Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Method
2.1. Materials
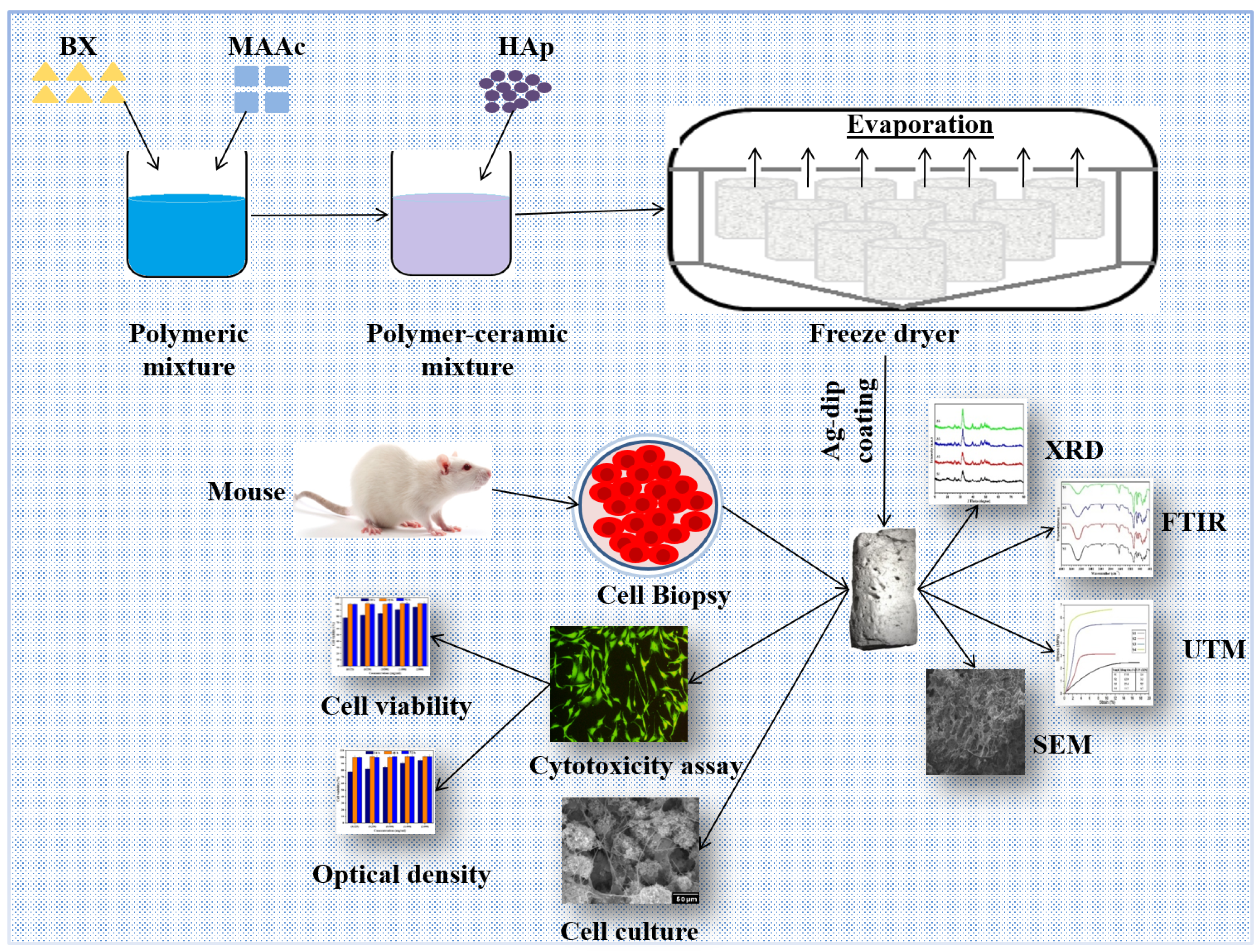
2.2. Bioactive Nanocomposite Scaffold Fabrication
2.3. Deposition of Silver Particles on Bioactive Nanocomposite Scaffolds
3. Characterizations
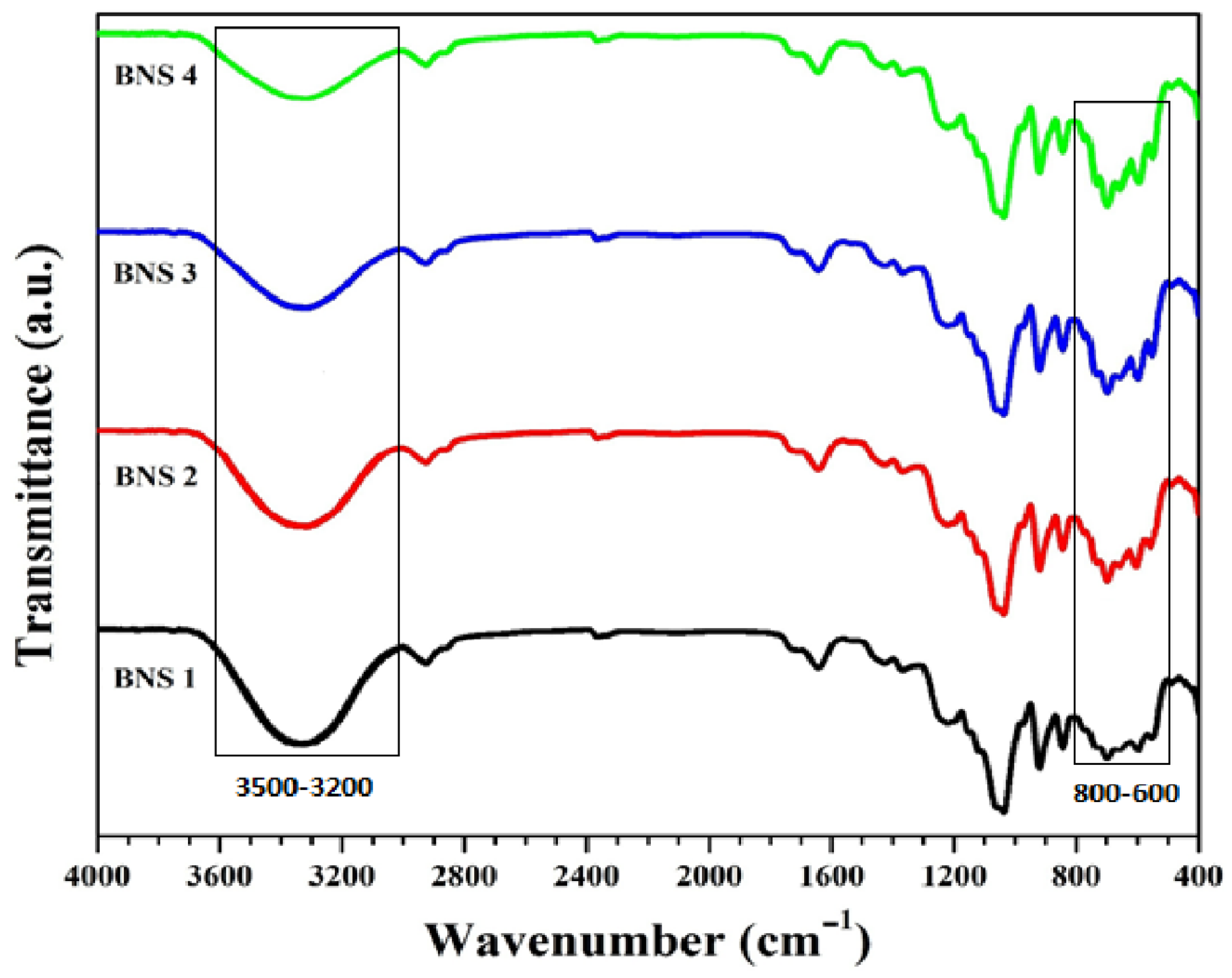
3.1. Fourier Transform Infrared Spectroscopy
3.2. X-ray Diffraction
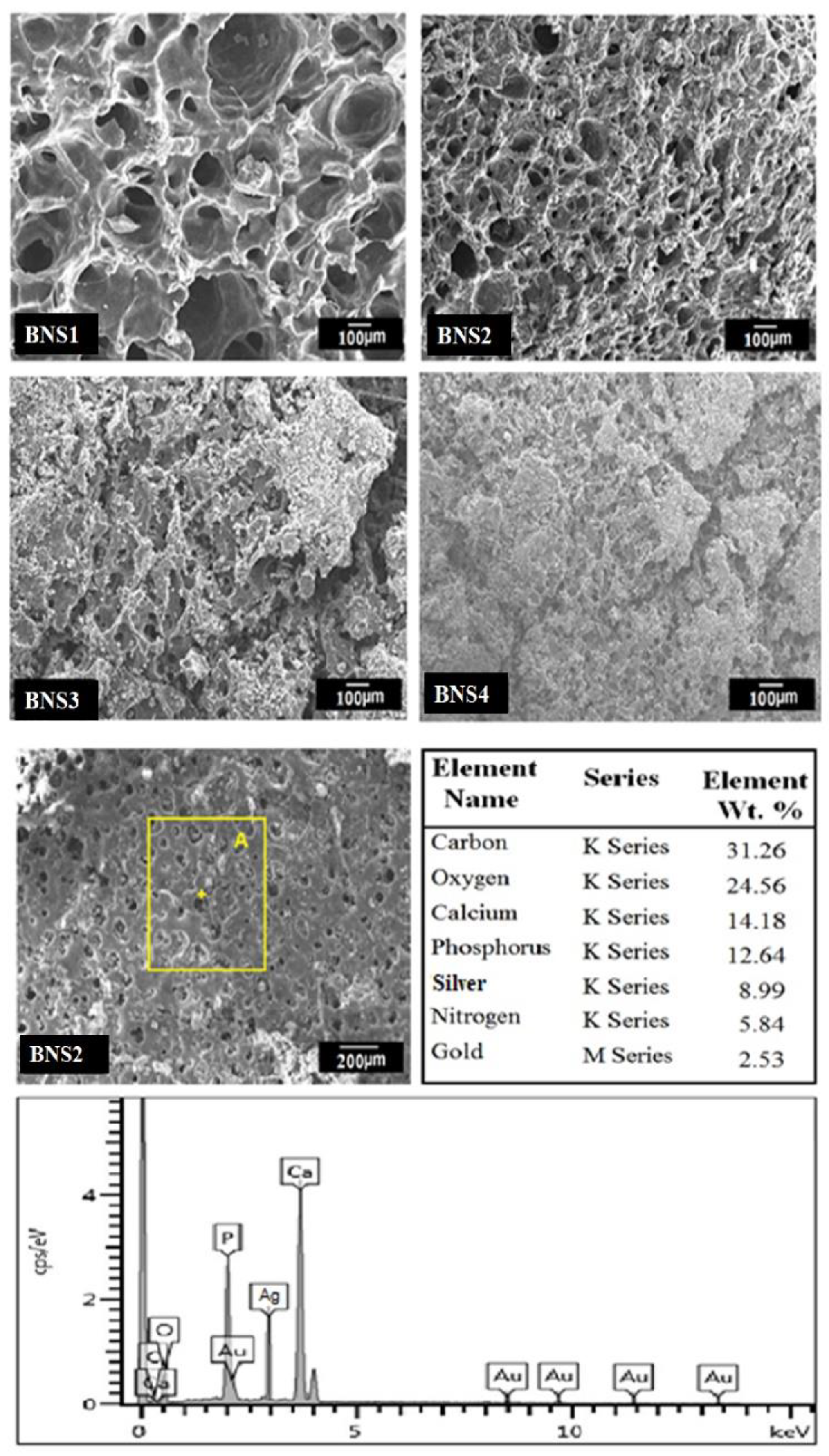
3.3. Scanning Electron Microscope/ Energy Dispersive Spectroscopy
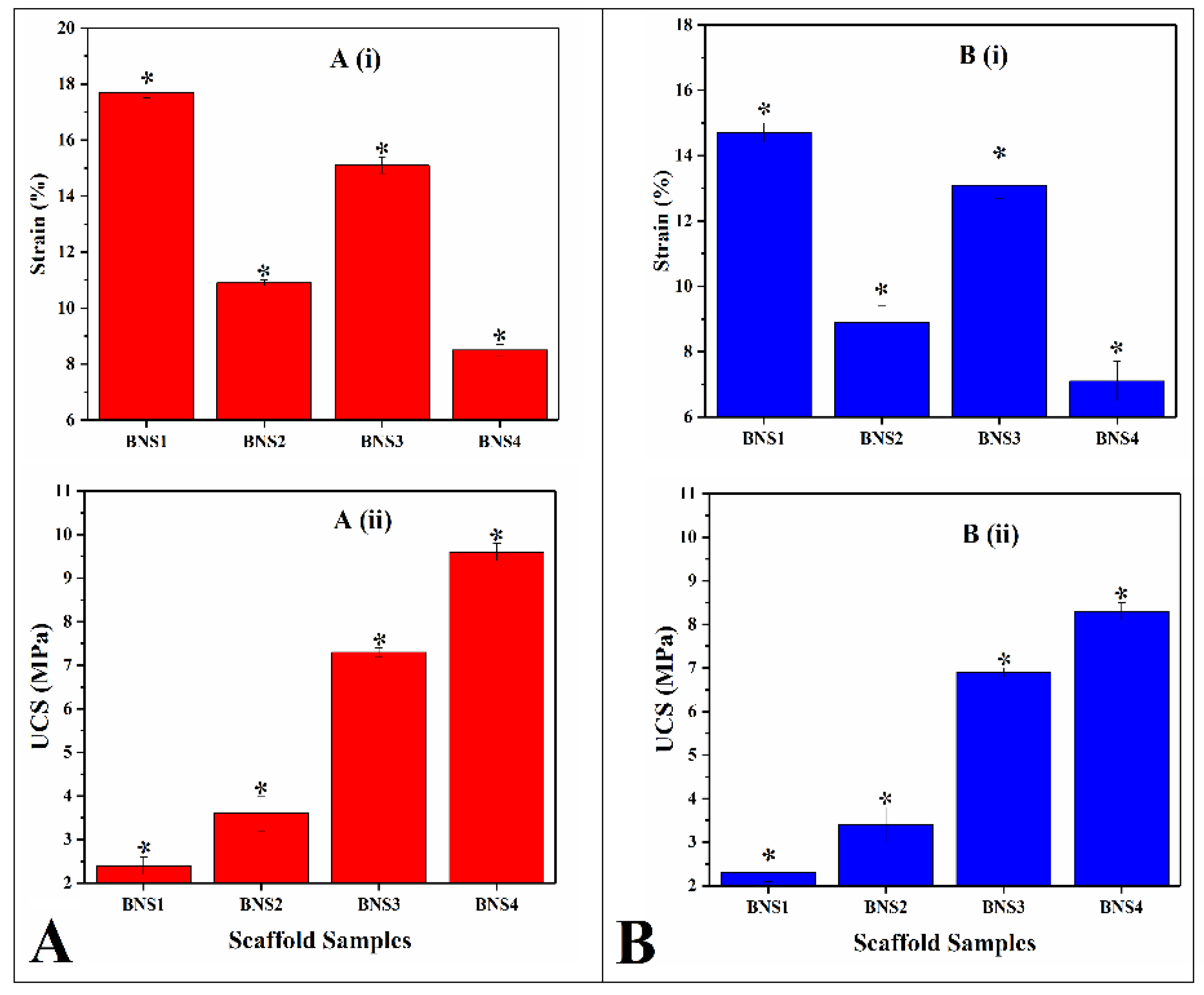
3.4. Mechanical Testing
3.5. Swelling Analysis
3.6. In Vitro Studies
3.6.1. Anti-Microbial Activities
3.6.2. Sample Preparation for Cell Culture
3.6.3. Cell Morphological Analysis
3.6.4. Cytotoxicity Using the Neutral Red Assay
3.6.5. SEM Sample Preparation
3.6.6. Statistical Analysis
4. Results and Discussion
4.1. FTIR
4.2. XRD
4.3. SEM–EDX
4.4. Mechanical Testing
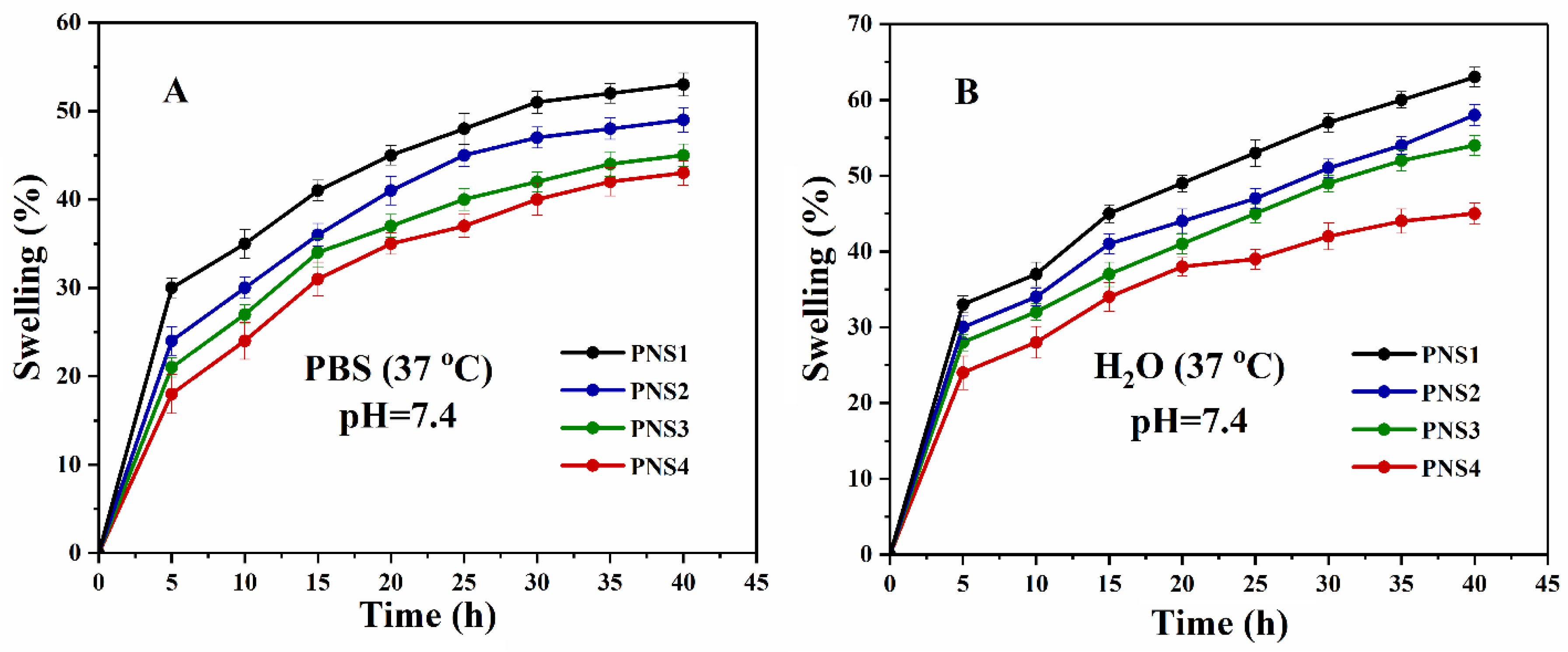
4.5. Swelling Analysis
4.6. In-Vitro Study
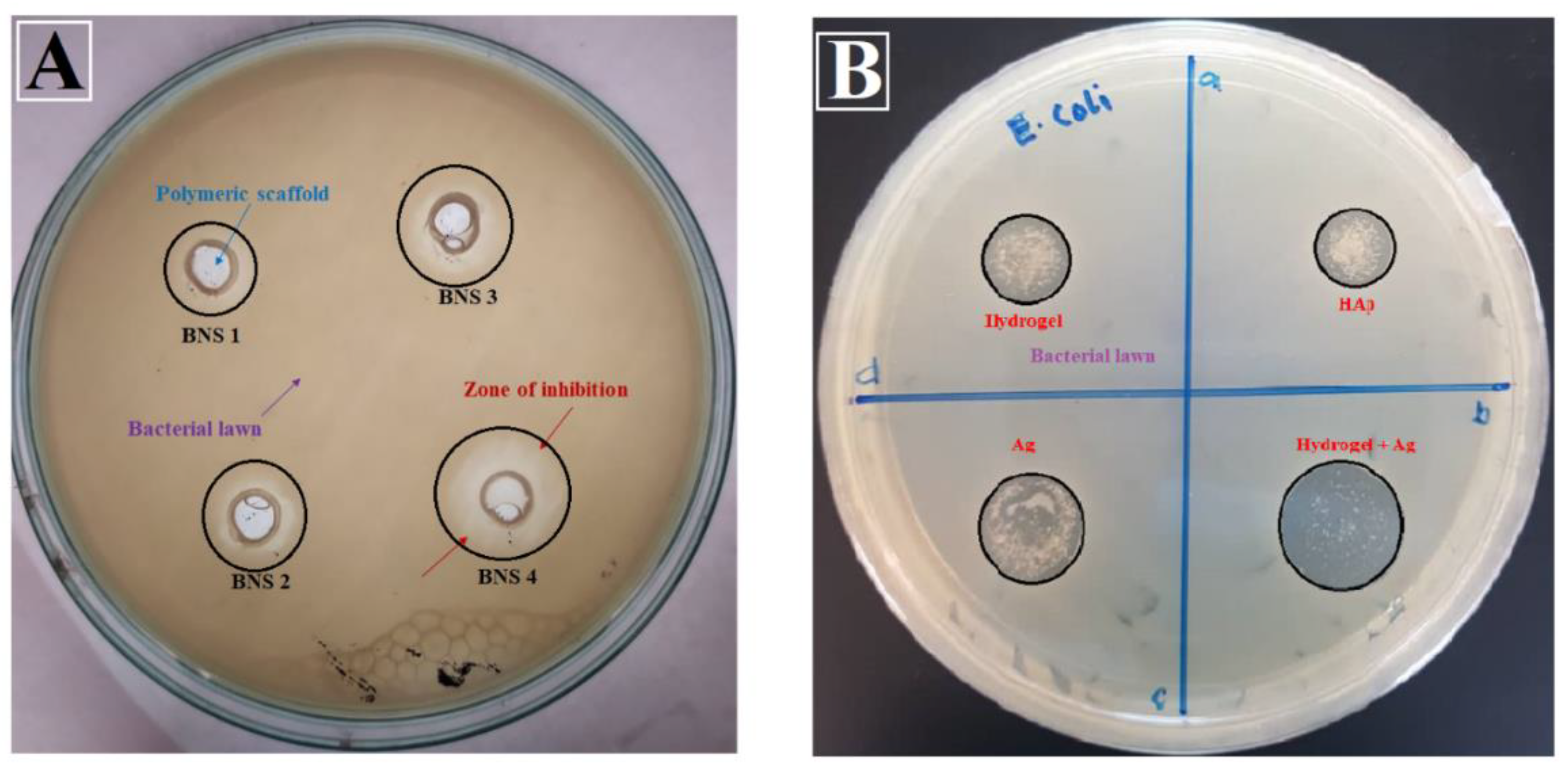
4.6.1. Anti-Microbial Activity
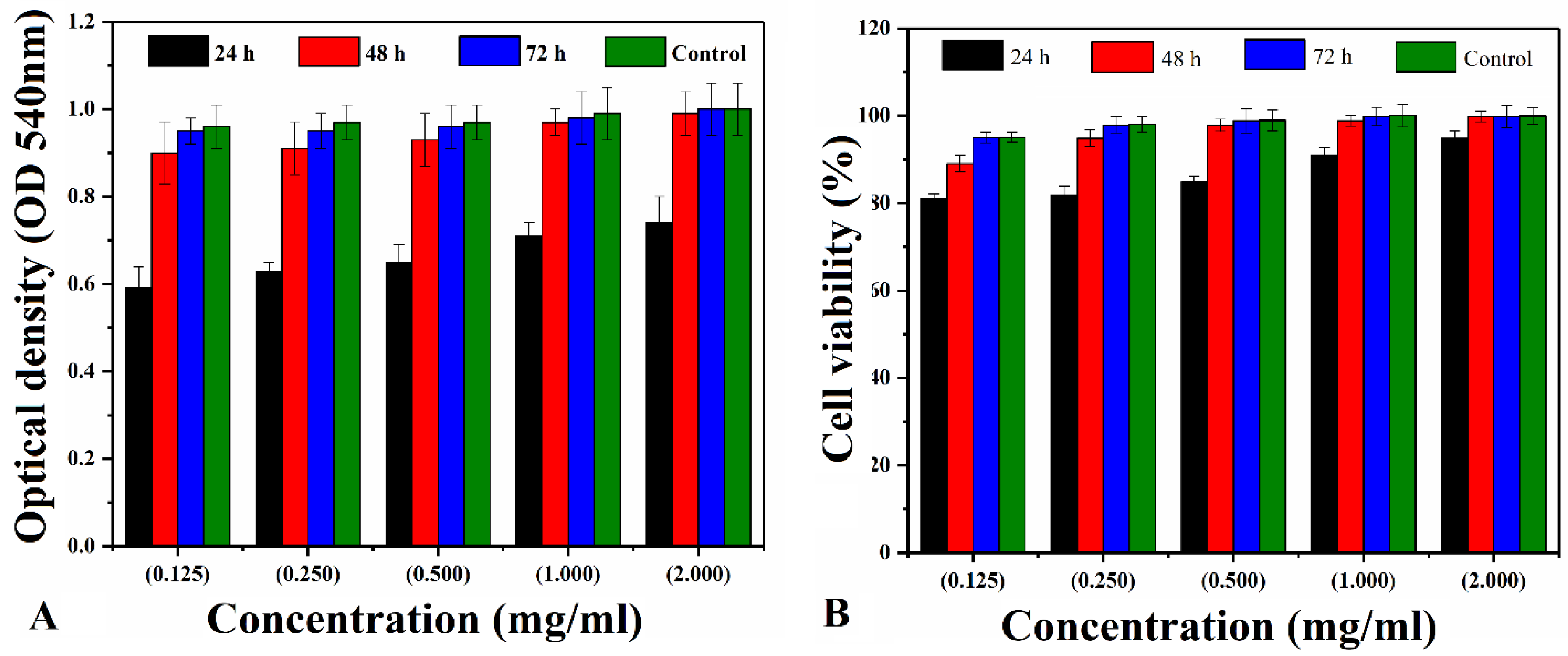
4.6.2. Cytotoxicity
4.6.3. Morphological Changes and Cell Attachment
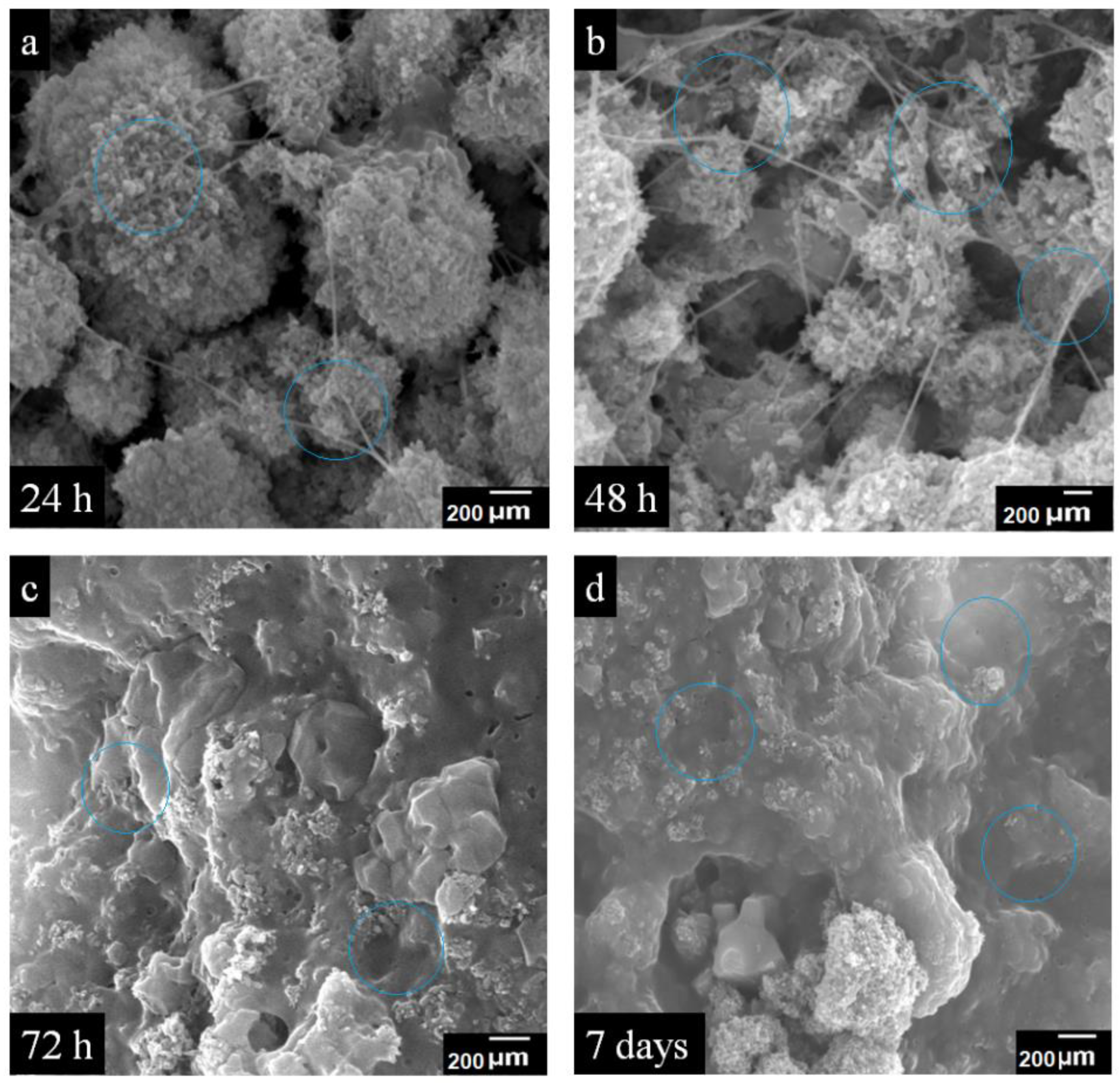
4.6.4. SEM Analysis of Cell Growth
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nettles, D.L.; Elder, S.H.; Gilbert, J.A. Potential use of chitosan as a cell scaffold material for cartilage tissue engineering. Tissue Eng. 2002, 8, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone tissue engineering: State of the art and future trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef] [PubMed]
- Ramseier, C.A.; Rasperini, G.; Batia, S.; Giannobile, W.V. Advanced reconstructive technologies for periodontal tissue repair. Periodontology 2012, 59, 185–202. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Y.; Yang, C.; Qi, X.; Li, S.; Liu, C.; Li, X. Mesoporous bioactive glass combined with graphene oxide scaffolds for bone repair. Int. J. Biol. Sci. 2019, 15, 2156. [Google Scholar] [CrossRef]
- Lasprilla, A.J.; Martinez, G.A.; Lunelli, B.H.; Jardini, A.L.; Maciel Filho, R. Poly-lactic acid synthesis for application in biomedical devices—A review. Biotechnol. Adv. 2012, 30, 321–328. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, R.B.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef]
- Quinlan, E.; López-Noriega, A.; Thompson, E.; Kelly, H.M.; Cryan, S.A.; O’Brien, F.J. Development of collagen–hydroxyapatite scaffolds incorporating PLGA and alginate microparticles for the controlled delivery of rhBMP-2 for bone tissue engineering. J. Control. Release 2015, 198, 71–79. [Google Scholar] [CrossRef]
- Elkhenany, H.; Amelse, L.; Lafont, A.; Bourdo, S.; Caldwell, M.; Neilsen, N.; Dervishi, E.; Derek, O.; Biris, A.S.; Anderson, D. Graphene supports in vitro proliferation and osteogenic differentiation of goat adult mesenchymal stem cells: Potential for bone tissue engineering. J. Appl. Toxicol. 2015, 35, 367–374. [Google Scholar] [CrossRef]
- Hussein, K.H.; Park, K.-M.; Kang, K.-S.; Woo, H.-M. Biocompatibility evaluation of tissue-engineered decellularized scaffolds for biomedical application. Mater. Sci. Eng. C 2016, 67, 766–778. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Rana, V.; Kennedy, J.F. Recent insights on applications of pullulan in tissue engineering. Carbohydr. Polym. 2016, 153, 455–462. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.; Blaker, J.; Boccaccini, A.R. Biodegradable and bioactive porous polymer/inorganic composite scaffolds for bone tissue engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ramay, H.R.; Hauch, K.D.; Xiao, D.; Zhang, M. Chitosan–alginate hybrid scaffolds for bone tissue engineering. Biomaterials 2005, 26, 3919–3928. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2019, 87, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yang, D.; Su, D.; Mu, X.; Kennedy, J.F.; Nie, J. Preparation and properties of water-soluble chitosan and polyvinyl alcohol blend films as potential bone tissue engineering matrix. Polym. Adv. Technol. 2010, 21, 189–195. [Google Scholar] [CrossRef]
- Fathi, M.; Hanifi, A.; Mortazavi, V. Preparation and bioactivity evaluation of bone-like hydroxyapatite nanopowder. J. Mater. Process. Technol. 2008, 202, 536–542. [Google Scholar] [CrossRef]
- Mondal, S.; Hoang, G.; Manivasagan, P.; Moorthy, M.S.; Nguyen, T.P.; Phan, T.T.V.; Kim, H.H.; Kim, M.H.; Nam, S.Y.; Oh, J. Nano-hydroxyapatite bioactive glass composite scaffold with enhanced mechanical and biological performance for tissue engineering application. Ceram. Int. 2018, 44, 15735–15746. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef]
- Khoshroo, K.; Kashi, T.S.J.; Moztarzadeh, F.; Tahriri, M.; Jazayeri, H.E.; Tayebi, L. Development of 3D PCL microsphere/TiO2 nanotube composite scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2017, 70, 586–598. [Google Scholar] [CrossRef]
- Shankar, S.; Jaiswal, L.; Aparna, R.; Prasad, R. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 2014, 137, 75–78. [Google Scholar] [CrossRef]
- Wu, X.; Li, J.; Wang, L.; Huang, D.; Zuo, Y.; Li, Y. The release properties of silver ions from Ag-nHA/TiO2/PA66 antimicrobial composite scaffolds. Biomed. Mater. 2010, 5, 044105. [Google Scholar] [CrossRef]
- Li, Y.; Luo, Y.; Hu, Y.; Zhu, D.-D.; Zhang, S.; Liu, Z.-J.; Gong, H.-B.; Zhu, H.-L. Design, synthesis and antimicrobial activities of nitroimidazole derivatives containing 1, 3, 4-oxadiazole scaffold as FabH inhibitors. Bioorganic Med. Chem. 2012, 20, 4316–4322. [Google Scholar] [CrossRef] [PubMed]
- Travan, A.; Marsich, E.; Donati, I.; Benincasa, M.; Giazzon, M.; Felisari, L.; Paoletti, S. Silver–polysaccharide nanocomposite antimicrobial coatings for methacrylic thermosets. Acta Biomater. 2011, 7, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Marsich, E.; Bellomo, F.; Turco, G.; Travan, A.; Donati, I.; Paoletti, S. Nano-composite scaffolds for bone tissue engineering containing silver nanoparticles: Preparation, characterization and biological properties. J. Mater. Sci. Mater. Med. 2013, 24, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; McHale, P.; Duffy, B. Preparation and rapid analysis of antibacterial silver, copper and zinc doped sol–gel surfaces. Colloids Surf. B Biointerfaces 2012, 94, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Das, K.; Bose, S.; Bandyopadhyay, A.; Karandikar, B.; Gibbins, B.L. Surface coatings for improvement of bone cell materials and antimicrobial activities of Ti implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 455–460. [Google Scholar] [CrossRef]
- Tyllianakis, M.; Dalas, E.; Christofidou, M.; Kallitsis, J.; Chrissanthopoulos, A.; Koutsoukos, P.; Bartzavali, C.; Gourdoupi, N.; Papadimitriou, K.; Oikonomou, E. Novel composites materials from functionalized polymers and silver coated titanium oxide capable for calcium phosphate induction, control of orthopedic biofilm infections: An “In Vitro” study. J. Mater. Sci. Mater. Med. 2010, 21, 2201–2211. [Google Scholar] [CrossRef] [PubMed]
- Sroka-Bartnicka, A.; Kimber, J.A.; Borkowski, L.; Pawlowska, M.; Polkowska, I.; Kalisz, G.; Belcarz, A.; Jozwiak, K.; Ginalska, G.; Kazarian, S.G. The biocompatibility of carbon hydroxyapatite/β-glucan composite for bone tissue engineering studied with Raman and FTIR spectroscopic imaging. Anal. Bioanal. Chem. 2015, 407, 7775–7785. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Li, X.; Wen, P.; Zhang, Y.; Long, Y.; Wang, X.; Guo, Y.; Xing, F.; Gao, J. Preparation of aligned porous gelatin scaffolds by unidirectional freeze-drying method. Acta Biomater. 2010, 6, 1167–1177. [Google Scholar] [CrossRef]
- Gibeaut, D.M.; Pauly, M.; Bacic, A.; Fincher, G.B. Changes in cell wall polysaccharides in developing barley (Hordeum vulgare) coleoptiles. Planta 2005, 221, 729–738. [Google Scholar] [CrossRef]
- Regand, A.; Chowdhury, Z.; Tosh, S.M.; Wolever, T.M.; Wood, P. The molecular weight, solubility and viscosity of oat beta-glucan affect human glycemic response by modifying starch digestibility. Food Chem. 2011, 129, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Choromanska, A.; Kulbacka, J.; Rembialkowska, N.; Pilat, J.; Oledzki, R.; Harasym, J.; Saczko, J. Anticancer properties of low molecular weight oat beta-glucan–an in vitro study. Int. J. Biol. Macromol. 2015, 80, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Hutmacher, D.W. Scaffolds in tissue engineering bone and cartilage. In The Biomaterials: Silver Jubilee Compendium; Elsevier: Amsterdam, The Netherlands, 2000; pp. 175–189. [Google Scholar]
- Christenson, E.M.; Anseth, K.S.; van den Beucken, J.J.; Chan, C.K.; Ercan, B.; Jansen, J.A.; Laurencin, C.T.; Li, W.J.; Murugan, R.; Nair, L.S. Nanobiomaterial applications in orthopedics. J. Orthop. Res. 2007, 25, 11–22. [Google Scholar] [CrossRef]
- Alzoreky, N.; Nakahara, K. Antibacterial activity of extracts from some edible plants commonly consumed in Asia. Int. J. Food Microbiol. 2003, 80, 223–230. [Google Scholar] [CrossRef]
- Repetto, G.; Del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, U.; Pattanayak, D.K.; Vijayalakshmi, U. Snail shell derived natural hydroxyapatite: Effects on NIH-3T3 cells for orthopedic applications. Mater. Manuf. Process. 2016, 31, 206–216. [Google Scholar] [CrossRef]
- Destainville, A.; Champion, E.; Bernache-Assollant, D.; Laborde, E. Synthesis, characterization and thermal behavior of apatitic tricalcium phosphate. Mater. Chem. Phys. 2003, 80, 269–277. [Google Scholar] [CrossRef]
- Rouahi, M.; Gallet, O.; Champion, E.; Dentzer, J.; Hardouin, P.; Anselme, K. Influence of hydroxyapatite microstructure on human bone cell response. J. Biomed. Mater. Res. Part A 2006, 78, 222–235. [Google Scholar] [CrossRef]
- Suchanek, W.L.; Byrappa, K.; Shuk, P.; Riman, R.E.; Janas, V.F.; TenHuisen, K.S. Preparation of magnesium-substituted hydroxyapatite powders by the mechanochemical–hydrothermal method. Biomaterials 2004, 25, 4647–4657. [Google Scholar] [CrossRef]
- Furuzono, T.; Yasuda, S.; Kimura, T.; Kyotani, S.; Tanaka, J.; Kishida, A. Nano-scaled hydroxyapatite/polymer composite IV. Fabrication and cell adhesion of a 3D scaffold made of composite material with a silk fibroin substrate to develop a percutaneous device. J. Artif. Organs 2004, 7, 137–144. [Google Scholar] [CrossRef]
- Kamal, H.; Abd-Elrahim, F.; Lotfy, S. Characterization and some properties of cellulose acetate-co-polyethylene oxide blends prepared by the use of gamma irradiation. J. Radiat. Res. Appl. Sci. 2014, 7, 146–153. [Google Scholar] [CrossRef]
- Srivastava, A.; Kumar, R. Synthesis and characterization of acrylic acid-g-(-carrageenan) copolymer and study of its application. Int. J. Carbohydr. Chem. 2013, 2013, 892615. [Google Scholar] [CrossRef]
- Sarker, B.; Papageorgiou, D.G.; Silva, R.; Zehnder, T.; Gul-E-Noor, F.; Bertmer, M.; Kaschta, J.; Chrissafis, K.; Detsch, R.; Boccaccini, A.R. Fabrication of alginate–gelatin crosslinked hydrogel microcapsules and evaluation of the microstructure and physico-chemical properties. J. Mater. Chem. B 2014, 2, 1470–1482. [Google Scholar] [CrossRef]
- Askari, M.B.; Banizi, Z.T.; Seifi, M.; Dehaghi, S.B.; Veisi, P. Synthesis of TiO2 nanoparticles and decorated multi-wall carbon nanotube (MWCNT) with anatase TiO2 nanoparticles and study of optical properties and structural characterization of TiO2/MWCNT nanocomposite. Opt. Int. J. Light Electron. Opt. 2017, 149, 447–454. [Google Scholar] [CrossRef]
- Abd-Khorsand, S.; Saber-Samandari, S.; Saber-Samandari, S. Development of nanocomposite scaffolds based on TiO2 doped in grafted chitosan/hydroxyapatite by freeze drying method and evaluation of biocompatibility. Int. J. Biol. Macromol. 2017, 101, 51–58. [Google Scholar] [CrossRef]
- Laurencin, C.T.; Nair, L.S. Nanotechnology and Regenerative Engineering: The Scaffold; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Saber-Samandari, S.; Yilmaz, O.; Yilmaz, E. Photoinduced graft copolymerization onto chitosan under heterogeneous conditions. J. Macromol. Sci. Part A 2012, 49, 591–598. [Google Scholar] [CrossRef]
- Ibrahim, S.; Sabudin, S.; Sahid, S.; Marzuke, M.; Hussin, Z.; Bashah, N.K.; Jamuna-Thevi, K. Bioactivity studies and adhesion of human osteoblast (hFOB) on silicon-biphasic calcium phosphate material. Saudi J. Biol. Sci. 2016, 23, S56–S63. [Google Scholar] [CrossRef]
- Dick, T.; Dos Santos, L. In Situ synthesis and characterization of hydroxyapatite/natural rubber composites for biomedical applications. Mater. Sci. Eng. C 2017, 77, 874–882. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Fan, Y.; Feng, Q.; Cui, F.Z.; Watari, F. Nanostructured scaffolds for bone tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 2424–2435. [Google Scholar] [CrossRef]
- Oh, S.H.; Park, I.K.; Kim, J.M.; Lee, J.H. In Vitro and in vivo characteristics of PCL scaffolds with pore size gradient fabricated by a centrifugation method. Biomaterials 2007, 28, 1664–1671. [Google Scholar] [CrossRef]
- Samanipour, F.; Bayati, M.; Zargar, H.; Golestani-Fard, F.; Troczynski, T.; Taheri, M. Electrophoretic enhanced micro arc oxidation of ZrO2–HAp–TiO2 nanostructured porous layers. J. Alloy. Compd. 2011, 509, 9351–9355. [Google Scholar] [CrossRef]
- Kaviyarasu, K.; Mariappan, A.; Neyvasagam, K.; Ayeshamariam, A.; Pandi, P.; Palanichamy, R.R.; Gopinathan, C.; Mola, G.T.; Maaza, M. Photocatalytic performance and antimicrobial activities of HAp-TiO2 nanocomposite thin films by sol-gel method. Surf. Interfaces 2017, 6, 247–255. [Google Scholar] [CrossRef]
- Tamaddon, M.; Samizadeh, S.; Wang, L.; Blunn, G.; Liu, C. Intrinsic Osteoinductivity of Porous Titanium Scaffold for Bone Tissue Engineering. Int. J. Biomater. 2017, 2017, 5093063. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Kaczmarek, B. Preparation and characterization of composites based on the blends of collagen, chitosan and hyaluronic acid with nano-hydroxyapatite. Int. J. Biol. Macromol. 2017, 102, 658–666. [Google Scholar] [CrossRef]
- Monfregola, L.; Bugatti, V.; Amodeo, P.; De Luca, S.; Vittoria, V. Physical and water sorption properties of chemically modified pectin with an environmentally friendly process. Biomacromolecules 2011, 12, 2311–2318. [Google Scholar] [CrossRef]
- Mohan, N.; Nair, P.D. Novel porous, polysaccharide scaffolds for tissue engineering applications. Trends Biomater. Artif. Organs 2005, 18, 219–224. [Google Scholar]
- Kim, C.H.; Khil, M.S.; Kim, H.Y.; Lee, H.U.; Jahng, K.Y. An improved hydrophilicity via electrospinning for enhanced cell attachment and proliferation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 78, 283–290. [Google Scholar] [CrossRef]
- Nazemi, K.; Moztarzadeh, F.; Jalali, N.; Asgari, S.; Mozafari, M. Synthesis and characterization of poly (lactic-co-glycolic) acid nanoparticles-loaded chitosan/bioactive glass scaffolds as a localized delivery system in the bone defects. BioMed Res. Int. 2014, 2014, 898930. [Google Scholar] [CrossRef]
- Bennet, D.; Marimuthu, M.; Kim, S.; An, J. Dual drug-loaded nanoparticles on self-integrated scaffold for controlled delivery. Int. J. Nanomed. 2012, 7, 3399. [Google Scholar]
- Maji, K.; Dasgupta, S.; Pramanik, K.; Bissoyi, A. Preparation and evaluation of gelatin-chitosan-nanobioglass 3D porous scaffold for bone tissue engineering. Int. J. Biomater. 2016, 2016, 9825659. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.; Cui, F.; Kim, T.; Kim, J. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef]
- Yamanaka, M.; Hara, K.; Kudo, J. Bactericidal actions of a silver ion solution on Escherichia coli, studied by energy-filtering transmission electron microscopy and proteomic analysis. Appl. Environ. Microbiol. 2005, 71, 7589–7593. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, X.; Zhou, X.; Wang, Q.; Shi, X.; Du, Y.; Deng, H.; Jiang, L. Characterization and cytotoxicity study of nanofibrous mats incorporating rectorite and carbon nanotubes. RSC Adv. 2014, 4, 33355–33361. [Google Scholar] [CrossRef]
- Zykwinska, A.; Tripon-Le Berre, L.; Sinquin, C.; Ropartz, D.; Rogniaux, H.; Colliec-Jouault, S.; Delbarre-Ladrat, C. Enzymatic depolymerization of the GY785 exopolysaccharide produced by the deep-sea hydrothermal bacterium Alteromonas infernus: Structural study and enzyme activity assessment. Carbohydr. Polym. 2018, 188, 101–107. [Google Scholar] [CrossRef] [PubMed]


| Samples | AgNO3 Concentration (M) | Pore Size (µm) | Porosity (%) |
|---|---|---|---|
| BNS1 | 0.15 | 175 ± 2 | 88.5 ± 2 |
| BNS2 | 0.30 | 135 ± 1 | 81.5 ± 1 |
| BNS3 | 0.45 | 115 ± 2 | 74.5 ± 2 |
| BNS4 | 0.60 | 92 ± 2 | 69.5 ± 1 |
| Sample | Angle (2ѳ) | FWMH | Lattice Strain (%) | d (Å) | D (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BNS1 | 31.1° | 39.69° | 0.70317 | 0.94232 | 0.0218 | 0.0115 | 3.620 | 2.45 | 12.25 | 9.36 | |
| HAp | Ag | ||||||||||
| BNS2 | 31.1° | 39.69° | 0.80141 | 0.96215 | 0.0208 | 0.0232 | 3.45 | 2.41 | 12.39 | 9.74 | |
| HAp | Ag | ||||||||||
| BNS3 | 31.1° | 39.69° | 0.8316 | 0.97137 | 0.0148 | 0.0212 | 3.36 | 2.32 | 12.95 | 9.82 | |
| HAp | Ag | ||||||||||
| BNS4 | 31.1° | 39.69° | 0.90317 | 0.99182 | 0.0217 | 0.0315 | 3.14 | 2.25 | 13.35 | 9.96 | |
| HAp | Ag | ||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, M.U.A.; Al-Thebaiti, M.A.; Hashmi, M.U.; Aftab, S.; Abd Razak, S.I.; Abu Hassan, S.; Abdul Kadir, M.R.; Amin, R. Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering. Materials 2020, 13, 971. https://doi.org/10.3390/ma13040971
Khan MUA, Al-Thebaiti MA, Hashmi MU, Aftab S, Abd Razak SI, Abu Hassan S, Abdul Kadir MR, Amin R. Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering. Materials. 2020; 13(4):971. https://doi.org/10.3390/ma13040971
Chicago/Turabian StyleKhan, Muhammad Umar Aslam, Mesfer A. Al-Thebaiti, Muhammad Uzair Hashmi, Saira Aftab, Saiful Izwan Abd Razak, Shukur Abu Hassan, Mohammed Rafiq Abdul Kadir, and Rashid Amin. 2020. "Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering" Materials 13, no. 4: 971. https://doi.org/10.3390/ma13040971
APA StyleKhan, M. U. A., Al-Thebaiti, M. A., Hashmi, M. U., Aftab, S., Abd Razak, S. I., Abu Hassan, S., Abdul Kadir, M. R., & Amin, R. (2020). Synthesis of Silver-Coated Bioactive Nanocomposite Scaffolds Based on Grafted Beta-Glucan/Hydroxyapatite via Freeze-Drying Method: Anti-Microbial and Biocompatibility Evaluation for Bone Tissue Engineering. Materials, 13(4), 971. https://doi.org/10.3390/ma13040971








