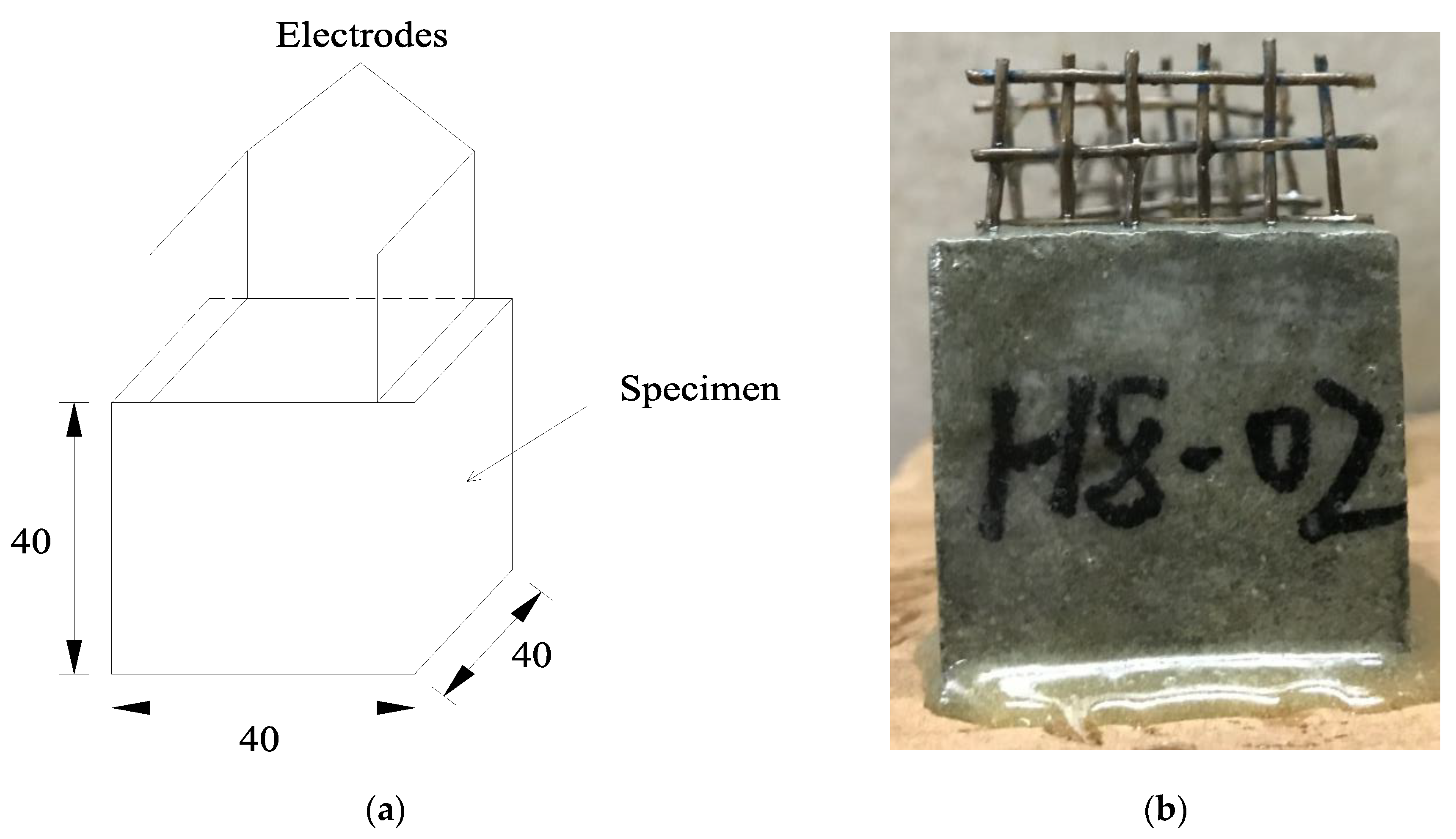
As mentioned before, the internal pores of ionically conductive mortar are filled with electrolyte solution. Since the solid matrix of the mortar is basically an insulating material, the impedance of the ionic conductive mortar is almost completely determined by its pore structure characteristics and the electrolyte solution inside mortar. Various ions in the electrolyte solution inside the mortar specimen, together with the capillary pores, gel pores, micro-cracks, micro-pores, and the solid matrix of the mortar, constitute a complex electrochemical system [
31]. We have determined the EIS of ionically conductive mortar with different porosity, concentration of electrolyte solution, and type of electrolyte solution using the electrochemical workstation. Additionally, equivalent internal circuit of ionically conductive mortar have been simulated. Based on this, the relationship between the element parameters (solution resistance, and charge transfer resistance, capacitance solid–liquid interface, etc.,) of equivalent circuit and the parameters of specimen (porosity, electrolyte solution types, electrolyte solution mass fraction) was stablished.
4.1. Circuit Model of Ionically Conductive Mortar
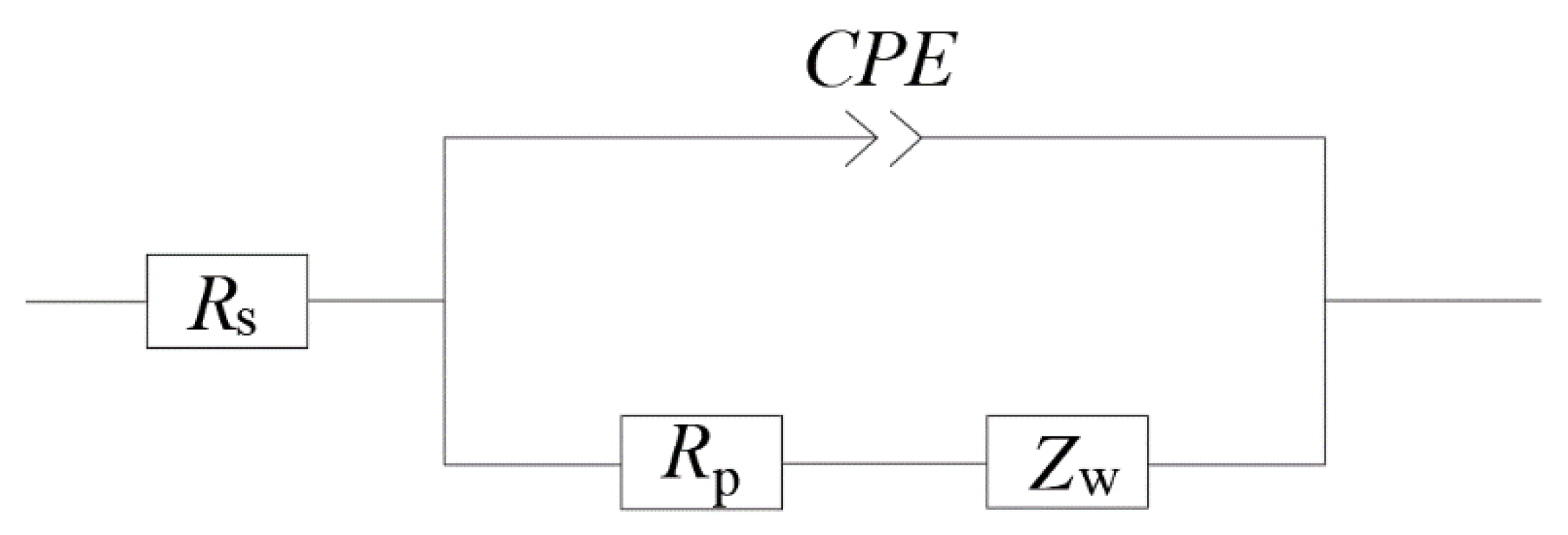
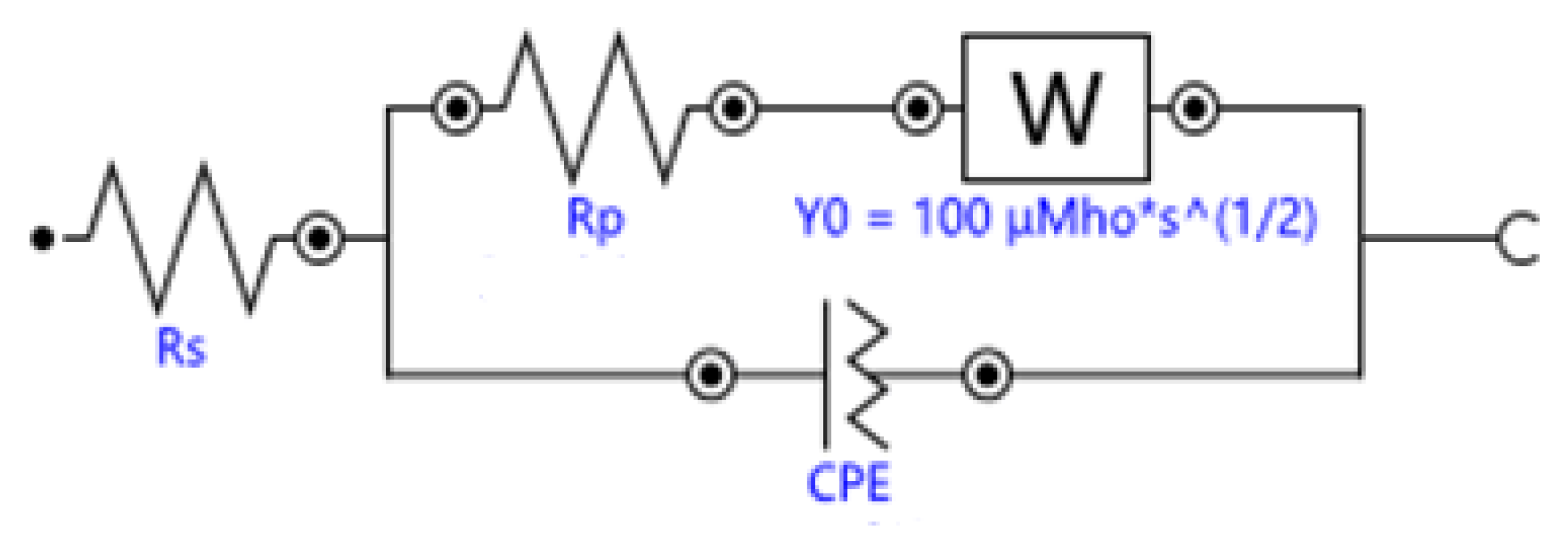
The equivalent circuit of the electrochemical system in ionically conductive mortar can be expressed as the circuit shown in
Figure 6, which is called quasi-Randles model, where,
is the solution resistance;
is the charge-transfer resistance, namely interface reaction resistance;
is the diffusion impedance;
is the capacitance in the solid–liquid interface.
An electrochemical system inside the mortar is essentially composed of two electrodes and the electrolyte between the electrodes. There are two processes proceeding synchronously: the capacitance in the system is charged and discharged with the change of electrode potential; and Faraday process when the electrode potential remains constant [
32]. Therefore, in the equivalent circuit, Faraday impedance can be expressed by the series consisting of charge transfer resistor
Rct and impedance
of the diffusion process. The diffusion impedance
can be divided into different types according to different electrochemical systems. The diffusion impedance of ionically conductive mortar is the semi-infinite diffusion impedance of the planar electrode, i.e. Warburg impedance [
32].
In circuit model, to avoid "dispersion effects," capacitors of electrochemical system are usually replaced with phase-angle elements CPE [
33]. Common electrochemical systems are composed of electrodes and electrolyte solutions, and the only factor that can cause the phase-constant angle element is the roughness of the electrode surface. However, for porous materials such as ionically conductive mortar, the internal complex pore structure can also cause the phase-constant angle element [
33]. In addition, compared with the electrode roughness factor, the influence of pore structure is much greater than the former [
34]. Therefore, for the electrochemical system inside the ionically conductive mortar, the influence of electrode surface roughness can be ignored.
Under electric field, parallel plate capacitor of electrochemical system is formed by directional arrangement of various ions on the solid–liquid interface, which is caused by the potential difference between the slurry solid matrix and pore solution [
26]. Thus, ionically conductive mortar also has electrochemical capacitors with solid–liquid interface, and the size of the capacitance is determined by the pore structure characteristics of the mortar.
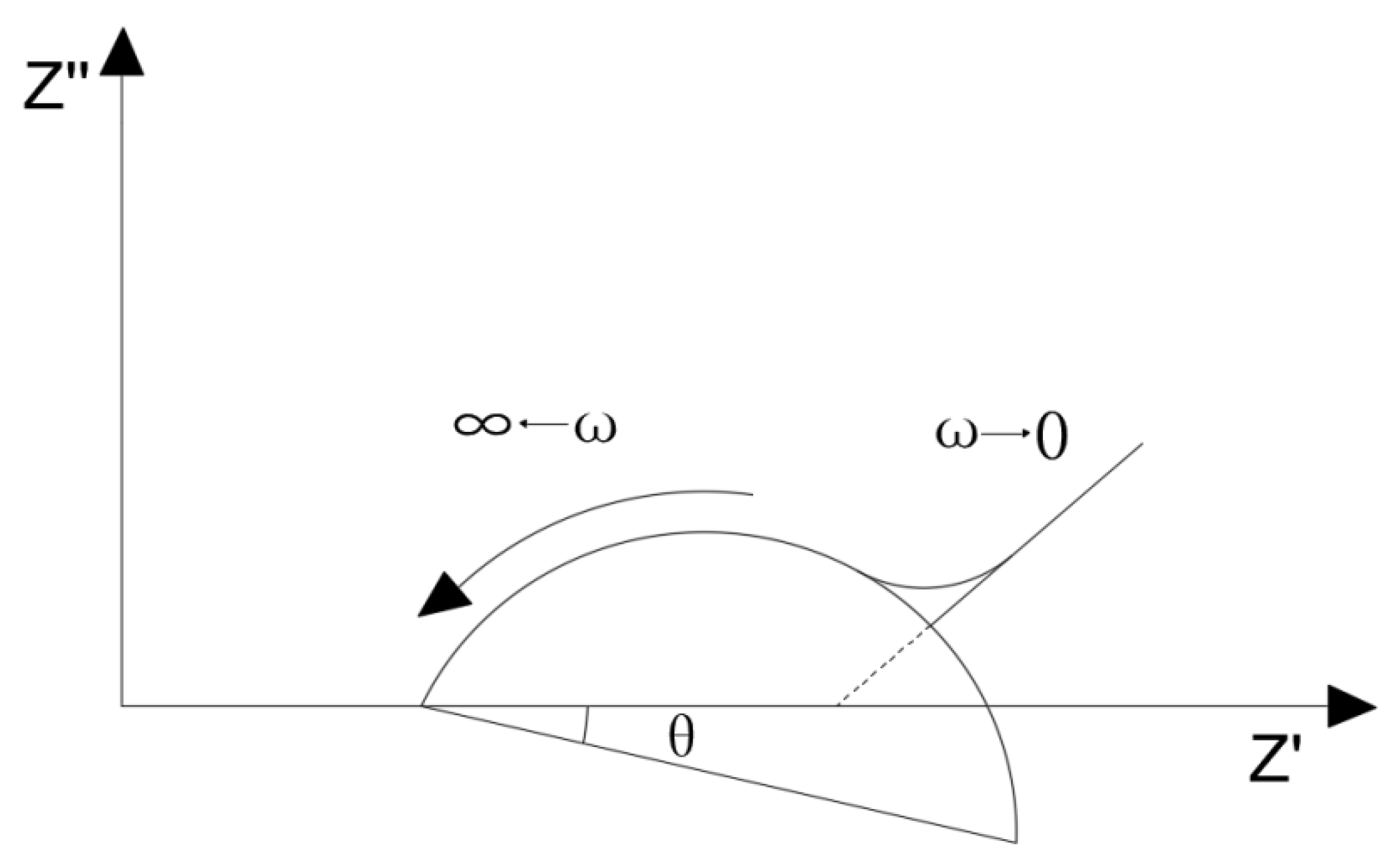
The Nyquist plot of the quasi-Randles model is shown in
Figure 7 [
31]. The Nyquist plot contains two parts: the semicircle (circular arc) in the high frequency region which is controlled by dynamics and the oblique line in the low frequency region which is controlled by diffusion. The intercept of curve with real axis
in high frequency region is solution resistance
, and the diameter of the semicircle is the charge-transfer resistance
, also known as the interface reaction resistance.
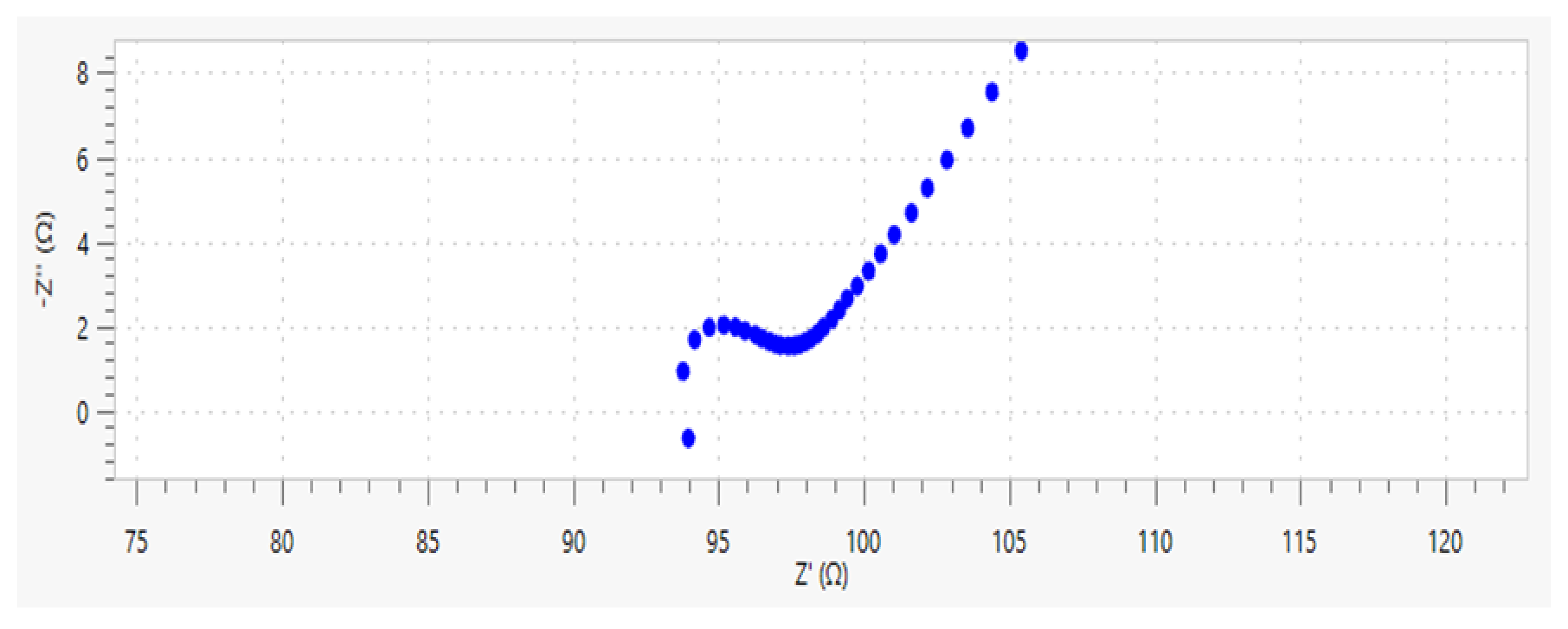
Figure 8 shows the typical Nyquist plot of ionically conductive mortar. It can be found that the Nyquist plot of ionically conductive mortar is also composed of the semicircle in the high frequency region and diffusion line in the low frequency region, which conforms to the quasi-Randles model. Thus, the quasi-Randles model was adopted in the following EIS research of the ionically conductive mortar.
4.3. EIS of Ionically Conductive Mortar with Different Porosity
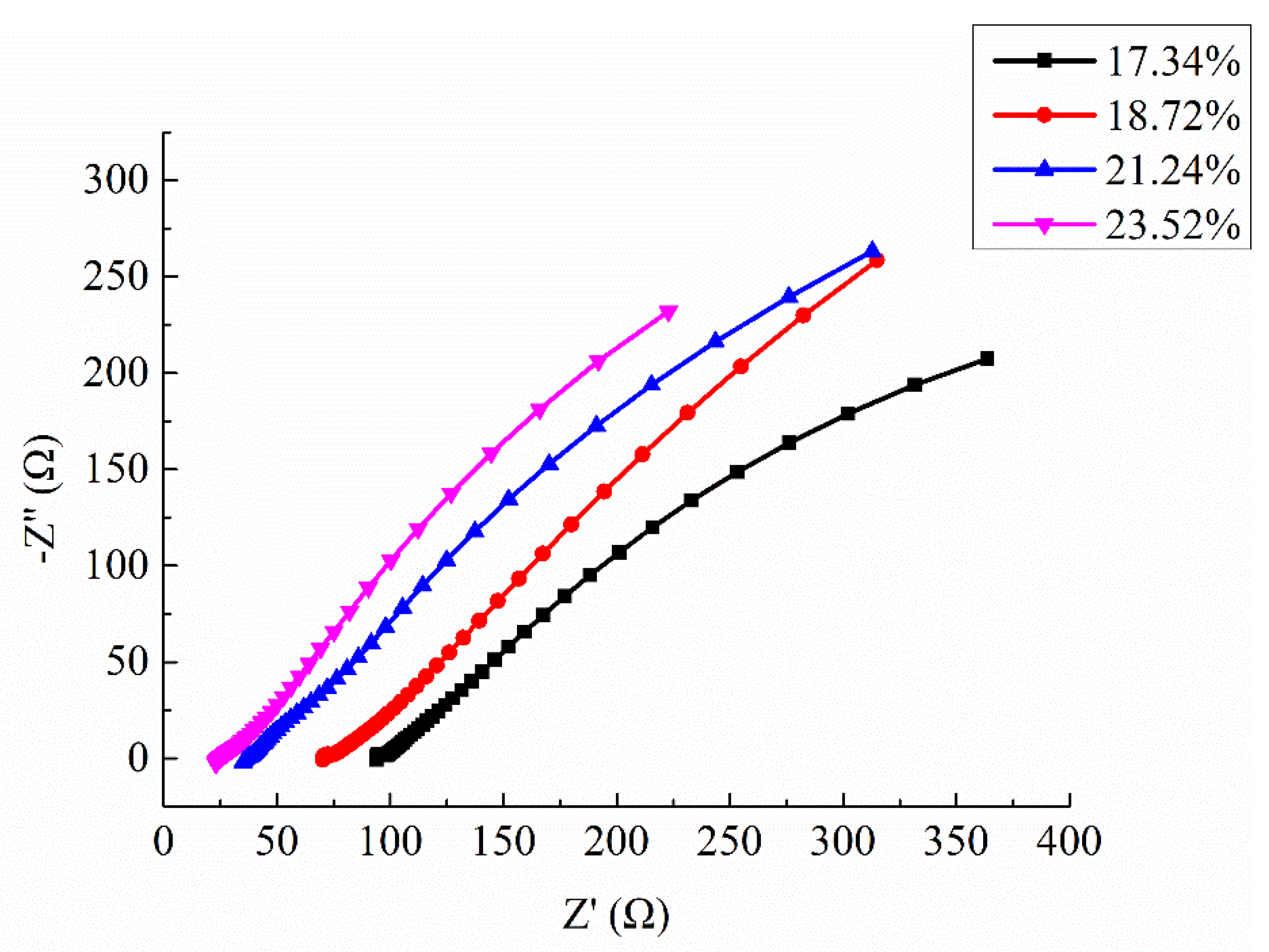
Four samples were selected in this test, with porosity of 17.34%, 18.72%, 21.24%, and 23.52%, respectively. The permeated electrolyte solution was 10% CaCl
2 solution (mass ratio), and the Nyquist plots of specimens are shown in
Figure 10. It is worth noting that the semicircle in the high frequency region just appears in the very beginning, with a small diameter, and then it starts to gradually diffuse. Therefore, the small semicircle in high frequency of Nyquist plots at this test is not very obvious.
As can be seen from
Figure 10, the shape and trend of the four curves are very similar which proves the stability of the test. However, the intercept between the extension line of semicircle in high frequency and the real axis
decreased with the increasing of porosity. As described in
Section 4.1, the intercept of curve with real axis
in high frequency region is solution resistance
. Thus, the results of test also means that the
decreased with the increasing of porosity. It has been proved by the permeating test that the penetration efficiency was improved with the increasing porosity. The electrolyte solution inside the mortar also increased with the increasing porosity. These are the reasons
decreased with the increasing porosity. The equivalent circuit of ionically conductive mortar also proves this rule.
According to the analysis in
Section 4.1, the basic equivalent circuit model of ionically conductive mortar is quasi-Randles model. Nova 2.1.3, the software installed in the electrochemical workstation of Autolab, was used to simulate the Nyquist plots of the above four samples. The equivalent circuit diagrams of ionically conductive mortar is shown in
Figure 11. The parameters of equivalent circuit and resistivity of specimens with different porosity are shown in
Table 8.
As can be seen from
Table 8 and
Figure 11, as the porosity of the mortar specimen increases, the solution resistance
and charge transfer resistance
both decrease, while the interface capacitance CPE increases.
As mentioned before, the impedance of ionically conductive mortar is almost entirely determined by its pore structure characteristics and electrolyte solution in the pores. With the increase of the porosity of the specimen, more electrolyte solution penetrates into the interior of the specimen, the number of ions in the pore solution also gradually increases, and the solution conductivity is enhanced by more ions moving in the direction of the electric field. It was proved that the solution resistance
depends on the concentration of the electrolyte in the liquid phase and the solid phase area [
25]. Thus, the change of
can reflect the change of ion concentration of the solution inside pore and porosity, and the porosity occupies a dominant position in the two factors, and the size of the resistance is inversely proportional to the specimen porosity [
35]. Therefore, the porosity of ionic conductive mortar decreases with the increase of porosity.
In fact, when the porosity of the specimens increases the size of internal pore also increases, so as to improve the inter-connectivity inside the mortar. The movement of ions inside the mortar become easier, which also means the transfer of electric charge is easier, the solid–liquid interface reaction is also increasingly fierce. Therefore, with the increase of specimen porosity, the charge-transfer resistance (interface reaction resistance) decreases. At the same time, greater porosity also means more electrolyte solution in the hole, which leads to the increase of charge at the solid–liquid interface and hence the increase of solid–liquid interface capacitance CPE.
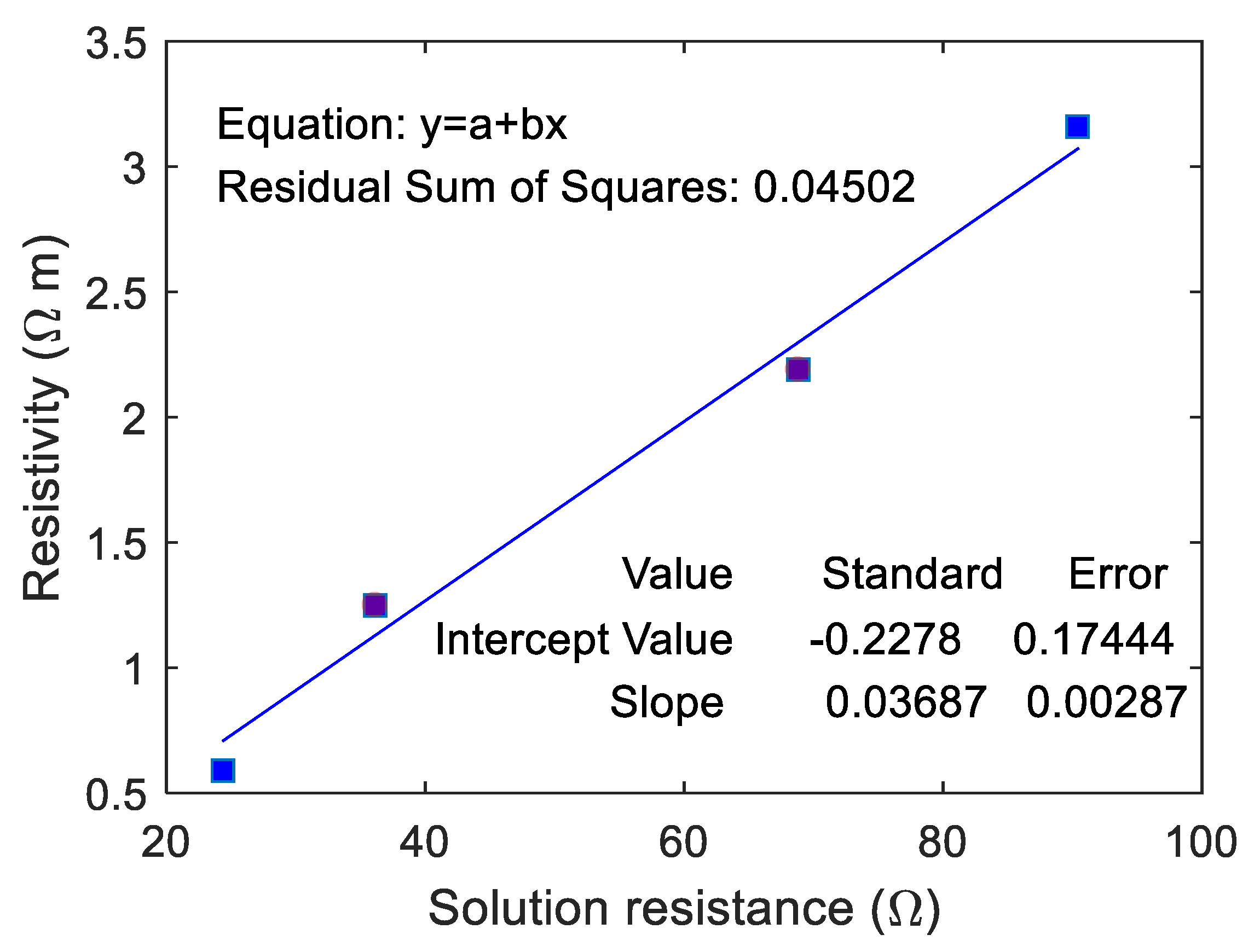
The correlation between the resistivity of ionically conductive mortar specimen and
was fitted, and the results are shown in
Figure 12. As can be seen from the figure, the resistivity of ionically conductive mortar and
are linearly correlated, and the correlation coefficient reaches 0.982. The fitting equation of the line is
. The real resistivity of the mortar specimen increases with the increase of
.
means the resistance of solution inside the pore and pore area. Thus, when the solution resistance
increases, it means that the resistivity of electrolyte solution increases and pore area decreases, consequently causing increase in the resistivity of the specimen. On the other hand, the linear correlation between the real resistivity of ionically conductive mortar specimen and
also proved the validity and effectiveness of the equivalent circuit.
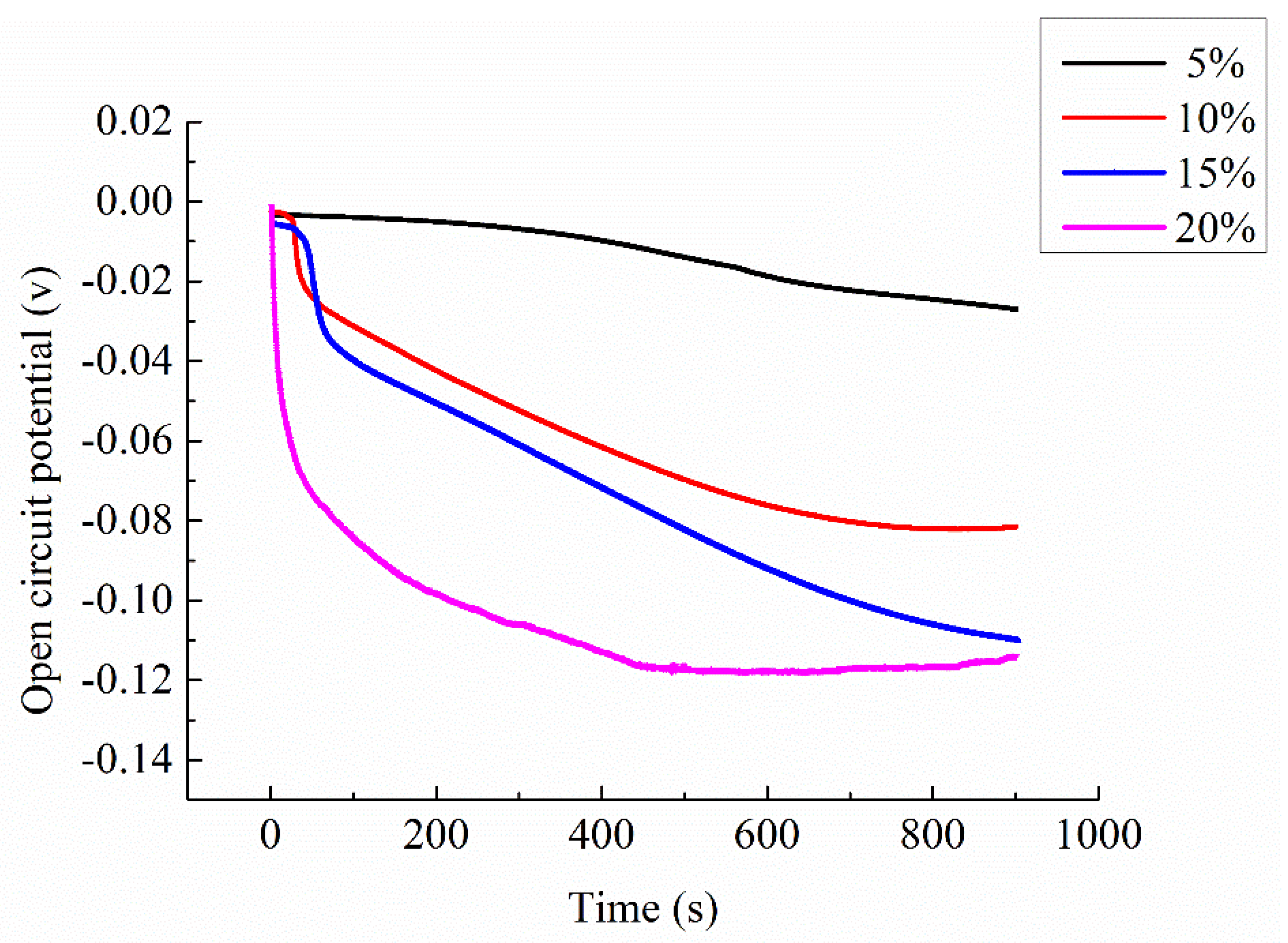
4.4. EIS of Ionically Conductive Mortar with Different Concentration of Electrolyte Solution
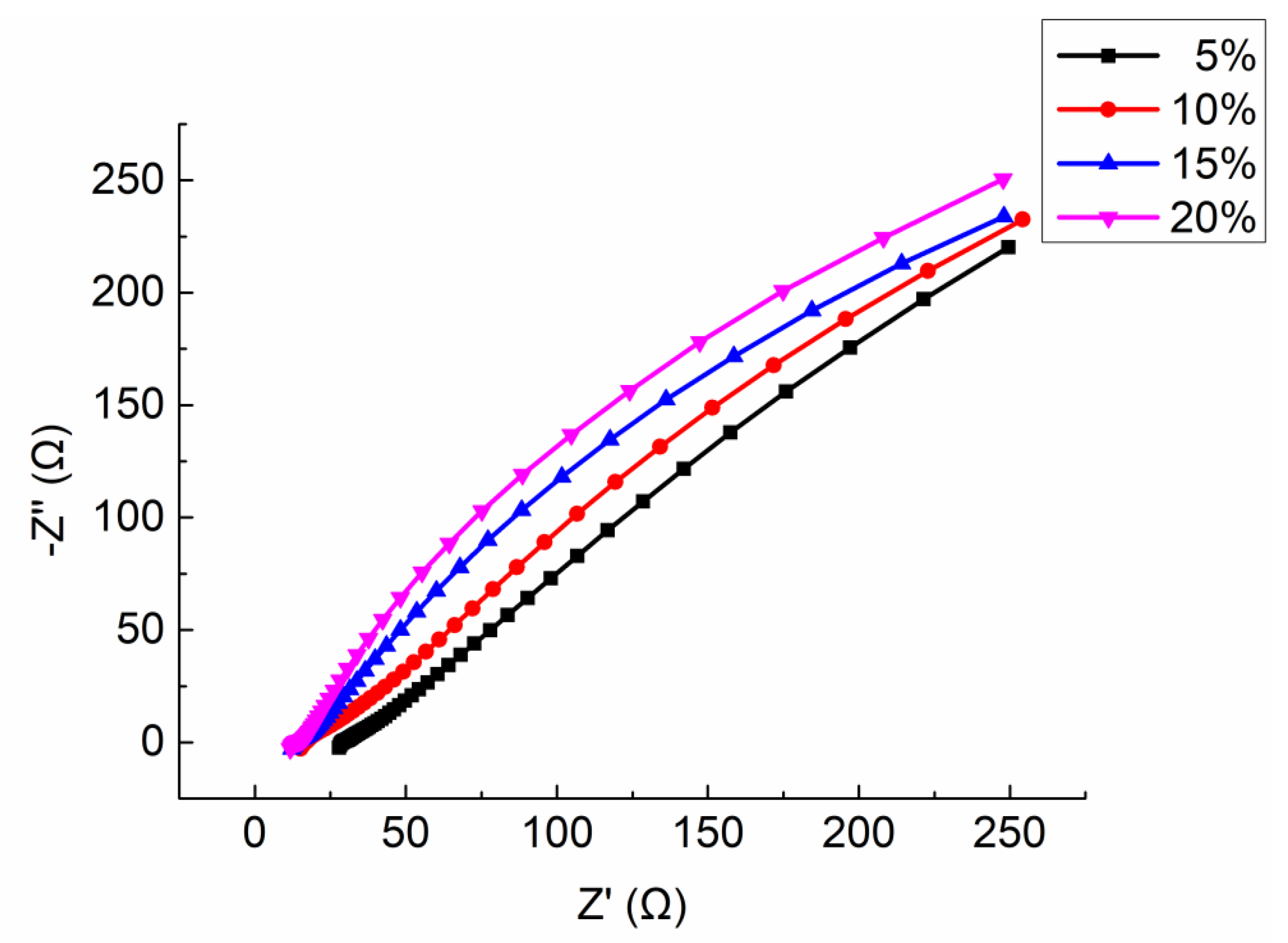
Four mortar specimens (porosity 23.3 ~ 23.7%) made in the same time and with the same formula were selected. The electrolyte solution was CaCl
2 with mass ratio 5%, 10%, 15%, and 20%, respectively. The Nyquist plots are shown in
Figure 13. The equivalent circuit diagrams are shown in
Figure 11. The parameters of equivalent circuit of specimen with different electrolyte solution concentration are shown in
Table 9 (including the measured resistivity of specimens).
It can be seen from the
Figure 13 that the shape and trend of the four curves are very similar which proves the stability of the test. However, the intercept between the extension line of semicircle in high frequency and the real axis
decrease with the increasing of the mass ratio of electrolyte solution. Combining the parameters shown in
Table 9, with the increase of the electrolyte solution mass ratio, the solution resistance
and the charge transfer resistance
both decrease, but the interface capacitance CPE increases.
As mentioned before, the resistivity of ionically conductive mortar is almost entirely determined by its pore structure characteristics and electrolyte solution inside pores. In this test, all the specimens were manufactured in the same time with the same mix ratio, thus, the porosity and internal pore structure characteristics were similar. Under this condition, the electrolyte solution inside the pores become the main factor affecting the resistivity of the specimen. Meanwhile, the solution resistance
is determined by the concentration of electrolyte solution in the specimen and the solid phase area [
34]. When the porosity and pore structure characteristics are similar, the value of
also is determined by the concentration of electrolyte solution. It has been proved that
is inversely proportional to the total concentration of ions inside pores [
35]. This is because the conductivity of the electrolyte solution is determined by the concentration of free moving ions in the solution and the charge they carry. The higher the concentration of free moving ions is, the more the charge the ions carry, the stronger the conductivity of the solution will be. It is worth noting that when the concentration of solution is lower than a certain level, the quantity of free ions dominates the conductivity of electrolyte solution, and the conductivity of solution is enhanced because of the increasing number of free ions. When the concentration of solution exceeds the certain level, the interaction force between ions increases, which declines the movement velocity of free ions in the solution. At this circumstance, the conductivity of solution decreases with the increase of concentration. Therefore, for electrolyte solution, there is a threshold value of concentration of electrolyte solution to its conductivity. It can be found in
Table 9 when the mass ratio of electrolyte solution increased from 5% to 10%, solution resistance
dropped from 29.13 Ω to 16.73 Ω, which proves the conductivity of electrolyte solution would increase when the concentration increases until reaching the threshold value. When the mass ratio increased from 10% to 15%, the change range of
became flat gradually. When the mass fraction increased from 15% to 20%,
only decreased from 13.82 Ω to 13.41 Ω. This is because that 20% mass ratio approaches the concentration of CaCl
2 solution. It can be predicted that if the solution concentration increased continually, the conductivity of CaCl
2 solution would decrease once the mass ratio exceed the threshold value, which also declines the conductivity of ionically conductive mortar.
However, the interfacial capacitance CPE increases with the increase of concentration of electrolyte solution. This is because with the increase of the concentration of electrolyte solution, the number of free ions increases, and the charge they carry also increases; these conditions make the solid–liquid interface reaction become fierce, consequently leading to the increase of the charge at the solid–liquid interface and hence the increase of solid-liquid interface capacitance CPE.
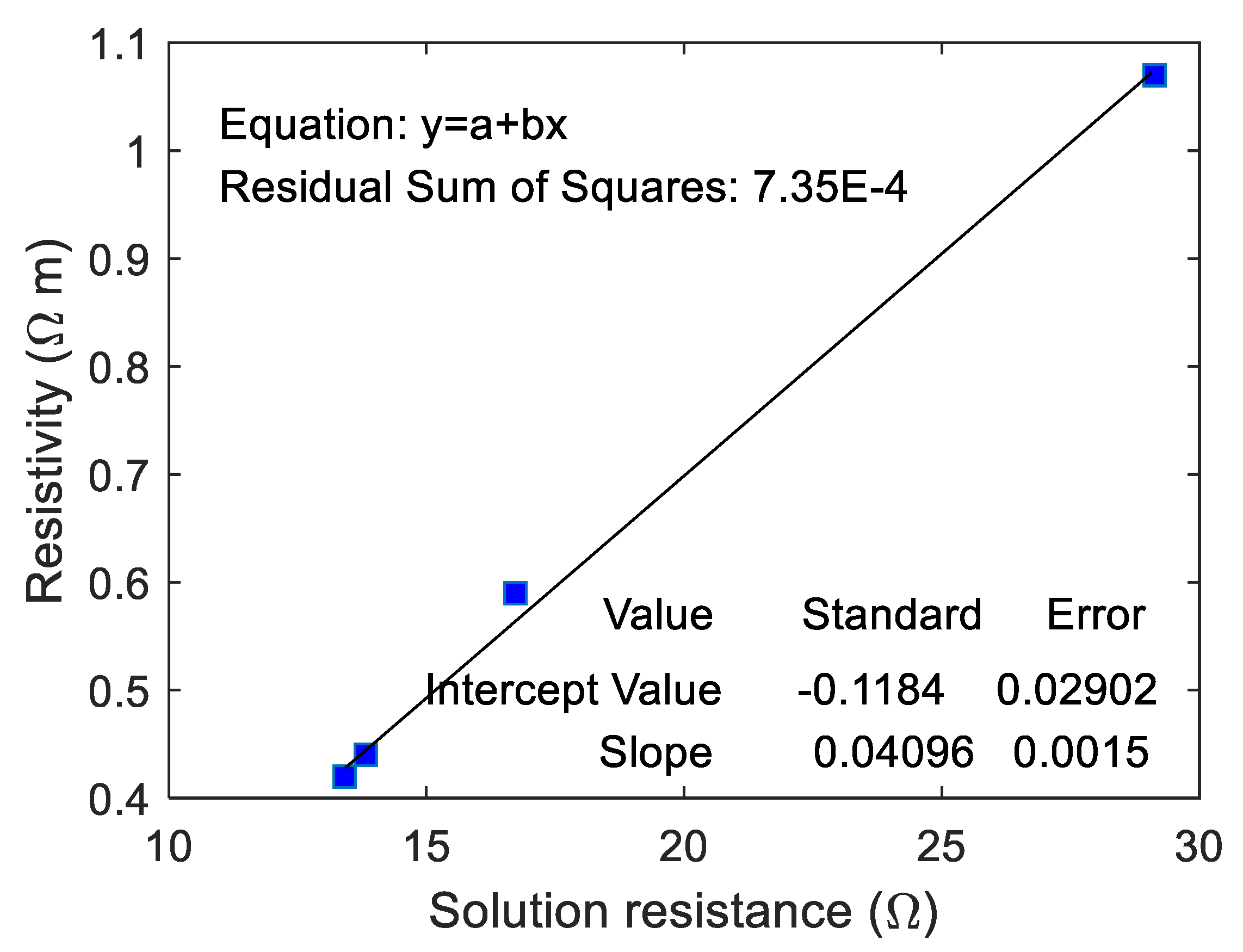
The correlation between the real resistivity of ionically conductive mortar specimens and
of the equivalent circuit was fitted, and the results are shown in
Figure 14. As can be seen from the figure, the resistivity of ionically conductive mortar and
are linearly correlated, and the correlation coefficient reaches 0.996. The fitting equation of the line is
. The real resistivity of the mortar specimen increases with the increase of
. The validity and effectiveness of equivalent circuit calculated by EIS has been proved again by this linear equation.
4.5. EIS of Ionically Conductive Mortar with Different Solute
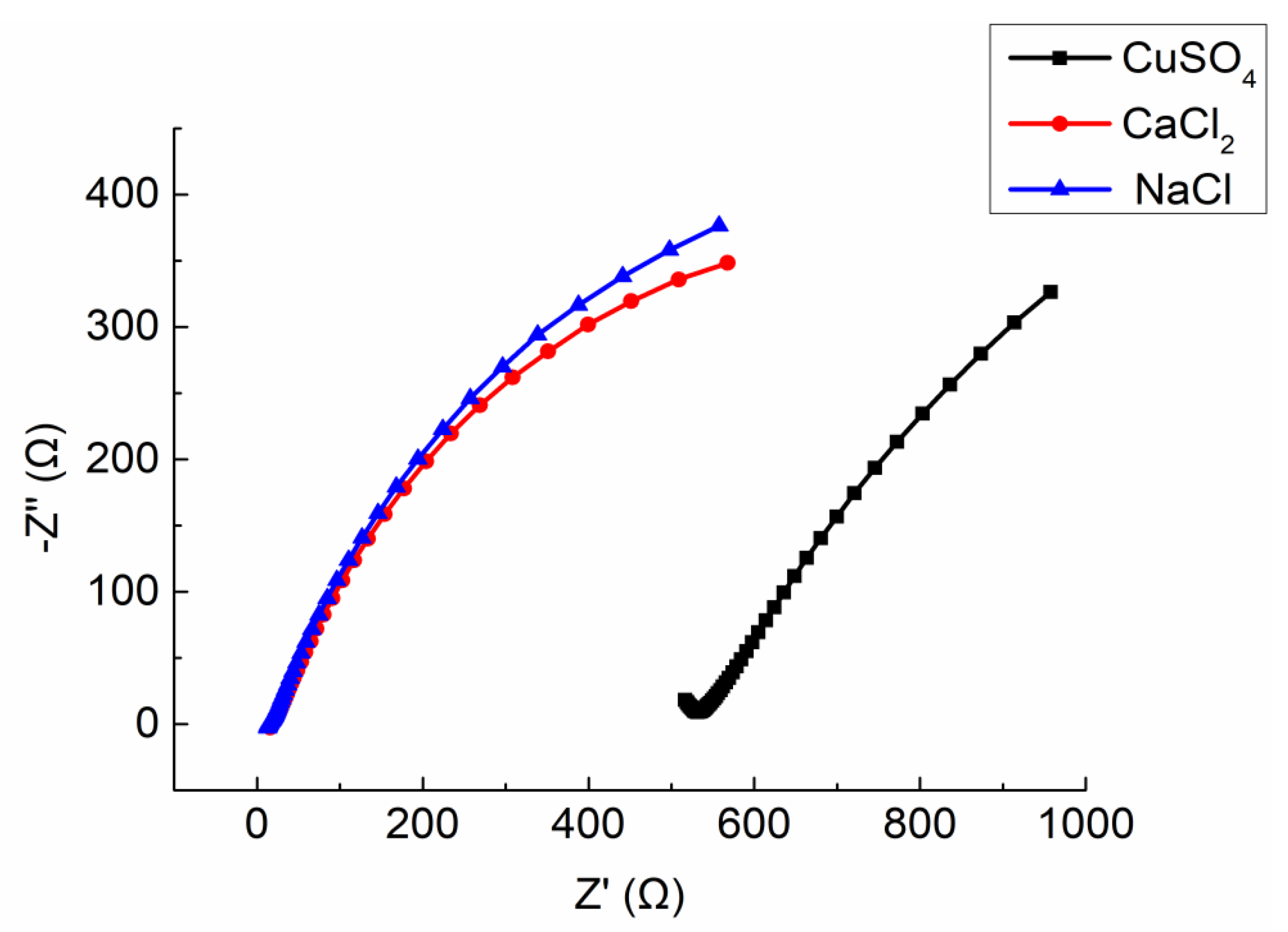
Three mortar specimens (porosity 23.3 ~ 23.7%) made in the same time and with the same mix ratio were selected. The solutes used to prepare electrolyte solution were CaCl
2, CuSO
4, and NaCl, respectively. The mass ratio of three electrolyte solution is 10%. The Nyquist plots are shown in
Figure 15. The equivalent circuit diagram of ionically conductive mortar is shown in
Figure 11. The parameters of equivalent circuits with different electrolyte solution are shown in
Table 10 (including the measured resistivity of specimens).
It can be seen from the
Figure 15 that the Nyquist plots of the specimens with CaCl
2 and NaCl solutions are similar, while the specimen with CuSO
4 solution are much different from the other two. The intercept between the extension line of semicircle in high frequency and the real axis
of the specimen with CuSO
4 solution is much larger than that of the specimen permeated by Cl
- solution. As shown in
Table 10, the different types of electrolyte solutions will lead to the differences in the solution resistance
, charge transfer resistance
, and interface capacitance CPE of equivalent circuit. Among them, the relationship between
and
is CuSO
4>CaCl
2>NaCl, while the CPE shows the opposite trend, which is NaCl > CaCl
2 > CuSO
4.
As described before, when the specimens were manufactured in the same time with the same mix ratio, the main factor affecting the resistivity of the specimen is the electrolyte solution inside pores. The conductivity of electrolyte solution is determined by the concentration and movement velocity of free ions in the solution. The mass ratio cannot accurately represent the concentration of ions in the solution since the type of electrolyte solutions in this section is different. Therefore, it is necessary to convert the mass fraction into the molarity of solute substances, so as to compare the concentration of different electrolyte solutions. The formula for the molarity of solute substances can be calculated as Equation (1) [
36]:
where,
is the density of electrolyte solution, which can be tested after solution being prepared,
w is the mass ratio of the solution, and
M is the molar mass of the solute substance. For the three solutions selected in this section, molarity of solute substances was calculated by Equation (1), the results are shown in
Table 11.
As shown in
Table 11, the molarity of the three solutes is: NaCl > CaCl
2 > CuSO
4, which means the solution of NaCl has largest amounts of free ions. Therefore, solution of NaCl has the strongest conductivity, while CuSO
4 solution is the weakest. As test results shown in
Table 10,
of specimen with NaCl solution is the smallest, only 14.44 Ω, the next is 17.77 Ω of the specimen with CaCl
2, and the
of specimen with CuSO
4 solution reached 411.96 Ω, far higher than the front two specimens.
The
and CPE of specimen also were influenced by the concentration of free ions in the solution. The
increases with the increase of the concentration of free ions in the solution, while the CPE decreases. The reason of this rule can be found in
Section 4.4. In this test, the order of concentration of free ions is NaCl > CaCl
2 >CuSO
4, the order of the
is CuSO
4 > CaCl
2 > NaCl, and the order of the CPE is NaCl > CaCl
2 > CuSO
4, all of the test results conform to the rule above.
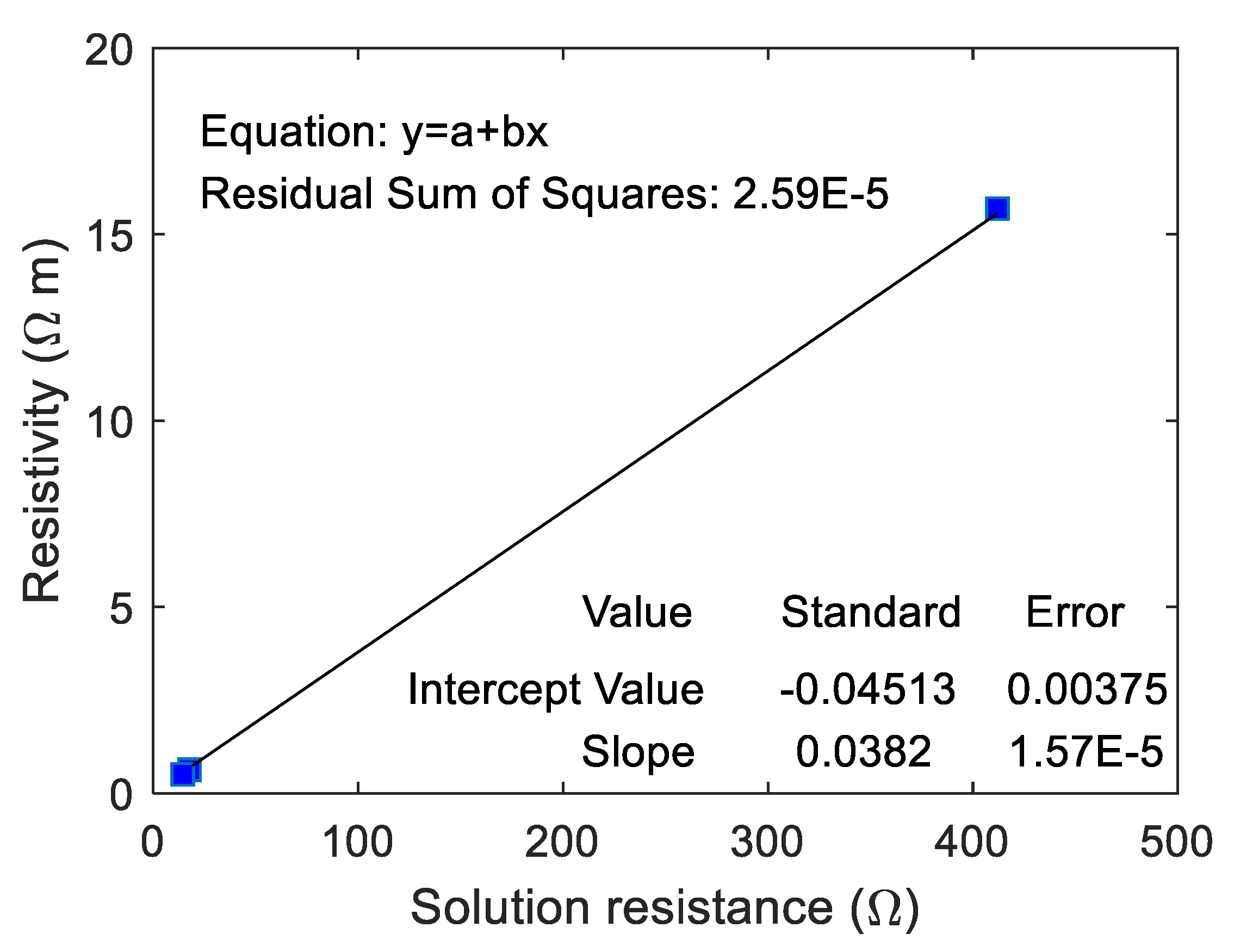
The correlation between the real resistivity of ionically conductive mortar specimen and the
was fitted, and the results are shown in
Figure 16. In this case, the real resistivity of ionically conductive mortar specimen and
are also linearly correlated. The correlation coefficient even reaches 1, and the equation of the fitting line is
. It shows that the equivalent circuit according to the EIS tested by electrochemical workstation can effectively reflect the true resistance of the ionically conductive mortar.