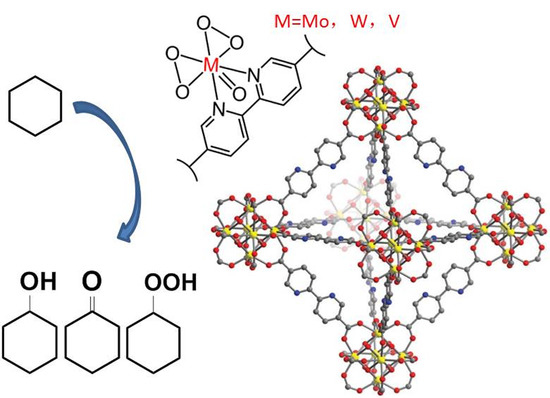
Transition Metal Oxodiperoxo Complex Modified Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Cyclohexane
Abstract
1. Introduction
2. Material and Methods
2.1. Material
2.2. Catalyst Preparation
2.2.1. Synthesis of UiO-67-bpydc
2.2.2. Synthesis of UiO-67-MoO(O2)2
2.2.3. Synthesis of UiO-67-WO(O2)2
2.2.4. Synthesis of UiO-67-KVO(O2)2
2.3. Catalytic Oxidation of Cyclohexane
2.4. Product Analysis
- (1)
- CyOH and Cyone
- (2)
- Peroxide
- (3)
- CHHP
3. Results and Discussion
3.1. Synthesis and Characterization
3.1.1. Synthesis
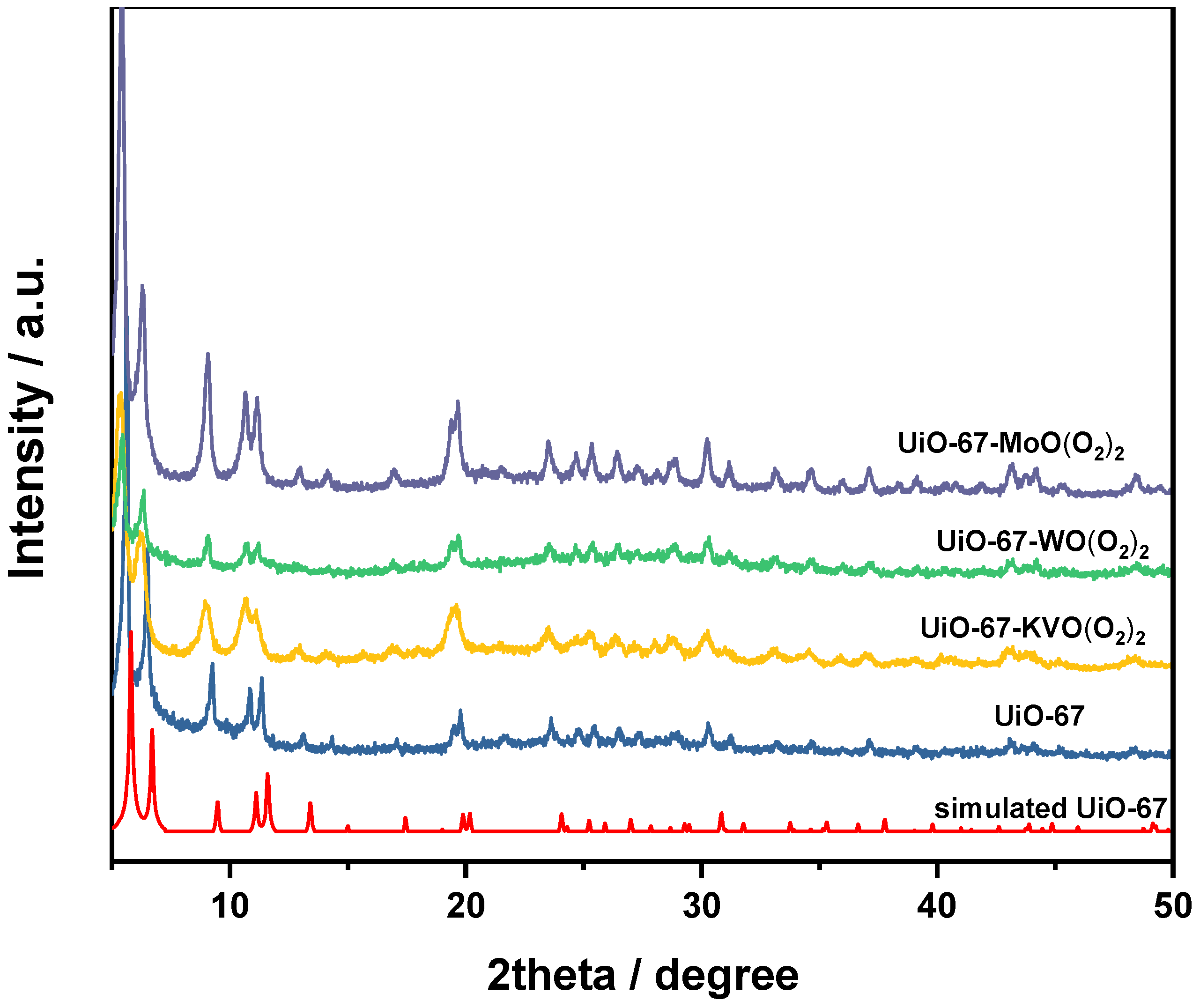
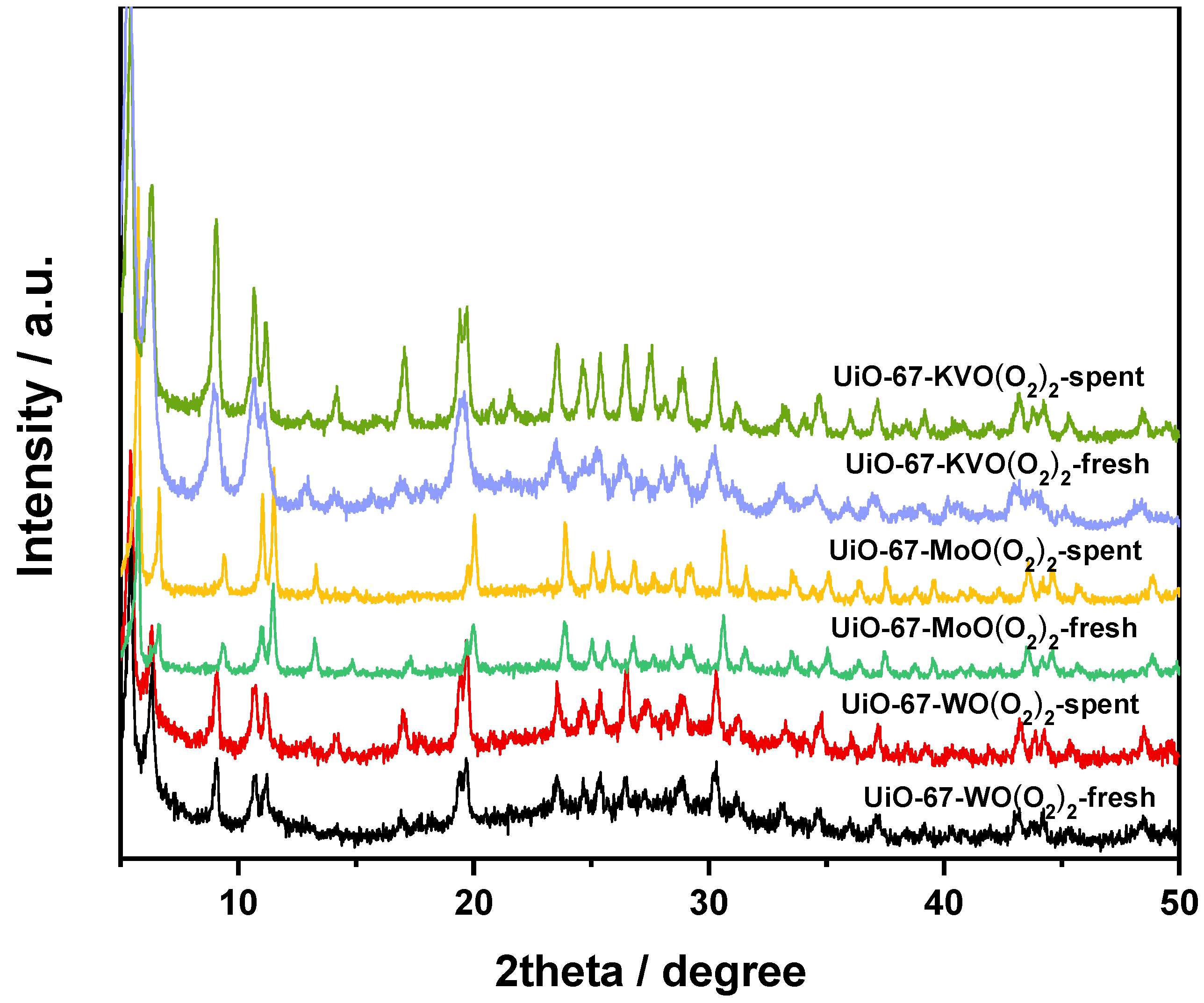
3.1.2. XRD Characterization
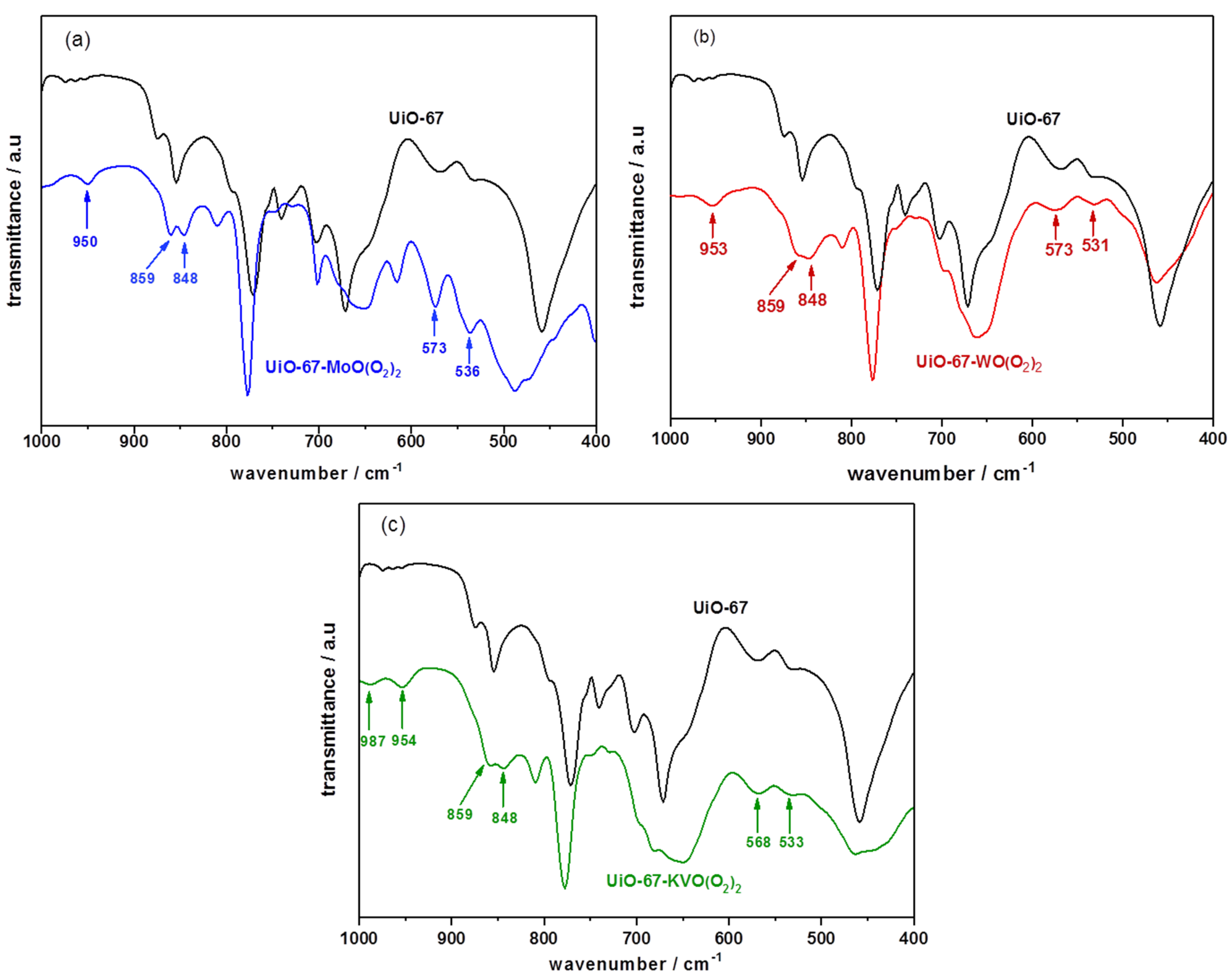
3.1.3. Nitrogen Physical Adsorption, ICP-AES, and FTIR Studies
3.1.4. SEM
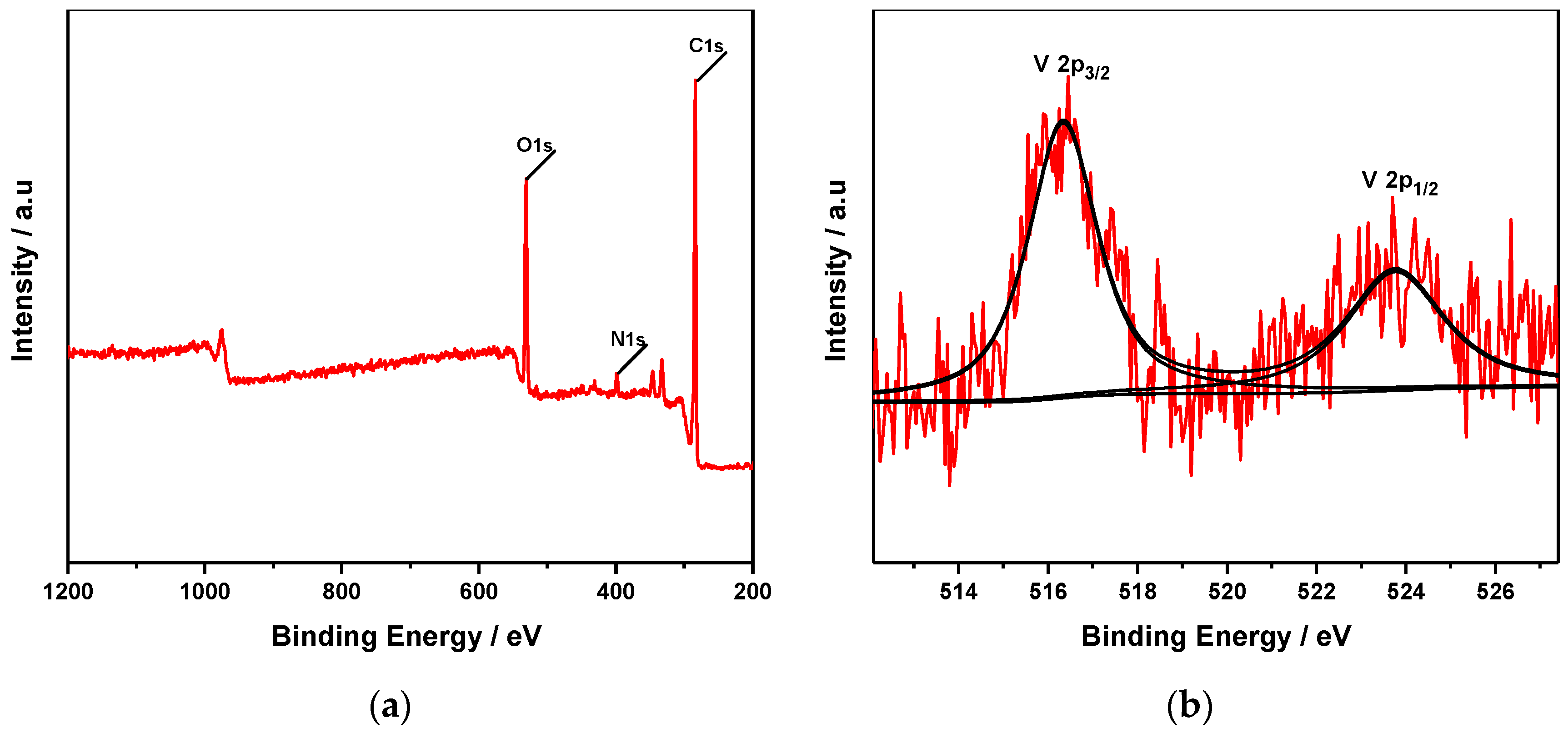
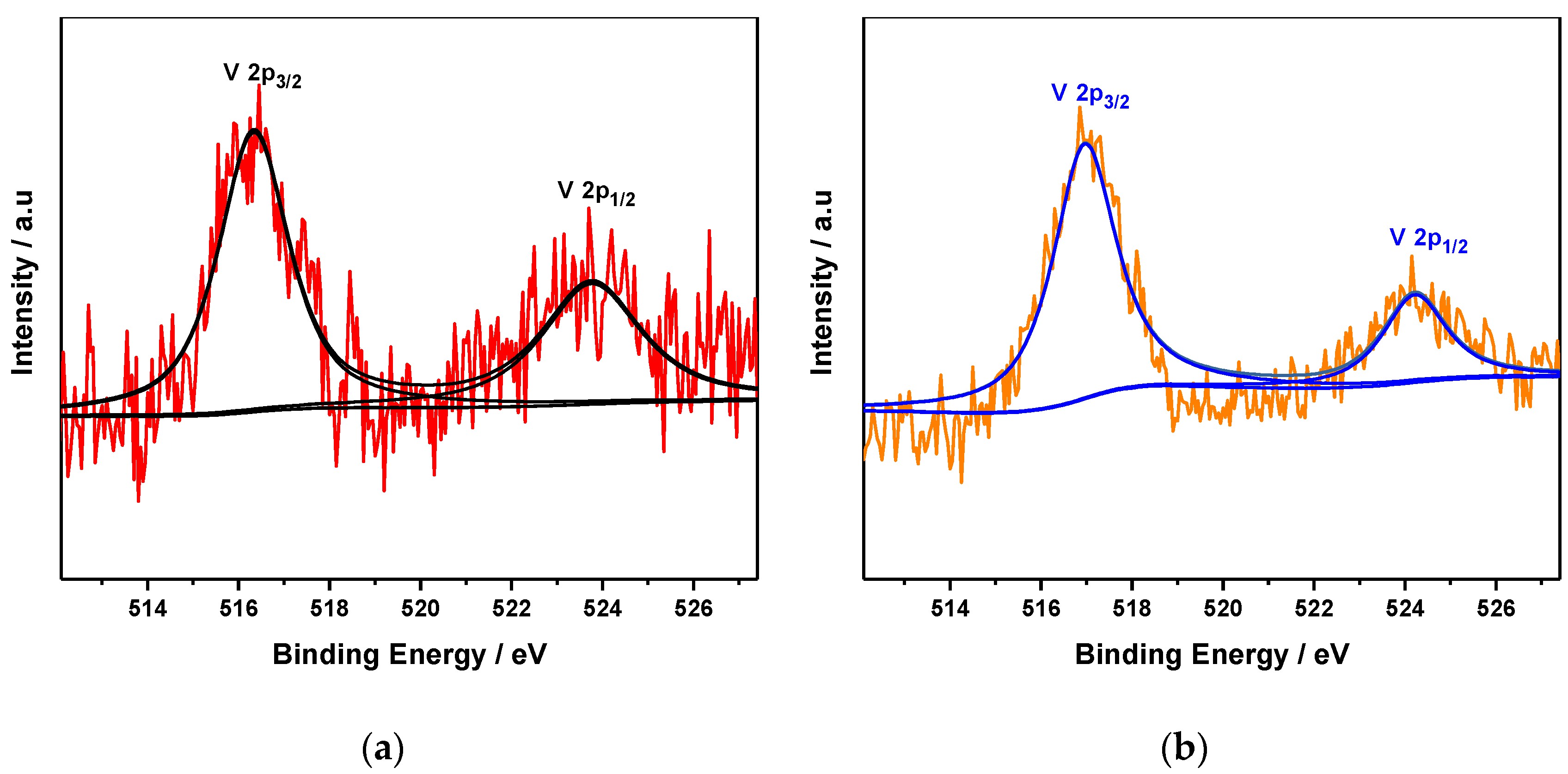
3.1.5. X-ray Photoelectron Spectroscopy
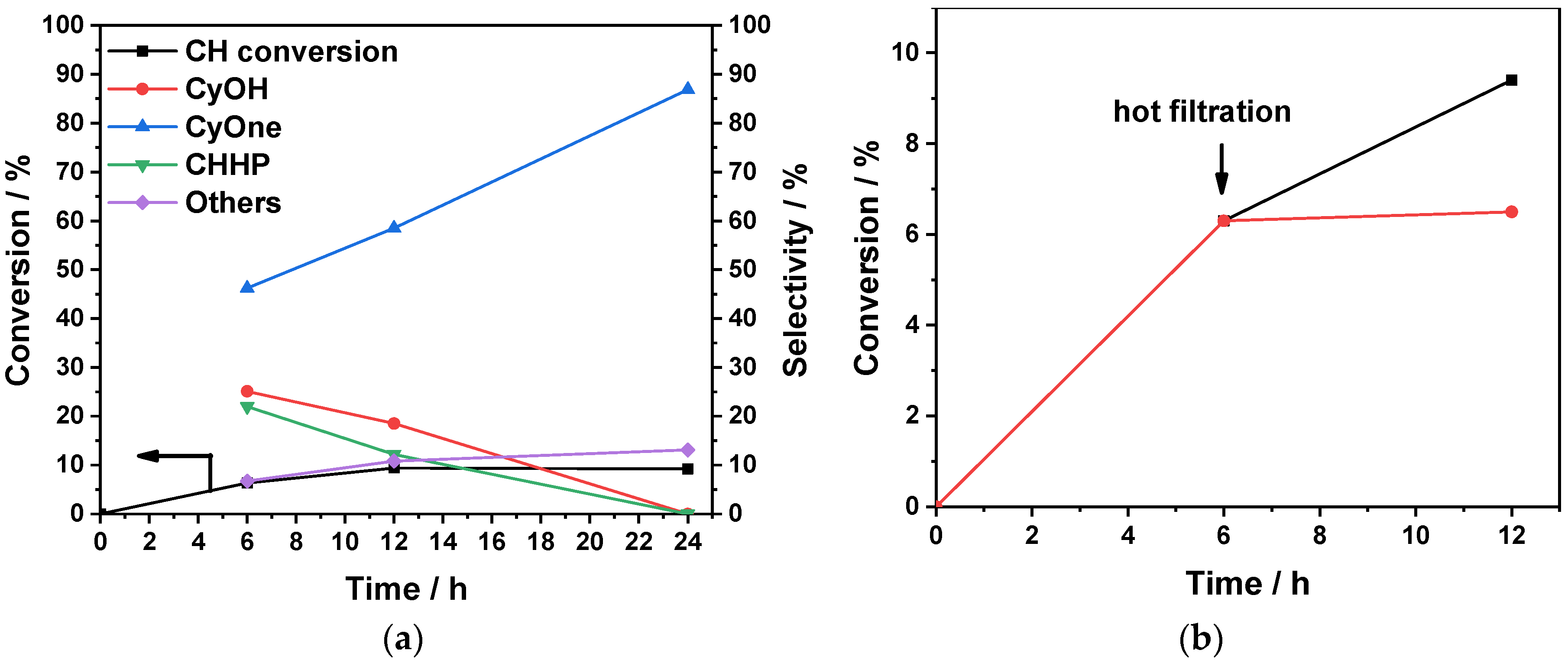
3.2. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Long, J.; Wang, L.; Gao, X.; Bai, C.; Jiang, H.; Li, Y. Activation of molecular oxygen by a metal–organic framework with open 2,2′-bipyridine for selective oxidation of saturated hydrocarbons. Chem. Commun. 2012, 48, 12109–12111. [Google Scholar] [CrossRef] [PubMed]
- Perkas, N.; Koltypin, Y.; Palchik, O.; Gedanken, A.; Chandrasekaran, S. Oxidation of cyclohexane with nanostructured amorphous catalysts under mild conditions. Appl. Catal. A Gen. 2001, 209, 125–130. [Google Scholar] [CrossRef]
- Kumar, R.; Sithambaram, S.; Suib, S.L. Cyclohexane oxidation catalyzed by manganese oxide octahedral molecular sieves—Effect of acidity of the catalyst. J. Catal. 2009, 262, 304–313. [Google Scholar] [CrossRef]
- Schuchardt, U.; Cardoso, D.; Sercheli, R.; Pereira, R.; Da Cruz, R.S.; Guerreiro, M.C.; Mandelli, D.; Spinacé, E.V.; Pires, E.L. Cyclohexane oxidation continues to be a challenge. Appl. Catal. A Gen. 2001, 211, 1–17. [Google Scholar] [CrossRef]
- Jing, B.; LI, J.; Qin, Z. Selective oxidation of cyclohexane over Co-APO-5: Effects of solvent and modification method on the catalytic performance. J. Fuel Chem. Technol. 2016, 44, 1249–1258. [Google Scholar] [CrossRef]
- Wang, J.; Ding, F.; Ma, J.; Liu, Q.; Cheng, J.; Dong, Y. Co(II)-MOF: A Highly Efficient Organic Oxidation Catalyst with Open Metal Sites. Inorg. Chem. 2015, 54, 10865–10872. [Google Scholar] [CrossRef]
- Wu, P.; Cao, Y.; Wang, Y.; Xing, W.; Zhong, Z.; Bai, P.; Yan, Z. Ultrastable bimetallic catalyst with tuned surface electronic properties for highly selective oxidation of cyclohexane. Appl. Surf. Sci. 2018, 457, 580–590. [Google Scholar] [CrossRef]
- Sun, L.; Liu, J.; Luo, W.; Yang, Y.; Wang, F.; Weerakkody, C.; Suib, S.L. Preparation of amorphous copper-chromium oxides catalysts for selective oxidation of cyclohexane. Mol. Catal. 2018, 460, 16–26. [Google Scholar] [CrossRef]
- Maksimchuk, N.V.; Kovalenko, K.A.; Fedin, V.P.; Kholdeeva, O.A. Cyclohexane selective oxidation over metal–organic frameworks of MIL-101 family: Superior catalytic activity and selectivity. Chem. Commun. 2012, 48, 6812–6814. [Google Scholar] [CrossRef]
- Acharyya, S.S.; Ghosh, S.; Adak, S.; Tripathi, D.; Bal, R. Fabrication of CuCr2O4 spinel nanoparticles: A potential catalyst for the selective oxidation of cycloalkanes via activation of Csp3–H bond. Catal. Commun. 2015, 59, 145–150. [Google Scholar] [CrossRef]
- Goldstein, A.S.; Beer, R.H.; Drago, R.S. Catalytic Oxidation of Hydrocarbons with O2 or H2O2 Using a Sterically Hindered Ruthenium Complex. J. Am. Chem. Soc. 1994, 116, 2424–2429. [Google Scholar] [CrossRef]
- Martins, N.M.R.; Pombeiro, A.J.L.; Martins, L.M.D.R.S. Green oxidation of cyclohexane catalyzed by recyclable magnetic transition-metal silica coated nanoparticles. Catal. Commun. 2019, 125, 15–20. [Google Scholar] [CrossRef]
- Hereijgers, B.P.C.; Weckhuysen, B.M. Aerobic oxidation of cyclohexane by gold-based catalysts: New mechanistic insight by thorough product analysis. J. Catal. 2010, 270, 16–25. [Google Scholar] [CrossRef]
- Graça, I.; Al-Shihri, S.; Chadwick, D. Selective oxidation of cyclohexane: Ce promotion of nanostructured manganese tungstate. Appl. Catal. A Gen. 2018, 568, 95–104. [Google Scholar] [CrossRef]
- Stultz, L.K.; Huynh, M.H.V.; Binstead, R.A.; Curry, M.; Meyer, T.J. Allylic Oxidation of Cyclohexene and Indene by cis-[RuIV(bpy)2(py)(O)]2+. J. Am. Chem. Soc. 2000, 122, 5984–5996. [Google Scholar] [CrossRef]
- Sutradhar, M.; Barman, T.; Pombeiro, A.; Martins, L. Catalytic Activity of Polynuclear vs. Dinuclear Aroylhydrazone Cu(II) Complexes in Microwave-Assisted Oxidation of Neat Aliphatic and Aromatic Hydrocarbons. Molecules 2019, 24, 47. [Google Scholar] [CrossRef]
- Wu, K.; Li, B.; Han, C.; Liu, J. Synthesis, characterization of MCM-41 with high vanadium content in the framework and its catalytic performance on selective oxidation of cyclohexane. Appl. Catal. A Gen. 2014, 479, 70–75. [Google Scholar] [CrossRef]
- Shylesh, S.; Singh, A. Heterogenized vanadyl cations over modified silica surfaces: A comprehensive understanding toward the structural property and catalytic activity difference over mesoporous and amorphous silica supports. J. Catal. 2006, 244, 52–64. [Google Scholar] [CrossRef]
- Santra, C.; Shah, S.; Mondal, A.; Pandey, J.K.; Panda, A.B.; Maity, S.; Chowdhury, B. Synthesis, characterization of VPO catalyst dispersed on mesoporous silica surface and catalytic activity for cyclohexane oxidation reaction. Microporous Mesoporous Mater. 2016, 223, 121–128. [Google Scholar] [CrossRef]
- Anand, R.; Hamdy, M.S.; Parton, R.; Maschmeyer, T.; Jansen, J.C.; Gläser, R.; Kapteijn, F.; Hanefeld, U. Metal-TUD-1 Catalyzed Aerobic Oxidation of Cyclohexane: A Comparative Study. Aust. J. Chem. 2009, 62, 360–365. [Google Scholar] [CrossRef]
- Alshehri, A.A.; Alhanash, A.M.; Eissa, M.; Hamdy, M.S. New catalysts with dual-functionality for cyclohexane selective oxidation. Appl. Catal. A Gen. 2018, 554, 71–79. [Google Scholar] [CrossRef]
- Anand, R.; Hamdy, M.S.; Hanefeld, U.; Maschmeyer, T. Liquid-Phase Oxidation of Cyclohexane over Co-TUD-1. Catal. Lett. 2004, 95, 113–117. [Google Scholar] [CrossRef]
- Hamdy, M.S.; Ramanathan, A.; Maschmeyer, T.; Hanefeld, U.; Jansen, J.C. Co-TUD-1: A Ketone-Selective Catalyst for Cyclohexane Oxidation. Chem. Eur. J. 2006, 12, 1782–1789. [Google Scholar] [CrossRef] [PubMed]
- Aboelfetoh, E.F.; Fechtelkord, M.; Pietschnig, R. Structure and catalytic properties of MgO-supported vanadium oxide in the selective oxidation of cyclohexane. J. Mol. Catal. A Chem. 2010, 318, 51–59. [Google Scholar] [CrossRef]
- Aboelfetoh, E.F.; Pietschnig, R. Preparation and Catalytic Performance of Al2O3, TiO2 and SiO2 Supported Vanadium Based-Catalysts for C-H Activation. Catal. Lett. 2009, 127, 83–94. [Google Scholar] [CrossRef]
- Pal, N.; Pramanik, M.; Bhaumik, A.; Ali, M. Highly selective and direct oxidation of cyclohexane to cyclohexanone over vanadium exchanged NaY at room temperature under solvent-free conditions. J. Mol. Catal. A Chem. 2014, 392, 299–307. [Google Scholar] [CrossRef]
- Noh, H.; Cui, Y.; Peters, A.W.; Pahls, D.R.; Ortuño, M.A.; Vermeulen, N.A.; Cramer, C.J.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. An Exceptionally Stable Metal-Organic Framework Supported Molybdenum(VI) Oxide Catalyst for Cyclohexene Epoxidation. J. Am. Chem. Soc. 2016, 138, 14720–14726. [Google Scholar] [CrossRef]
- Li, Y.; Wei, Z.; Liu, L.; Gao, M.; Han, Z. Ag nanoparticles supported on UiO-66 for selective oxidation of styrene. Inorg. Chem. Commun. 2018, 88, 47–50. [Google Scholar] [CrossRef]
- Stock, N.; Biswas, S. Synthesis of Metal-Organic Frameworks (MOFs): Routes to Various MOF Topologies, Morphologies, and Composites. Chem. Rev. 2012, 112, 933–969. [Google Scholar] [CrossRef]
- Liu, Y.; Leus, K.; Grzywa, M.; Weinberger, D.; Strubbe, K.; Vrielinck, H.; Van Deun, R.; Volkmer, D.; Van Speybroeck, V.; Van Der Voort, P. Synthesis, Structural Characterization, and Catalytic Performance of a Vanadium-Based Metal-Organic Framework (COMOC-3). Eur. J. Inorg. Chem. 2012, 2819–2827. [Google Scholar] [CrossRef]
- Fei, H.; Cohen, S.M. A robust, catalytic metal–organic framework with open 2,2′-bipyridine sites. Chem. Commun. 2014, 50, 4810–4812. [Google Scholar] [CrossRef]
- Liu, Y.; Leus, K.; Sun, Z.; Li, X.; Depauw, H.; Wang, A.; Zhang, J.; Van Der Voort, P. Catalytic oxidative desulfurization of model and real diesel over a molybdenum anchored metal-organic framework. Microporous Mesoporous Mater. 2019, 277, 245–252. [Google Scholar] [CrossRef]
- Leus, K.; Liu, Y.; Meledina, M.; Turner, S.; Van Tendeloo, G.; Van Der Voort, P. A MoVI grafted Metal Organic Framework: Synthesis, characterization and catalytic investigations. J. Catal. 2014, 316, 201–209. [Google Scholar] [CrossRef]
- Manna, K.; Zhang, T.; Greene, F.X.; Lin, W. Bipyridine- and Phenanthroline-Based Metal-Organic Frameworks for Highly Efficient and Tandem Catalytic Organic Transformations via Directed C–H Activation. J. Am. Chem. Soc. 2015, 137, 2665–2673. [Google Scholar] [CrossRef]
- Wang, S.; Sun, Z.; Zou, X.; Zhang, Z.; Fu, G.; Li, L.; Zhang, X.; Luo, F. Enhancing catalytic aerobic oxidation performance of cyclohexane via size regulation of mixed-valence {V16} cluster-based metal-organic frameworks. New J. Chem. 2019, 43, 14527–14535. [Google Scholar] [CrossRef]
- Rimoldi, M.; Howarth, A.J.; DeStefano, M.R.; Lin, L.; Goswami, S.; Li, P.; Hupp, J.T.; Farha, O.K. Catalytic Zirconium/Hafnium-Based Metal–Organic Frameworks. ACS Catal. 2017, 7, 997–1014. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A New Zirconium Inorganic Building Brick Forming Metal Organic Frameworks with Exceptional Stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.W.; Moon, S.; Wagner, G.W.; Hall, M.G.; De Coste, J.B.; Hupp, J.T.; Farha, O.K. Tailoring the Pore Size and Functionality of UiO-Type Metal–Organic Frameworks for Optimal Nerve Agent Destruction. Inorg. Chem. 2015, 54, 9684–9686. [Google Scholar] [CrossRef] [PubMed]
- Szeto, K.C.; Kongshaug, K.O.; Jakobsen, S.; Tilset, M.; Lillerud, K.P. Design, synthesis and characterization of a Pt-Gd metal-organic framework containing potentially catalytically active sites. Dalton Trans. 2008, 2054–2060. [Google Scholar] [CrossRef]
- Tang, J.; Dong, W.; Wang, G.; Yao, Y.; Cai, L.; Liu, Y.; Zhao, X.; Xu, J.; Tan, L. Efficient molybdenum(vi) modified Zr-MOF catalysts for epoxidation of olefins. RSC Adv. 2014, 4, 42977–42982. [Google Scholar] [CrossRef]
- Das, S.P.; Boruah, J.J.; Sharma, N.; Islam, N.S. New polymer-immobilized peroxotungsten compound as an efficient catalyst for selective and mild oxidation of sulfides by hydrogen peroxide. J. Mol. Catal. A Chem. 2012, 356, 36–45. [Google Scholar] [CrossRef]
- Yu, X.; Yi, P.; Ji, D.; Zeng, B.; Li, X.; Xu, X. Study of the coordination and solution structures for the interaction systems between diperoxidovanadate complexes and 4-(pyridin-2-yl)pyrimidine-like ligands. Dalton Trans. 2012, 41, 3684–3694. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cohen, S.M. Postsynthetic modification of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1315–1329. [Google Scholar] [CrossRef]
- Sarmah, S.; Kalita, D.; Hazarika, P.; Borah, R.; Islam, N.S. Synthesis of new dinuclear and mononuclear peroxovanadium(V) complexes containing biogenic co-ligands: A comparative study of some of their properties. Polyhedron 2004, 23, 1097–1107. [Google Scholar] [CrossRef]
- Veisi, H.; Sajjadifar, S.; Biabri, P.M.; Hemmati, S. Oxo-vanadium complex immobilized on chitosan coated-magnetic nanoparticles (Fe3O4): A heterogeneous and recyclable nanocatalyst for the chemoselective oxidation of sulfides to sulfoxides with H2O2. Polyhedron 2018, 153, 240–247. [Google Scholar] [CrossRef]
- Silversmit, G.; Depla, D.; Poelman, H.; Marin, G.B.; De Gryse, R. Determination of the V2p XPS binding energies for different vanadium oxidation states (V5+ to V0+). J. Electron Spectrosc. Relat. Phenom. 2004, 135, 167–175. [Google Scholar] [CrossRef]
- Horvath, B.; Strutz, J.; Geyer-Lippmann, J.; Horvath, E.G. Preparation, Properties, and ESCA Characterization of Vanadium Surface Compounds on Silicagel. I. Z. Anorg. Allg. Chem. 1981, 483, 181–192. [Google Scholar] [CrossRef]
- Occhiuzzi, M.; Tuti, S.; Cordischi, D.; Dragone, R.; Indovina, V. Characterisation of VOx/ZrO2 catalysts by electron paramagnetic resonance and X-ray photoelectron spectroscopy. J. Chem. Soc. Faraday Trans. 1996, 92, 4337–4345. [Google Scholar] [CrossRef]
- Mahdavi, V.; Hasheminasab, H.R. Liquid-phase efficient oxidation of cyclohexane over cobalt promoted VPO catalyst using tert-butylhydroperoxide. J. Taiwan Inst. Chem. Eng. 2015, 51, 53–62. [Google Scholar] [CrossRef]

| MOFs | Metal Loading | Metal Loading | Metal/MOF | SBET |
|---|---|---|---|---|
| wt% | mmol/g | Molar Ratio | m2/g | |
| UiO-67 | - | - | - | 1562 |
| UiO-67-MoO(O2)2 | 14.1 (Mo) | 1.47 (Mo) | 4.2 (Mo) | 583 |
| UiO-67-WO(O2)2 | 6.3 (W) | 0.34 (W) | 0.8 (W) | 656 |
| UiO-67-KVO(O2)2 | 2.7 (V) | 0.53 (V) | 1.2 (V) | 305 |
| Catalyst | Time/h | Conversion b/% | Selectivity c/% | ||||
|---|---|---|---|---|---|---|---|
| CyOH | Cyone | KA oil | CHHP | Others d | |||
| No catalyst | 12 | <1 | - | - | - | - | - |
| UiO-67 | 12 | 1.3 | 13.4 | 35.8 | 49.2 | 50.8 | - |
| UiO-67-MoO(O2)2 | 12 | <1 | - | 33.2 | 33.2 | 66.8 | - |
| UiO-67-WO(O2)2 | 12 | 1.6 | 16.2 | 23.2 | 39.4 | 60.6 | - |
| UiO-67-KVO(O2)2 | 12 | 9.4 | 18.5 | 58.5 | 77.0 | 12.2 | 10.8 |
| UiO-67-KVO(O2)2 | 24 | 9.2 | - | 86.9 | 86.9 | - | 13.1 |
| UiO-67-KVO(O2)2 | 6 | 6.3 | 25.1 | 46.2 | 71.3 | 22.0 | 6.7 |
| UiO-67-KVO(O2)2 after hot filtration e | 12 | 6.5 | 22.4 | 49.3 | 71.7 | 21.8 | 6.5 |
| H2bpydc-KVO(O2)2 | 12 | 7.4 | 18.8 | 68.3 | 87.1 | 9.2 | 3.8 |
| Catalyst | Time/h | Temp./°C | Conv. a/% | Selectivity | Refs | ||||
|---|---|---|---|---|---|---|---|---|---|
| CyOH | Cyone | KA oil | CHHP | Others | |||||
| UiO-67-KVO(O2)2 | 12 | 70 | 9.4 | 18.5 | 58.5 | 77.0 | 13.1 | 10.8 | This work |
| V-Co-TUD-1 c | 8 | 70 | 15.9 | 62 | 25.9 | 87.9 | 2.4 | 9.7 | [21] |
| VPO | 8 | 90 | 10 | 33 | 22 | 55 | - | 45 b | [49] |
| VPO-Co(0.10) | 8 | 90 | 65 | 20 | 33 | 53 | - | 47 b | [49] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, Y.; Peng, J.; Sun, Z.; Yu, Z.; Wang, A.; Wang, Y.; Liu, Y.-Y.; Xu, F.; Sun, L.-X. Transition Metal Oxodiperoxo Complex Modified Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Cyclohexane. Materials 2020, 13, 829. https://doi.org/10.3390/ma13040829
Hong Y, Peng J, Sun Z, Yu Z, Wang A, Wang Y, Liu Y-Y, Xu F, Sun L-X. Transition Metal Oxodiperoxo Complex Modified Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Cyclohexane. Materials. 2020; 13(4):829. https://doi.org/10.3390/ma13040829
Chicago/Turabian StyleHong, Yuechao, Jie Peng, Zhichao Sun, Zhiquan Yu, Anjie Wang, Yao Wang, Ying-Ya Liu, Fen Xu, and Li-Xian Sun. 2020. "Transition Metal Oxodiperoxo Complex Modified Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Cyclohexane" Materials 13, no. 4: 829. https://doi.org/10.3390/ma13040829
APA StyleHong, Y., Peng, J., Sun, Z., Yu, Z., Wang, A., Wang, Y., Liu, Y.-Y., Xu, F., & Sun, L.-X. (2020). Transition Metal Oxodiperoxo Complex Modified Metal-Organic Frameworks as Catalysts for the Selective Oxidation of Cyclohexane. Materials, 13(4), 829. https://doi.org/10.3390/ma13040829