A Tactile Device Generating Repulsive Forces of Various Human Tissues Fabricated from Magnetic-Responsive Fluid in Porous Polyurethane
Abstract
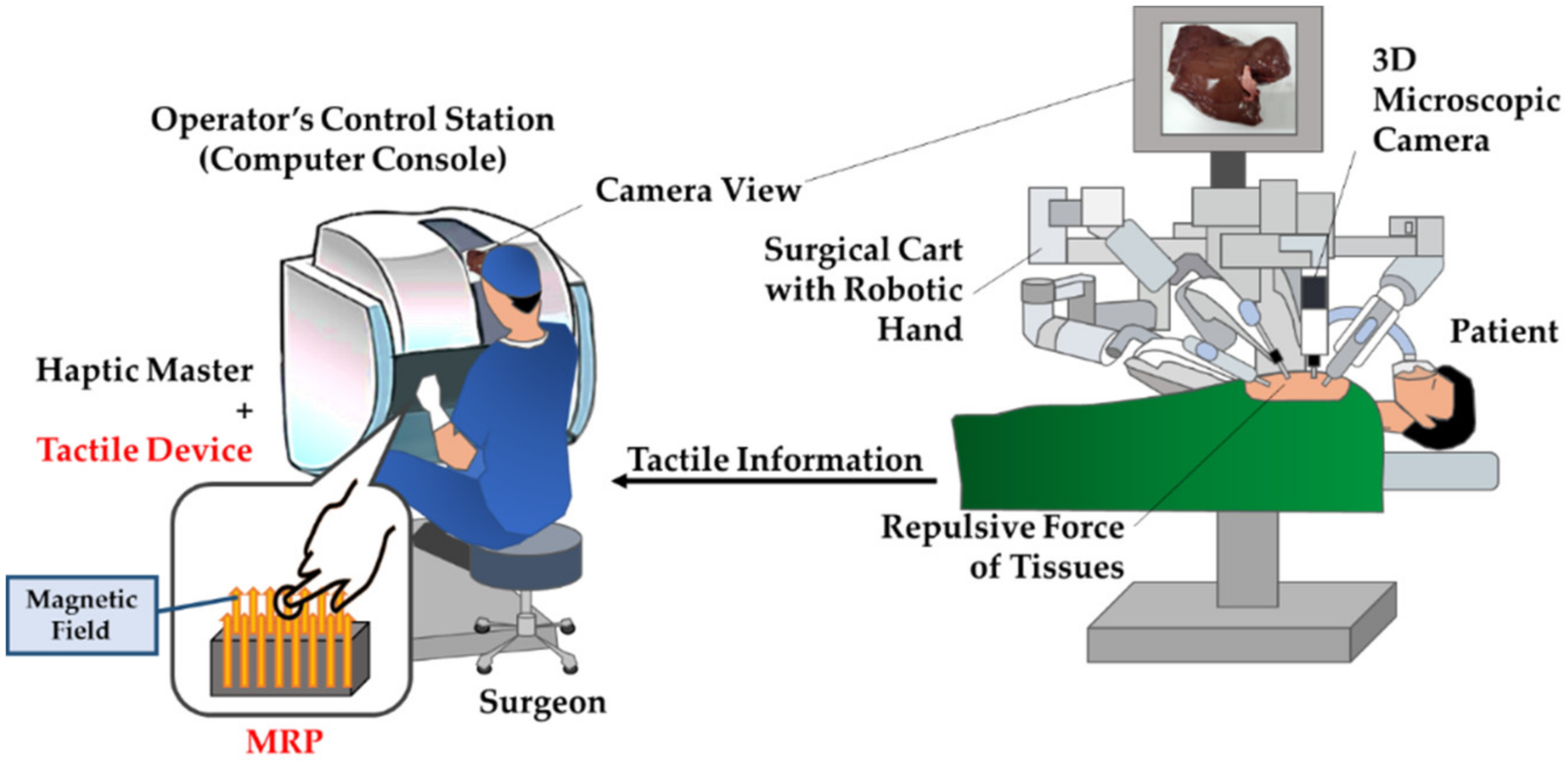
1. Introduction
2. Fabrication of MRP Samples
2.1. Operating Principle
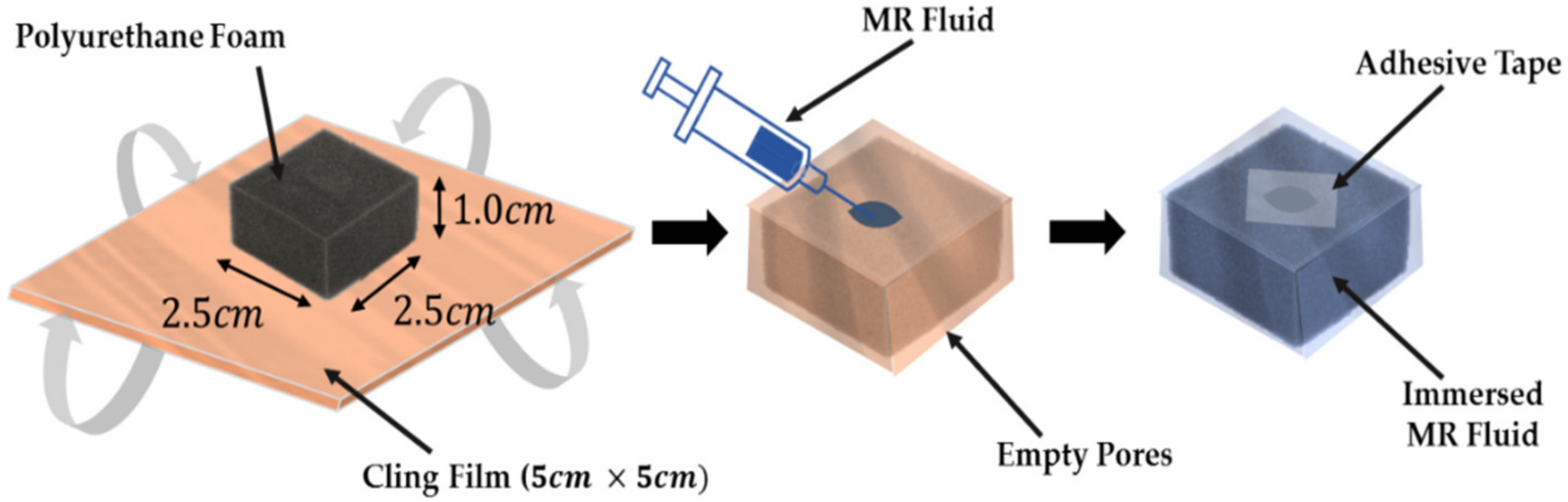
2.2. Fabrication Procedures
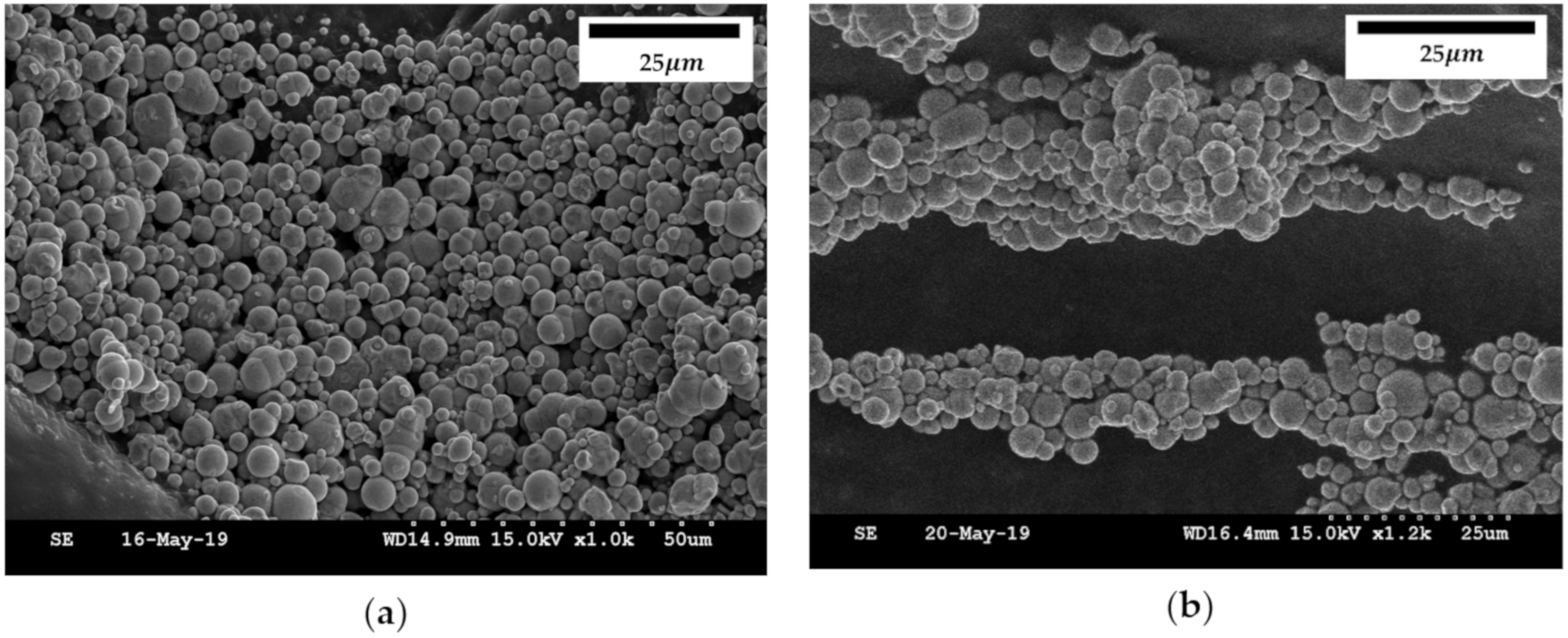
2.3. Microscopic Observation
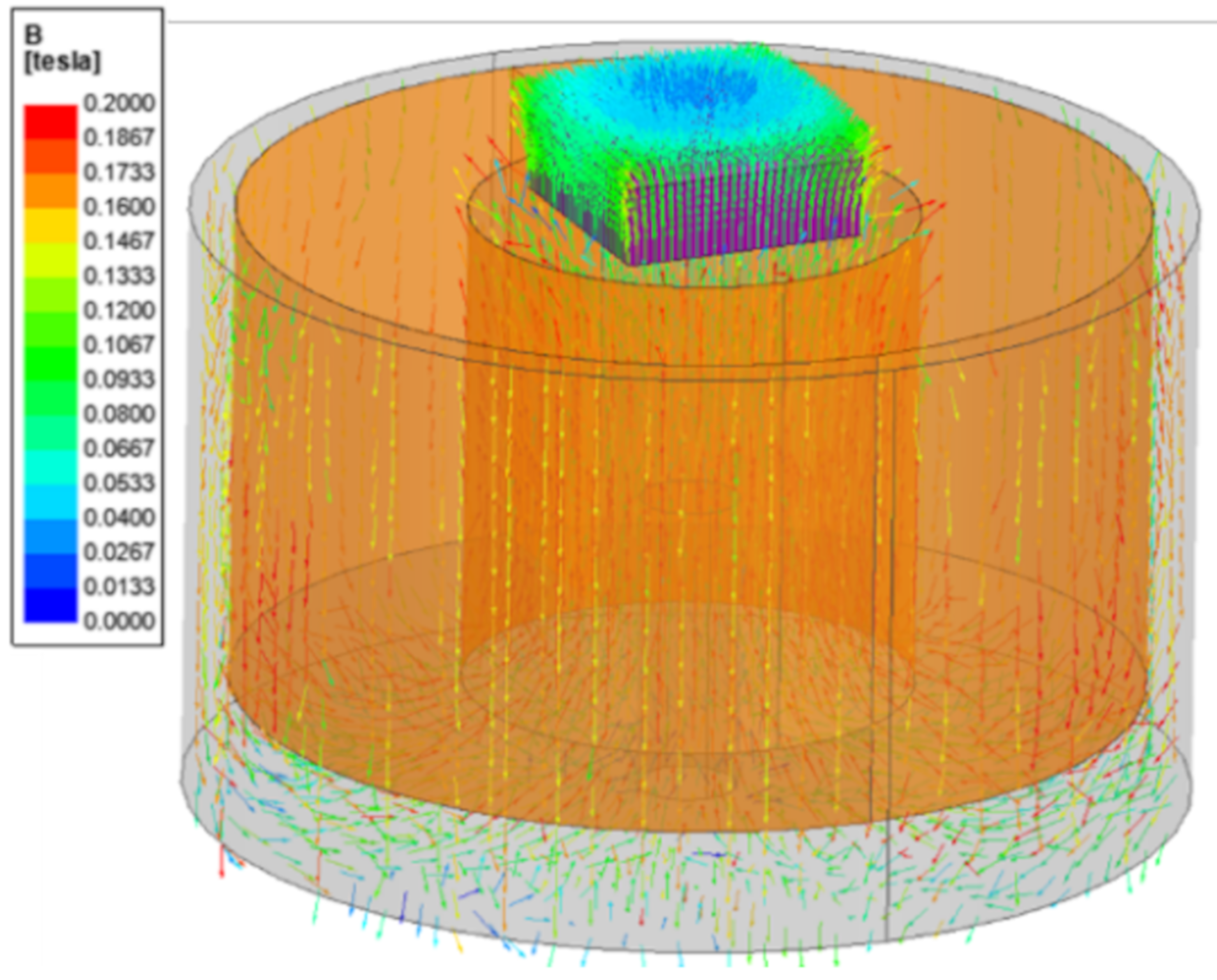
2.4. Magnetic Analysis
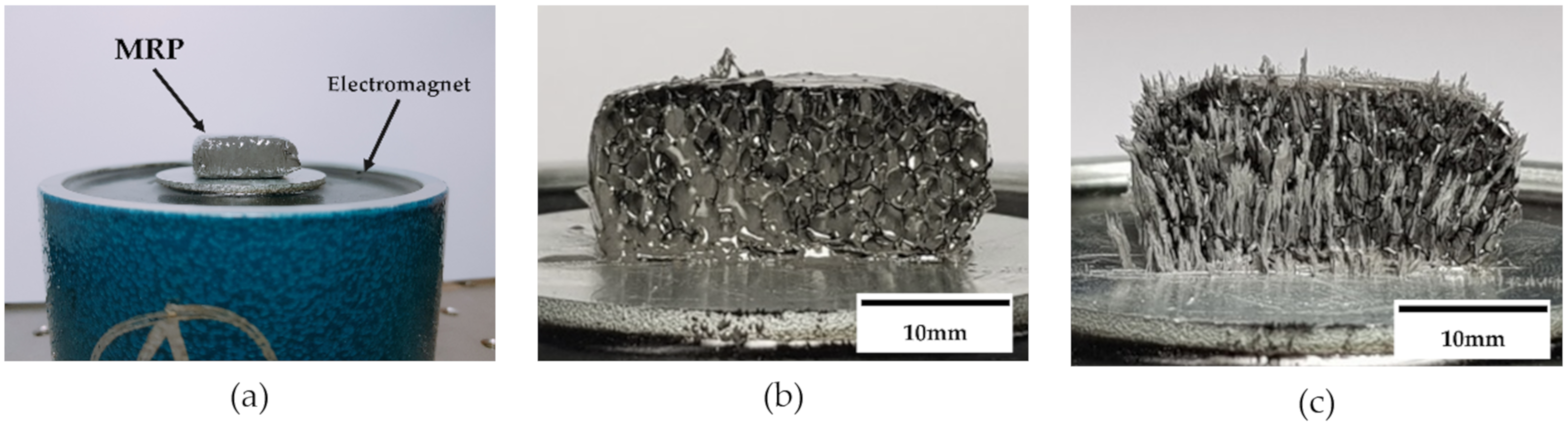
2.5. Fabricated Samples
3. Repulsive Force Measurements
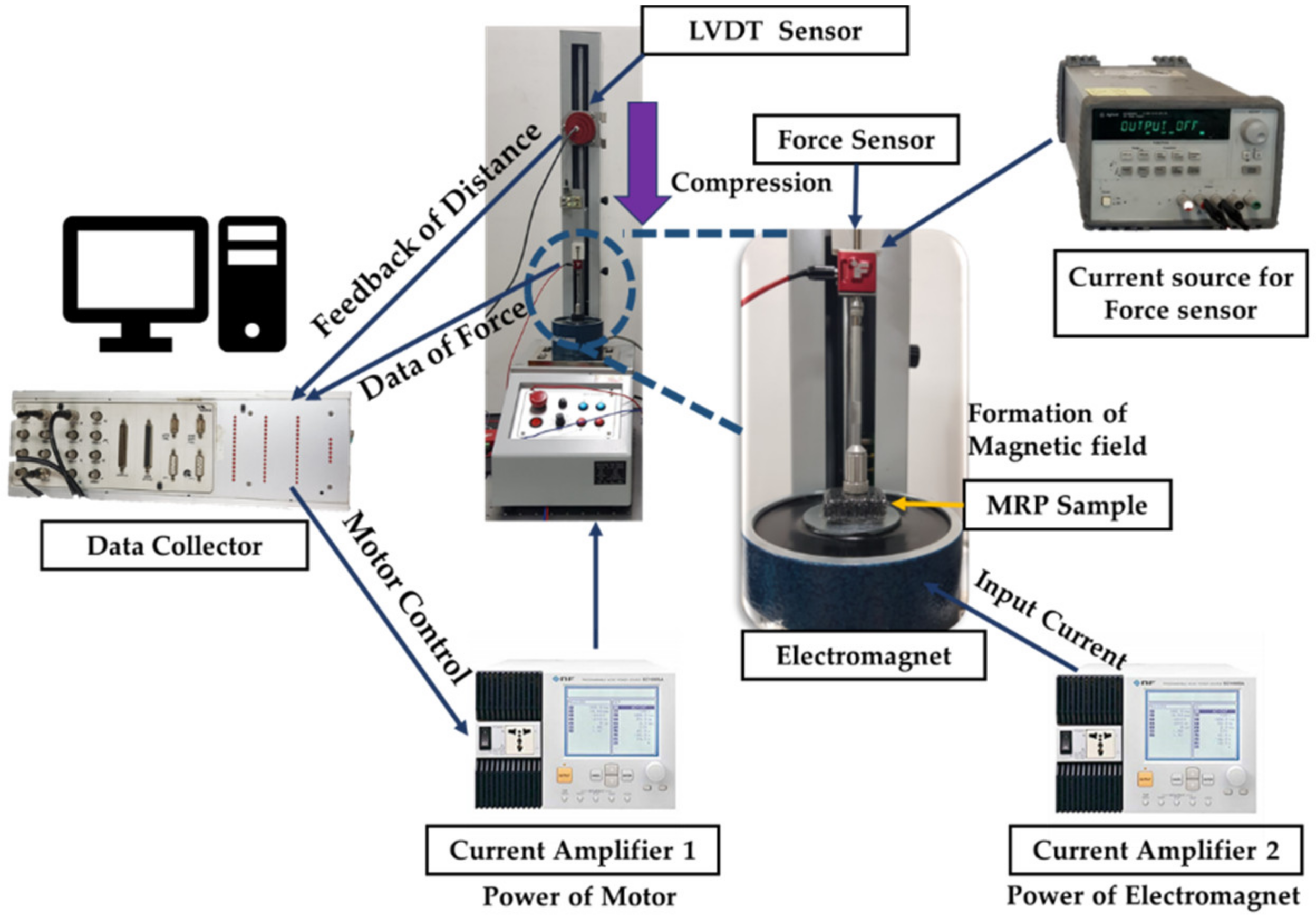
3.1. Experimental Apparatus
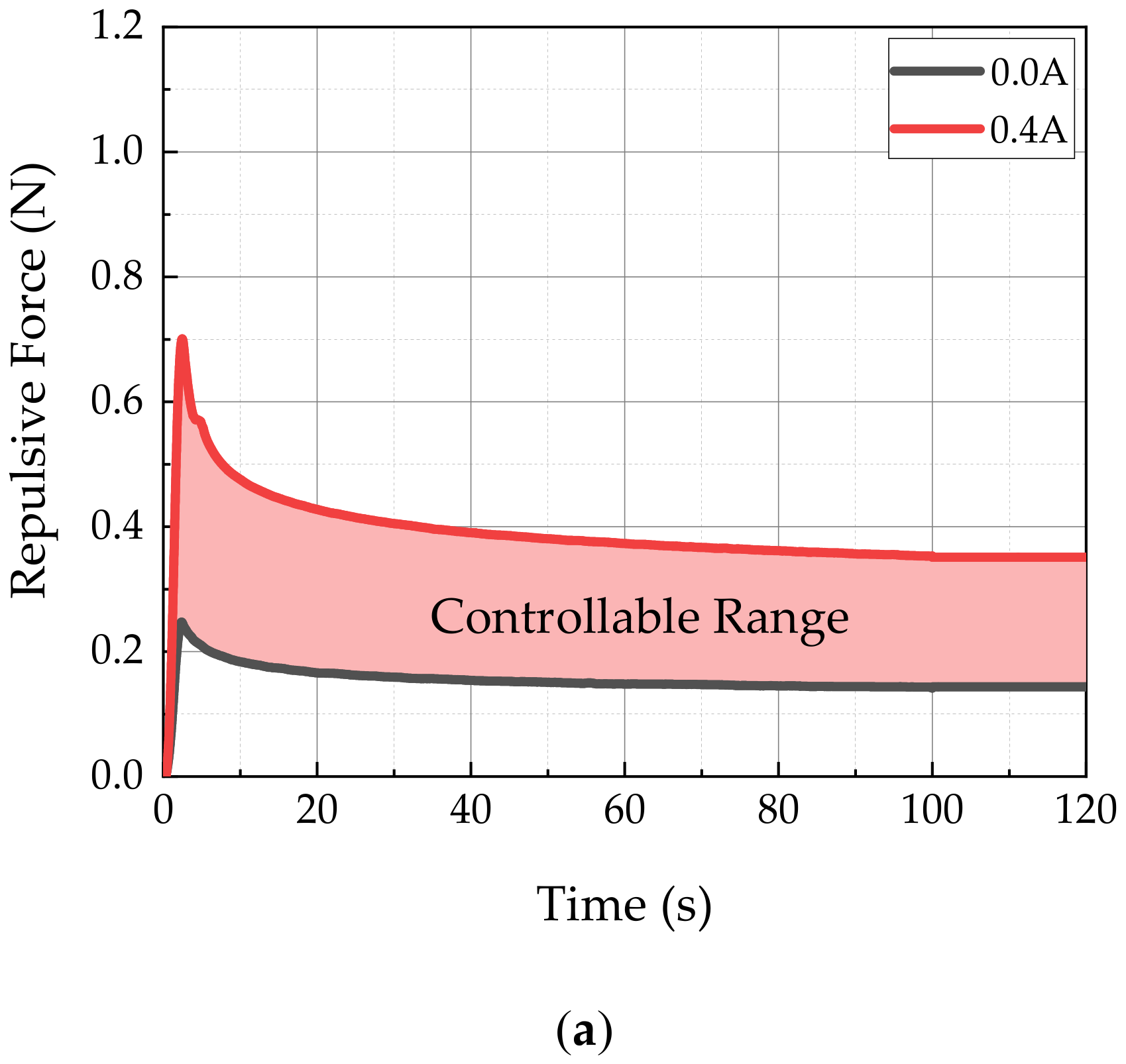
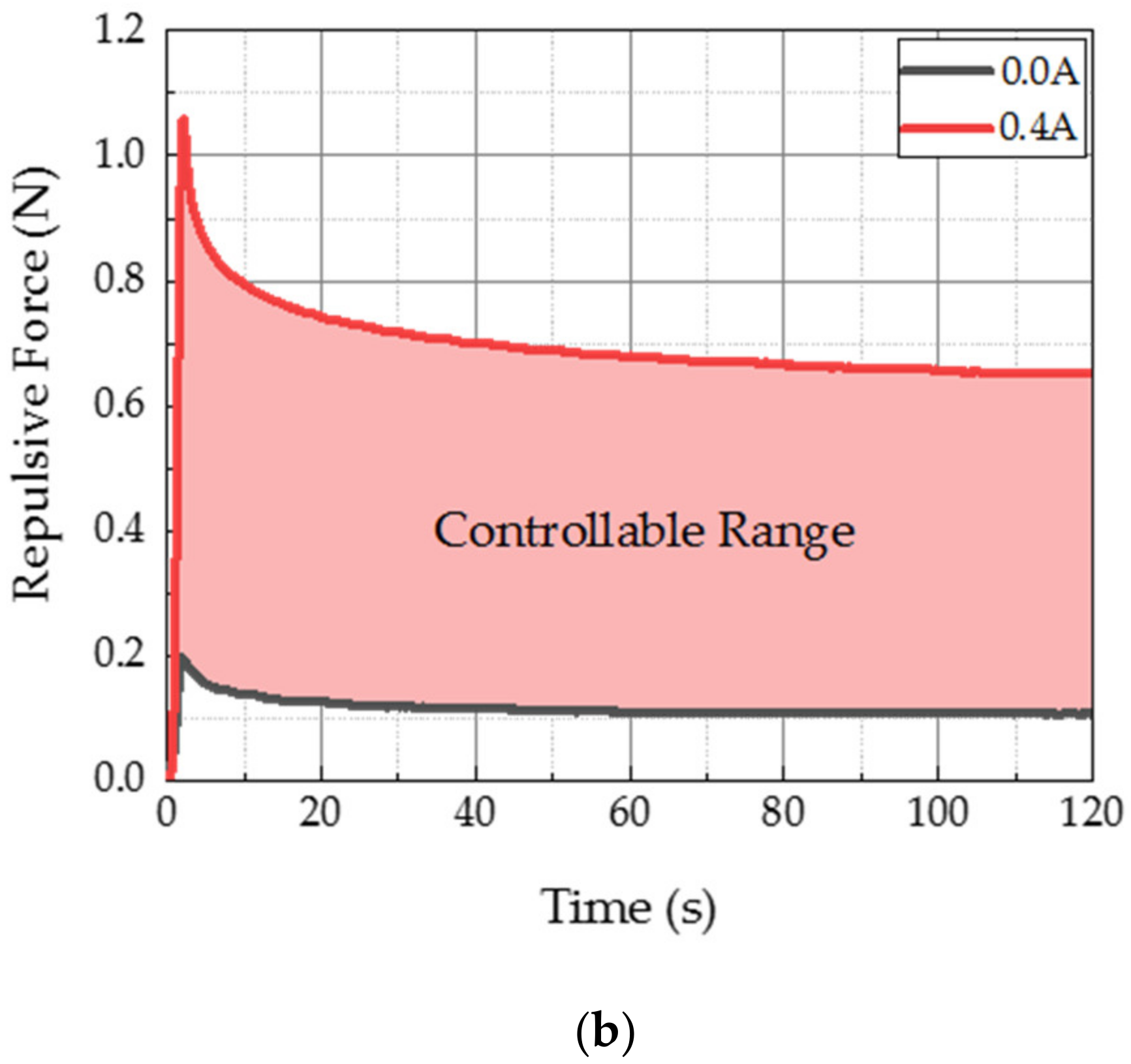
3.2. Results and Discussion
3.3. Validation of Practical Use
3.4. Psychophysical Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mack, M.J. Minimally invasive and robotic surgery. JAMA 2001, 285, 568–572. [Google Scholar] [CrossRef]
- Kim, W.W.; Kim, J.S.; Hur, S.M.; Kim, S.H.; Lee, S.-K.; Choi, J.H.; Kim, S.M.; Lee, J.E.; Kim, J.-H.; Nam, S.J.; et al. Is robotic surgery superior to endoscopic and open surgeries in thyroid cancer? World J. Surg. 2011, 35, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Park, A.; Birch, D.W.; Lovrics, P. Laparoscopic and open incisional hernia repair: A comparison study. Surgery 1998, 124, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.; Chae, B.J.; Park, H.S.; Kim, K.H.; Kim, S.H.; Song, B.J.; Jung, S.S.; Bae, J.S. Comparison of surgical outcomes between endoscopic and robotic thyroidectomy. J Surg. Oncol. 2012, 105, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S. Early experience with single incision laparoscopic surgery: Eliminating the scar from abdominal operations. J. Pediatr. Surg. 2009, 44, 1741–1745. [Google Scholar] [CrossRef]
- Dogangil, G.; Davies, B.L.; Rodriguez y Baena, F. A review of medical robotics for minimally invasive soft tissue surgery. Proc. Inst. Mech. Eng. H 2010, 224, 653–679. [Google Scholar] [CrossRef]
- Lederman, S.J.; Klatzky, R.L. Sensing and displaying spatially distributed fingertip forces in haptic interfaces for teleoperator and virtual environment systems. Presence 1999, 8, 86–103. [Google Scholar] [CrossRef]
- Brown, S.M.; Tabaee, A.; Singh, A.; Schwartz, T.H.; Anand, V.K. Three-dimensional endoscopic sinus surgery: Feasibility and technical aspects. Otolaryngol. Head Neck Surg. 2008, 138, 400–402. [Google Scholar] [CrossRef]
- Alaraimi, B.; El Bakbak, W.; Makkiyah, S.; Al-Marzoug, A.; Goriparthi, R.; Bouhelal, A.; Quan, V.; Patel, B. A randomized prospective study comparing acquisition of laparoscopic skills in three-dimensional (3D) vs. two-dimensional (2D) laparoscopy. World J. Surg. 2014, 38, 2746–2752. [Google Scholar] [CrossRef]
- Chan, A.C.W.; Chung, S.C.S.; Yim, A.P.C.; Lau, J.Y.W.; Ng, E.K.W.; Li, A.K.C. Comparison of two-dimensional vs three-dimensional camera systems in laparoscopic surgery. Surg. Endosc. 1997, 11, 438–440. [Google Scholar] [CrossRef]
- Currò, G.; La Malfa, G.; Caizzone, A.; Rampulla, V.; Navarra, G. Three-dimensional (3D) versus two-dimensional (2D) laparoscopic bariatric surgery: A single-surgeon prospective randomized comparative study. Obes. Surg. 2015, 25, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Okamura, A.M.; Simone, V.; O’Leary, M.D. Force modeling for needle insertion into soft tissue. IEEE Trans. Biomed. Eng. 2004, 51, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Rosen, J.; Kim, Y.S.; Chang, L.; Sinanan, M.N.; Hannaford, B. In-vivo and in-situ compressive properties of porcine abdominal soft tissues. Stud. Health Technol. Inform. 2003, 94, 26–32. [Google Scholar] [PubMed]
- Westwood, J.D. Quantifying surgeon grasping mechanics in laparoscopy using the blue DRAGON system. In Medicine Meets Virtual Reality 12: Building a Better You: The Next Tools for Medical Education, Diagnosis, and Care; IOS Press: Amsterdam, The Netherlands, 2004; Volume 98, p. 34. [Google Scholar]
- Sarver, J.J.; Robinson, P.S.; Elliott, D.M. Methods for quasi-linear viscoelastic modeling of soft tissue: Application to incremental stress-relaxation experiments. J. Biomech. Eng. 2003, 125, 754–758. [Google Scholar] [CrossRef]
- Palevski, A.; Glaich, I.; Portnoy, S.; Linder-Ganz, E.; Gefen, A. Stress relaxation of porcine gluteus muscle subjected to sudden transverse deformation as related to pressure sore modeling. J. Biomech. Eng. 2006, 128, 782–787. [Google Scholar] [CrossRef]
- Julkunen, P.; Wilson, W.; Jurvelin, J.S.; Rieppo, J.; Qu, C.-J.; Lammi, M.J.; Korhonen, R.K. Stress–relaxation of human patellar articular cartilage in unconfined compression: Prediction of mechanical response by tissue composition and structure. J. Biomech. 2008, 41, 1978–1986. [Google Scholar] [CrossRef]
- Duenwald, S.E.; Vanderby, R.; Lakes, R.S. Stress relaxation and recovery in tendon and ligament: Experiment and modeling. Biorheology 2010, 47, 1–14. [Google Scholar] [CrossRef]
- Alipour-Haghighi, F.; Titze, I.R. Viscoelastic modeling of canine vocalis muscle in relaxation. J. Acoust. Soc. Am. 1985, 78, 1939–1943. [Google Scholar] [CrossRef]
- King, C.-H.; Culjat, M.O.; Franco, M.L.; Bisley, J.W.; Carman, G.P.; Dutson, E.P.; Grundfest, W.S. A Tactile Feedback System for Robotic Surgery. In Proceedings of the 2008 30th Annual International IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 1930–1934. [Google Scholar]
- Culjat, M.; King, C.-H.; Franco, M.; Bisley, J.; Grundfest, W.; Dutson, E. Pneumatic balloon actuators for tactile feedback in robotic surgery. Ind. Robot 2008, 35, 449–455. [Google Scholar] [CrossRef]
- Benali-Khoudja, M.; Hafez, M.; Alexandre, J.-M.; Kheddar, A. Tactile Interfaces: A State-of-the-Art Survey. In Proceedings of the 35th International Symposium on Robotics, Paris, France, 23–26 March 2004; pp. 23–26. [Google Scholar]
- Hasser, C.J. Preliminary Evaluation of a Shape-Memory Alloy Tactile Feed-Back Display; ASME: New York, NY, USA, 1993; pp. 73–80. [Google Scholar]
- Kontarinis, D.A.; Howe, R.D. Tactile display of vibratory information in teleoperation and virtual environments. Presence 1995, 4, 387–402. [Google Scholar] [CrossRef]
- Alex, M.; Koo, J.-H.; Yang, T.-H. A compact and compliant electrorheological actuator for generating a wide range of haptic sensations. Smart Mater. Struct. 2020. [Google Scholar] [CrossRef]
- Han, Y.M.; Oh, J.-S.; Kim, J.-K.; Choi, S.-B. Design and experimental evaluation of a tactile display featuring magnetorheological fluids. Smart Mater. Struct. 2014, 23, 077001. [Google Scholar] [CrossRef]
- Kciuk, M.; Turczyn, R. Properties and application of magnetorheological fluids. J. Achiev. Mater. Manuf. Eng. 2006, 18, 127–130. [Google Scholar]
- Handorf, A.M.; Zhou, Y.; Halanski, M.A.; Lee, W.-J. Tissue stiffness dictates development, homeostasis, and disease progression. Organogenesis 2015, 11, 1–15. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Liang, X.; Boppart, S.A. Biomechanical properties of in vivo human skin from dynamic optical coherence elastography. IEEE Trans. Biomed. Eng. 2009, 57, 953–959. [Google Scholar] [CrossRef]


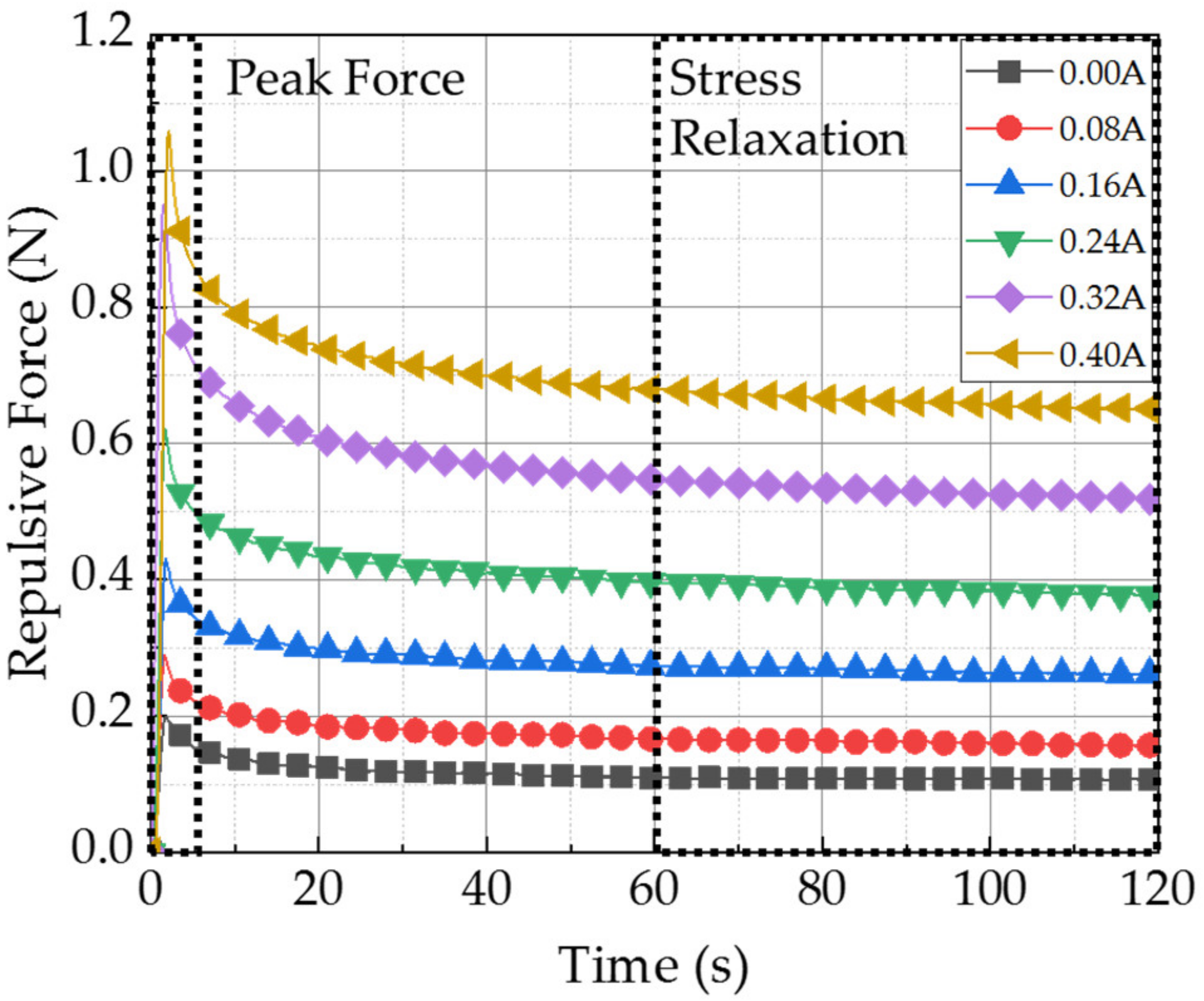
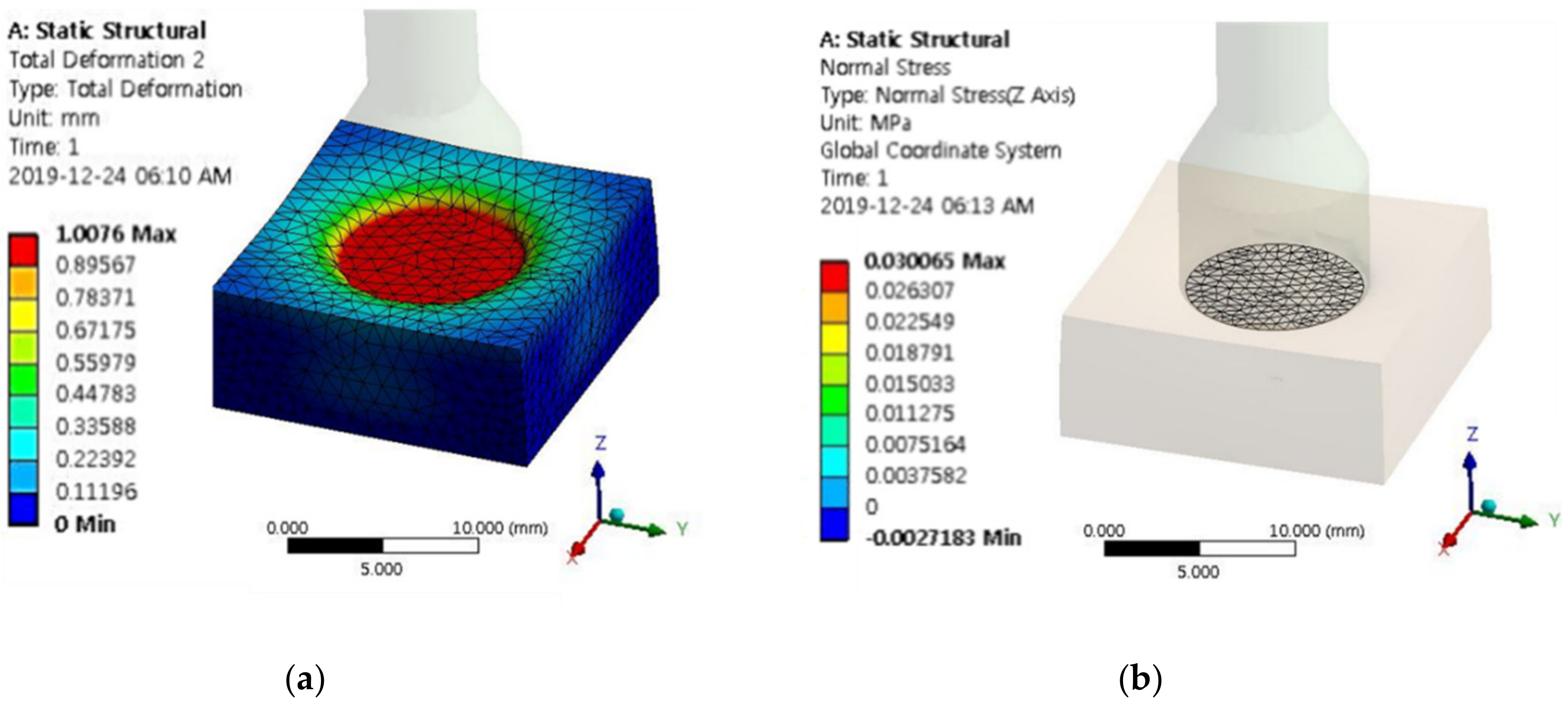

| Input Current | MRP2 | Input Current | MRP2 | ||
|---|---|---|---|---|---|
| Maximum Peak Force | 0.00 A | 0.20 N | Stress Relaxation | 0.00 A | 0.11 N |
| 0.08 A | 0.29 N | 0.08 A | 0.16 N | ||
| 0.16 A | 0.43 N | 0.16 A | 0.26 N | ||
| 0.24 A | 0.62 N | 0.24 A | 0.38 N | ||
| 0.32 A | 0.95 N | 0.32 A | 0.52 N | ||
| 0.40 A | 1.06 N | 0.40 A | 0.65 N |
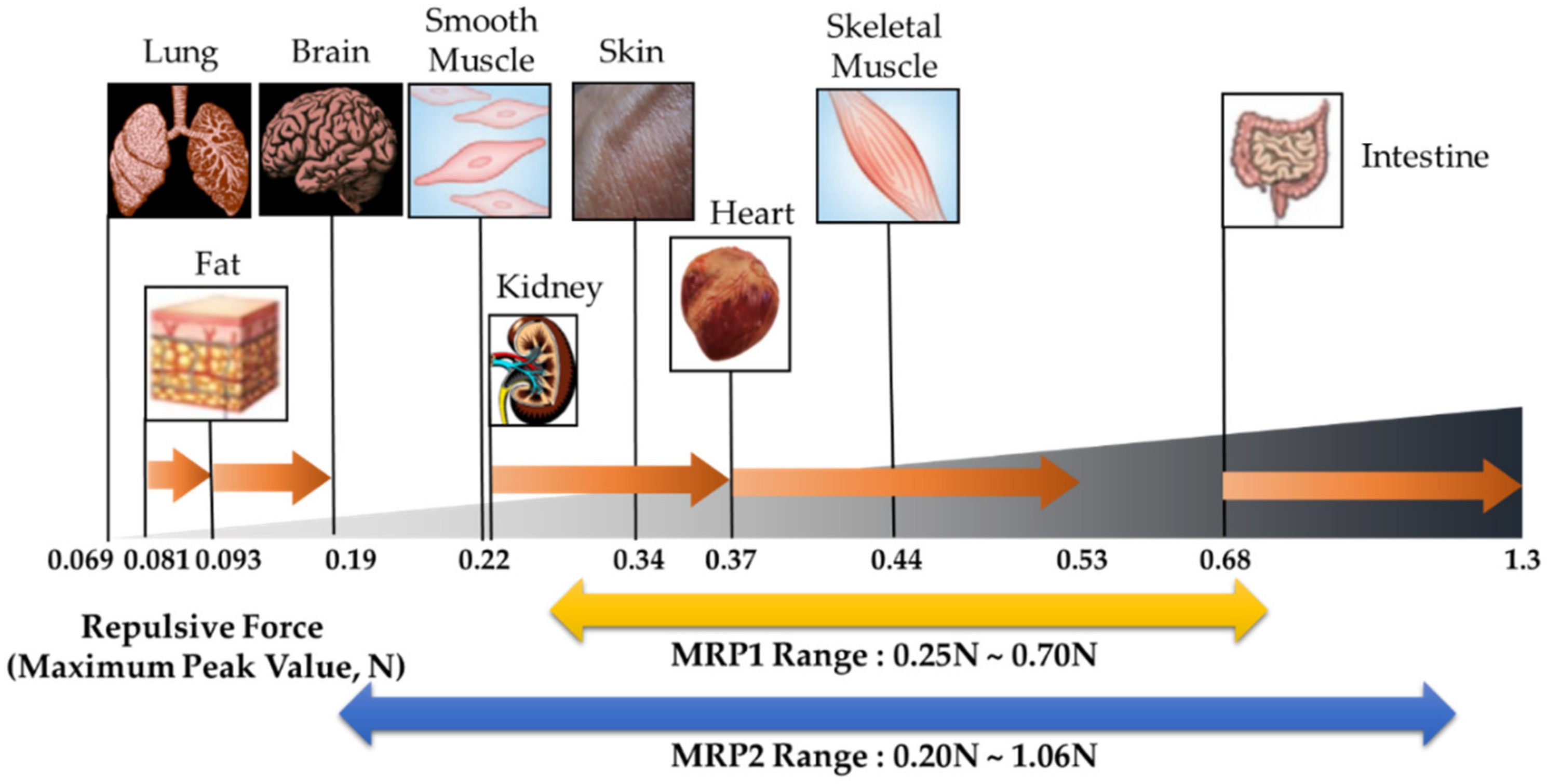
| Young’s Modulus of Human Tissues (Pa) | Calculated Maximum Repulsive Force (N) | Magnetic Flux Density Applied to MRP2 (T) | |
|---|---|---|---|
| Lung | 200 | 0.069 | Not defined |
| Fat | 500–1000 | 0.081–0.093 | Not defined |
| Brain | 1000–4000 | 0.097–0.19 | Not defined |
| Smooth Muscle | 5000 | 0.22 | 0.0089 |
| Kidney | 5000–10,000 | 0.22–0.37 | 0.0089–0.063 |
| Skin | 9000 | 0.34 | 0.054 |
| Skeleton Muscle | 12,000 | 0.44 | 0.082 |
| Heart | 10,000–15,000 | 0.37–0.53 | 0.063–0.10 |
| Intestine | 20,000–40,000 | 0.68–1.3 | 0.13–0.2 (1.07N–1.3N is not defined) |
| Question | No. of Volunteers | Mean | Standard Deviation |
|---|---|---|---|
| Q.1 Can you recognize the difference between repulsive forces? | 20 | 4.25 | 0.85 |
| Q.2. Do you agree that the repulsive force of each organ can be realized using the proposed sample? | 20 | 4.10 | 0.91 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, Y.-J.; Yoon, J.-Y.; Kang, B.-H.; Kim, G.-W.; Choi, S.-B. A Tactile Device Generating Repulsive Forces of Various Human Tissues Fabricated from Magnetic-Responsive Fluid in Porous Polyurethane. Materials 2020, 13, 1062. https://doi.org/10.3390/ma13051062
Park Y-J, Yoon J-Y, Kang B-H, Kim G-W, Choi S-B. A Tactile Device Generating Repulsive Forces of Various Human Tissues Fabricated from Magnetic-Responsive Fluid in Porous Polyurethane. Materials. 2020; 13(5):1062. https://doi.org/10.3390/ma13051062
Chicago/Turabian StylePark, Yu-Jin, Ji-Young Yoon, Byung-Hyuk Kang, Gi-Woo Kim, and Seung-Bok Choi. 2020. "A Tactile Device Generating Repulsive Forces of Various Human Tissues Fabricated from Magnetic-Responsive Fluid in Porous Polyurethane" Materials 13, no. 5: 1062. https://doi.org/10.3390/ma13051062
APA StylePark, Y.-J., Yoon, J.-Y., Kang, B.-H., Kim, G.-W., & Choi, S.-B. (2020). A Tactile Device Generating Repulsive Forces of Various Human Tissues Fabricated from Magnetic-Responsive Fluid in Porous Polyurethane. Materials, 13(5), 1062. https://doi.org/10.3390/ma13051062






