Evaluating Methane Adsorption Characteristics of Coal-Like Materials
Abstract
1. Introduction
2. Device and Experimental Design
2.1. Experimental Materials
2.2. Experimental Design
2.2.1. Different Influencing Factors
2.2.2. Experimental Design and Steps
3. Results and Analysis
3.1. Pore Structure Results
3.2. The Test Results of Adsorption Coefficients
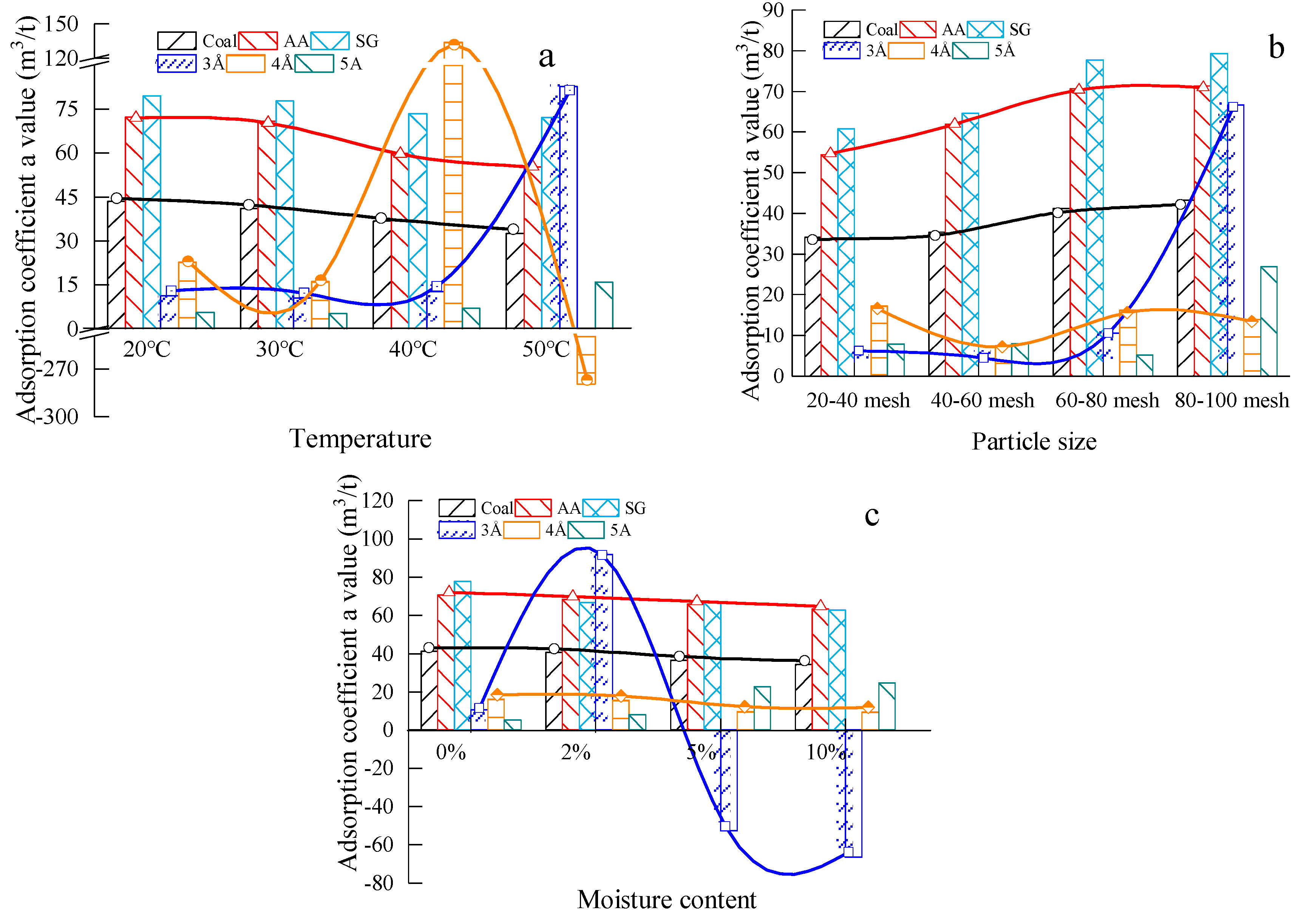
3.2.1. The Test Result of Adsorption Coefficient
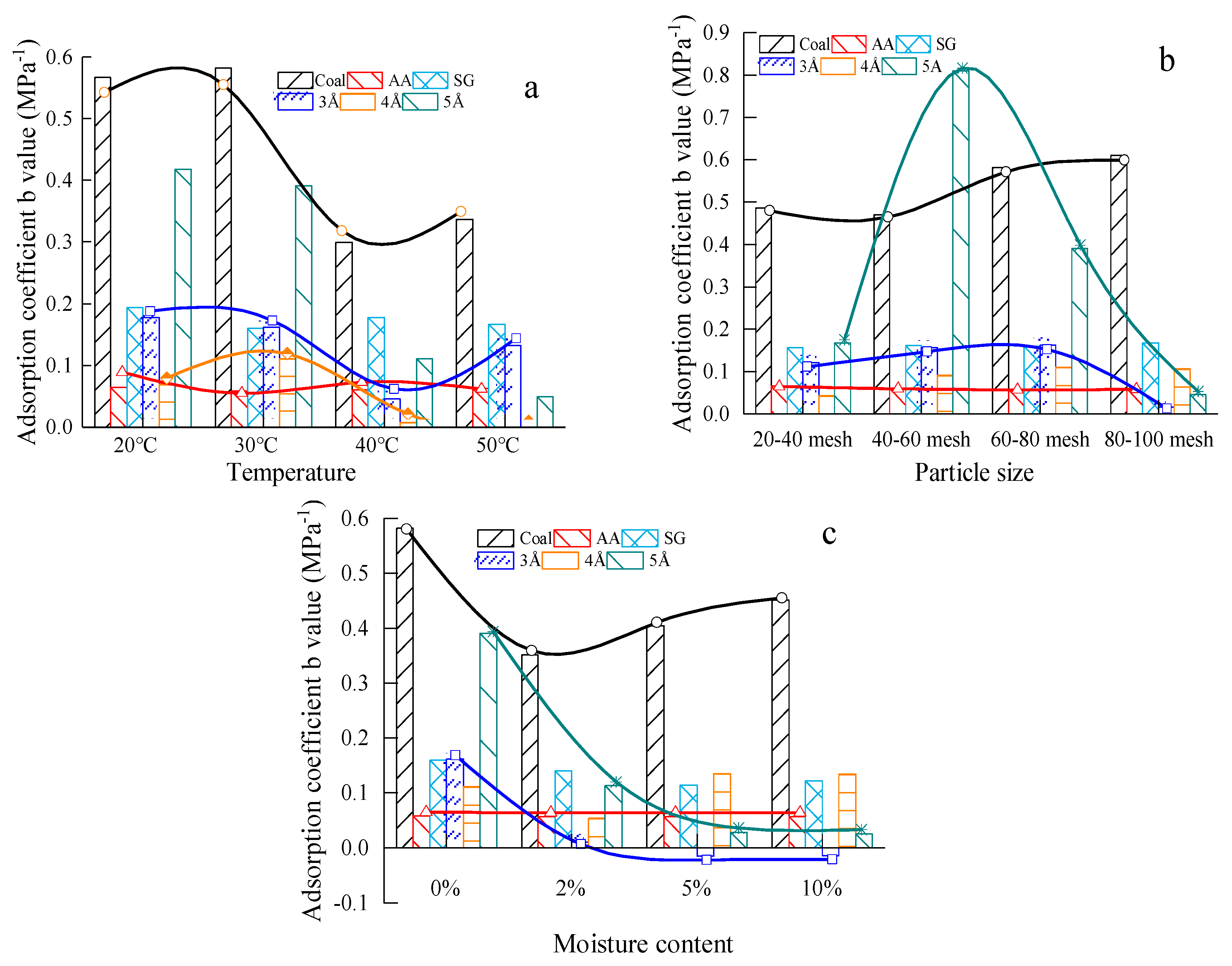
3.2.2. The Test Result of Adsorption Coefficient B Values
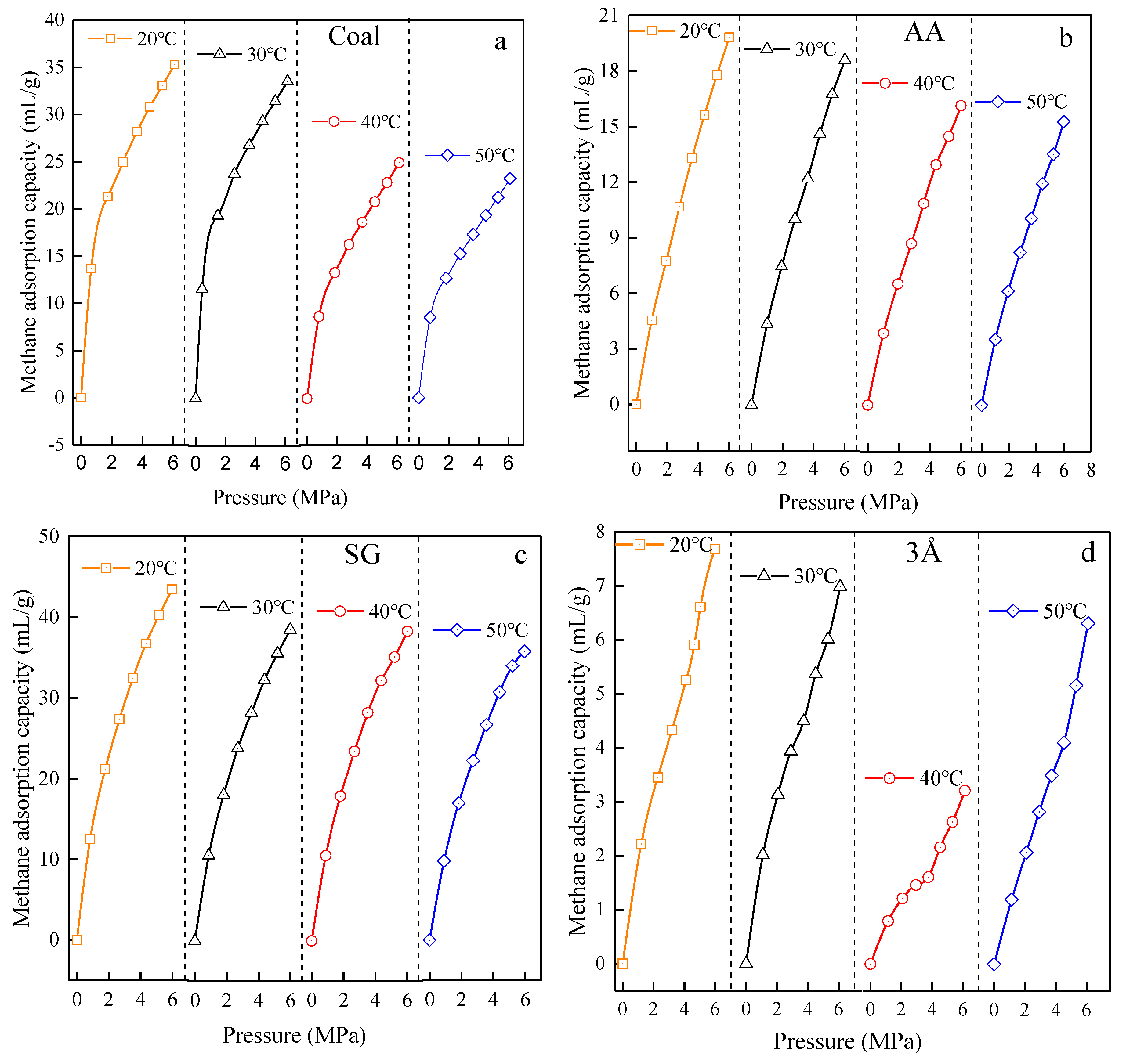
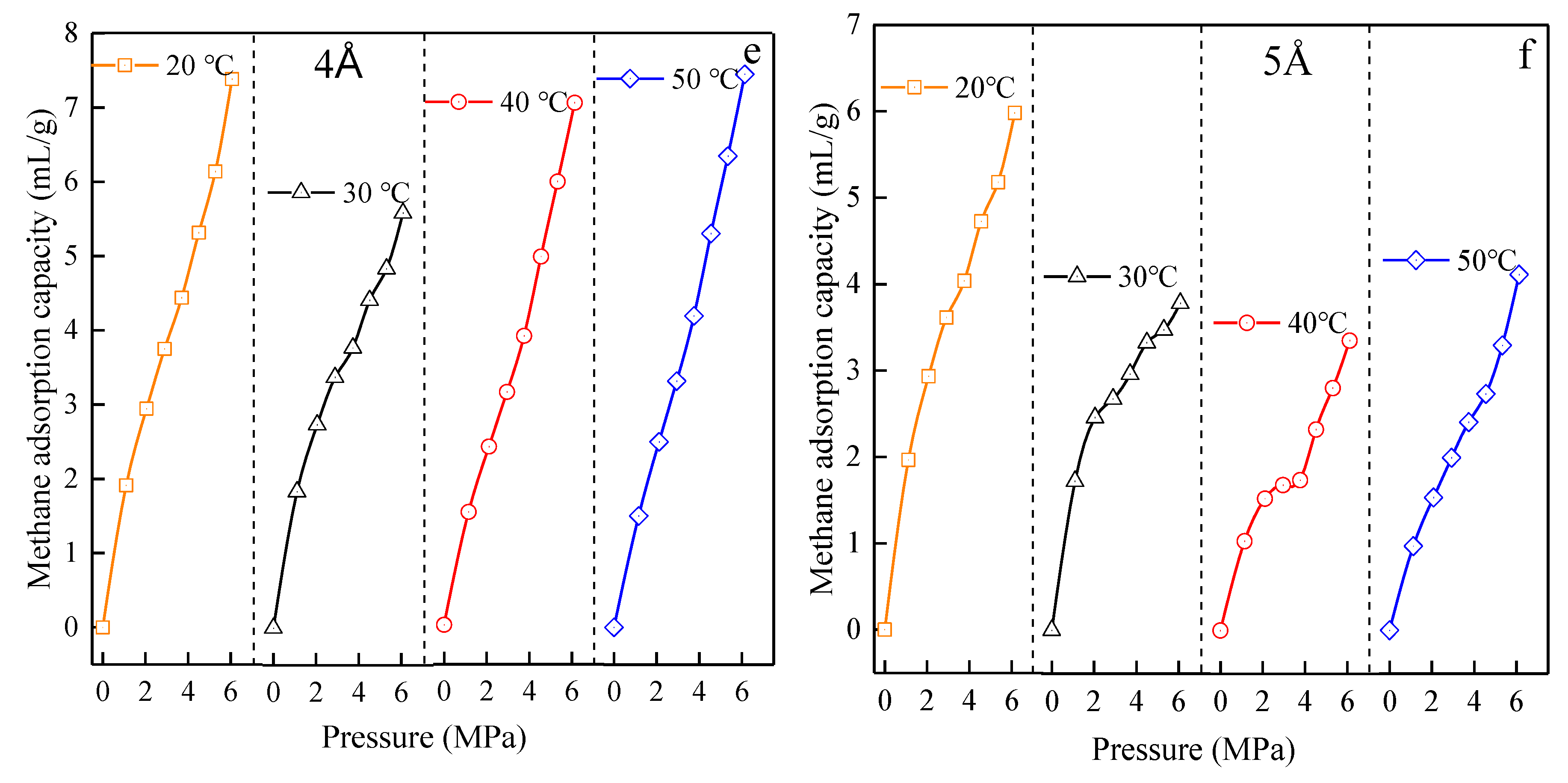
3.3. Adsorption Isotherm Test Results
3.3.1. Test Results of Adsorption Isotherms of Test Materials at Different Temperatures
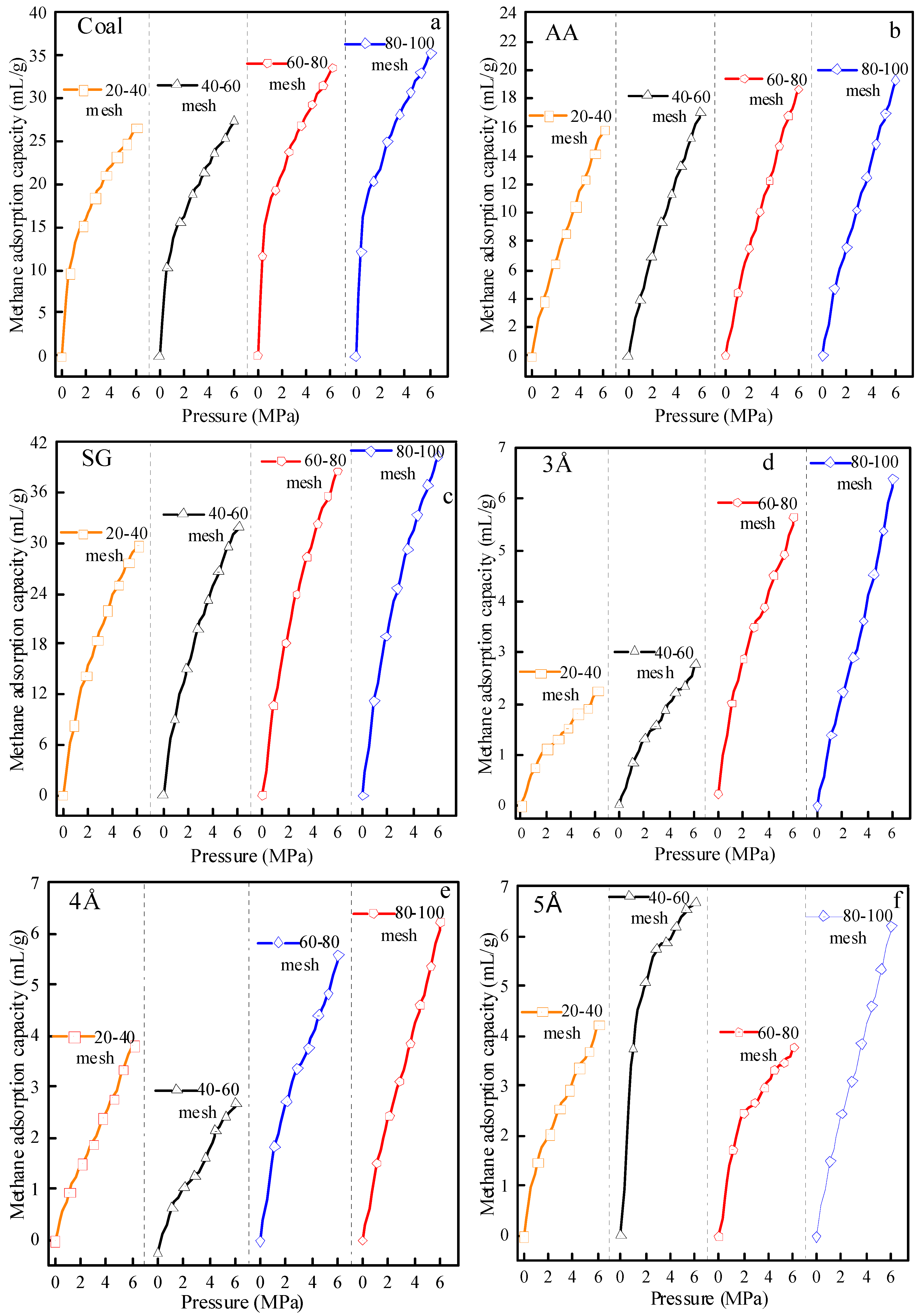
3.3.2. Test Results of Adsorption Isotherms of Test Materials at Different Particle Sizes
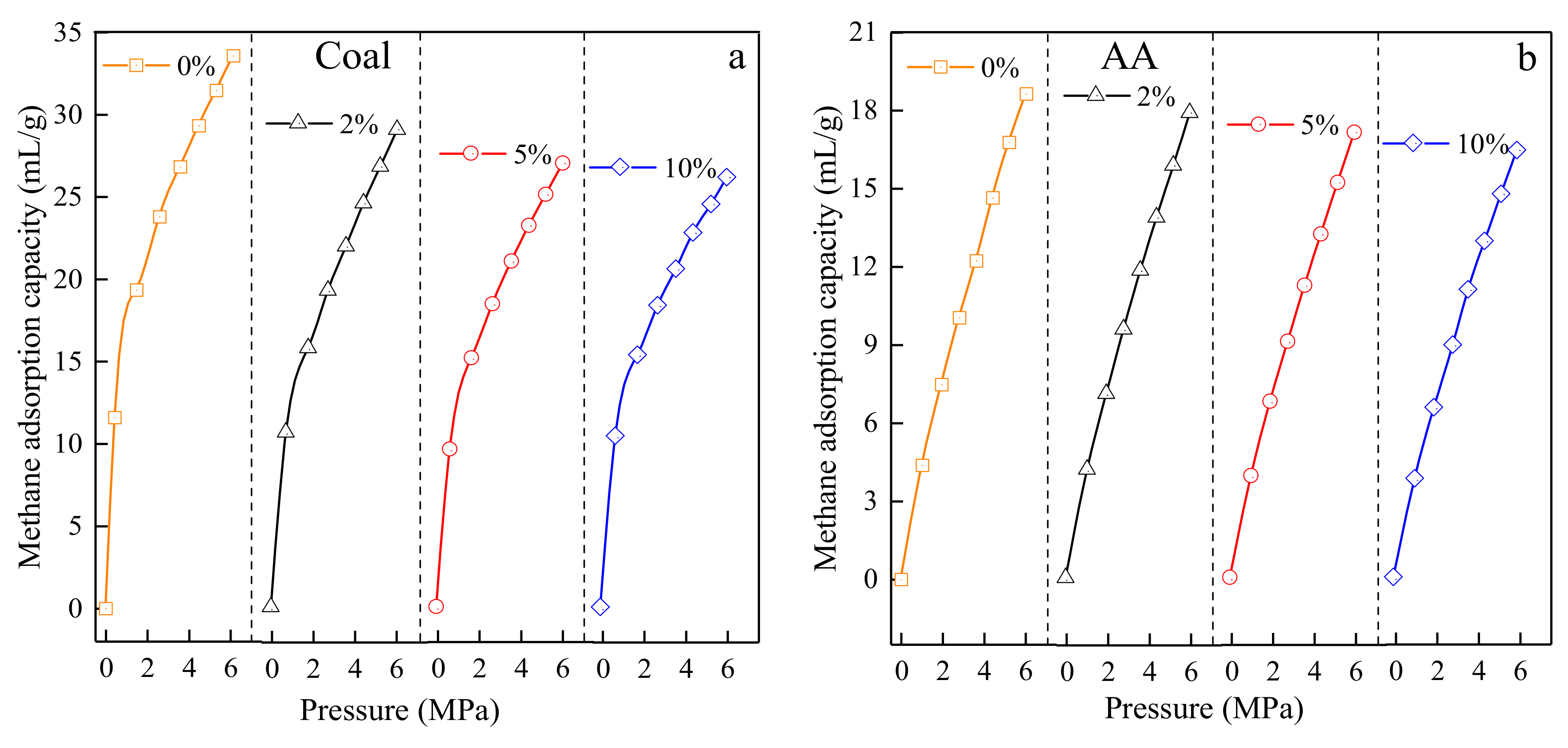
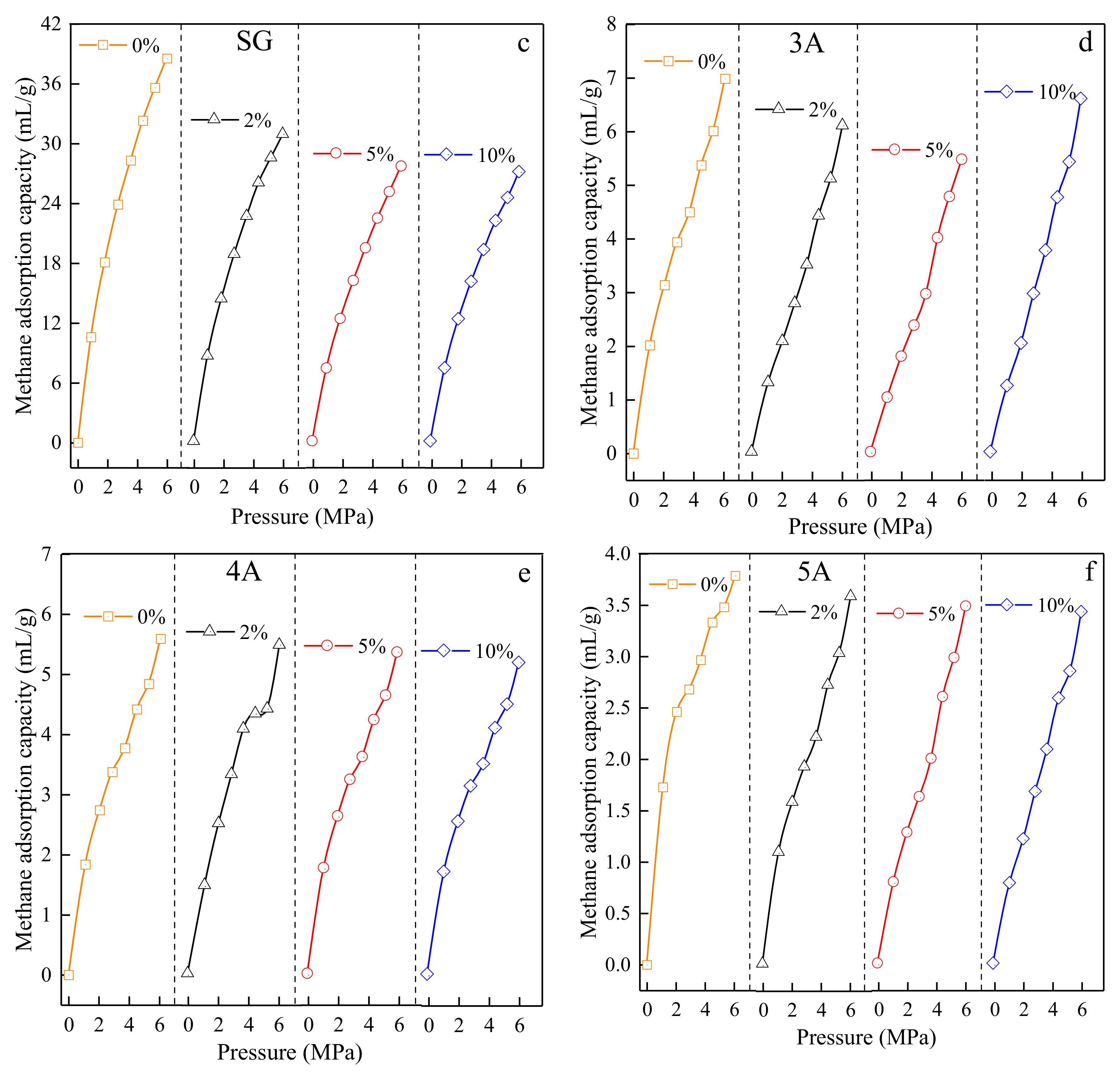
3.3.3. Test Results of Adsorption Isotherms of Test Materials with Different Moisture Content
4. Discussion
4.1. Analysis of Pore Structure Results and Langmuir Model Fit
4.1.1. Analysis of Pore Structure Results
4.1.2. Langmuir Model Fitting Analysis
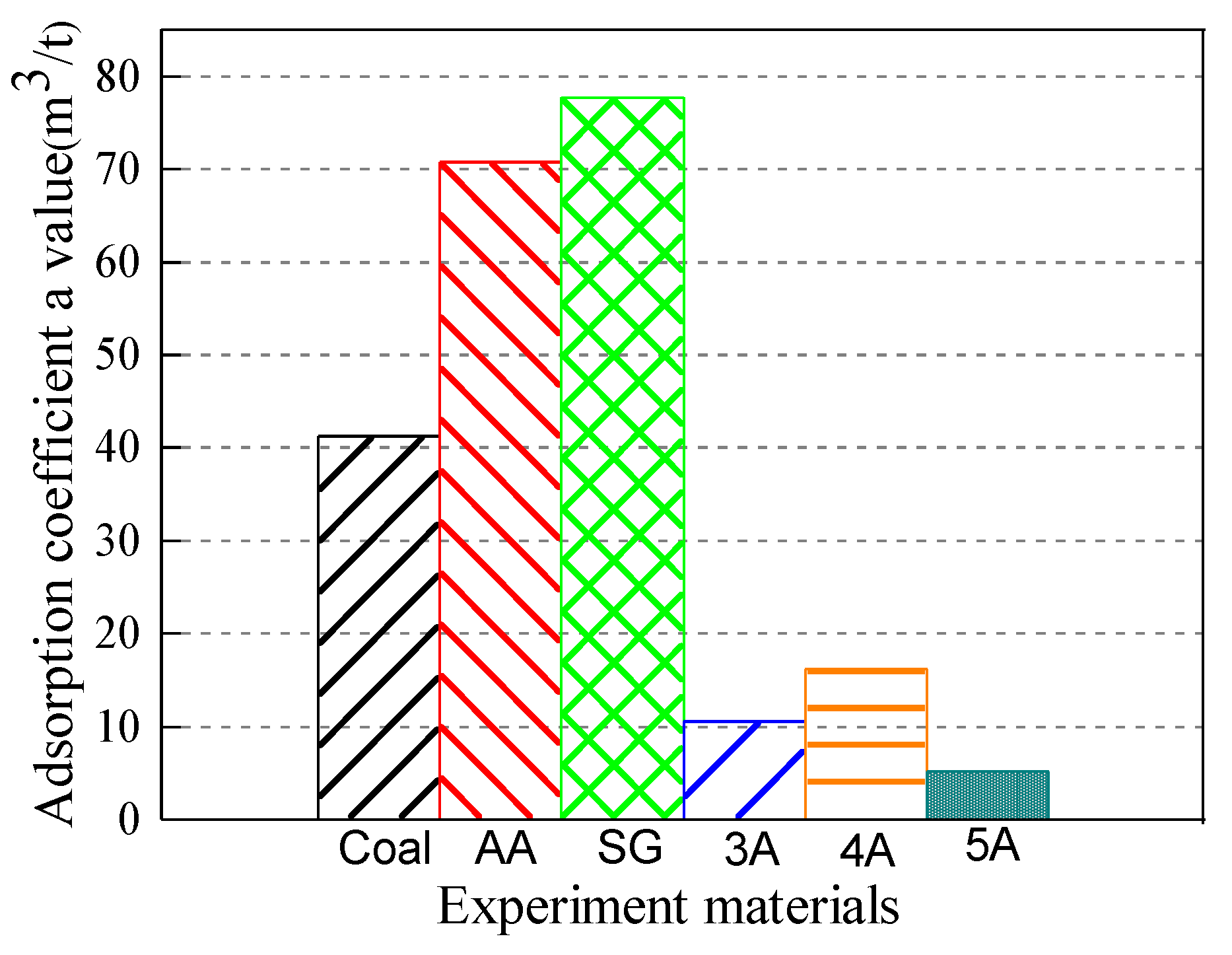
4.2. Analysis of Ultimate Adsorption Capacity and Adsorption Properties of Materials
4.2.1. Comparative Analysis of Material and Ultimate Adsorption Capacity of Coal Methane
4.2.2. Analysis of Material and Coal Methane Adsorption Change Properties
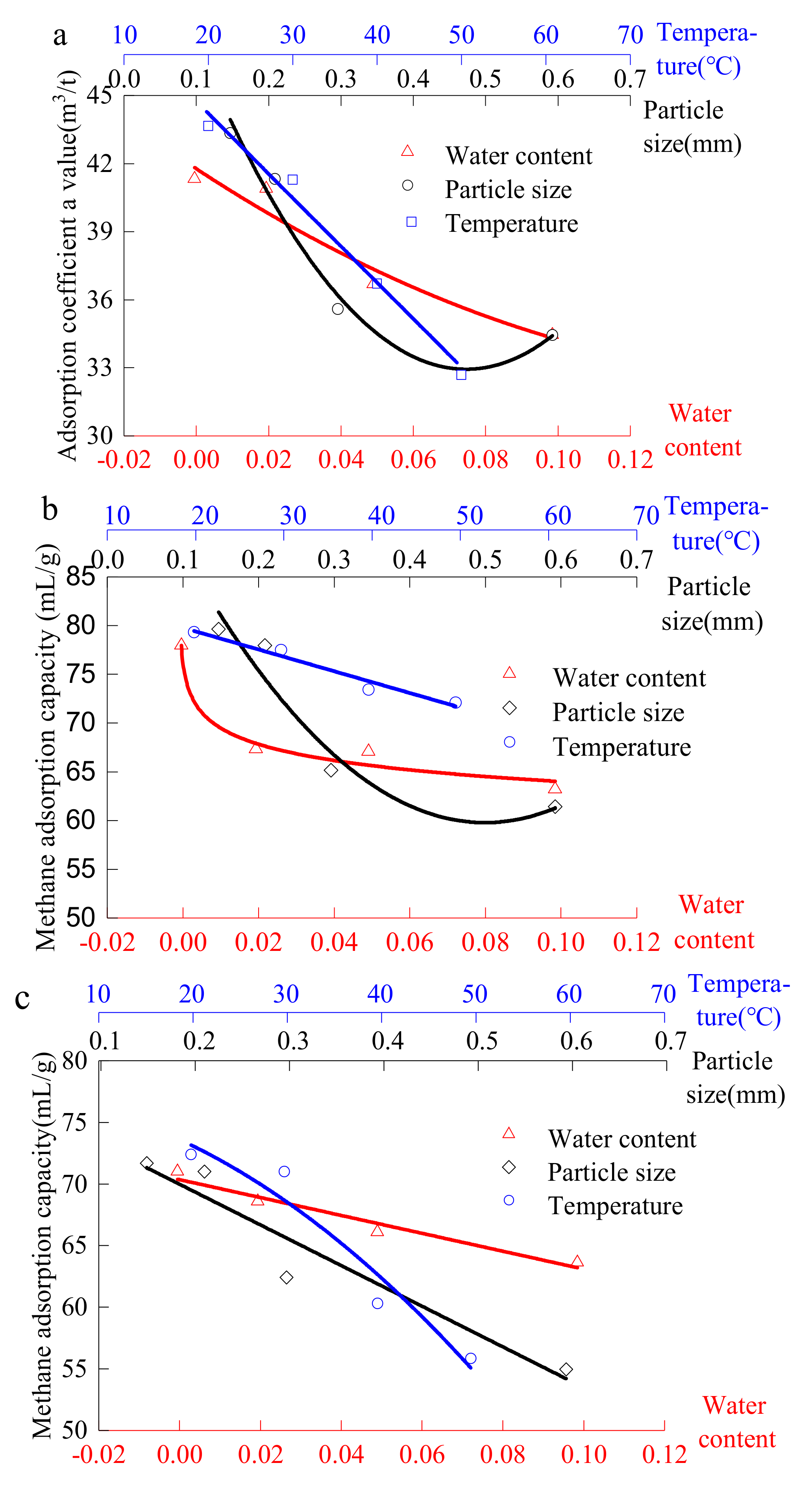
4.3. Analysis of Factors Affecting the Ultimate Adsorption of Materials
4.3.1. Analysis of the Influence Principles of Material Ultimate Methane Adsorption
4.3.2. Sensitivity Analysis of Methane Adsorption Ultimate of The Coal-Like Material
5. Conclusions
- (1)
- The adsorption process of silica gel and activated alumina under the influence of particle size, temperature and moisture have good agreement with the Langmuir adsorption model; it is consistent with the methane adsorption properties of raw coal under the three different factors. The adsorption processes of the three molecular sieves under the influence of particle size, temperature and moisture are quite different, and seemed not to possess a good fit with the Langmuir adsorption model; they cannot be used as adsorption materials.
- (2)
- The specific pore surfaces of the micropores and transition pores of silica gel and activated alumina are large, so the adsorption coefficient a has a large value. As the pressure increases, the methane adsorption ratio gradually decreases and then it tends to be stabilized. The adsorption amount and the change of the adsorption capacity of different pressures are in line with the methane adsorption characteristics of coal-like materials simulating raw coal.
- (3)
- Temperature, particle size and moisture content have a significant influence on the methane adsorption of the #1890 working face coal sample in Aiweiergou, the silica gel and activated alumina. However, the sensitivity of the materials to different factors is different. By analyzing the change trend of the adsorption amount of the #1890 working face coal sample in Aiweiergou, silica gel and activated alumina, the change trend equation is obtained.
Author Contributions
Funding
Conflicts of Interest
References
- Martin, A.; Idrus Alhamid, M.; Nasruddin Suryawan, B.; Soong Loh, W.; Bin Ismail, A.; Chun, W.; Choon Ng, K. High-Pressure Adsorption Isotherms of Carbon Dioxide and Methane on Activated Carbon from Low-Grade Coal of Indonesia. Heat Transf. Eng. 2017, 38, 396–402. [Google Scholar] [CrossRef]
- Sun, F.; Gao, J.H.; Yang, Y.Q.; Zhu, Y.W.; Wang, L.J.; Pi, X.X.; Liu, X.; Qu, Z.B.; Wu, S.H.; Qin, Y.K. One-step ammonia activation of Zhundong coal generating nitrogen-doped microporous carbon for gas adsorption and energy storage. Carbon 2016, 109, 747–754. [Google Scholar] [CrossRef]
- Zhang, J.; Duan, Y.F.; Zhou, Q.; Zhu, C.; She, M.; Ding, W.K. Adsorptive removal of gas-phase mercury by oxygen non-thermal plasma modified activated carbon. Chem. Eng. J. 2016, 294, 281–289. [Google Scholar] [CrossRef]
- Wojtacha-Rychter, K.; Smolinski, A. Multi-component gas mixture transport through porous structure of coal. Fuel 2018, 233, 37–44. [Google Scholar] [CrossRef]
- Bae, J.S.; Jin, Y.G.; Huynh, C.; Su, S. Biomass-derived carbon composites for enrichment of dilute methane from underground coal mines. J. Environ. Manag. 2018, 217, 373–380. [Google Scholar] [CrossRef]
- Naveen, P.; Asif, M.; Ojha, K. Integrated fractal description of nanopore structure and its effect on CH4, adsorption on Jharia coals, India. Fuel 2018, 232, 190–204. [Google Scholar] [CrossRef]
- Zhang, L.; Aziz, N.; Ren, T.; Nemcik, J.; Tu, S.H. Influence of coal particle size on coal adsorption and desorption characteristics. Arch. Min. Sci. 2014, 59, 807–820. [Google Scholar] [CrossRef]
- Strydom, C.A.; Campbell, Q.P.; le Roux, M.; Preez, S.M.D. Validation of using a modified BET model to predict the moisture adsorption behavior of bituminous coal. Int. J. Coal Prep. Util. 2016, 36, 28–43. [Google Scholar] [CrossRef]
- Wang, C.J.; Yang, S.Q.; Li, J.H.; Li, X.W.; Jiang, C.L. Influence of coal moisture on initial gas desorption and gas-release energy characteristics. Fuel 2018, 232, 351–361. [Google Scholar] [CrossRef]
- Chen, M.Y.; Cheng, Y.P.; Li, H.R.; Wang, L.; Jin, K.; Dong, J. Impact of inherent moisture on the methane adsorption characteristics of coals with various degrees of metamorphism. J. Nat. Gas Sci. Eng. 2018, 55, 312–320. [Google Scholar] [CrossRef]
- Dardona, S.; Peles, A.; Wrobel, G.; Piech, M.; Gao, P.X. Gas adsorption and high-emission current induced degradation of field emission characteristics in solution-processed ZnO nanoneedles. J. Appl. Phys. 2010, 108, 6004. [Google Scholar] [CrossRef]
- Guo, H.J.; Cheng, Y.P.; Wang, L.; Lu, S.Q.; Jin, K. Experimental study on the effect of moisture on low-rank coal adsorption characteristics. J. Nat. Gas Sci. Eng. 2015, 24, 245–251. [Google Scholar] [CrossRef]
- Kim, Y. Water adsorption on the surface of Ni- and Co-based layer-structured cathode materials for lithium-ion batteries. Int. J. Quantum Chem. 2018, 118, e25591. [Google Scholar] [CrossRef]
- Crosdale, P.J.; Moore, T.A.; Mares, T.E. Influence of moisture content and temperature on methane adsorption isotherm analysis for coals from a low-rank, biogenically-sourced gas reservoir. Int. J. Coal Geol. 2008, 76, 166–174. [Google Scholar] [CrossRef]
- Pribylov, A.A.; Murdmaa, K.O. Estimation of the average heat of gas adsorption from the adsorption isotherm measured at supercritical temperatures and pressures. Russ. Chem. Bull. 2017, 66, 849–856. [Google Scholar] [CrossRef]
- Fianu, J.; Gholinezhad, J.; Hassan, M. Comparison of Temperature-Dependent Gas Adsorption Models and Their Application to Shale Gas Reservoirs. Energy Fuels 2018, 32, 4763–4771. [Google Scholar] [CrossRef]
- Reddy, K.S.K.; al Shoaibi, A.; Srinivasakannan, C. Gas-phase mercury removal through sulfur impregnated porous carbon. J. Ind. Eng. Chem. 2014, 20, 2969–2974. [Google Scholar] [CrossRef]
- Stirling, R.J.; Snape, C.E.; Meredith, W. The impact of hydrothermal carbonisation on the char reactivity of biomass. Fuel Process. Technol. 2018, 177, 152–158. [Google Scholar] [CrossRef]
- Rao, V.U.S. Evaluation of particle-size measurement techniques for dispersed iron catalysts. Energy Fuels 1994, 8, 44–47. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhang, Q.H.; Yuan, L. Development of a similar material for methane-bearing coal and its application to outburst experiment. Rock Soil Mech. 2015, 36, 1676–1682. [Google Scholar] [CrossRef]
- Li, S.G.; Zhao, P.X.; Lin, H.F.; Xiao, P. Study on character of physical simulation similar material of coal-rock and gas solid-gas coupling. J. China Coal Soc. 2015, 40, 80–86. [Google Scholar] [CrossRef]
- Salahudeen, N.; Ahmed, A.S.; Al-Muhtaseb, A.H.; Dauda, M.; Waziri, S.M.; Jibril, B.Y.; Al-Sabahi, J. Synthesis, characterization and adsorption study of nano-sized activated alumina synthesized from kaolin using novel method. Powder Technol. 2015, 280, 266–272. [Google Scholar] [CrossRef]
- Koohsaryan, E.; Anbia, M. Nanosized and hierarchical zeolites: A short review. Chin. J. Catal. 2016, 37, 447–467. [Google Scholar] [CrossRef]
- Ahmed, M.J.; Theydan, S.K. Temperature distribution studies for light hydrocarbons (propane, n-butane, and iso-butane) adsorption on 5A molecular sieve zeolite. Heat Mass Transf. 2015, 51, 33–38. [Google Scholar] [CrossRef]
- Uebersfeld, J.; Tienne, É.A. Paramagnetic resonance, a new property of coal-like materials. Nature 1954, 174, 614. [Google Scholar] [CrossRef]
- Zhang, T.J.; Xu, H.J.; Li, S.G.; Ren, S.X. The effect of particle size on adsorption of methane on coal. J. Hunan Univ. Sci. Technol. 2009, 24, 9–12. [Google Scholar] [CrossRef]
- Yue, G.W.; Wang, Z.F.; Kang, B. Prediction for isothermal adsorption curve of coal/CH4 based on adsorption heat theory. Nat. Gas Geosci. 2015, 26, 148–153. [Google Scholar] [CrossRef]
- Clarkson, C.R.; Bustin, R.M. Binary gas adsorption/desorption isotherms: Effect of moisture and coal composition upon carbon dioxide selectivity over methane. Int. J. Coal Geol. 2000, 42, 241–271. [Google Scholar] [CrossRef]
- Rathgeber, C.; Schmit, H.; Hiebler, S.; Voigt, W. Application of the modified BET model to concentrated salt solutions with relatively high water activities: Predicting solubility phase diagrams of NaCl + H2O, NaCl + LiCl + H2O, and NaCl + CaCl2 + H2O. Calphad 2019, 66, 101633. [Google Scholar] [CrossRef]
- Qiu, F.G.; Li, B.B.; Fu, K.M.; Xu, J.T.; Wang, J.L. The Changes of pore structure and specific surface area in water treatment residual before and after phosphorus adsorption. Ion Exchang. Adsorpt. 2019, 35, 60–70. [Google Scholar] [CrossRef]
- OuYang, M.; Shao, Y.; Zhao, X.L. Discussion on key steps in determination of true relative density of coal. Coal Qual. Technol. 2013, 38–39, 46. (In Chinese) [Google Scholar] [CrossRef]
- Dao, T.U.; Nguyen, H.T.; Sy, D.T.; Nguyen, K.H.; Nguyen, A.T.; Nguyen, T.T.; Nguyen, T.D. Adsorption isotherms and kinetic models for congo red adsorption on Ca-Al layer double hydroxide adsorbent. Solid State Phenom. 2019, 4837, 128–132. [Google Scholar] [CrossRef]
- Chen, X.J.; Liu, J.; Wang, L. Influence of pore size distribution of different metamorphic grade of coal on adsorption constant. J. China Coal Soc. 2013, 38, 294–300. [Google Scholar] [CrossRef]
- Li, Y.G.; Qing, Q.; Wu, C.X.; Luo, X.; Yu, X.; Chen, M.J. The inappropriate application of the regression Langmuir Qm for adsorption capacity comparison. Sci. Total Environ. 2020, 699, 134222. [Google Scholar] [CrossRef]
- Zhao, P.X.; Liu, H.; Li, S.G.; Lin, H.F.; Jia, Y.Y.; Yan, M.; Yuan, M.Q.; Lin, J. Experimental Investigation of the Adsorption Characteristics of Mixed Coal and Variations of Specific Surface Areas before and after CH4 Adsorption. Appl. Sci. 2019, 9, 524–541. [Google Scholar] [CrossRef]
- Zhao, Z.; Ma, X.; Zhang, Z.H.; Li, Y.X. In-situ transition of amorphous gels to Na-P1 zeolite in geopolymer: Mechanical and adsorption properties. Constr. Build. Mater. 2019, 202, 851–860. [Google Scholar] [CrossRef]
- Lin, J.; Ren, T.; Wang, G.D.; Booth, P.; Nemcilk, J. Experimental investigation of N2 injection to enhance gas drainage in CO2-rich low permeable seam. Fuel 2018, 215, 665–674. [Google Scholar] [CrossRef]
- Yao, Y.B.; Liu, D.M.; Tang, D.Z.; Tang, S.H.; Huang, W.H. Fractal characterization of adsorption-pores of coals from North China: An investigation on CH4 adsorption capacity of coals. Int. J. Coal Geol. 2007, 73, 27–42. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef]



| Experiment Material | 1890 | Activated Alumina | Silica Gel | 3Å Molecular Sieve | 4Å Molecular Sieve | 5Å Molecular Sieve | |
|---|---|---|---|---|---|---|---|
| Temperature | 20 °C | 0.97 | 0.98 | 0.998 | 0.89 | 0.85 | 0.28 |
| 30 °C | 0.96 | 0.99 | 0.99 | 0.97 | 0.94 | 0.99 | |
| 40 °C | 0.98 | 0.95 | 0.99 | 0.52 | 0.22 | 0.71 | |
| 50 °C | 0.96 | 0.99 | 0.99 | 0.16 | 0.19 | 0.74 | |
| Particle size | #20–#40 sieve | 0.98 | 0.98 | 0.99 | 0.95 | 0.83 | 0.97 |
| #40–#60 sieve | 0.97 | 0.99 | 0.99 | 0.96 | 0.86 | 1.00 | |
| #60–#80 sieve | 0.96 | 0.98 | 0.99 | 0.97 | 0.94 | 0.99 | |
| #80–#100 sieve | 0.96 | 0.95 | 0.99 | 0.45 | 0.88 | 0.88 | |
| Moisture content | 0% | 0.96 | 0.99 | 0.99 | 0.98 | 0.94 | 0.97 |
| 2% | 0.97 | 0.98 | 0.99 | 0.37 | 0.27 | 0.90 | |
| 5% | 0.97 | 0.99 | 0.99 | 0.52 | 0.98 | 0.62 | |
| 10% | 0.97 | 0.96 | 0.99 | 0.65 | 0.98 | 0.71 | |
| Experiment Material | Factor | Formula | Fit |
|---|---|---|---|
| Aiweiergou 1890# working face coal sample | Moisture content | Q = 41.697 − 105.665w − 292.827w2 | 0.947 |
| Particle size | Q = 56.332 − 98.7d + 102.88d2 | 0.964 | |
| Temperature | Q = 51.704 − 0.375t | 0.985 | |
| Silica gel | Moisture content | Q = 58.185(w + 4.920 × 10−4)−0.038 | 0.975 |
| Particle size | Q = 103.559 − 175.386d + 173.252d2 | 0.925 | |
| Temperature | Q = 84.905 − 0.262t | 0.961 | |
| Activated alumina | Moisture content | Q = 70.778 − 73.024w | 0.961 |
| Particle size | Q = 76.842 − 38.720d | 0.920 | |
| Temperature | Q = 77.272 − 0.061t − 0.008t2 | 0.938 |
| Numbering | Moisture Content | Particle Size | Temperature |
|---|---|---|---|
| 1 | 41.194 | 34.196 | 43.649 |
| 2 | 40.75 | 35.36 | 41.194 |
| 3 | 36.475 | 41.194 | 36.701 |
| 4 | 34.185 | 43.254 | 32.682 |
| Range | 7.009 | 9.058 | 10.967 |
| Numbering | Moisture Content | Particle Size | Temperature |
|---|---|---|---|
| 1 | 70.721 | 54.405 | 72.111 |
| 2 | 68.261 | 61.979 | 70.721 |
| 3 | 65.709 | 70.721 | 59.845 |
| 4 | 63.205 | 71.399 | 55.298 |
| Range | 7.516 | 16.994 | 16.813 |
| Numbering | Moisture Content | Particle Size | Temperature |
|---|---|---|---|
| 1 | 77.699 | 60.841 | 79.524 |
| 2 | 66.826 | 64.646 | 77.699 |
| 3 | 66.593 | 77.699 | 73.523 |
| 4 | 62.672 | 79.361 | 72.179 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, P.; Liu, H.; Ho, C.-H.; Li, S.; Liu, Y.; Lin, H.; Yan, M. Evaluating Methane Adsorption Characteristics of Coal-Like Materials. Materials 2020, 13, 751. https://doi.org/10.3390/ma13030751
Zhao P, Liu H, Ho C-H, Li S, Liu Y, Lin H, Yan M. Evaluating Methane Adsorption Characteristics of Coal-Like Materials. Materials. 2020; 13(3):751. https://doi.org/10.3390/ma13030751
Chicago/Turabian StyleZhao, Pengxiang, Hui Liu, Chun-Hsing Ho, Shugang Li, Yanqun Liu, Haifei Lin, and Min Yan. 2020. "Evaluating Methane Adsorption Characteristics of Coal-Like Materials" Materials 13, no. 3: 751. https://doi.org/10.3390/ma13030751
APA StyleZhao, P., Liu, H., Ho, C.-H., Li, S., Liu, Y., Lin, H., & Yan, M. (2020). Evaluating Methane Adsorption Characteristics of Coal-Like Materials. Materials, 13(3), 751. https://doi.org/10.3390/ma13030751





