Enhancing the Corrosion Resistance Performance of Mg-1.8Zn-1.74Gd-0.5Y-0.4Zr Biomaterial via Solution Treatment Process
Abstract
1. Introduction
2. Experimental Details
3. Results and Discussion
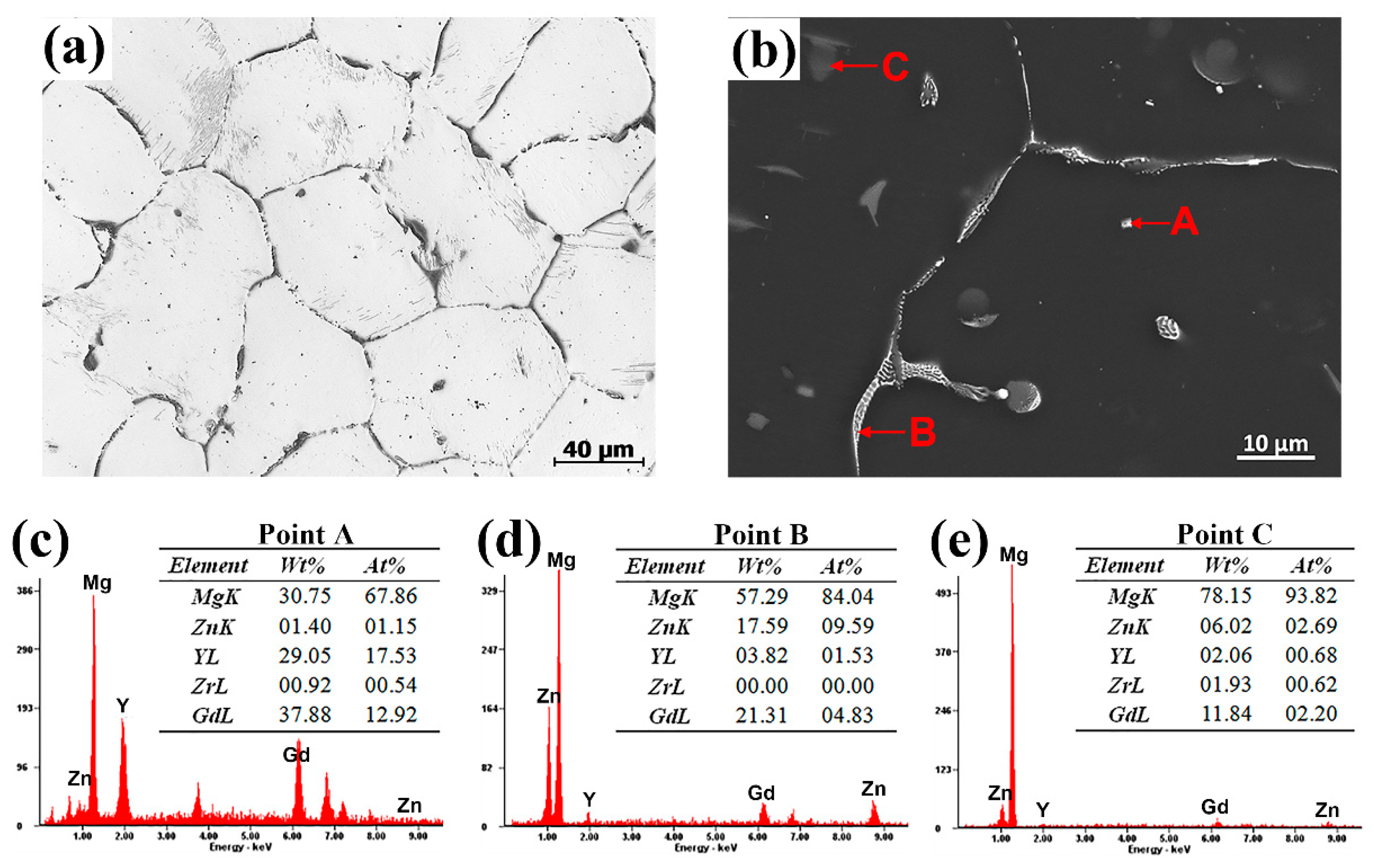
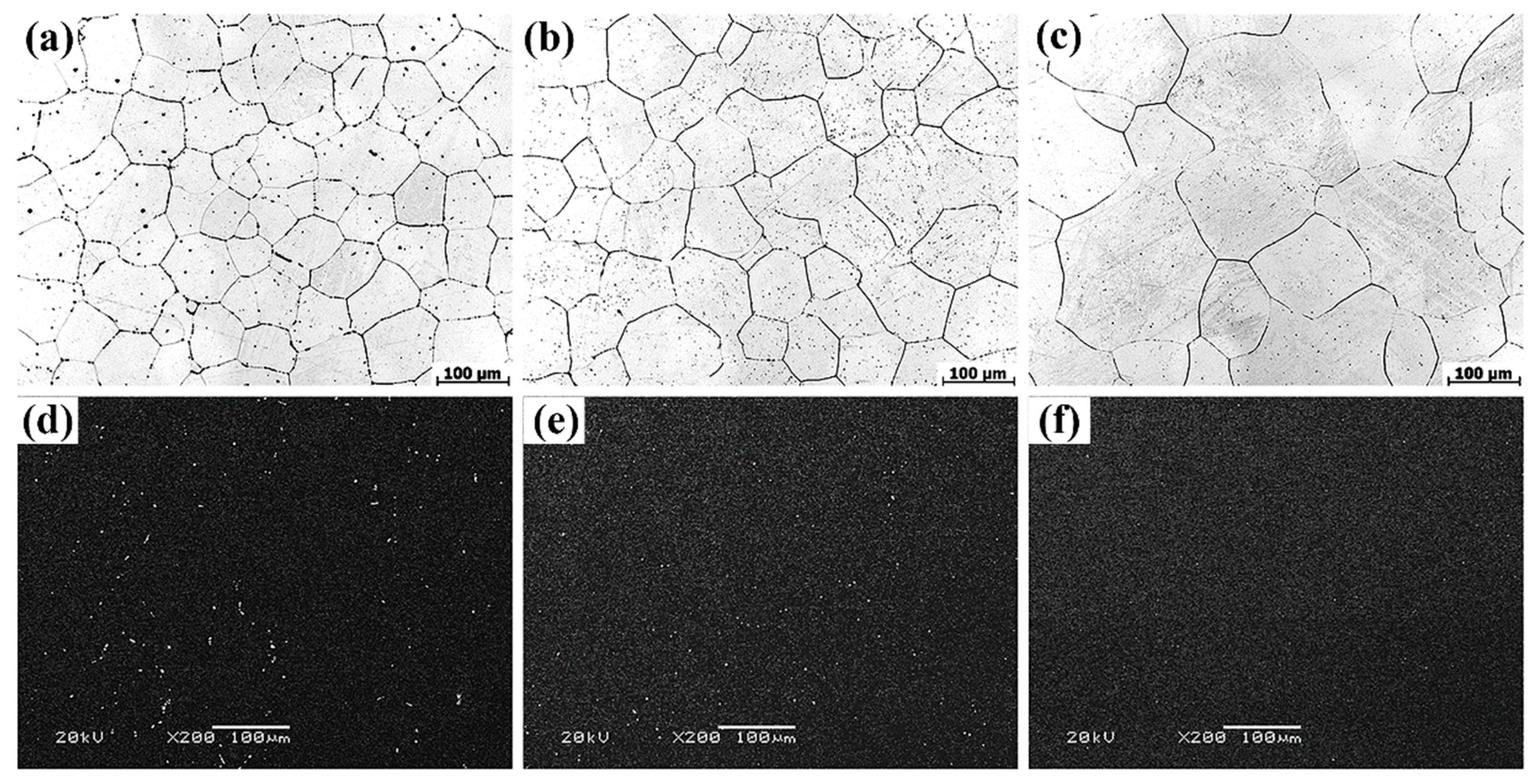
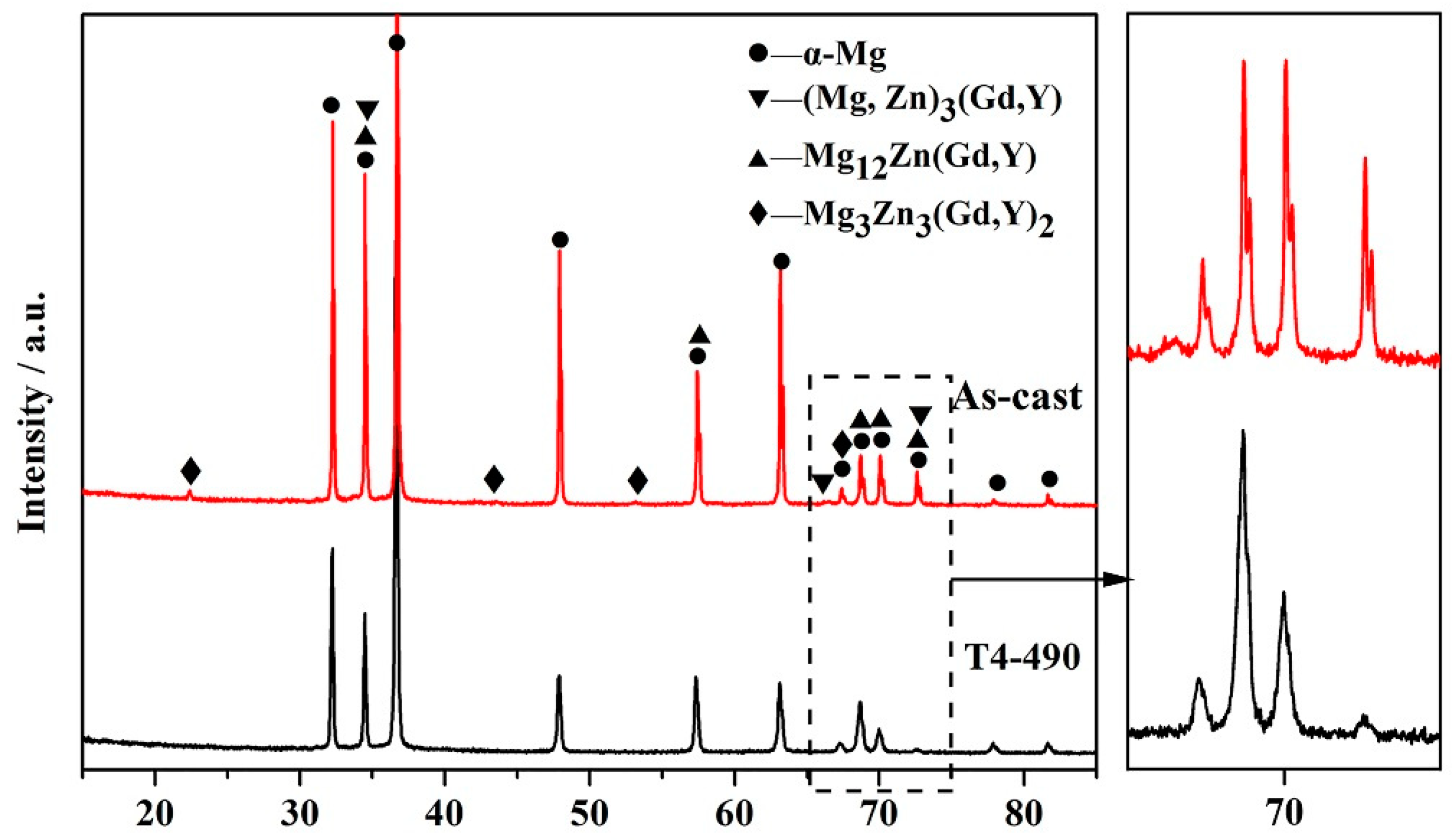
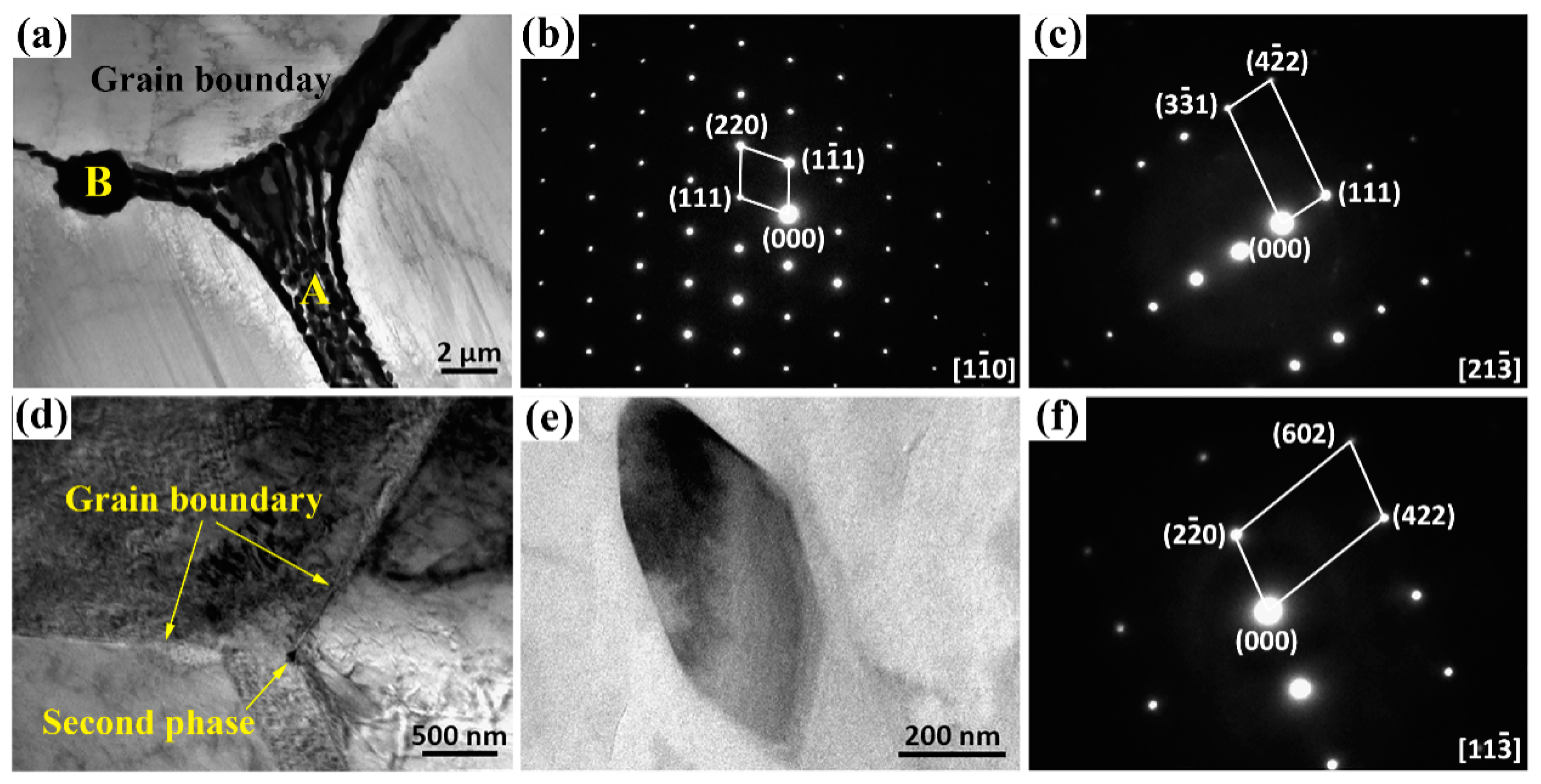
3.1. Microstructures Analysis
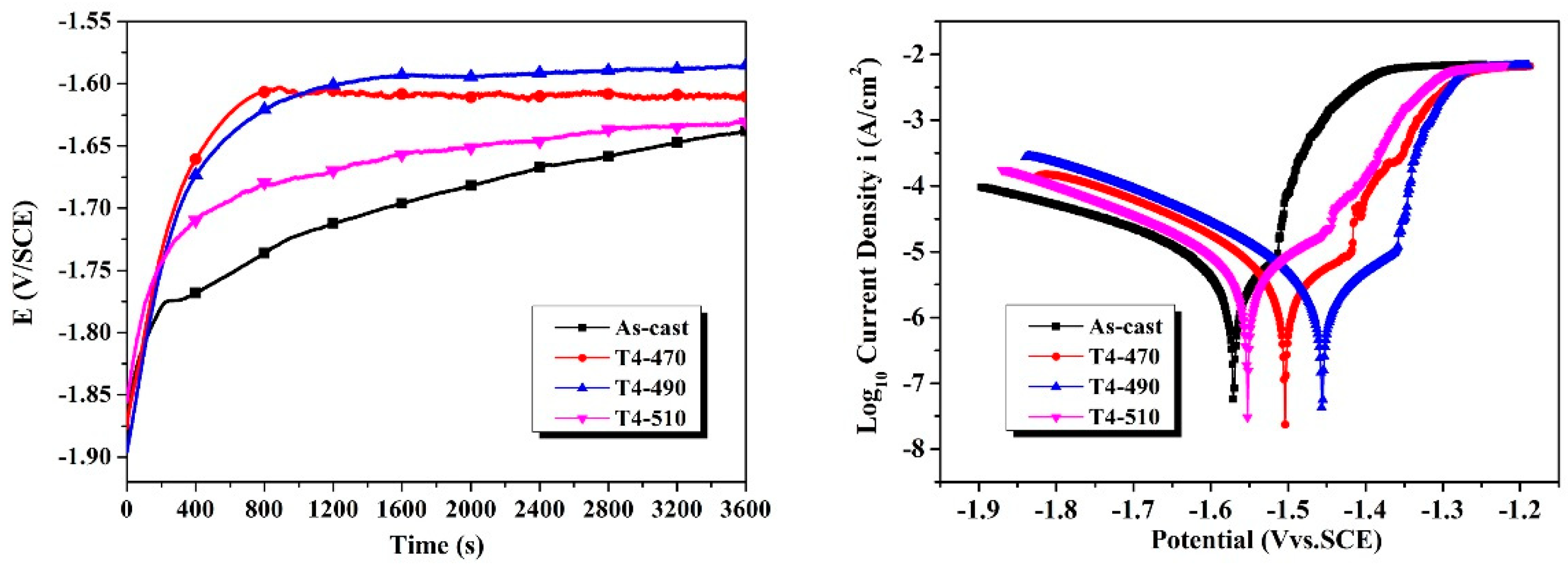
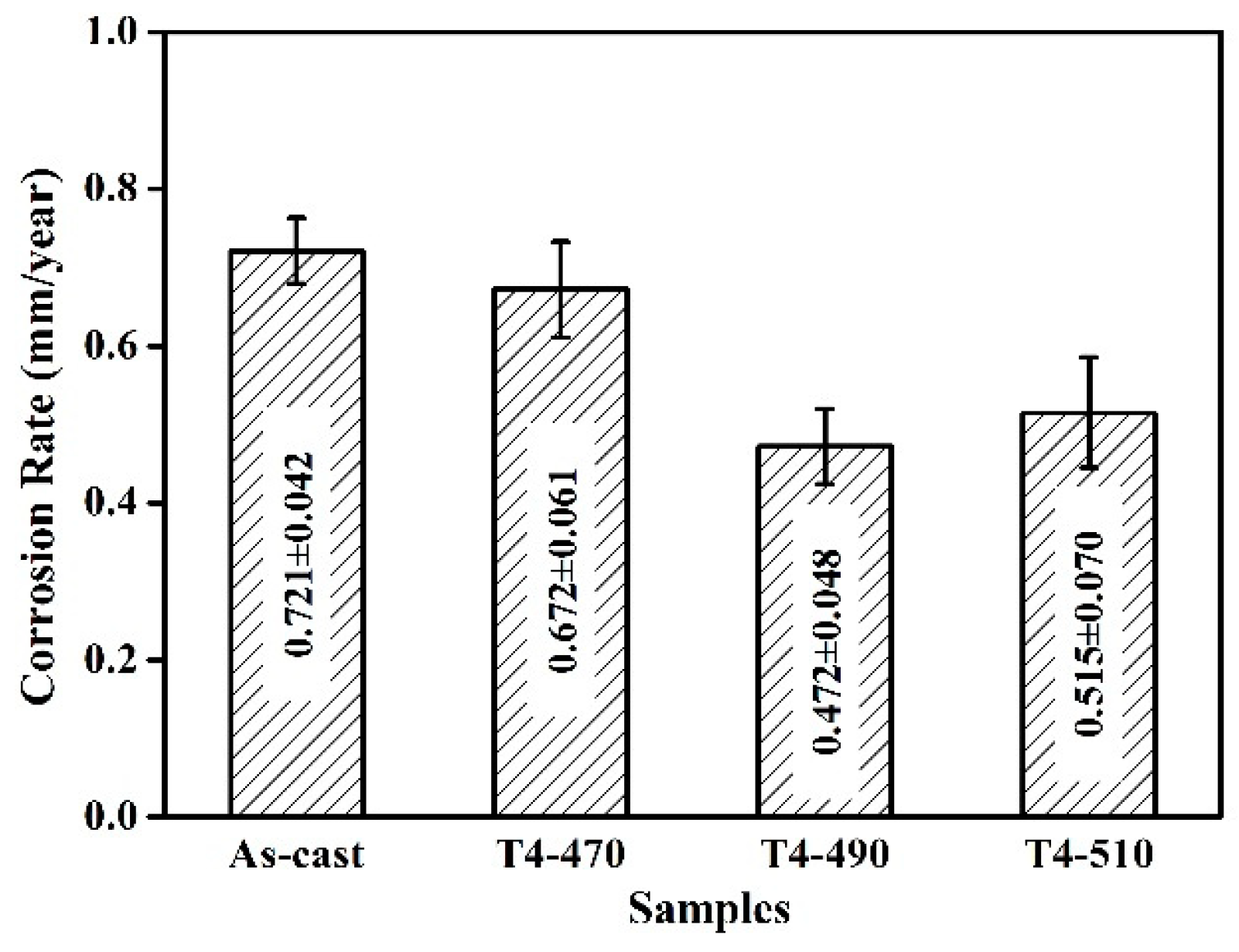
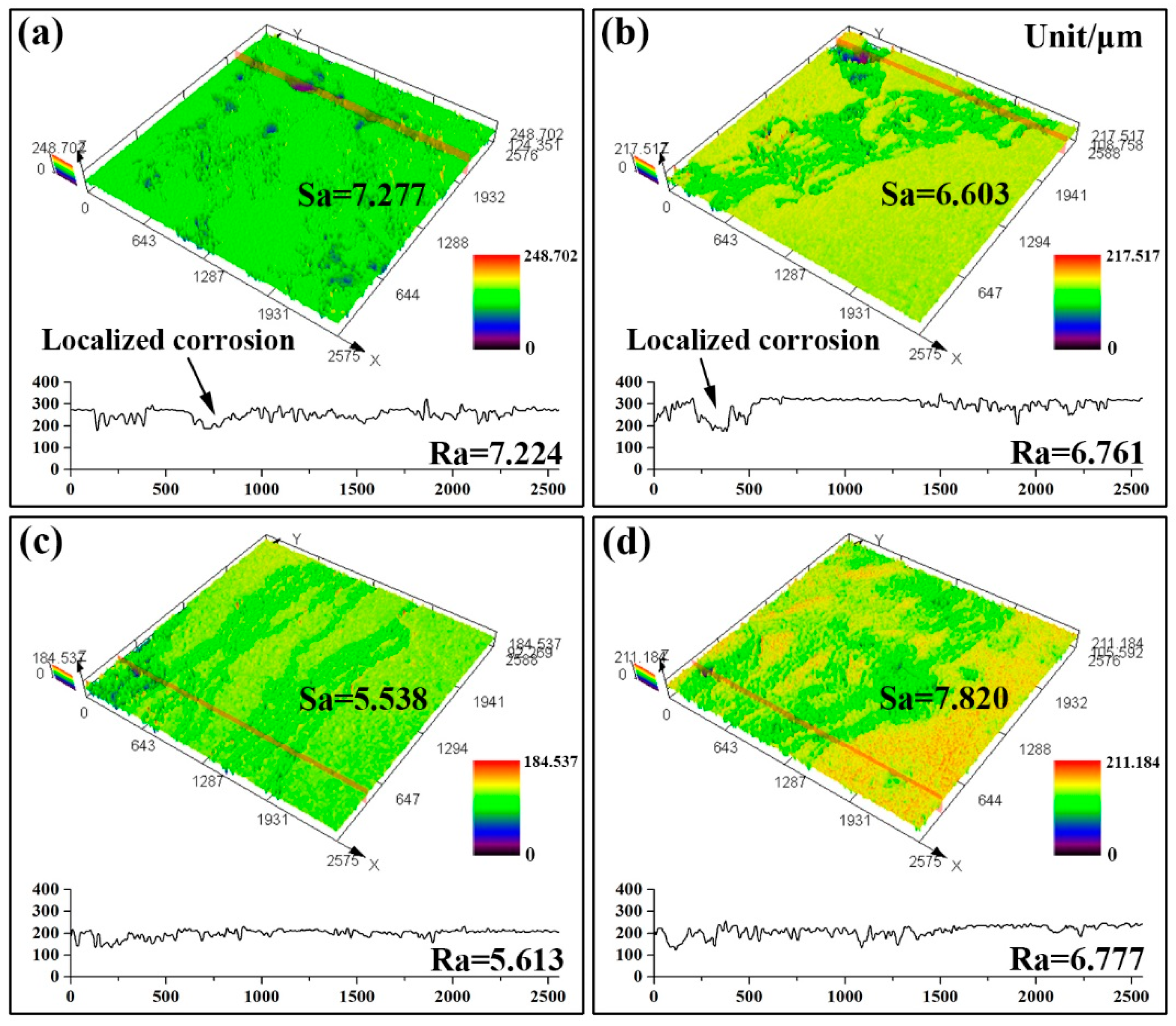
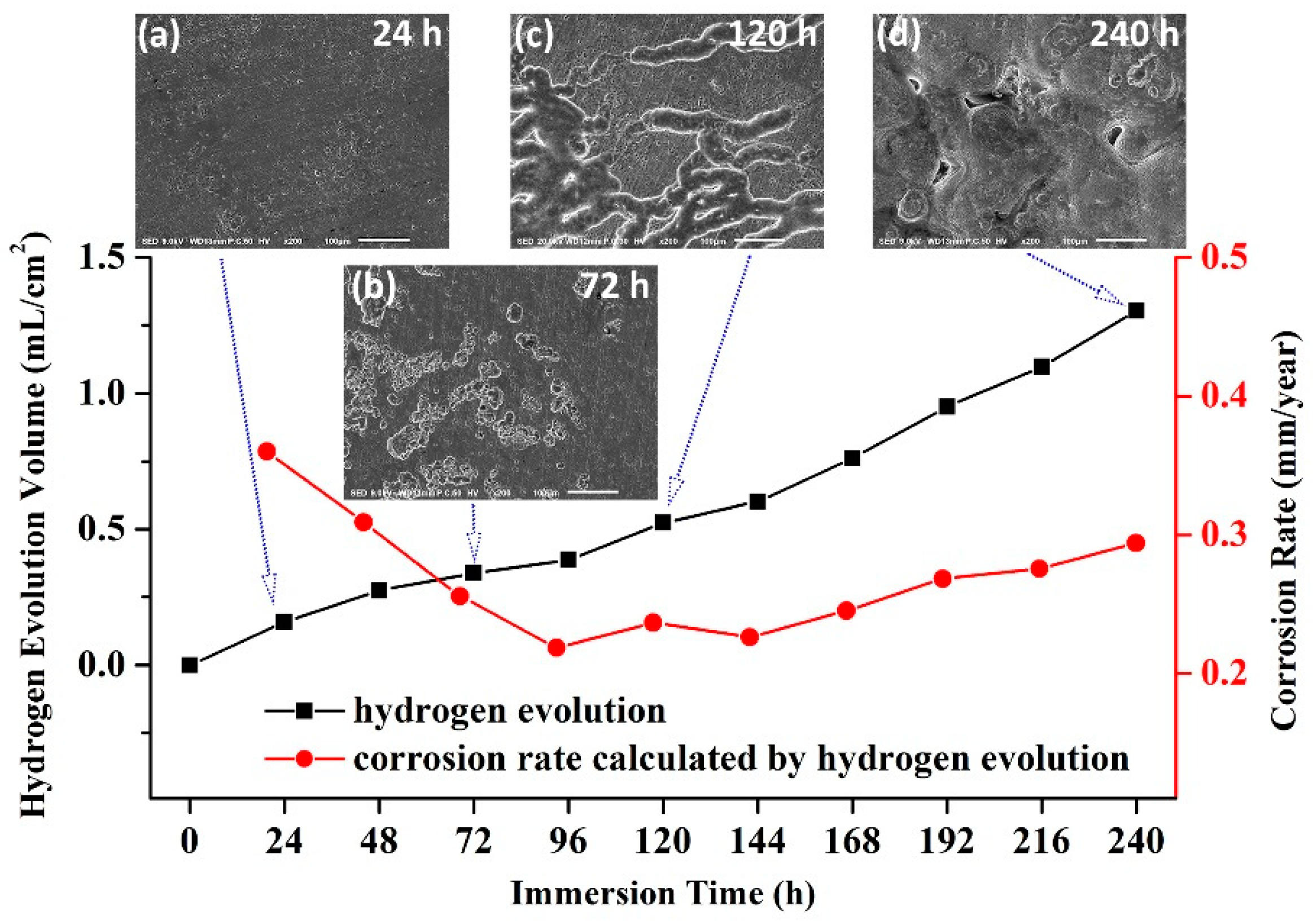
3.2. Electrochemical Tests and Immersion Tests
4. Conclusions
- (1)
- The as-cast Mg-Zn-Gd-Y-Zr alloy is mainly composed of α-Mg, lamellar structure Mg12Zn(Gd,Y) phase, skeleton-like Mg3Zn3(Gd,Y)2 phase and cubic-like (Mg,Zn)3(Gd,Y) phase. At the range of 470 °C~510 °C, Mg12Zn(Gd,Y) phase completely dissolves with a small amount of residual similar ellipse phase left. The volume fraction of second phase decreases significantly from 3.07% ± 0.37% of as-cast alloy to 0.70% ± 0.23% of T4-470, 0.34% ± 0.13% of T4-490 and 0.11% ± 0.03% of T4-510.
- (2)
- After solution treatment, with the increase of the matrix potential and the decrease of the residual second phase, the sample shows better corrosion resistance. The remaining phase of solution-treated at 490 °C for 8 h exhibits relatively homogeneous dissolution. The corrosion rate and surface roughness are 0.472 ± 0.048 mm/year and 5.538 μm, respectively.
- (3)
- For all the investigated samples, the T4-490 sample exhibits much better corrosion resistance and more uniform corrosion characteristic. With the extension of immersing time to 240 h, corrosion rate tends to be stable with a more uniform corrosion morphology.
Author Contributions
Funding
Conflicts of Interest
References
- Song, M.S.; Zeng, R.C.; Ding, Y.F.; Li, R.W.; Easton, M.; Cole, I.; Birbilis, N.; Chen, X.B. Recent advances in biodegradation controls over Mg alloys for bone fracture management: A review. J. Mater. Sci. Technol. 2019, 35, 535–544. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Daroonparvar, M.; Saud, S.N.; Abdul-kadir, M.R. Bi-layer nano-TiO2/FHA composite coatings on Mg–Zn–Ce alloy prepared by combined physical vapour deposition and electrochemical deposition methods. Vacuum 2014, 110, 127–135. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, H.; Wang, L.; Zhu, S.; Guan, S. Microstructure, mechanical properties and deformation mechanisms of an as-cast Mg–Zn–Y–Nd–Zr alloy for stent applications. J. Mater. Sci. Technol. 2019, 35, 1211–1217. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Liaw, P.K.; Xu, Y.; Lai, H. Effects of heat treatment on the mechanical properties and corrosion behaviour of the Mg-2Zn-0.2Mn-xNd alloys. J. Alloys Compd. 2018, 769, 552–565. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Sheng, Y.; Wang, X.; Zhao, X.; Liu, H.; Li, W. Effect of the Microstructure and Distribution of the Second Phase on the Stress Corrosion Cracking of Biomedical Mg-Zn-Zr-xSr Alloys. Materials 2018, 11, 551. [Google Scholar] [CrossRef]
- Yao, H.; Wen, J.; Xiong, Y.; Liu, Y.; Lu, Y.; Cao, W. Microstructures, mechanical properties, and corrosion behavior of as-cast Mg-2.0Zn-0.5Zr-xGd (wt.%) biodegradable alloys. Materials 2018, 11, 1564. [Google Scholar] [CrossRef]
- Jafari, H.; Rahimi, F.; Sheikhsofla, Z. In vitro corrosion behavior of Mg-5Zn alloy containing low Y contents. Mater. Corros. 2016, 67, 396–405. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Li, J. Microstructure, mechanical properties, corrosion behavior and film formation mechanism of Mg-Zn-Mn-xNd in Kokubo’s solution. J. Alloys Compd. 2018, 730, 458–470. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Hamzah, E.; Kasiri-Asgarani, M.; Saud, S.N.; Yaghoubidoust, F.; Akbari, E. Structure, corrosion behavior, and antibacterial properties of nanosilicagraphene oxide coating on biodegradable magnesium alloy for biomedical applications. Vacuum 2016, 131, 106–110. [Google Scholar] [CrossRef]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drucker, H.; Vogt, C.; Hort, N. Evaluation of short-term effects of rare earth and other elements used in magnesium alloys on primary cells and cell lines. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- Jamesh, M.I.; Wu, G.S.; Zhao, Y.; McKenzie, D.R.; Bilek, M.M.M.; Chu, P.K. Effects of zirconium and oxygen plasma ion implantation on the corrosion behavior of ZK60 Mg in simulated body fluids. Corros. Sci. 2014, 82, 7–26. [Google Scholar] [CrossRef]
- Witte, F.; Fischer, J.; Nellesen, J.; Crostack, H.; Kaese, V.; Pisch, A.; Beckmann, F.; Windhagen, H. In vitro and in vivo corrosion measurements of magnesium alloys. Biomaterials 2006, 27, 1013–1018. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Liu, Y.; Ding, W.; Lu, C. Effects of extrusion on the microstructure and mechanical properties of Mg-Zn-Gd alloy reinforced with quasicrystalline particles. Mater. Sci. Eng. A 2008, 474, 348–354. [Google Scholar] [CrossRef]
- Yang, J.; Wang, L.D.; Wang, L.M.; Zhang, H.J. Microstructures and mechanical properties of the Mg–4.5Zn–xGd (x = 0, 2, 3 and 5) alloys. J. Alloys Comp. 2008, 459, 274–280. [Google Scholar] [CrossRef]
- Liu, M.; Schmutz, P.; Uggowitzer, P.; Song, G.; Atrens, A. The influence of yttrium (Y) on the corrosion of Mg-Y binary alloys. Corros. Sci. 2010, 52, 3687–3701. [Google Scholar] [CrossRef]
- Ding, Y.; Wen, C.; Hodgson, P.; Li, Y. Effects of alloying elements on the corrosion behavior and biocompatibility of biodegradable magnesium alloys: A review. J. Mater. Chem. B 2014, 2, 1912–1933. [Google Scholar] [CrossRef]
- Barylski, A.; Kupka, M.; Aniołek, K.; Rak, J. The effect of precipitation hardening on the structure and mechanical and tribological properties of magnesium alloy WE54. Vacuum 2017, 129, 77–86. [Google Scholar] [CrossRef]
- Jafari, H.; Heidari, E.; Barabi, A.; Dashti Kheirabadi, M. Effect of Phase Transformation During Long-Term Solution Treatment on Microstructure, Mechanical Properties, and Bio-corrosion Behavior of Mg-5Zn-1.5Y Cast Alloy. Acta Metall. Sin. 2018, 31, 561–574. [Google Scholar] [CrossRef]
- Li, J.; Barrirero, J.; Sha, G.; Aboulfadl, H.; Mücklich, F.; Schumacher, P. Precipitation hardening of an Mg-5Zn-2Gd-0.4Zr (wt.%) alloy. Acta Mater. 2016, 108, 207–218. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Wu, S.; Lü, S. Effects of heat treatment on microstructure evolution and mechanical properties of Mg-6Zn-1.4Y-0.6Zr alloy. China Foundry 2017, 14, 199–204. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, J.; Zong, X.; Liu, W.; Xu, C. Spheroidizing Behavior and Spheroidizing Kinetics of W-phase During Solid-Solution Treatment in Mg-Zn-Y-Mn-(B) Alloys. Acta Metall. Sin. 2017, 30, 464–469. [Google Scholar] [CrossRef]
- Liu, S.J.; Yang, G.Y.; Luo, S.F.; Jie, W.Q. Microstructure and mechanical properties of sand mold cast Mg-4.58Zn-2.6Gd-0.18Zr magnesium alloy after different heat treatments. J. Alloys Compd. 2015, 644, 846–853. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Q.; Chen, F.; Wu, Y.; Wang, Z.; Wang, Q. Relation between LPSO structure and biocorrosion behavior of biodegradable GZ51K alloy. Mater. Lett. 2015, 138, 212–215. [Google Scholar] [CrossRef]
- Saha, P.; Roy, M.; Datta, M.K.; Lee, B.; Kumta, P.N. Effects of grain refinement on the biocorrosion and in vitro bioactivity of magnesium. Mater. Sci. Eng. A 2015, 57, 294–303. [Google Scholar] [CrossRef]
- Alvarez-Lopez, M.; Pereda, M.D.; Valle, J.A.; Fernandez-Lorenzo, M.; Garcia-Alonso, M.C.; Ruano, O.A.; Escudero, M.L. Corrosion behavior of AZ31 magnesium alloy with different grain sizes in simulated biological fluids. Acta Biomater. 2010, 6, 1763–1771. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.; He, J.; Li, H. Enhanced mechanical properties and corrosion resistance of biodegradable Mg-Zn-Zr-Gd alloy by Y microalloying. J. Mater. Sci. 2020, 55, 1813–1825. [Google Scholar] [CrossRef]
- Wu, Y.J.; Xu, C.; Zheng, F.Y.; Peng, L.M.; Zhang, Y.; Ding, W.J. Formation and characterization of microstructure of as-cast Mg–6Gd–4Y–xZn–0.5Zr (x = 0.3, 0.5 and 0.7 wt.%) alloys. Mater. Charact. 2013, 79, 93–99. [Google Scholar] [CrossRef]
- Liu, X.B.; Shan, D.Y.; Song, Y.W.; Chen, R.S.; Han, E.N. Influences of the quantity of Mg2Sn phase on the corrosion behavior of Mg–7Sn magnesium alloy. Electrochim. Acta 2011, 56, 2582–2590. [Google Scholar] [CrossRef]
- Miao, H.; Huang, H.; Shi, Y.; Zhang, H.; Pei, J.; Yuan, G. Effects of solution treatment before extrusion on the microstructure, mechanical properties and corrosion of Mg-Zn-Gd alloy in vitro. Corros. Sci. 2017, 122, 90–99. [Google Scholar] [CrossRef]
- Janbozorgi, M.; Taheri, K.K.; Taheri, A.K. Microstructural evolution, mechanical properties, and corrosion resistance of a heat-treated Mg alloy for the bio-medical application. J. Magnes. Alloys 2019, 7, 80–89. [Google Scholar] [CrossRef]
- Zhang, X.B.; Yuan, G.Y.; Fang, X.X.; Wang, Z.Z.; Zhang, T. Effects of solution treatment on yield ratio and biocorrosion behaviour of as-extruded Mg–2.7Nd–0.2Zn–0.4Zr alloy for cardiovascular stent application. Mater. Technol. 2013, 28, 155–158. [Google Scholar] [CrossRef]
- Li, J.; Zhang, N.; Wang, X.; Wu, D.; Chen, R. Effect of Solution Treatment on Microstructure and Corrosion Properties of Mg-4Gd-1Y-1Zn-0.5Ca-1Zr Alloy. Acta Metall. Sin. 2018, 31, 189–198. [Google Scholar] [CrossRef]
- Cai, C.; Song, R.; Wang, L.; Li, J. Surface corrosion behavior and reaction product film deposition mechanism of Mg-Zn-Zr-Nd alloys during degradation process in Hank’s solution. Surf. Coat. Technol. 2018, 342, 57–68. [Google Scholar] [CrossRef]
| Samples | As-cast | T4-470 | T4-490 | T4-510 |
|---|---|---|---|---|
| Grain size (μm) | 68.1 ± 6.6 | 79.0 ± 1.4 | 95.1 ± 3.8 | 112.5 ± 10.5 |
| Volume fraction of second phase (%) | 3.07 ± 0.37 | 0.70 ± 0.23 | 0.34 ± 0.13 | 0.11 ± 0.03 |
| Samples | Ecorr (V) | Eb (V) | −βc (V/dec) | Icorr (μA/cm2) | Pi (mm/year) |
|---|---|---|---|---|---|
| As-cast | −1.571 | −1.514 | 0.167 | 4.688 | 0.107 |
| T4-470 | −1.504 | −1.423 | 0.160 | 4.338 | 0.099 |
| T4-490 | −1.457 | −1.361 | 0.149 | 3.102 | 0.071 |
| T4-510 | −1.522 | −1.472 | 0.169 | 5.334 | 0.122 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Wen, J.; Yao, H.; He, J.; Li, H. Enhancing the Corrosion Resistance Performance of Mg-1.8Zn-1.74Gd-0.5Y-0.4Zr Biomaterial via Solution Treatment Process. Materials 2020, 13, 836. https://doi.org/10.3390/ma13040836
Liu Y, Wen J, Yao H, He J, Li H. Enhancing the Corrosion Resistance Performance of Mg-1.8Zn-1.74Gd-0.5Y-0.4Zr Biomaterial via Solution Treatment Process. Materials. 2020; 13(4):836. https://doi.org/10.3390/ma13040836
Chicago/Turabian StyleLiu, Ya, Jiuba Wen, Huai Yao, Junguang He, and Huan Li. 2020. "Enhancing the Corrosion Resistance Performance of Mg-1.8Zn-1.74Gd-0.5Y-0.4Zr Biomaterial via Solution Treatment Process" Materials 13, no. 4: 836. https://doi.org/10.3390/ma13040836
APA StyleLiu, Y., Wen, J., Yao, H., He, J., & Li, H. (2020). Enhancing the Corrosion Resistance Performance of Mg-1.8Zn-1.74Gd-0.5Y-0.4Zr Biomaterial via Solution Treatment Process. Materials, 13(4), 836. https://doi.org/10.3390/ma13040836





