Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Fly Ashes
2.1.1. Density
- Vm—volume of pulverized sample, dm3
- mm—mass of pulverized sample, g
- Vch—volume of sealed chamber, dm3
- Pch—the pressure within the sample chamber, Pa
- Vr—reference chamber of known volume, dm3
- Pr—charged pressure, Pa
- Psys—system pressure, Pa
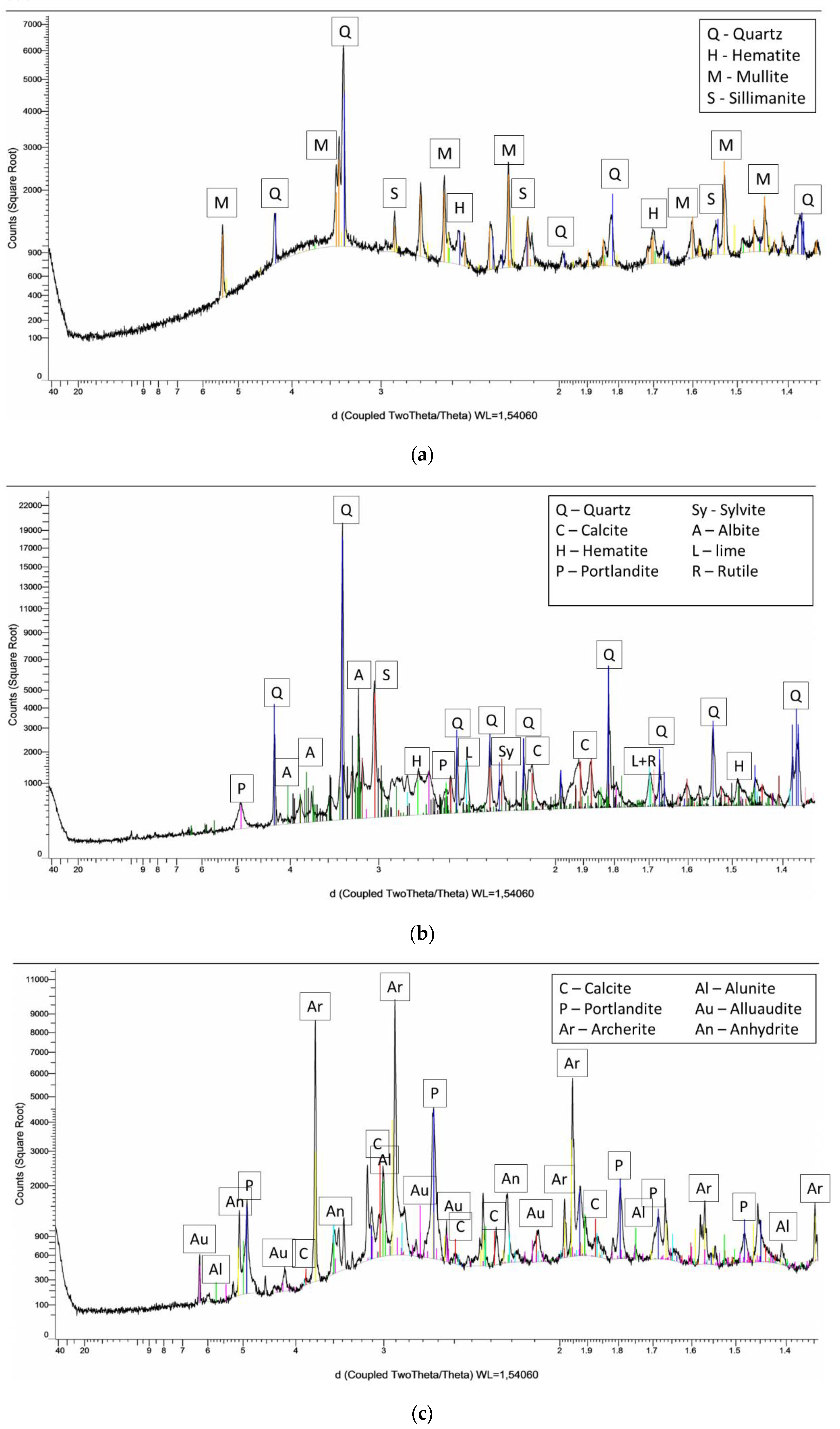
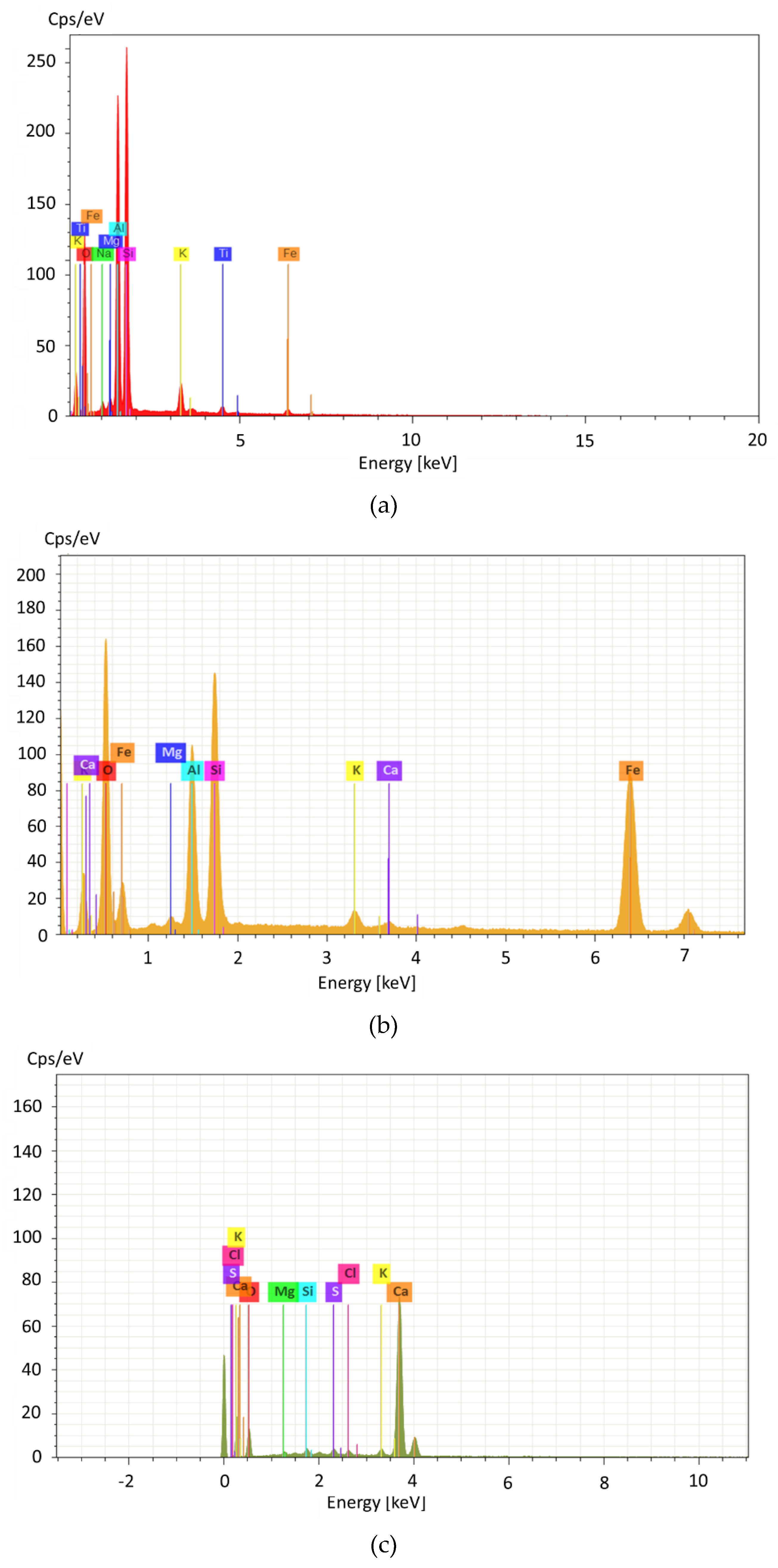
2.1.2. XRF and XRD
2.1.3. Loss on Ignition (LOI)
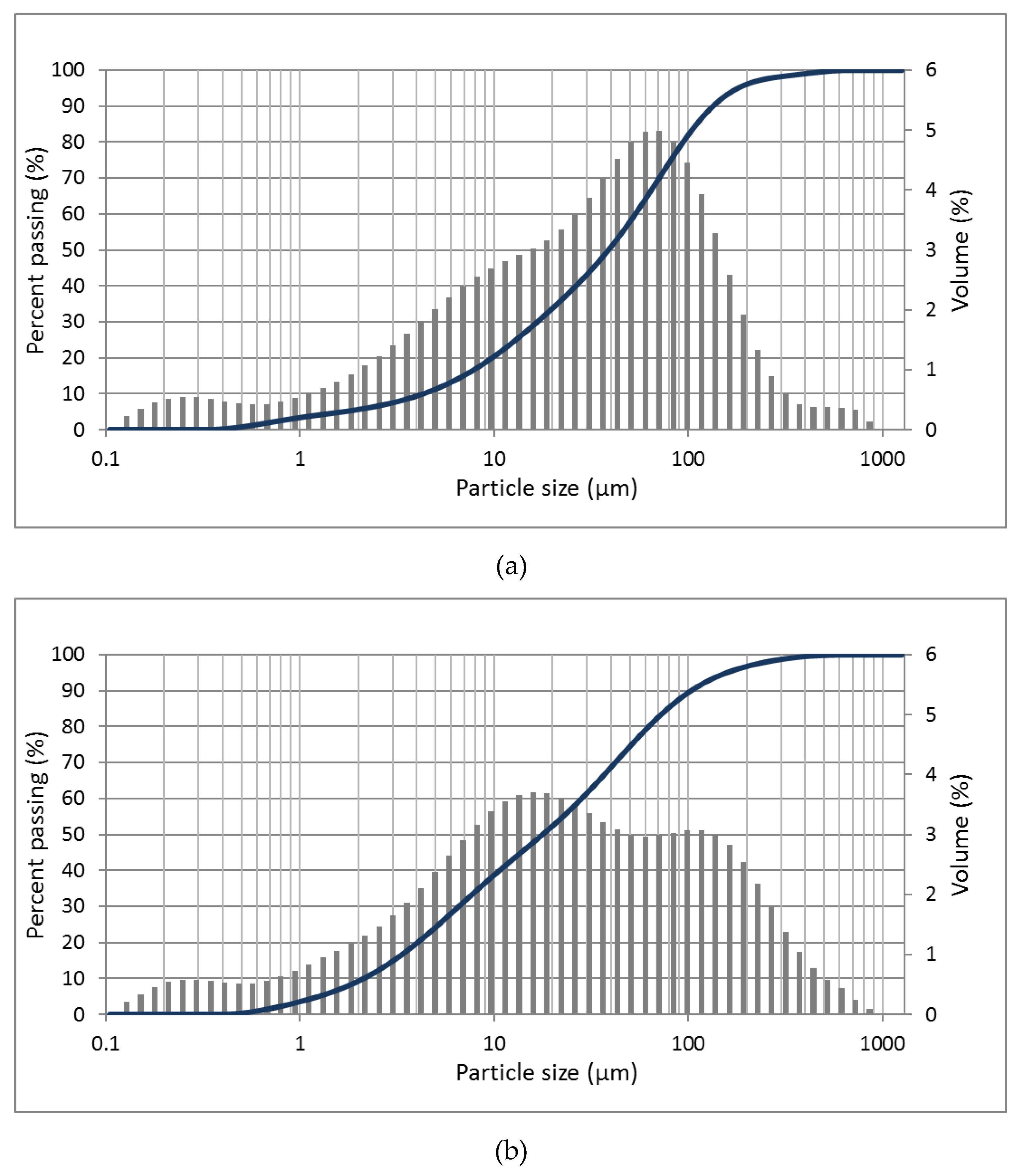
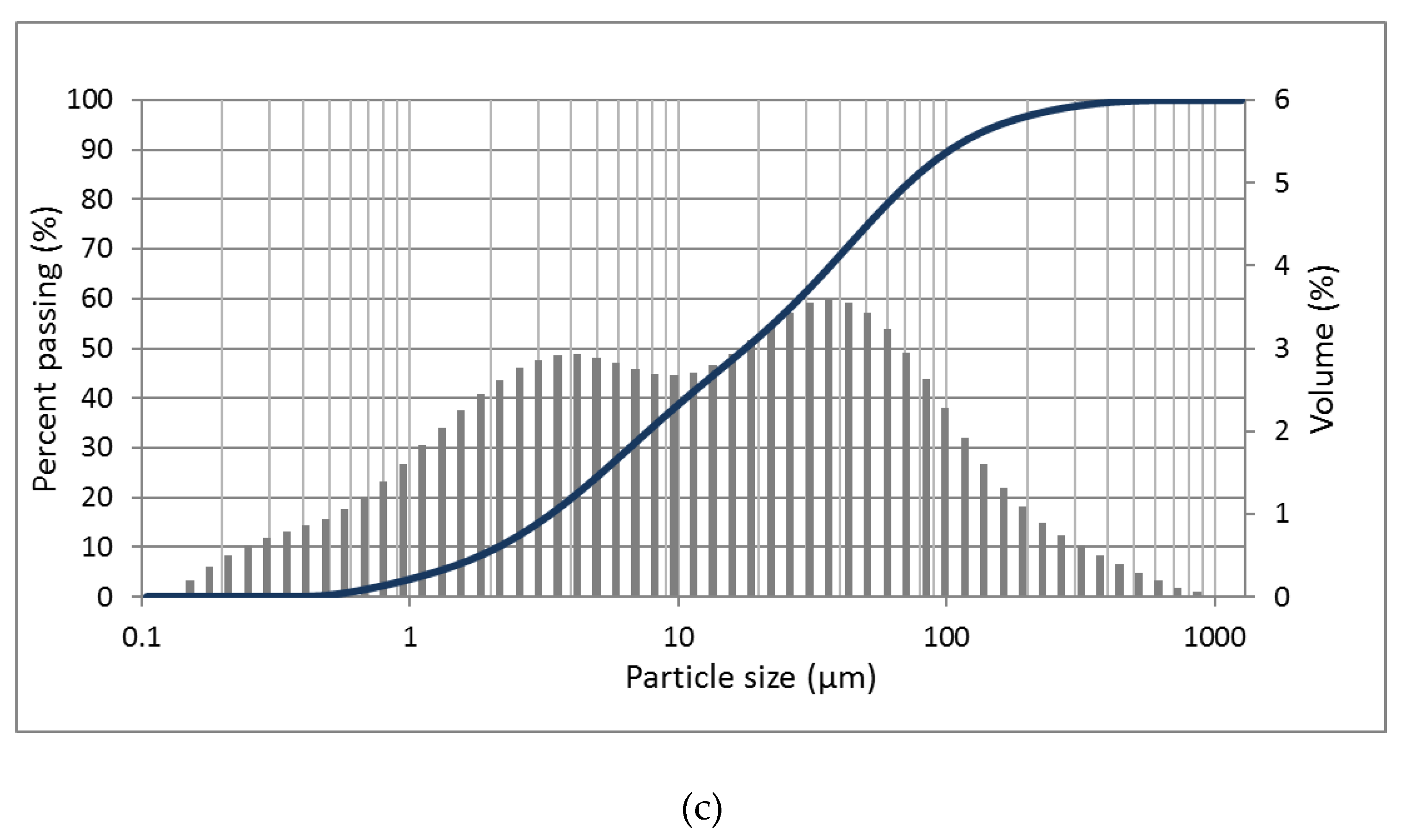
2.1.4. Particle Size Distributions
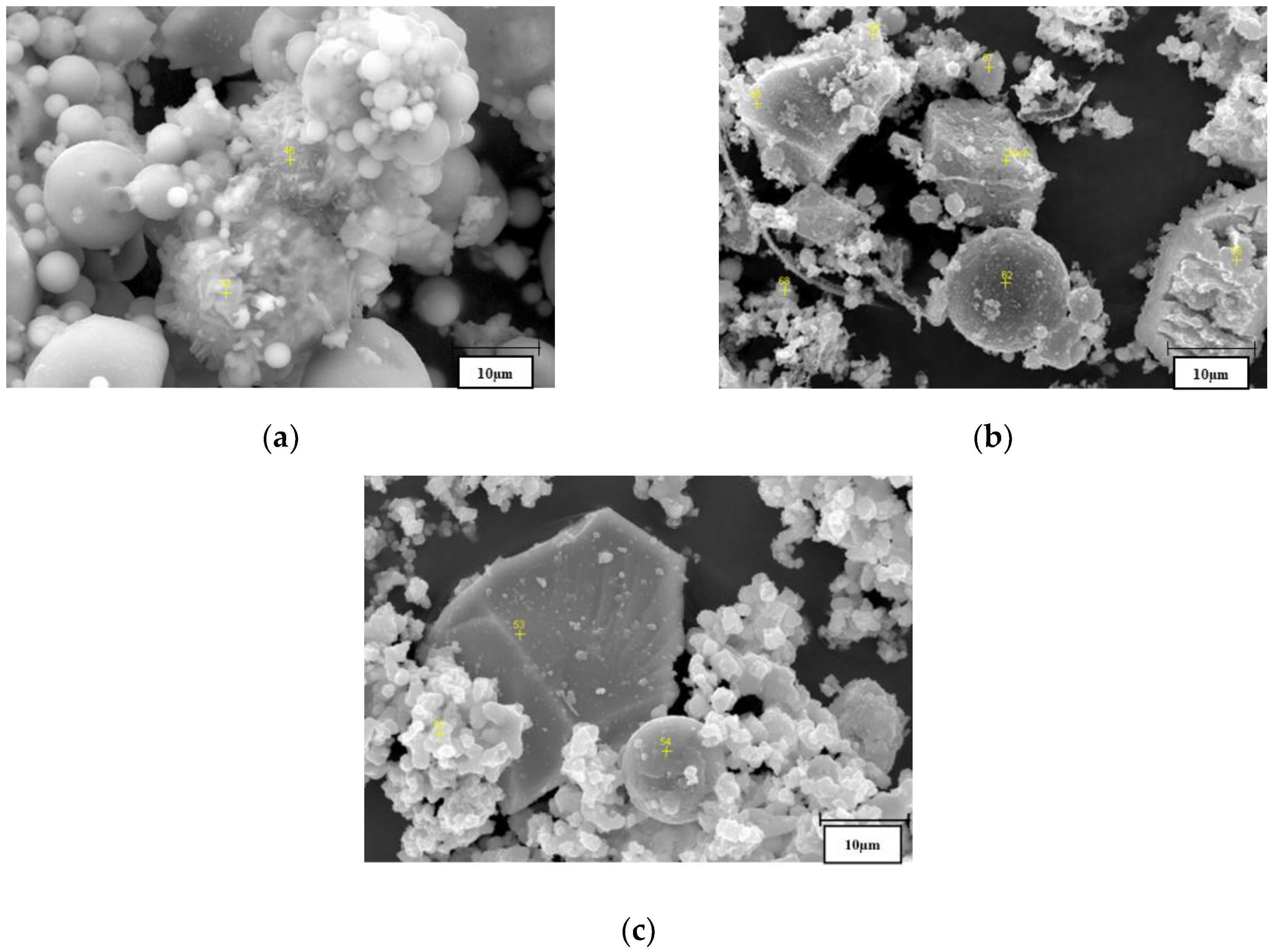
2.1.5. SEM Image Analysis
2.2. Alkali Activated Mortars
- −
- Aluminosilicate precursors: fly ashes;
- −
- Alkaline activators: sodium hydroxide, sodium silicate, and quicklime;
- −
- Fine aggregate: sand.
- −
- Adding aluminosilicate precursor to the mixer pan and activating the mixer at low RPM (rotational movement - 140 ± 5 RPM-1).
- −
- Pouring the sand in at an even rate for the first 30 s of mixing.
- −
- Adding alkaline activator solution at an even rate for the next 30 s of mixing (the “zero time” for setting time measurement).
- −
- Switching the mixer to high RPM (rotational movement 285 ± 10 RPM-1) and continuing mixing for 30 s more.
- −
- Stopping the mixer after a total of 90 s and following the EN 196-1 standard procedure for preparation of mortars.
2.2.1. Compressive and Flexural Strengths
2.2.2. TGA
2.2.3. Calorimetry
3. Results and Discussion
3.1. Fly Ashes
3.1.1. The Chemical Compositions, Mineral Phase Characterizations, and Physical Characteristics
3.1.2. Particle Size Distributions
3.1.3. SEM Analysis
3.2. Alkali Activated Mortars
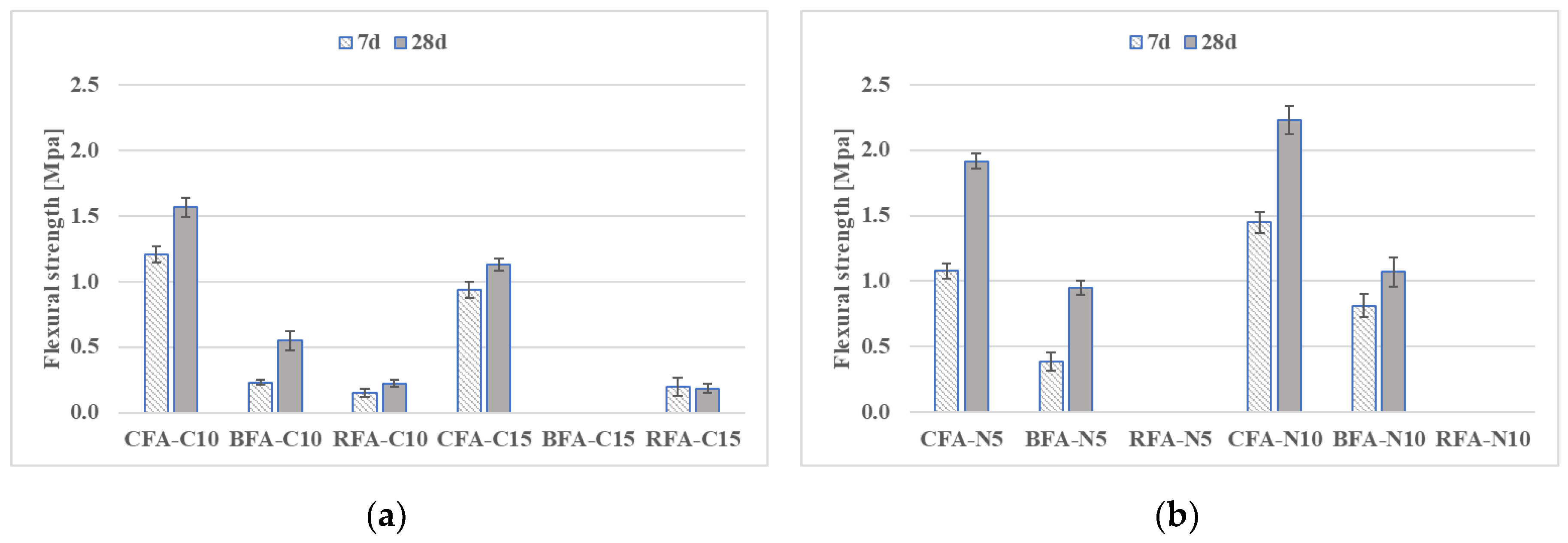
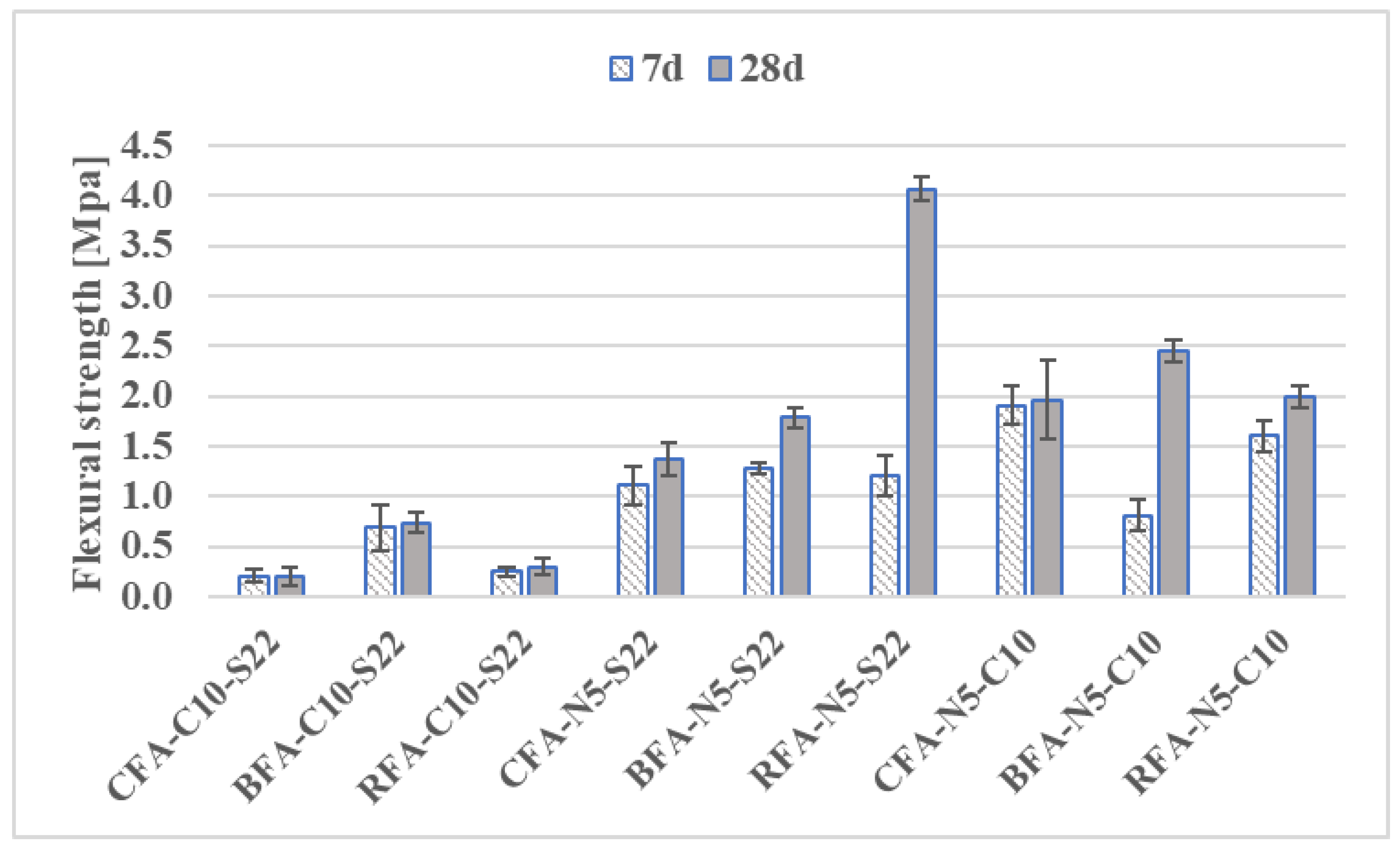
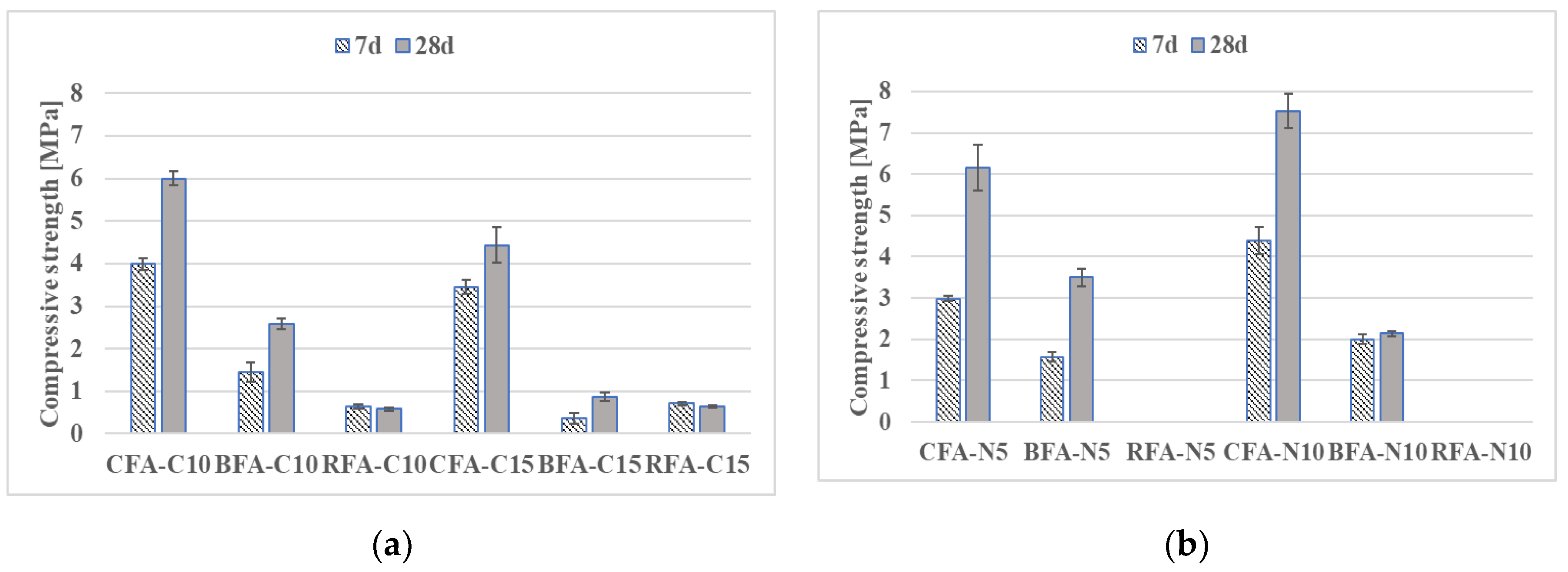
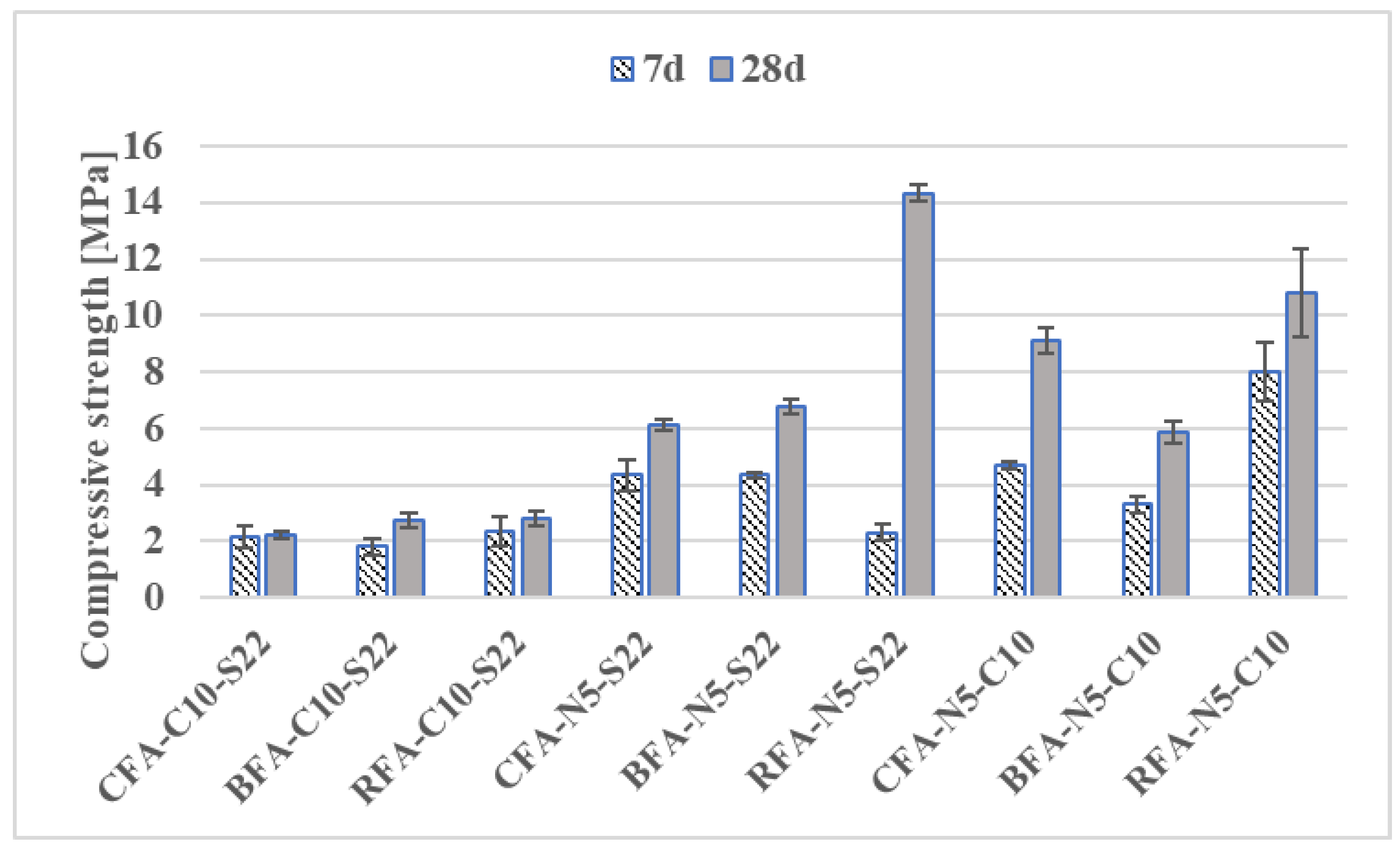
3.2.1. Flexural and Compressive Strengths
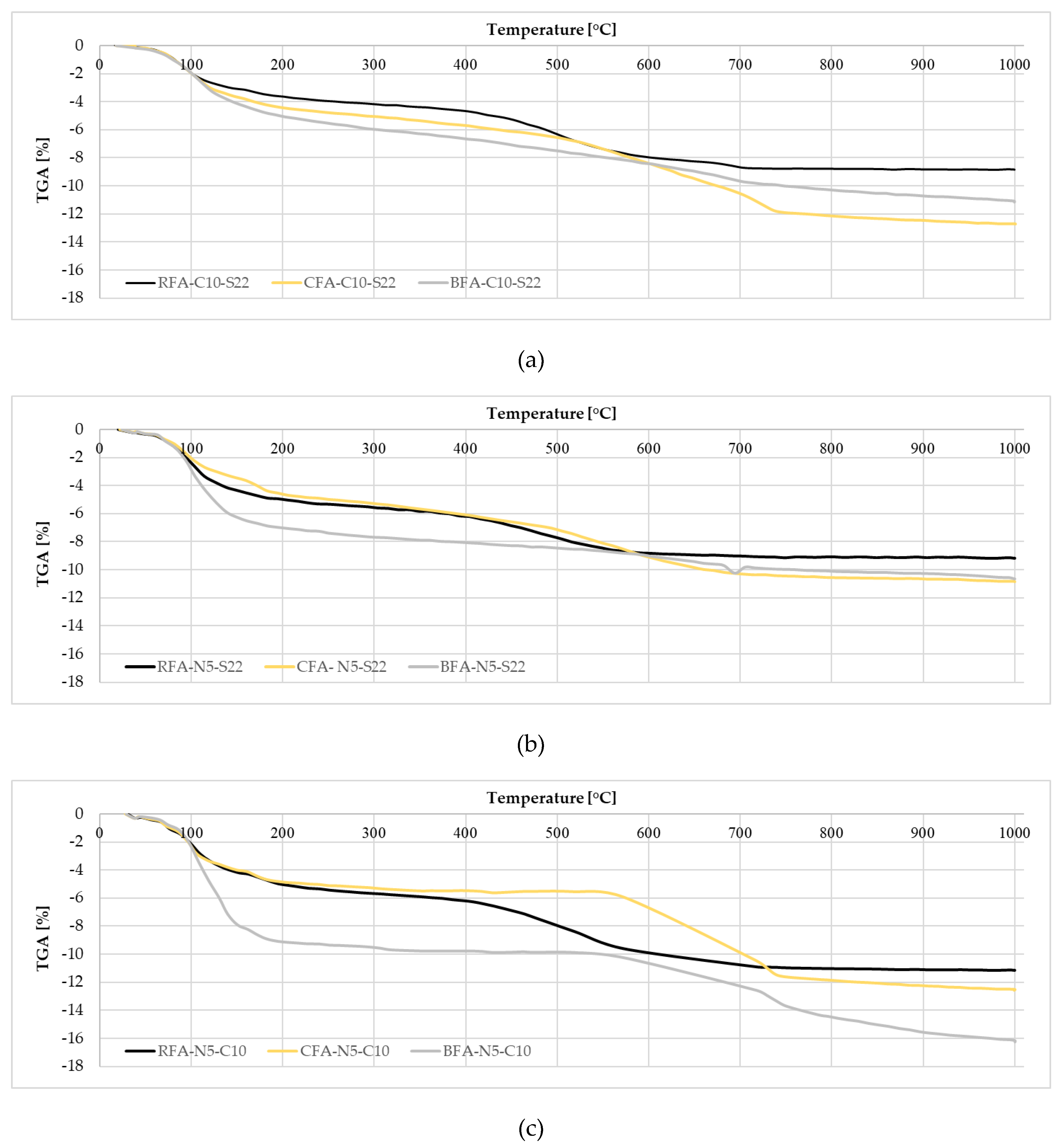
3.2.2. TGA
3.2.3. Calorimetry
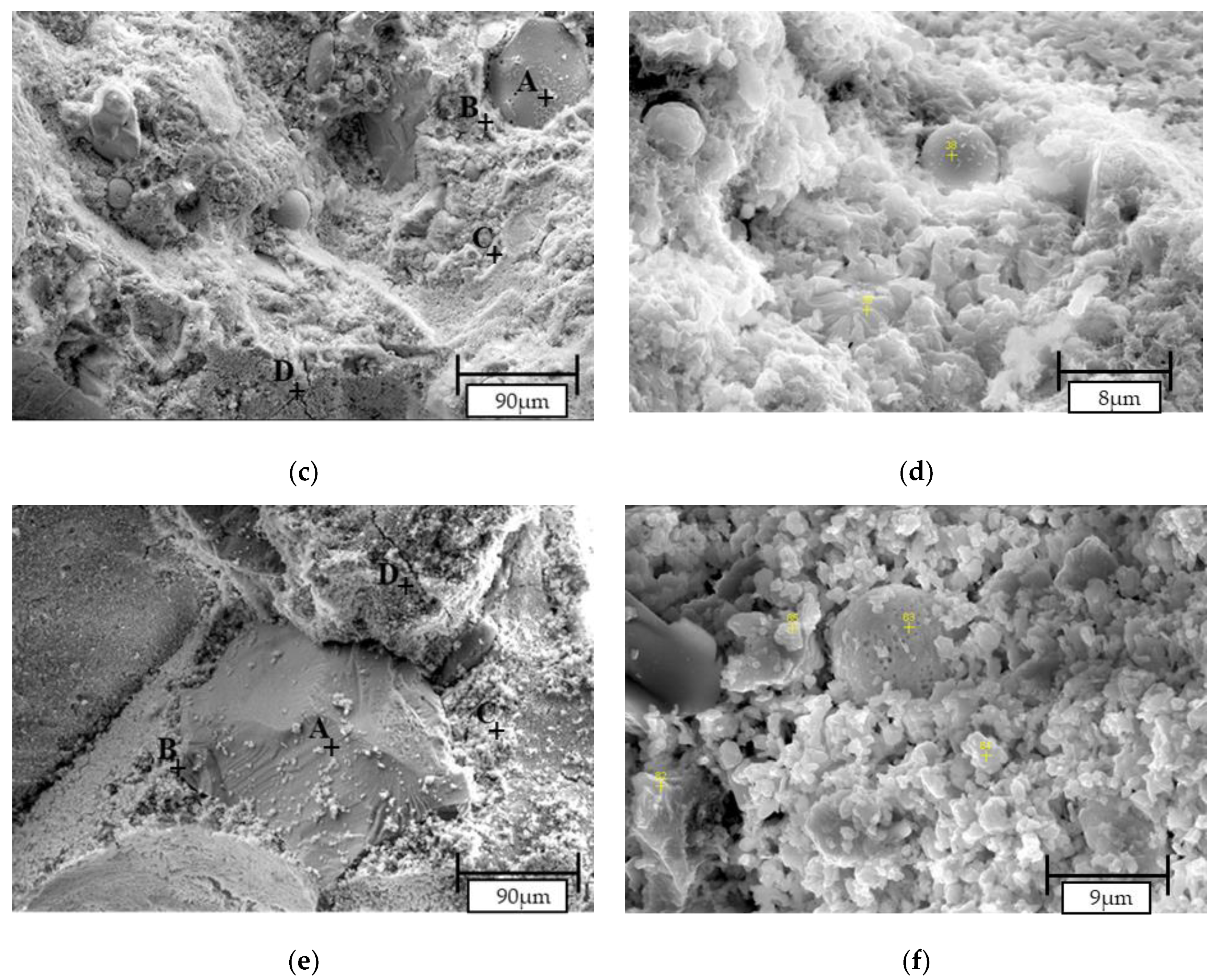
3.2.4. SEM
4. Conclusions
- Mortars activated with NaOH and Sodium Silicate (N5-S22) and NaOH with CaO (N5-C10) induced the highest flexural and compressive strengths for all the AAM. At 28 days, compressive strength results of CFA mortars with CaO addition were at least 71% higher than for other samples,
- The increase of NaOH molar concentration had a positive effect on mechanical properties of CFA mortars by improving its flexural and compressive strength results by 15% and 22%, respectively,
- Compressive strengths of geopolymer mortars RFA-N5-S22 and RFA-N5-C10 with equal 14.3 MPa and 10.8 MPa after 28 days were the highest of all mixes. The higher glassy content, a lower amount of unburned carbon in RFA with a more visually homogenous gel structure as well as a denser, less porous matrix of RFA-based AAM when compared to CFA, BFA, and their mortars, which have possibly contributed to its high strength after activation,
- Using the activator had a significant influence on the maximum temperature of the alkali-activated pastes setting. The N5-C10 in comparison to the N5-S22 activator increased the setting temperature by more than 45% for CFA and almost 36% for RFA. The activators from the most to the least effective on the setting temperature are arranged as: N5-C10 > C10-S22 >N5-S22,
- The Biomass Fly Ash (BFA) due to low alumina and silica content is not adequate for alkali-activation. The main chemical structures were connected with hydration of active CaO and creation of CaCO3. Solidification in an acidic environment may be analyzed due to a high level of P2O5.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Acronyms
| AAM | Alkali–Activated Materials |
| BFA | Biomass Fly Ash (derived from combustion of agricultural biomass) |
| C-(A)-S-H | calcium-(alumino)-silicate-hydrate |
| CC | Calcium Carbonate(CaCO3) |
| CFA | Co-combustion Fly Ash (derived from combustion of wooden biomass and coal) |
| CH | Calcium Hydroxide (Ca(OH)2 |
| C10, C15 | 10% or 15% substitution by mass of fly ash with quicklime |
| EDS | Energy Dispersive X-Ray Spectroscopy |
| FA | Fly Ash |
| FE | SEM-Field Emission Scanning Electron Microscope |
| H | Bound H2O |
| LOI | Lost on Ignition measurement |
| N-A-S-H | sodium-alumino-silicate-hydrate |
| N5, N10 | 5 M or 10 M solution of NaOH |
| OPC | Ordinary Portland Cement |
| PSDs | Particle Size Distributions |
| SSA | Specific Surface Area |
| S22 | 100 g addition of sodium silicate to alkaline activator |
| TGA | Thermogravimetric analysis |
| XRD | X-ray Diffraction Test |
| XRF | X-ray Fluorescence Test |
References
- Cembureau. The European Cement Association Activity Report 2017; Cembureau: Bruxelles, Belgium, 2018; p. 46. [Google Scholar]
- PBL Netherlands EAA. Trends in Global CO2 Emissions. 2016 Report; Joint Research Center: Hague, Denmark, 2016; pp. 62–66. [Google Scholar]
- Robbie, M.A. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar]
- Meyer, C. The greening of the concrete industry. Cement Concrete Compos. 2009, 31, 601–605. [Google Scholar] [CrossRef]
- The, S.H.; Wiedmann, T.; Castel, A.; de Burgh, J. Hybrid life cycle assessment of greenhouse gas emissions from cement, concrete and geopolymer concrete in Australia. J. Clean. Prod. 2017, 152, 312–320. [Google Scholar]
- BP p.l.c. BP Statistical Review of World Energy; BP p.l.c: London, UK, 2017; p. 50. [Google Scholar]
- Jones, N.; Johnson, K.; Sutti, E. Potential and Implications of Using Biomass for Energy in the European Union; Building Research Establishment ltd: Liverpool, UK, 23 October 2015. [Google Scholar]
- Millera, S.A.; Johnb, V.M.; Paccac, S.A.; Horvathd, A. Carbon dioxide reduction potential in the global cement industry by 2050. Cement Concrete Res. 2018, 114, 115–124. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Al-Majidi, M.H.; Lampropoulos, A.; Cundy, A.; Meikle, S. Development of geopolymer mortar under ambient temperature for in situ applications. Constr. Build. Mater. 2016, 120, 198–211. [Google Scholar] [CrossRef]
- Huseiena, G.F.; Mirzaa, J.; Ismaila, M.; Ghoshalc, S.K.; Husseina, A.A. Geopolymer mortars as sustainable repair material: A comprehensive review. Renew. Sustain. Energy Rev. 2017, 80, 54–74. [Google Scholar] [CrossRef]
- Rangan, B.V. Low-calcium fly ash-based geopolymer concrete. In Concrete Construction Engineering Handbook, 2nd ed.; Nawy, E.G., Ed.; CRC Press: New York, NY, USA, 2007; p. 1584. [Google Scholar]
- Singh, B.; Rahman, M.R.; Paswan, R.; Bhattacharyya, S.K. Effect of activator concentration on the strength, ITZ and drying shrinkage of fly ash/slag geopolymer concrete. Constr. Build. Mater. 2016, 118, 171–179. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. Chemistry & Application, 3rd ed.; Geopolymer Institute: Saint-Quentin, France, 2011; p. 613. [Google Scholar]
- Puligilla, S.; Mondal, P. Role of slag in microstructural development and hardening of fly ash-slag geopolymer. Cement Concrete Res. 2013, 43, 70–80. [Google Scholar] [CrossRef]
- Cho, Y.K.; Yoo, S.W.; Jung, S.H.; Lee, K.M.; Kwo, S.J. Effect of Na2O content, SiO2/Na2O molar ratio, and curing conditions on the compressive strength of FA-based geopolymer. Constr. Build. Mater. 2017, 145, 253–260. [Google Scholar] [CrossRef]
- de Souza Azevedoa, A.G.; Streckerb, K. Brazilian fly ash based inorganic polymers production using different alkali activator solutions. Ceram. Int. 2017, 43, 9012–9018. [Google Scholar] [CrossRef]
- Yuan, J.; He, P.; Jia, D.; Yang, C.; Yan, S.; Yang, Z.; Duan, X.; Wang, S.; Zhou, Y. Effect of curing temperature and SiO2/K2O molar ratio on the performance of metakaolin-based geopolymers. Ceram. Int. 2016, 42, 16184–16190. [Google Scholar] [CrossRef]
- Zhang, M. A multiscale investigation of reaction kinetics, phase formation, and mechanical properties of metakaolin geopolymer. Cement Concrete Comp. 2017, 78, 21–32. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Xu, J.; Wang, P.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- Singh, G.B.; Kolluru, V.L. Subramaniam Evaluation of sodium content and sodium hydroxide molarity on compressive strength of alkali activated low-calcium fly ash. Cement Concrete Comp. 2017, 81, 122–132. [Google Scholar] [CrossRef]
- Siyala, A.A.; Azizlia, K.A.; Mana, Z.; Ullaha, H. Effects of Parameters on the Setting Time of Fly Ash Based Geopolymers Using Taguchi Method. Procedia Eng. 2016, 148, 302–307. [Google Scholar] [CrossRef]
- Narayanan, A.; Shanmugasundaram, P. An Experimental Investigation on Flyash-based Geopolymer Mortar under different curing regime for Thermal Analysis. Energy Build. 2017, 138, 539–545. [Google Scholar] [CrossRef]
- Češnovar, M.; Traven, K.; Horvat, B.; Ducman, V. The Potential of Ladle Slag and Electric Arc Furnace Slag use in Synthesizing Alkali Activated Materials; the Influence of Curing on Mechanical Properties. Materials 2019, 12, 1173. [Google Scholar] [CrossRef]
- Sun, Z.; Lin, X.; Vollpracht, A. Pervious concrete made of alkali activated slag and geopolymers. Constr. Build. Mater. 2018, 189, 797–803. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, D.; Zhou, W.; Lu, H.; Wang, L. Effect of activator and curing mode on fly ash-based geopolymers. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 17, 711–715. [Google Scholar] [CrossRef]
- Canfield, G.M.; Eichler, J.; Griffith, K.; Hearn, J.D. The role of calcium in blended fly ash geopolymers. J. Mater. Sci. 2014, 17, 5922–5933. [Google Scholar] [CrossRef]
- Gum, S.R.; Young, B.L.; Kyung, T.K.; Chung, Y.S. The mechanical properties of fly ash–based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar]
- Jaarsveld van, J.; Deventer van, J. Effect of the Alkali Metal Activator on the Properties of Fly Ash-Based Geopolymers. Eng. Chem. Res. 1999, 38, 3932–3941. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Morsy, M.S.; Alsayed, S.H.; Al-Salloum, Y.; Almusallam, T. ffect of Sodium Silicate to Sodium Hydroxide Ratios on Strength and Microstructure of Fly Ash Geopolymer Binder. Arabian J. Sci. Eng. 2014, 39, 4333–4339. [Google Scholar] [CrossRef]
- Vickers, L.; van Riessen, A.; Rickard, W.D.A. Fire-Resistant Geopolymers. Role of Fibres and Fillers to Enhance Thermal Properties. In Briefs in Materials; Springer: Berlin, Germany, 2014; Volume 7, pp. 39–52. [Google Scholar]
- Fernández-Jiménez, A.; Torre de la, A.G.; Palomo, A.; López-Olmo, G.; Alonso, M.M.; Aranda, M.A.G. Quantitative determination of phases in the alkaline activation of fly ash. Part II: Degree of reaction. Fuel 2006, 85, 1960–1969. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Provis, J.L.; van Deventer, J.S. Alkali Activated Materials. State of the Art Report of RILEM TC 224-AAM; Springer/RILEM: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Provis, J.L. Gepolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Shearer, C.R.; Provis, J.L.; Bernal, S.A.; Kurtis, K.E. Alkali-activation potential of biomass-coal co-fired fly ash. Cement Concrete Comp. 2016, 73, 62–74. [Google Scholar] [CrossRef]
- Shearer, C.R.; Kurtis, K.E. Use of biomass and co-fired fly ash in concrete. ACI Mater. J. 2015, 112, 209–218. [Google Scholar] [CrossRef]
- Deschner, F.; Winnefeld, F.; Lothenbach, B.; Seufert, S.; Schwesig, P.; Dittrich, S.; Goetz-Neunhoeffer, F.; Neubauer, J. Hydration of Portland cement with high replacement by siliceous fly ash. Cement Concrete Res. 2012, 42, 1389–1400. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cement Concrete Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Perris, E.; Amahjour, F. Thermogravimetric Methods for Determining Carbon Content in Fly Ashes. Cement Concrete Res. 1998, 28, 675–686. [Google Scholar] [CrossRef]
- Bernal, S.A.; Juenger, M.C.; Ke, X.; Matthes, W.; Lothenbach, B.; De Belie, N.; Provis, J.L. Characterization of supplementary cementitious materials by thermal analysis. Mater. Struct. 2017, 50, 26. [Google Scholar] [CrossRef]
- Zhuang, X.Y.; Chen, L.; Komarneni, S.; Zhou, C.H.; Tong, D.S.; Yang, H.M.; Yu, W.H.; Wang, H. Fly ash-based geopolymer: Clean production, properties and Application. J. Clean. Prod. 2016, 126, 253–267. [Google Scholar] [CrossRef]
- Soutsos, M.; Boyle, A.P.; Vinai, R.; Hadjierakleous, A.; Barnett, S.J. Factors influencing the compressive strength of fly ash based geopolymers. Constr. Build. Mater. 2016, 110, 355–368. [Google Scholar] [CrossRef]
- Tosti, L.; van Zomeren, A.; Pels Jan, R.; Comans, R.N.J. Technical and environmental performance of lower carbon footprint cement mortars containing biomass fly ash as a secondary cementitious material. Resour. Conserv. Recycl. 2018, 134, 25–33. [Google Scholar] [CrossRef]
- Belviso, C. State-of-the-art applications of fly ash from coal and biomass: A focus on zeolite synthesis processes and issues. Prog. Energy Combust. 2018, 65, 109–135. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the chemical composition of biomass. Fuel 2010, 89, 913–933. [Google Scholar] [CrossRef]
- van Loo, S.; Koppejan, J. The Handbook of Biomass Combustion & Cofiring; Earthscan: Washington, DC, USA, 2010. [Google Scholar]
- Baxter, L.L.; Miles, T.R.; Miles, T.R., Jr.; Jenkins, B.M.; Milne, T.; Dayton, D.; Bryers, R.W.; Oden, L.L. The behavior of inorganic material in biomass-fired power boilers: Field and laboratory experiences. Fuel Process. Technol. 1998, 54, 47–78. [Google Scholar] [CrossRef]
- Dahlquist, E. Biomass as Energy Source: Resources, Systems and Applications; CRC Press, Auerbach: Boca Raton, FL, USA, 2013. [Google Scholar]
- Robl, T.; Oberlink, A.; Jones, R. Coal Combustion Products (CCPs): Their Nature, Utilization and Beneficiation; Woodhead Publishing: Sawston, UK, 2017. [Google Scholar]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Li, S.; Cooke, R.A.; Wang, L.; Ma, F.; Bhattarai, R. Characterization of fly ash ceramic pellet for phosphorus removal. J. Environ. Manag. 2017, 189, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Sevim, Ö.; Demir, İ. Optimization of fly ash particle size distribution for cementitious systems with high compactness. Constr. Build. Mater. 2019, 195, 104–114. [Google Scholar] [CrossRef]
- Hasler, P.H.; Nussbaumer, T.H. Particle Size Distribution Of The Fly Ash From Biomass Combustion, Biomass for Energy and Industry, In Proceedings of the 10th European Conference and Technology Exhibition, Würzburg, Germany, 8–11 June 1998.
- Aughenbaugh, K.L.; Stutzman, P.; Juenger, M.C.G. Identifying Glass Compositions in Fly Ash. Front. Mater. 2016, 3, 1–10. [Google Scholar] [CrossRef]
- Yeboah, N.N.N.; Shearer, C.R.; Burns, S.E.; Kurtis, K.E. Characterization of biomass and high carbon content coal ash for productive reuse applications. Fuel 2014, 116, 438–447. [Google Scholar] [CrossRef]
- Purbasari, A.; Samadhi, T.W.; Binda, Y. The Effect of Alkaline Activator Types on Strength and Microstructural Properties of Geopolymer from Co-Combustion Residuals of Bamboo and Kaolin. Indones. J. Chem. 2018, 18, 397–402. [Google Scholar] [CrossRef]
- Hamidi, R.M.; Man, Z.; Azizli, K.A. Concentration of NaOH and the Effect on the Properties of Fly Ash Based Geopolymer. Procedia Eng. 2016, 148, 189–193. [Google Scholar] [CrossRef]
- Temuujin, J.; Riessen van, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Faris, M.A.; Abdullah, M.M.A.; Sandu, A.V.; Ismail, K.N.; Moga, L.M.; Neculai, O.; Muniandy, R. Assessment of alkali activated geopolymer binders as an alternative of portlant cement. Mater. Plast. 2017, 54, 145–154. [Google Scholar] [CrossRef]
- Djobo, J.N.Y.; Elimbi, A.; Tchakouté, H.K.; Kumar, S. Mechanical activation of volcanic ash for geopolymer synthesis: Effect on reaction kinetics, gel characteristics, physical and mechanical properties. RSC Adv. 2016, 6, 39106–39117. [Google Scholar] [CrossRef]
- Nath, S.K.; Kumar, S. Role of particle fineness on engineering properties and microstructure of fly ash derived geopolymer. Constr. Build. Mater. 2020, 233, 117294. [Google Scholar] [CrossRef]
- Mucsi, G.; Kumar, S.; Csőke, B.; Kumar, R.; Molnár, Z.; Rácz, Á.; Mádai, F.; Debreczeni, Á. Control of geopolymer properties by grinding of land filled fly ash. Int. J. Miner. Process. 2015, 143, 50–58. [Google Scholar] [CrossRef]
- Guades, E.J. Experimental investigation of the compressive and tensile strengths of geopolymer mortar: The effect of sand/fly ash (S/FA) ratio. Constr. Build. Mater. 2016, 127, 484–493. [Google Scholar] [CrossRef]
- Patankar, S.V.; Ghugal, Y.M.; Jamkar, S.S. Effect of Concentration of Sodium Hydroxide and Degree of Heat Curing on Fly Ash-Based Geopolymer Mortar. Indian J. Mater. Sci. 2014, 2014, 6. [Google Scholar] [CrossRef]
- Hadi, M.N.S.; Al-Azzawi, M.; Yu, T. Effects of fly ash characteristics and alkaline activator components on compressive strength of fly ash-based geopolymer mortar. Constr. Build. Mater. 2018, 175, 41–54. [Google Scholar] [CrossRef]
- de Vargas, A.S.; Dal Molin, D.C.; Masuero, Â.B.; Vilela, A.C.; Castro-Gomes, J.; de Gutierrez, R.M. Strength development of alkali-activated fly ash produced with combined NaOH and Ca(OH)2 activators. Cement Concr. Compos. 2014, 53, 341–349. [Google Scholar] [CrossRef]
- Gorhan, G.; Kurklu, G. The influence of the NaOH solution on the properties of the fly ash-based geopolymer mortar cured at different temperatures. Compos. Pt. B 2014, 58, 371–377. [Google Scholar] [CrossRef]
- Atiş, C.D.; Görür, E.B.; Karahan, O.; Bilim, C.; İlkentapar, S.; Luga, E. Very high strength (120MPa) class F fly ash geopolymer mortar activated at different NaOH amount, heat curing temperature and heat curing duration. Constr. Build. Mater. 2015, 96, 673–678. [Google Scholar]
- Fernández-Jiménez, A.; Palomo, A. Composition and microstructure of alkali activated fly ash binder: Effect of the activator. Cement Concrete Res. 2005, 35, 1984–1992. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Mäkelä, M.; Paananen, T.; Kokkonen, T.; Makkonen, H.; Heino, J.; Dahl, O. Preliminary Evaluation of Fly Ash and Lime for Use as Supplementary Cementing Materials in Cold-Agglomerated Blast Furnace Briquetting. ISIJ Int. 2011, 51, 776–781. [Google Scholar] [CrossRef]
- Gao, Y.M.; Kulaots, G.; Chen, X.; Suuberg, E.M.; Hurt, R.H.; Veranth, J.M. The effect of solid fuel type and combustion conditions on residual carbon properties and fly ash quality. Proc. Combus. Inst. 2002, 29, 475–483. [Google Scholar] [CrossRef]
- Dey, T. Thermal Properties Of A Sustainable Cement Material: Effect Of Cure Conditions. Ceram. Silik. 2014, 58, 275–281. [Google Scholar]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Inorganics polymeric new materials. J. Thermal Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Yang, N.; Tran, H.M.; Scott, J.; Dhakal, R.P.; Watson, M.; Shi, C.J. Properties Of Magnesium Based Cements. In Proceedings of the The New Zeland Concrete Industry Conference, Te Papa, Wellington, New Zealand, 12–14 October 2017. [Google Scholar]
- Yang, N.; Shi, C.; Yang, J.; Chang, Y. Research Progresses in Magnesium Phosphate Cement–Based Materials. J. Mater. Civ. Eng. 2014, 26, 4014–4071. [Google Scholar] [CrossRef]
- Jumrat, S.; Chatveera, B.; Rattanadecho, P. Dielectric properties and temperature profile of fly ash-based geopolymer mortar. Int. Commun. Heat Mass. 2011, 38, 242–248. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; MacKenzie, K.J.D. Preparation and characterisation of fly ash based geopolymer mortars. Constr. Build. Mater. 2010, 24, 1906–1910. [Google Scholar] [CrossRef]
- Shindunata; van Deventer, J.S.J.; Lukey, G.C.; Xu, H. Effect of curing temperature and silicate concentration on fly ash based geopolymerization. Ind. Eng. Chem. Res 2006, 45, 3559–3568. [Google Scholar] [CrossRef]
- Cuneyt, A. Amorphous Calcium Phosphate (Acp) Nanoparticles: Biomimetic Synthesis. Mater. Sci. Eng. C Mater. 2012, 32, 1097–1106. [Google Scholar]


| Mix Type | Raw Material (g) | Sand (g) | NaOH Solution (g) | Silicate Solution (g) | CaO (g) | Water (g) |
|---|---|---|---|---|---|---|
| RFA-N5 | 450 | 1350 | 225 | 0 | 0 | 0 |
| CFA-N5 | 450 | 1350 | 225 | 0 | 0 | 0 |
| BFA-N5 | 450 | 1350 | 225 | 0 | 0 | 0 |
| RFA-N10 | 450 | 1350 | 225 | 0 | 0 | 0 |
| CFA-N10 | 450 | 1350 | 225 | 0 | 0 | 0 |
| BFA-N10 | 450 | 1350 | 225 | 0 | 0 | 0 |
| RFA-C10 | 405 | 1350 | 0 | 0 | 45 | 225 |
| CFA-C10 | 405 | 1350 | 0 | 0 | 45 | 225 |
| BFA-C10 | 405 | 1350 | 0 | 0 | 45 | 225 |
| RFA-C15 | 382 | 1350 | 0 | 0 | 68 | 225 |
| CFA-C15 | 382 | 1350 | 0 | 0 | 68 | 225 |
| BFA-C15 | 382 | 1350 | 0 | 0 | 68 | 225 |
| RFA-C10-S22 | 405 | 1350 | 0 | 100 | 45 | 225 |
| CFA-C10-S22 | 405 | 1350 | 0 | 100 | 45 | 225 |
| BFA-C10-S22 | 405 | 1350 | 0 | 100 | 45 | 225 |
| RFA-N5-S22 | 450 | 1350 | 225 | 100 | 0 | 0 |
| CFA-N5-S22 | 450 | 1350 | 225 | 100 | 0 | 0 |
| BFA-N5-S22 | 450 | 1350 | 225 | 100 | 0 | 0 |
| RFA-N5-C10 | 405 | 1350 | 225 | 0 | 45 | 0 |
| CFA-N5-C10 | 405 | 1350 | 225 | 0 | 45 | 0 |
| BFA-N5-C10 | 405 | 1350 | 225 | 0 | 45 | 0 |
| Chemical Composition (%) and Physical Characteristic | RFA | CFA | BFA |
|---|---|---|---|
| SiO2 | 50.56 | 46.57 | 0.45 |
| Al2O3 | 26.20 | 4.15 | 0.12 |
| Fe2O3 | 6.21 | 3.40 | 0.22 |
| MnO | 0.06 | 0.66 | 0.02 |
| MgO | 2.63 | 3.27 | 1.19 |
| CaO | 2.70 | 21.90 | 30.95 |
| Na2O | 0.94 | 0.98 | 1.20 |
| K2O | 3.51 | 5.58 | 16.19 |
| TiO2 | 1.13 | 0.21 | 0.00 |
| P2O5 | 0.55 | 3.01 | 27.68 |
| Loss On Ignition, LOI (%) | 5.51 | 7.65 | 17.98 |
| Total of XRF | 100.01 | 97.37 | 96.00 |
| ∑ (SiO2 + Al2O3 + Fe2O3) | 82.97 | 54.12 | 0.79 |
| Real density (g/cm3) | 2.188 | 2.732 | 2.453 |
| Mean particle size (μm) | 56.02 | 57.96 | 38.47 |
| BET SSA * (m2/g) | 3.4489 | 5.5573 | 2.0971 |
| Mineral Composition (%) | |||
| Quartz | 7.02 | 18.72 | – |
| Mullite | 14.63 | – | – |
| Calcite | – | 10.6 | – |
| Portlandite | – | 3.45 | 15.51 |
| Microcline | – | 3.33 | – |
| Orthoclase | – | 3.18 | – |
| Microcline | – | – | – |
| Alunite | – | – | 2.4 |
| Anhydrite | – | – | 2.41 |
| Arcanite | – | – | 10.96 |
| Alite | – | – | – |
| Archerite | – | – | 23.61 |
| Amor | 76.91 | 53.13 | 41.35 |
| Mix | Free H2O, % | H *, %w | CH *, %w | CC *, %w | Total |
|---|---|---|---|---|---|
| <105 °C | 105 °C–550 °C | 410 °C–550 °C | 550 °C–800 °C | %w | |
| CFA-C10-S22 | 2.33 | 5.56 | 1.70 | 4.31 | 12.70 |
| BFA-C10-S22 | 2.30 | 5.96 | 1.25 | 2.13 | 11.15 |
| RFA-C10-S22 | 2.23 | 5.33 | 2.58 | 1.21 | 8.84 |
| CFA-N5-S22 | 2.39 | 5.92 | 1.90 | 2.30 | 10.88 |
| BFA-N5-S22 | 3.57 | 5.51 | 0.71 | 1.15 | 10.66 |
| RFA-N5-S22 | 2.77 | 5.75 | 2.12 | 0.59 | 9.18 |
| CFA-N5-C10 | 3.11 | 3.21 | 0.47 | 5.65 | 12.51 |
| BFA-N5-C10 | 3.81 | 7.01 | 0.48 | 3.96 | 16.26 |
| RFA-N5-C10 | 3.06 | 6.70 | 3.21 | 1.29 | 11.16 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prochon, P.; Zhao, Z.; Courard, L.; Piotrowski, T.; Michel, F.; Garbacz, A. Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars. Materials 2020, 13, 1033. https://doi.org/10.3390/ma13051033
Prochon P, Zhao Z, Courard L, Piotrowski T, Michel F, Garbacz A. Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars. Materials. 2020; 13(5):1033. https://doi.org/10.3390/ma13051033
Chicago/Turabian StyleProchon, Piotr, Zengfeng Zhao, Luc Courard, Tomasz Piotrowski, Frédéric Michel, and Andrzej Garbacz. 2020. "Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars" Materials 13, no. 5: 1033. https://doi.org/10.3390/ma13051033
APA StyleProchon, P., Zhao, Z., Courard, L., Piotrowski, T., Michel, F., & Garbacz, A. (2020). Influence of Activators on Mechanical Properties of Modified Fly Ash Based Geopolymer Mortars. Materials, 13(5), 1033. https://doi.org/10.3390/ma13051033







