Control of Peri-Implant Mucous Inflammation by Using Chlorhexidine or Ultraviolet C Radiation for Cleaning Healing Abutments. Double-Blind Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Description
2.1.1. Recruitment
2.1.2. Sample Size
2.1.3. Inclusion Criteria
2.1.4. Exclusion Criteria
2.2. Study Protocol
2.3. Randomization
2.4. Blinding
2.5. Statistical Analysis
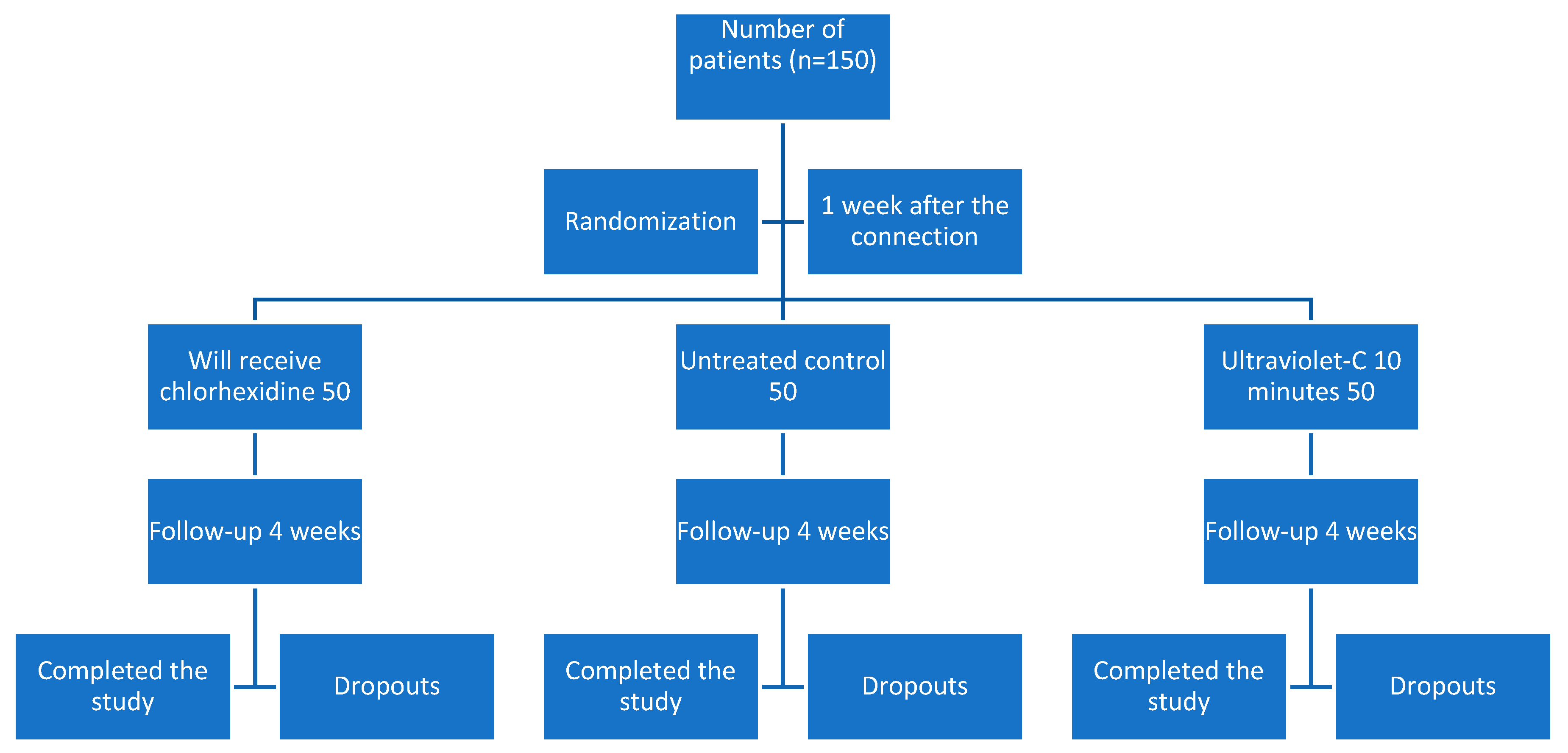
3. Results
- Group 1 (treated with chlorhexidine gel) had an average age of 59.3 (C.I. 56.1–62.6), consisting of 29 women with an average age of 59.6 (C.I. 55.2–64.0) and 21 men with an average age of 59.0 (C.I. 53.7–64.3). Inflammation was 1.4 (C.I.1.2–1.6) at the time of impression, 0.8 (C.I. 0.6–1.0) at metal try-in, 0.6 (C.I. 0.4–0.8) in the ceramic delivery and 0.5 (C.I. 0.3–0.7) at the time of prosthesis cementation. The plaque index at the end of monitoring was 0.9 (C.I. 0.7–1.2). Two patients withdrew from the study voluntarily.
- Group 2 (control without modification) had an average age of 62.2 (C.I. 59.2–65.2), consisting of 27 women with an average age of 62.3 (C.I. 59.4–65.3) and 23 men with an average age of 62.1 (C.I. 56.2–68.0). Inflammation was 1.4 (C.I. 1.1–1.6) at the time of impression, 1.0 (C.I. 0.7–1.2) when metal try-in, 0.8 (C.I. 0.6–1.0) in the ceramic delivery follow up and 0.7 (C.I. 0.5–0.9) at the visit of the prosthesis cementation. The plaque index at the end of monitoring was 0.9 (C.I. 0.6–1.1). There were six dropouts of the study: one missed appointment, two failures, and three withdrawals.
- Group 3 (treated with UV-C at 254 nm and 80 W) had an average of 60.4 years (C.I. 57.5–63.3), consisting of 32 women with an average age of 59.6 (C.I. 55.8–63.4) and 18 men with an average age of 61.9 (C.I. 57.0–66.8). Inflammation was 1.2 (C.I. 1.0–1.5) at the time of impression, 0.7 (C.I. 0.5–1.0) at the metal try-in, 0.7 (C.I. 0.4–0.9) at the ceramic delivery visit and 0.4 (C.I. 0.3–0.7) at the visit for cementation of the prosthesis. The plaque index at the end of monitoring was 0.5 (C.I. 0.3–0.6). There were nine drop-outs from the study: seven for non-compliance with appointments, and two failures.
4. Discussion
5. Conclusions
- No treatment showed a statistically significant decrease in the degree of clinically recorded inflammation.
- The worst result in terms of plaque accumulation was observed in the chlorhexidine treated group.
- The best result regarding plaque accumulation was presented by the abutments that were UV-C treated at 254 nm for 15 min, with statistically significant differences.
- Peri-implant tissue maturation improves with time.
- Since the most important changes take place during the first two weeks, we recommend taking impressions at this time, although for the best aesthetic results, we recommend waiting four weeks for soft tissue maturation.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Berglundh, T.; Lindhe, J.; Ericsson, I.; Marinello, C.P.; Liljenberg, B.; Thomsen, P. The Soft TissueBarrier at Implants and Teeth. Clin. Oral Implants Res. 1991, 2, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Penarrocha-Oltra, D.; Marchionni, S.; Bagán, L.; Peñarrocha-Diago, M.-A.; Micarelli, C. Soft Tissue Cell Adhesion to Titanium Abutments after Different Cleaning Procedures: Preliminary Results of a Randomized Clinical Trial. Med. Oral Patol. Oral Cir. Bucal 2014, 19, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Schoolfield, J.D.; Schenk, R.K.; Buser, D.; Cochran, D.L. Influence of the Size of the Microgap on Crestal Bone Changes around Titanium Implants. A Histometric Evaluation of Unloaded Non-Submerged Implants in the Canine Mandible. J. Periodontol. 2001, 72, 1372–1383. [Google Scholar] [CrossRef] [PubMed]
- Cochran, D.L.; Hermann, J.S.; Schenk, R.K.; Higginbottom, F.L.; Buser, D. Biologic Width Around Titanium Implants. A Histometric Analysis of the Implanto-Gingival Junction Around Unloaded and Loaded Nonsubmerged Implants in the Canine Mandible. J. Periodontol. 1997, 68, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Cochran, D.L.; Nummikoski, P.V.; Buser, D. Crestal Bone Changes around Titanium Implants. A Radiographic Evaluation of Unloaded Nonsubmerged and Submerged Implants in the Canine Mandible. J. Periodontol. 1997, 68, 1117–1130. [Google Scholar] [CrossRef]
- King, G.N.; Hermann, J.S.; Schoolfield, J.D.; Buser, D.; Cochran, D.L. Influence of the Size of the Microgap on Crestal Bone Levels in Non-Submerged Dental Implants: A Radiographic Study in the Canine Mandible. J. Periodontol. 2002, 73, 1111–1117. [Google Scholar] [CrossRef]
- Romanos, G.E. Tissue preservation strategies for fostering long-term soft and hard tissue stability. Int. J. Perio. Rest. Dent. 2015, 35, 363–371. [Google Scholar] [CrossRef]
- Romanos, G.E.; Aydin, E.; Gärtner, K.; Nentwig, G.H. Long-term results after subcrestal or crestal placement of delayed loaded implants. Clin. Implant Dent. Relat. Res. 2015, 17, 133–141. [Google Scholar] [CrossRef]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.; Schenk, R.K.; Buser, D.; Cochran, D.L. Peri-Implant Inflammation Defined by the Implant-Abutment Interface. J. Dent. Res. 2006, 85, 473–478. [Google Scholar] [CrossRef]
- Broggini, N.; McManus, L.M.; Hermann, J.S.; Medina, R.U.; Oates, T.W.; Schenk, R.K.; Buser, D.; Mellonig, J.T.; Cochran, D.L. Persistent Acute Inflammation at the Implant-Abutment Interface. J. Dent. Res. 2003, 82, 232–237. [Google Scholar] [CrossRef]
- Cardaropoli, G.; Lekholm, U.; Wennström, J.L. Tissue Alterations at Implant-Supported Single-Tooth Replacements: A 1-Year Prospective Clinical Study. Clin. Oral Implants Res. 2006, 17, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Micarelli, C.; Iannello, G. Microscopical and Chemical Surface Characterization of the Gingival Portion and Connection of an Internal Hexagon Abutment before and after Different Technical Stages of Preparation. Clin. Oral Implants Res. 2013, 24, 606–611. [Google Scholar] [CrossRef] [PubMed]
- Penarrocha-Oltra, D.; Rossetti, P.H.O.; Covani, U.; Galluccio, F.; Canullo, L. Microbial Leakage at the Implant/Abutment Connection Due to Implant Insertion Maneuvers: Cross-Sectional Study 5 Years Post Loading in Healthy Patients. J. Oral Implantol. 2014, 41, 292–296. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Bollen, C.M.; Eyssen, H.; van Steenberghe, D. Microbial Penetration along the Implant Components of the Brånemark System. An in Vitro Study. Clin. Oral Implants Res. 1994, 5, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Cochran, D.L. Crestal Bone Changes around Titanium Implants. A Histometric Evaluation of Unloaded Non-Submerged and Submerged Implants in the Canine Mandible. J. Periodontol. 2000, 71, 1412–1424. [Google Scholar] [CrossRef]
- Buser, D.; Mericske-Stern, R.; Dula, K.; Lang, N.P. Clinical Experience with One-Stage, Non-Submerged Dental Implants. Adv. Dent. Res. 1999, 13, 153–161. [Google Scholar] [CrossRef]
- Besimo, C.E.; Guindy, J.S.; Lewetag, D.; Meyer, J. Prevention of Bacterial Leakage into and from Prefabricated Screw-Retained Crowns on Implants in vitro. Int. J. Oral Maxillofac. Implants 1999, 14, 654–660. [Google Scholar]
- Piattelli, A.; Scarano, A.; Paolantonio, M.; Assenza, B.; Leghissa, G.C.; Di Bonaventura, G.; Catamo, G.; Piccolomini, R. Fluids and Microbial Penetration in the Internal Part of Cement-Retained Versus Screw-Retained Implant-Abutment Connections. J. Periodontol. 2001, 72, 1146–1150. [Google Scholar] [CrossRef]
- Rimondini, L.; Marin, C.; Brunella, F.; Fini, M. Internal Contamination of a 2-Component Implant System After Occlusal Loading and Provisionally Luted Reconstruction With or Without a Washer Device. J. Periodontol. 2001, 72, 1652–1657. [Google Scholar] [CrossRef]
- Larrucea Verdugo, C.; Jaramillo Núñez, G.; Acevedo Avila, A.; Larrucea San Martín, C. Microleakage of the Prosthetic Abutment/Implant Interface with Internal and External Connection: In Vitro Study. Clin. Oral Implants Res. 2014, 25, 1078–1083. [Google Scholar] [CrossRef]
- Larrucea, C.; Conrado, A.; Olivares, D.; Padilla, C.; Barrera, A.; Lobos, O. Bacterial Microleakage at the Abutment-Implant Interface, in Vitro Study. Clin. Implant Dent. Relat. Res. 2018, 20, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; Suárez, F.; OValle, F.; Spinato, S.; Catena, A. Prosthetic Abutment Height Is a Key Factor in Peri-Implant Marginal Bone Loss. J. Dent. Res. 2014, 93, 80S–85S. [Google Scholar] [CrossRef]
- Krozer, A.; Hall, J.; Ericsson, I. Chemical Treatment of Machined Titanium Surfaces. An in vitro Study. Clin. Oral Implants Res. 1999, 10, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Bressan, E.; Tessarolo, F.; Sbricoli, L.; Caola, I.; Nollo, G.; Di Fiore, A. Effect of Chlorhexidine in Preventing Plaque Biofilm on Healing Abutment: A Crossover Controlled Study. Implant Dent. 2014, 23, 64–68. [Google Scholar] [CrossRef]
- Sennerby, L.; Lekholm, U. The Soft Tissue Response to Titanium Abutments Retrieved from Humans and Reimplanted in Rats. A Light Microscopic Study. Clin. Oral Implants Res. 1993, 4, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Vezeau, P.; Koorbusch, G.; Draughn, R.; Keller, J. Effects of Multiple Sterilization on Surface Characteristics and in Vitro Biologic Responses to Titanium. J. Oral Maxillofac. Surg. 1996, 54, 738–746. [Google Scholar] [CrossRef]
- Keller, J.C.; Draughn, R.A.; Wightman, J.P.; Dougherty, W.J.; Meletiou, S.D. Characterization of Sterilized CP Titanium Implant Surfaces. Int. J. Oral Maxillofac. Implants 1990, 5, 360–367. [Google Scholar]
- Vezeau, P.J.; Keller, J.C.; Wightman, J.P. Reuse of Healing Abutments: An in Vitro Model of Plasma Cleaning and Common Sterilization Techniques. Implant Dent. 2000, 9, 236–246. [Google Scholar] [CrossRef]
- Rowland, S.A.; Shalaby, S.W.; Latour, R.A.; von Recum, A.F. Effectiveness of Cleaning Surgical Implants: Quantitative Analysis of Contaminant Removal. J. Appl. Biomater. Off. J. Soc. Biomater. 1995, 6, 1–7. [Google Scholar] [CrossRef]
- Zöller, G.O.; Zentner, A. Initial Attachment of Human Gingival Fibroblast-like Cells in vitro to Titanium Surfaces Pretreated with Saliva and Serum. Clin. Oral Implants Res. 1996, 7, 311–315. [Google Scholar] [CrossRef]
- Steinemann, S.G. Titanium—The Material of Choice? Periodontology 1998, 17, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Barboza, E.P.; Caúla, A.L.; Carvalho, W.R. Crestal Bone Loss around Submerged and Exposed Unloaded Dental Implants: A Radiographic and Microbiological Descriptive Study. Implant Dent. 2002, 11, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E.; Domken, O.; Degidi, M.; Pontes, A.E.F.; Piattelli, A. The Effect of Material Characteristics, of Surface Topography and of Implant Components and Connections on Soft Tissue Integration: A Literature Review. Clin. Oral Implants Res. 2006, 2, 55–67. [Google Scholar] [CrossRef] [PubMed]
- General Assembly of the World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Mombelli, A.; van Oosten, M.A.C.; Schürch, E.; Lang, N. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Altman, D.G. Better Reporting of Randomised Controlled Trials: The CONSORT Statement. BMJ 1996, 313, 570–571. [Google Scholar] [CrossRef]
- Quirynen, M.; Bollen, C.M.; Papaioannou, W.; Van Eldere, J.; van Steenberghe, D. The Influence of Titanium Abutment Surface Roughness on Plaque Accumulation and Gingivitis: Short-Term Observations. Int. J. Oral Maxillofac. Implants 1996, 11, 169–178. [Google Scholar]
- Quirynen, M.; van Steenberghe, D. Bacterial Colonization of the Internal Part of Two-Stage Implants. An in vivo Study. Clin. Oral Implants Res. 1993, 4, 158–161. [Google Scholar] [CrossRef]
- Persson, L.G.; Lekholm, U.; Leonhardt, A.; Dahlén, G.; Lindhe, J. Bacterial Colonization on Internal Surfaces of Brånemark System Implant Components. Clin. Oral Implants Res. 1996, 7, 90–95. [Google Scholar] [CrossRef]
- Jansen, V.K.; Conrads, G.; Richter, E.J. Microbial Leakage and Marginal Fit of the Implant-Abutment Interface. Int. J. Oral Maxillofac. Implants 1997, 12, 527–540. [Google Scholar]
- Guindy, J.S.; Besimo, C.E.; Besimo, R.; Schiel, H.; Meyer, J. Bacterial Leakage into and from Prefabricated Screw-Retained Implant-Borne Crowns in vitro. J. Oral Rehabil. 1998, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Gross, M.; Abramovich, I.; Weiss, E.I. Microleakage at the Abutment-Implant Interface of Osseointegrated Implants: A Comparative Study. Int. J. Oral Maxillofac. Implants 1999, 14, 94–100. [Google Scholar] [PubMed]
- Mombelli, A.; Lang, N.P. The Diagnosis and Treatment of Peri-Implantitis. Periodontology 1998, 17, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Mericske-Stern, R. Microbiological Features of Stable Osseointegrated Implants Used as Abutments for Overdentures. Clin. Oral Implants Res. 1990, 1, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Piatelli, A.; Polimeni, A.; Di Iorio, D.; Carinci, F. Bacterial adhesion on commercially pure titanium and anatase-coated titanium healing screws: An in vivo human study. J. Periodontol. 2010, 81, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Carinci, F.; Grecchi, E.; Bignozzi, C.A.; Murmura, G.; Piatelli, A.; Scarano, A. Bactercline® coated implants: Clinical results up to 1 year after loading from a controlled clinical trial. Dent. Res. J. 2012, 9, S142–S146. [Google Scholar]
- Rismanchian, M.; Hatami, M.; Badrian, H.; Khalighinejad, N.; Goroohi, H. Evaluation of Microgap Size and Microbial Leakage in the Connection Area of 4 Abutments with Straumann (ITI) Implant. J. Oral Implantol. 2012, 38, 677–685. [Google Scholar] [CrossRef]
- Waerhaug, J. The Angular Bone Defect and Its Relationship to Trauma from Occlusion and Downgrowth of Subgingival Plaque. J. Clin. Periodontol. 1979, 6, 61–82. [Google Scholar] [CrossRef]
- Garant, P.R. Ultrastructural Studies of Inflammation Induced in Rats by Injection of Antigen-Antibody Precipitates. Changes in Palatal Bone and Periosteum to a Single Exposure. J. Periodontal Res. 1979, 14, 26–38. [Google Scholar] [CrossRef]
- Graves, D.T.; Cochran, D. The Contribution of Interleukin-1 and Tumor Necrosis Factor to Periodontal Tissue Destruction. J. Periodontol. 2003, 74, 391–401. [Google Scholar] [CrossRef]
- Abrahamsson, I.; Berglundh, T.; Lindhe, J. The Mucosal Barrier Following Abutment Dis/Reconnection. An Experimental Study in Dogs. J. Clin. Periodontol. 1997, 24, 568–572. [Google Scholar] [CrossRef] [PubMed]
- Koutouzis, T.; Koutouzis, G.; Gadalla, H.; Neiva, R. The Effect of Healing Abutment Reconnection and Disconnection on Soft and Hard Peri-Implant Tissues: A Short-Term Randomized Controlled Clinical Trial. Int. J. Oral Maxillofac. Implants 2013, 28, 807–814. [Google Scholar] [CrossRef]
- Koutouzis, T. Implant-abutment Connection as Contributing Factor to Peri-implant Diseases. Periodontology 2019, 81, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Rompen, E. The Impact of the Type and Configuration of Abutments and Their (Repeated) Removal on the Attachment Level and Marginal Bone. Eur. J. Oral Implantol. 2012, 5, 83–90. [Google Scholar]
- Bressan, E.; Grusovin, M.G.; D’Avenia, F.; Neumann, K.; Sbricoli, L.; Luongo, G.; Esposito, M. The Influence of Repeated Abutment Changes on Peri-Implant Tissue Stability: 3-Year Post-Loading Results from a Multicentre Randomised Controlled Trial. Eur. J. Oral Implantol. 2017, 10, 373–390. [Google Scholar] [PubMed]
- Zipprich, H.; Weigl, P.; Ratka, C.; Lange, B.; Lauer, H.-C. The Micromechanical Behavior of Implant-Abutment Connections under a Dynamic Load Protocol. Clin. Implant Dent. Relat. Res. 2018, 20, 814–823. [Google Scholar] [CrossRef]
- Ericsson, I.; Nilner, K.; Klinge, B.; Glantz, P.O. Radiographical and Histological Characteristics of Submerged and Nonsubmerged Titanium Implants. An Experimental Study in the Labrador Dog. Clin. Oral Implants Res. 1996, 7, 20–26. [Google Scholar] [CrossRef]
- Brånemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindström, J.; Ohlsson, A. Intra-Osseous Anchorage of Dental Prostheses. I. Experimental Studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Buser, D.; Weber, H.P.; Donath, K.; Fiorellini, J.P.; Paquette, D.W.; Williams, R.C. Soft Tissue Reactions to Non-Submerged Unloaded Titanium Implants in Beagle Dogs. J. Periodontol. 1992, 63, 225–235. [Google Scholar] [CrossRef]
- Comut, A.A.; Weber, H.P.; Shortkroff, S.; Cui, F.Z.; Spector, M. Connective Tissue Orientation around Dental Implants in a Canine Model. Clin. Oral Implants Res. 2001, 12, 433–440. [Google Scholar] [CrossRef]
- Welander, M.; Abrahamsson, I.; Berglundh, T. The Mucosal Barrier at Implant Abutments of Different Materials. Clin. Oral Implants Res. 2008, 19, 635–641. [Google Scholar] [PubMed]
- Abrahamsson, I.; Berglundh, T.; Glantz, P.O.; Lindhe, J. The Mucosal Attachment at Different Abutments. An Experimental Study in Dogs. J. Clin. Periodontol. 1998, 25, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, I.; Zitzmann, N.U.; Berglundh, T.; Linder, E.; Wennerberg, A.; Lindhe, J. The Mucosal Attachment to Titanium Implants with Different Surface Characteristics: An Experimental Study in Dogs. J. Clin. Periodontol. 2002, 29, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Linkevicius, T.; Apse, P. Influence of Abutment Material on Stability of Peri-Implant Tissues: A Systematic Review. Int. J. Oral Maxillofac. Implants 2008, 23, 449–456. [Google Scholar]
- Glauser, R.; Schüpbach, P.; Gottlow, J.; Hämmerle, C.H.F. Periimplant Soft Tissue Barrier at Experimental One-Piece Mini-Implants with Different Surface Topography in Humans: A Light-Microscopic Overview and Histometric Analysis. Clin. Implant Dent. Relat. Res. 2005, 7, 44–51. [Google Scholar] [CrossRef]
- Koutouzis, T.; Wallet, S.; Calderon, N.; Lundgren, T. Bacterial Colonization of the Implant–Abutment Interface Using an in vitro Dynamic Loading Model. J. Periodontol. 2011, 82, 613–618. [Google Scholar] [CrossRef]
- Berglundh, T.; Lindhe, J. Dimension of the Periimplant Mucosa. Biological Width Revisited. J. Clin. Periodontol. 1996, 23, 971–973. [Google Scholar] [CrossRef]
- Brägger, U.; Häfeli, U.; Huber, B.; Hämmerle, C.H.; Lang, N.P. Evaluation of Postsurgical Crestal Bone Levels Adjacent to Non-Submerged Dental Implants. Clin. Oral Implants Res. 1998, 9, 218–224. [Google Scholar] [CrossRef]
- Canullo, L.; Bignozzi, I.; Cocchetto, R.; Cristalli, M.P.; Iannello, G. Immediate Positioning of a Definitive Abutment versus Repeated Abutment Replacements in Post-Extractive Implants: 3-Year Follow-up of a Randomised Multicentre Clinical Trial. Eur. J. Oral Implantol. 2010, 3, 285–296. [Google Scholar]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, 1–247. [Google Scholar] [CrossRef]
- Paolantonio, M.; Perinetti, G.; D’Ercole, S.; Graziani, F.; Catamo, G.; Sammartino, G.; Piccolomini, R. Internal Decontamination of Dental Implants: An in Vivo Randomized Microbiologic 6-Month Trial on the Effects of a Chlorhexidine Gel. J. Periodontol. 2008, 79, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Sinjari, B.; D’Addazio, G.; De Tullio, I.; Traini, T.; Caputi, S. Peri-Implant Bone Resorption during Healing Abutment Placement: The Effect of a 0.20% Chlorhexidine Gel vs. Placebo—A Randomized Double Blind Controlled Human Study. Biomed. Res. Int. 2018, 2018, 5326340. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Biltucci, M.T.; Kokaras, A.; Paster, B.J. Bacterial Composition at the Implant-Abutment Connection under Loading in Vivo. Clin. Implant Dent. Relat. Res. 2016, 18, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E.; Malmstrom, H.; Feng, C.; Ercoli, C.; Caton, J. Immediately loaded platform-switched implants in the anterior mandible with fixed prostheses: A randomized, split-mouth, masked prospective trial. Clin. Implant Dent. Relat. Res. 2014, 16, 884–892. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Lan, C.; Barbosa, J.; Lill, K.; Chen, R.; Rudney, J.; Aparicio, C. Antimicrobial Agents Used in the Treatment of Peri-Implantitis Alter the Physicochemistry and Cytocompatibility of Titanium Surfaces. J. Periodontol. 2016, 87, 809–819. [Google Scholar] [CrossRef]
- Hori, N.; Ueno, T.; Suzuki, T.; Yamada, M.; Att, W.; Okada, S.; Ohno, A.; Aita, H.; Kimoto, K.; Ogawa, T. Ultraviolet Light Treatment for the Restoration of Age-Related Degradation of Titanium Bioactivity. Int. J. Oral Maxillofac. Implants 2010, 25, 49–62. [Google Scholar]
- Iwasa, F.; Hori, N.; Ueno, T.; Minamikawa, H.; Yamada, M.; Ogawa, T. Enhancement of Osteoblast Adhesion to UV-Photofunctionalized Titanium via an Electrostatic Mechanism. Biomaterials 2010, 31, 2717–2727. [Google Scholar] [CrossRef]
- Park, K.H.; Koak, J.Y.; Kim, S.K.; Han, C.H.; Heo, S.J. The Effect of Ultraviolet-C Irradiation via a Bactericidal Ultraviolet Sterilizer on an Anodized Titanium Implant: A Study in Rabbits. Int. J. Oral Maxillofac. Implants 2013, 28, 57–66. [Google Scholar] [CrossRef][Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanchez-Perez, A.; Nicolas-Silvente, A.I.; Sanchez-Matas, C.; Cascales-Pina, E.; Macia-Manresa, V.; Romanos, G.E. Control of Peri-Implant Mucous Inflammation by Using Chlorhexidine or Ultraviolet C Radiation for Cleaning Healing Abutments. Double-Blind Randomized Clinical Trial. Materials 2020, 13, 1124. https://doi.org/10.3390/ma13051124
Sanchez-Perez A, Nicolas-Silvente AI, Sanchez-Matas C, Cascales-Pina E, Macia-Manresa V, Romanos GE. Control of Peri-Implant Mucous Inflammation by Using Chlorhexidine or Ultraviolet C Radiation for Cleaning Healing Abutments. Double-Blind Randomized Clinical Trial. Materials. 2020; 13(5):1124. https://doi.org/10.3390/ma13051124
Chicago/Turabian StyleSanchez-Perez, Arturo, Ana I. Nicolas-Silvente, Carmen Sanchez-Matas, Elena Cascales-Pina, Vanesa Macia-Manresa, and Georgios E. Romanos. 2020. "Control of Peri-Implant Mucous Inflammation by Using Chlorhexidine or Ultraviolet C Radiation for Cleaning Healing Abutments. Double-Blind Randomized Clinical Trial" Materials 13, no. 5: 1124. https://doi.org/10.3390/ma13051124
APA StyleSanchez-Perez, A., Nicolas-Silvente, A. I., Sanchez-Matas, C., Cascales-Pina, E., Macia-Manresa, V., & Romanos, G. E. (2020). Control of Peri-Implant Mucous Inflammation by Using Chlorhexidine or Ultraviolet C Radiation for Cleaning Healing Abutments. Double-Blind Randomized Clinical Trial. Materials, 13(5), 1124. https://doi.org/10.3390/ma13051124





