] [CrossRef]
- Subrahmanyam, A.; Karuppasamy, A. Optical and electrochromic properties of oxygen sputtered tungsten oxide (WO3) thin films. Sol. Energy Mater. Sol. Cells 2007, 91, 266–274. [Google Scholar] [CrossRef]
- Liang, L.; Zhang, J.J.; Zhou, Y.Y.; Xie, J.F.; Zhang, X.D.; Guan, M.L.; Pan, B.C.; Xie, Y. High-performance flexible electrochromic device based on facile semiconductor-to-metal transition realized by WO3 center dot 2H2O ultrathin nanosheets. Sci. Rep. 2013, 3, 1936. [Google Scholar] [CrossRef] [PubMed]
- Cai, G.F.; Darmawan, P.; Cui, M.Q.; Chen, J.W.; Wang, X.; Eh, A.L.S.; Magdassi, S.; Lee, P.S. Inkjet-printed all solid-state electrochromic devices based on NiO/WO3 nanoparticle complementary electrodes. Nanoscale 2016, 8, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Deepa, M.; Singh, D.P.; Shivaprasad, S.M.; Agnihotry, S.A. A comparison of electrochromic properties of sol-gel derived amorphous and nanocrystalline tungsten oxide films. Curr. Appl. Phys. 2007, 7, 220–229. [Google Scholar] [CrossRef]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Sun, B.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; Zhang, W.; Zhang, J. A Solar-Driven Flexible Electrochromic Supercapacitor. Materials 2020, 13, 1206. https://doi.org/10.3390/ma13051206
Zhang D, Sun B, Huang H, Gan Y, Xia Y, Liang C, Zhang W, Zhang J. A Solar-Driven Flexible Electrochromic Supercapacitor. Materials. 2020; 13(5):1206. https://doi.org/10.3390/ma13051206
Chicago/Turabian StyleZhang, Danni, Baolin Sun, Hui Huang, Yongping Gan, Yang Xia, Chu Liang, Wenkui Zhang, and Jun Zhang. 2020. "A Solar-Driven Flexible Electrochromic Supercapacitor" Materials 13, no. 5: 1206. https://doi.org/10.3390/ma13051206
APA StyleZhang, D., Sun, B., Huang, H., Gan, Y., Xia, Y., Liang, C., Zhang, W., & Zhang, J. (2020). A Solar-Driven Flexible Electrochromic Supercapacitor. Materials, 13(5), 1206. https://doi.org/10.3390/ma13051206






