Hybrid TiO2-ZnO Nanomaterials Prepared Using Laser Ablation in Liquid
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. XRD Analysis
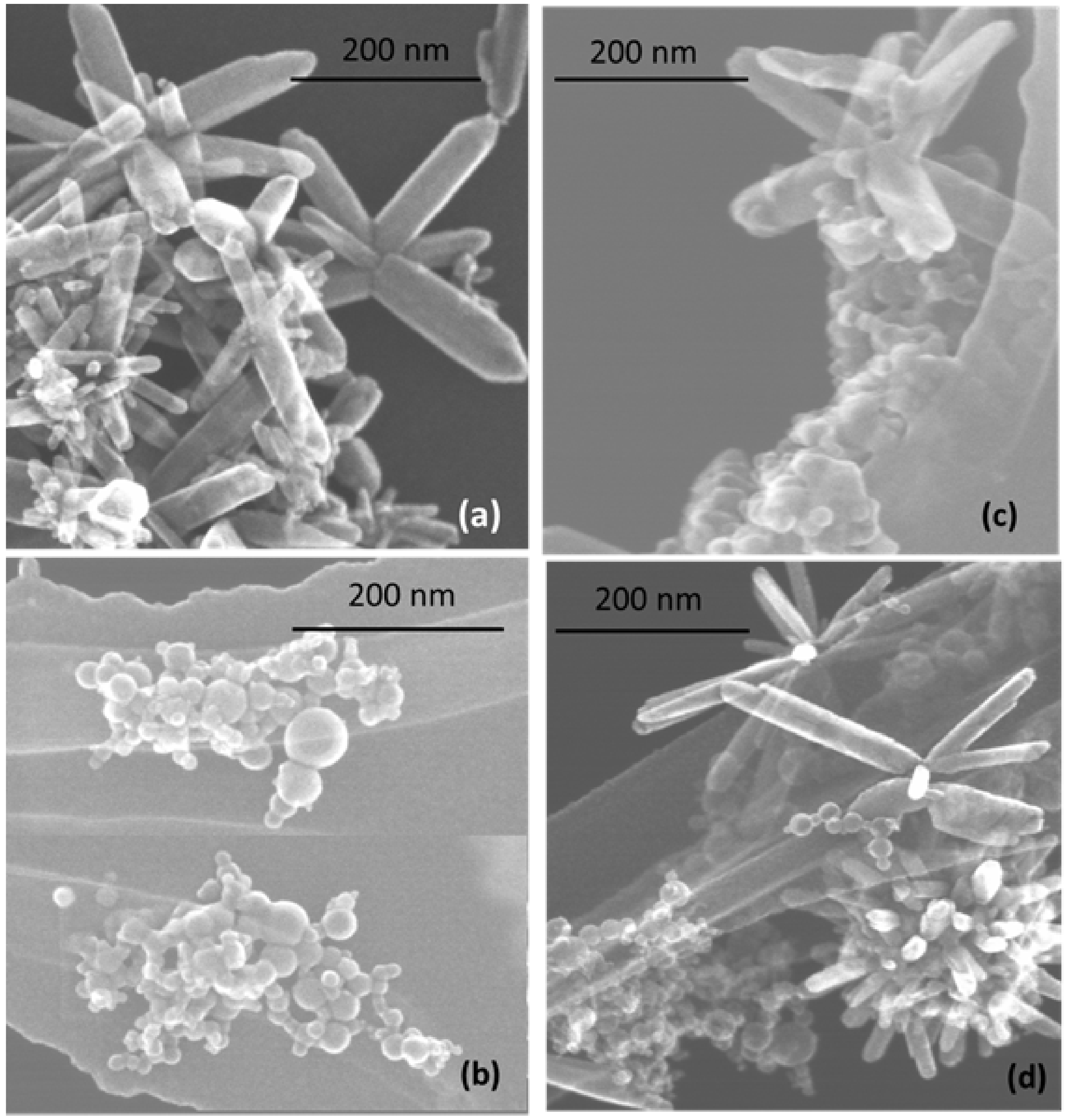
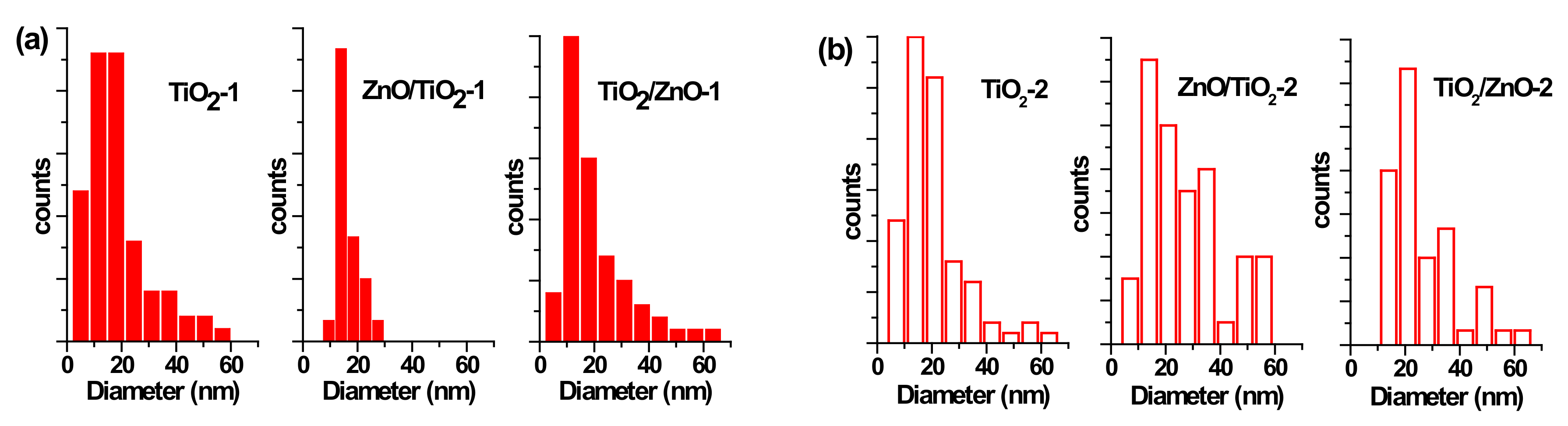
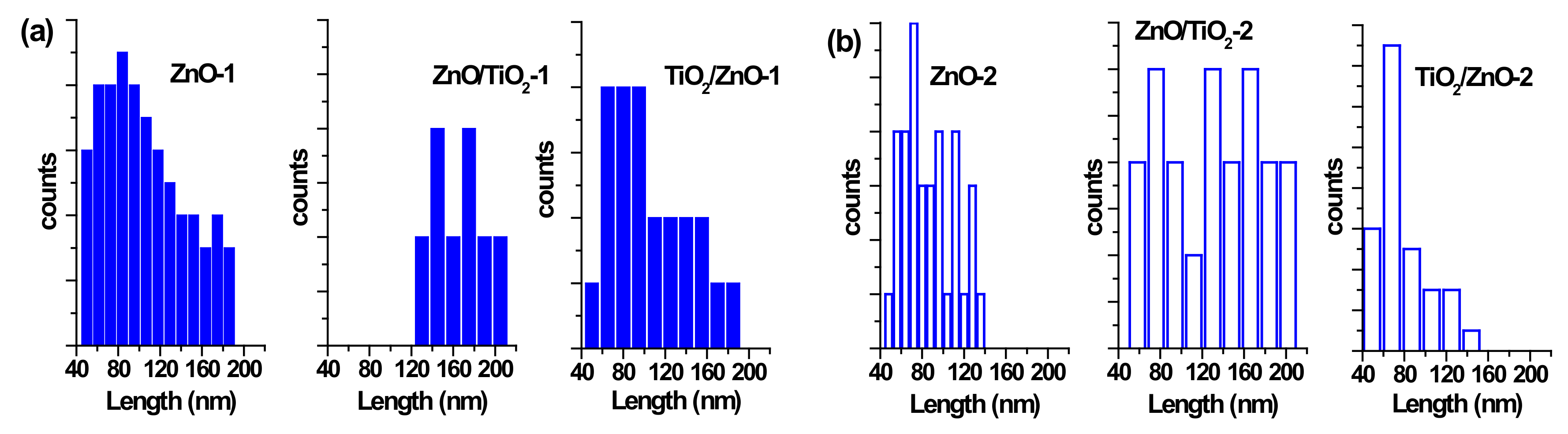
3.2. TEM and Size Distribution
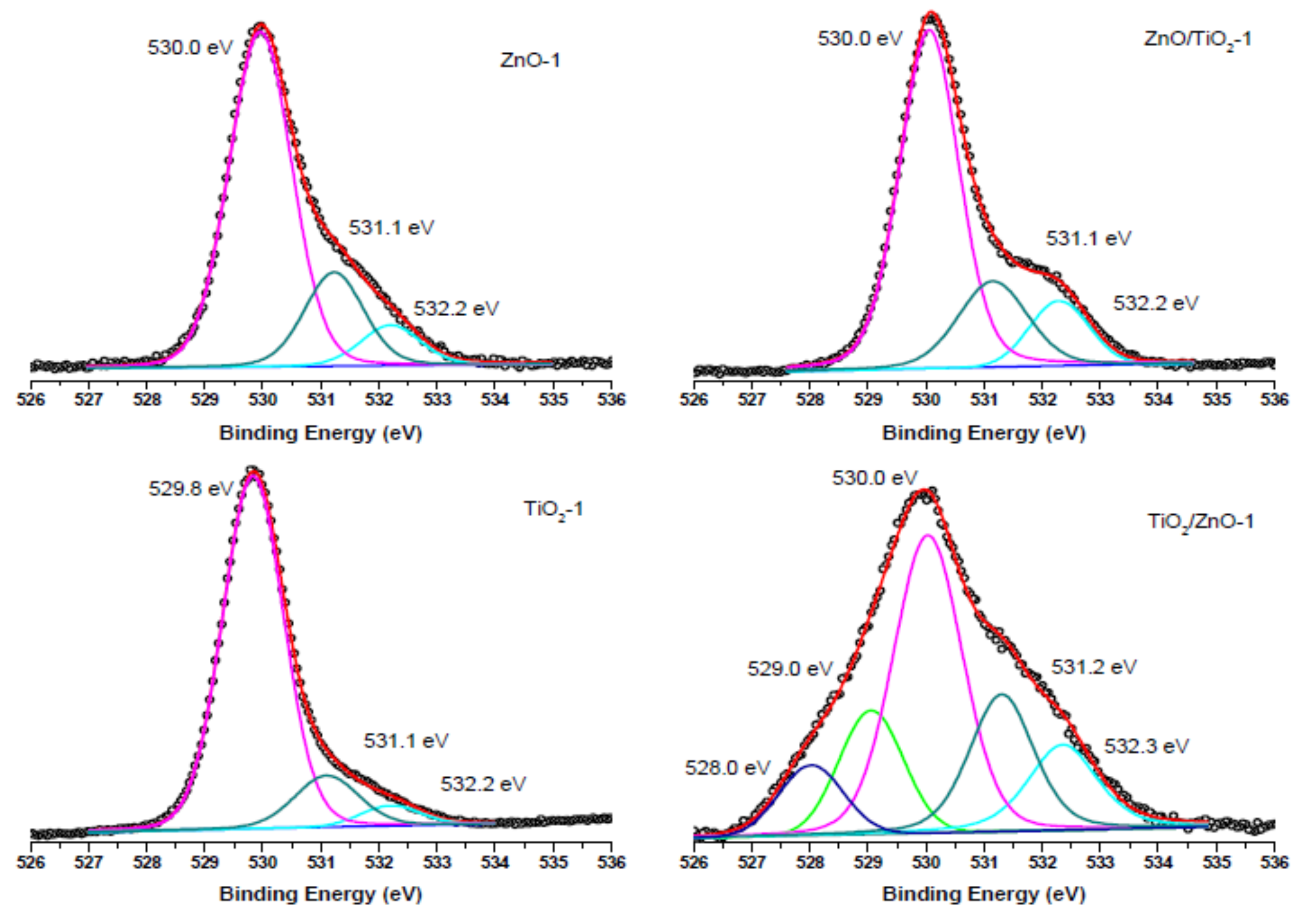
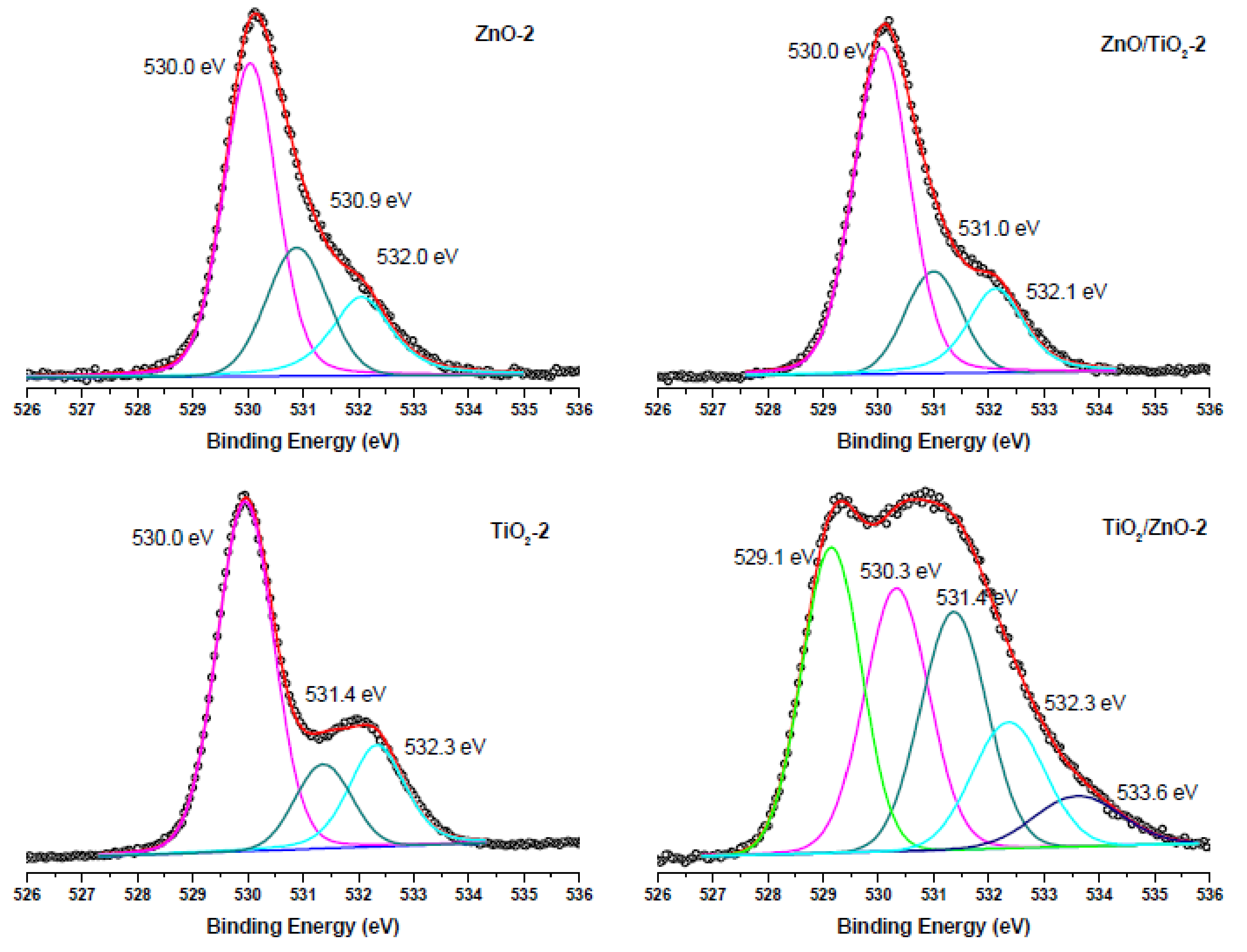
3.3. XPS Analysis
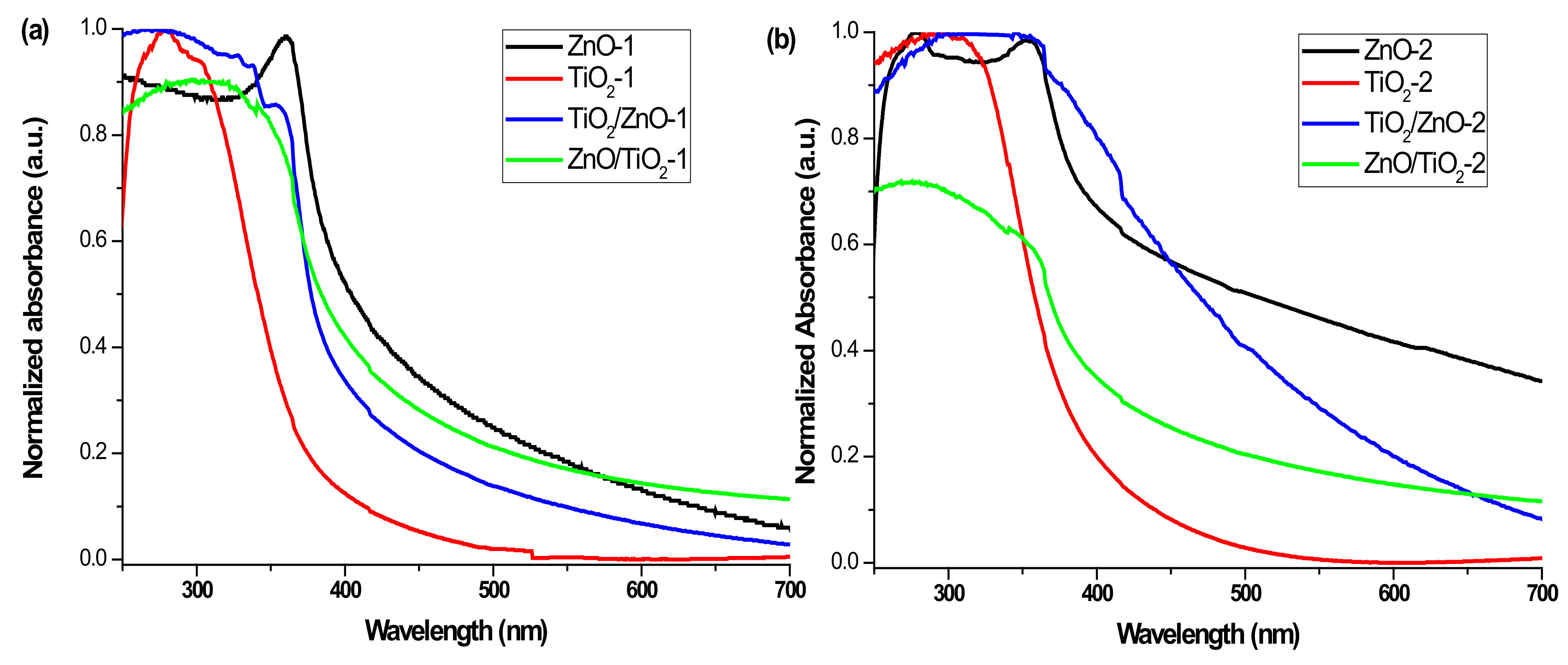
3.4. UV-Vis Absorption Spectra
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Amans, D.; Cai, W.; Barcikowski, S. Status and demand of research to bring laser generation of nanoparticles in liquids to maturity. Appl. Surf. Sci. 2019, 488, 445–454. [Google Scholar] [CrossRef]
- Zeng, H.B.; Du, X.W.; Singh, S.C.; Kulinich, S.A.; Yang, S.K.; He, J.P.; Cai, W.P. Nanomaterials via laser ablation/ irradiation in liquid: A review. Adv. Funct. Mater. 2012, 22, 1333–1353. [Google Scholar] [CrossRef]
- Niu, K.Y.; Yang, J.; Kulinich, S.A.; Sun, J.; Li, H.; Du, X.W. Morphology control of nanostructures via surface reaction of metal nanodroplets. J. Am. Chem. Soc. 2010, 132, 9814–9819. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Liu, P.; Wang, C.X.; Yang, G.W. External field-assisted laser ablation in liquid: An efficient strategy for nanocrystal synthesis and nanostructure assembly. Prog. Mater. Sci. 2017, 87, 140–220. [Google Scholar] [CrossRef]
- Zhang, J.; Claverie, J.; Chaker, M.; Ma, D. Colloidal metal nanoparticles prepared by laser ablation and their applications. ChemPhysChem 2017, 18, 986–1006. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chaker, M.; Ma, D. Pulsed laser ablation based synthesis of colloidal metal nanoparticles for catalytic applications. J. Colloid Interface Sci. 2017, 489, 138–149. [Google Scholar] [CrossRef]
- Reichenberger, S.; Marzun, G.; Muhler, M.; Barcikowski, S. Perspective of surfactant-free colloidal nanoparticles in heterogeneous catalysis. ChemCatChem 2019, 11, 4489–4518. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, J.; Li, P.; Tian, Z.; Liang, C. Recent advances in surfactant-free, surface-charged, and defect-rich catalysts developed by laser ablation and processing in liquids. ChemNanoMat 2017, 3, 512–533. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Z.; Liu, H.; Dong, C.; Wang, J.; Kulinich, S.A.; Du, X.W. Laser-prepared CuZn alloy catalyst for selective electrochemical reduction of CO2 to ethylene. Langmuir 2018, 34, 13544–13549. [Google Scholar] [CrossRef] [PubMed]
- Mintcheva, N.; Srinivasan, P.; Rayappan, J.B.B.; Kuchmizhak, A.A.; Gurbatov, S.; Kulinich, S.A. Room-temperature gas sensing of laser-modified anatase TiO2 decorated with Au nanoparticles. Appl. Surf. Sci. 2020, 507, 145169. [Google Scholar] [CrossRef]
- Niu, K.Y.; Kulinich, S.A.; Yang, J.; Zhu, A.L.; Du, X.W. Galvanic replacement reactions of active metal nanoparticles. Chem.-Eur. J. 2012, 18, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, E.A.; Goncharova, D.A.; Lapin, I.N.; Nemoykina, A.L.; Svetlichnyi, V.A.; Aljulaih, A.A.; Mintcheva, N.; Kulinich, S.A. Comparative study of physicochemical and antibacterial properties of ZnO nanoparticles prepared by laser ablation of Zn target in water and air. Materials 2019, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Mintcheva, N.; Aljulaih, A.A.; Bito, S.; Honda, M.; Kondo, T.; Iwamori, S.; Kulinich, S.A. Nanomaterials produced by laser beam ablating Sn-Zn alloy in water. J. Alloys Compd. 2018, 747, 166–175. [Google Scholar] [CrossRef]
- Wang, H.B.; Wang, J.Q.; Mintcheva, N.; Wang, M.; Li, S.; Mao, J.; Liu, H.; Dong, C.K.; Kulinich, S.A.; Du, X.W. Laser synthesis of iridium nanospheres for overall water splitting. Materials 2019, 12, 3028. [Google Scholar] [CrossRef]
- Liu, P.; Cai, W.; Fang, M.; Li, Z.; Zeng, H.; Hu, J.; Luo, X.; Jing, W. Room temperature synthesized rutile TiO2 nanoparticles induced by laser ablation in liquid and their photocatalytic activity. Nanotechnology 2009, 20, 285707. [Google Scholar] [CrossRef]
- Zimbone, M.; Cacciato, G.; Buccheri, M.A.; Sanz, R.; Piluso, N.; Reitano, R.; La Via, F.; Grimaldi, M.G.; Privitera, V. Photocatalytical activity of amorphous hydrogenated TiO2 obtained by pulsed laser ablation in liquid. Mater. Sci. Semicond. Proc. 2016, 42, 28–31. [Google Scholar] [CrossRef]
- Pan, S.S.; Lu, W.; Zhao, Y.H.; Tong, W.; Li, M.; Jin, L.M.; Choi, J.Y.; Qi, F.; Chen, S.G.; Fei, L.F.; et al. Self-doped rutile titania with high performance for direct and ultrafast assay of H2O2. ACS Appl. Mater. Interfaces 2013, 5, 12784–12788. [Google Scholar] [CrossRef]
- Amin, M.; Tomko, J.; Naddeo, J.J.; Jimenez, R.; Bubb, D.M.; Steiner, M.; Fitz-Gerald, J.; O’Malley, S.M. Laser-assisted synthesis of ultra-small anatase TiO2 nanoparticles. Appl. Surf. Sci. 2015, 348, 30–37. [Google Scholar] [CrossRef]
- Huang, C.N.; Bow, J.S.; Zheng, Y.; Chen, S.Y.; Ho, N.; Shen, P. Nonstoichiometric titanium oxides via pulsed laser ablation in water. Nanoscale Res. Lett. 2010, 5, 972–985. [Google Scholar] [CrossRef]
- Nikolov, A.S.; Atanasov, P.A.; Milev, D.R.; Stoyanchov, T.R.; Deleva, A.D.; Peshev, Z.Y. Synthesis and characterization of TiOx nanoparticles prepared by pulsed-laser ablation of Ti target in water. Appl. Surf. Sci. 2009, 255, 5351–5354. [Google Scholar] [CrossRef]
- García Guillén, G.; Shaji, S.; Mendivil, I.; Avellaneda, D.; Castillo, G.; Roy, T.; Garcia-Gutierrez, D.; Krishnan, B. Effects of ablation energy and post-irradiation on the structure and properties of titanium dioxide nanomaterials. Appl. Surf. Sci. 2017, 405, 183–194. [Google Scholar] [CrossRef]
- Hong, S.M.; Lee, S.; Jung, H.J.; Yu, Y.; Shin, J.H.; Kwon, K.Y.; Choi, M.Y. Simple preparation of anatase TiO2 nanoparticles via pulsed laser ablation in liquid. Bull. Korean Chem. Soc. 2013, 34, 279–282. [Google Scholar] [CrossRef]
- Singh, S.C.; Swarnkar, R.K.; Gopal, R. Synthesis of titanium dioxide nanomaterial by pulsed laser ablation in water. J. Nanosci. Nanotechnol. 2009, 9, 5367–5371. [Google Scholar] [CrossRef]
- Barreca, F.; Acacia, N.; Barletta, E.; Spadaro, D.; Curro, G.; Neri, F. Small size TiO2 nanoparticles prepared by laser ablation in water. Appl. Surf. Sci. 2010, 256, 6408–6412. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Joshi, M.P.; Mondal, P.; Sinha, A.K.; Srivastava, A.K. Growth of anatase and rutile phase TiO2 nanoparticles using pulsed laser ablation in liquid: Influence of surfactant addition and ablation time variation. Appl. Surf. Sci. 2017, 396, 303–309. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Rodríguez-González, B.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Production of TiO2 crystalline nanoparticles by laser ablation in ethanol. Appl. Surf. Sci. 2012, 258, 9484–9486. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Rodríguez-González, B.; del Val, J.; Comesaña, R.; Lusquiños, F.; Pou, J. Laser-assisted production of spherical TiO2 nanoparticles in water. Nanotechnology 2011, 22, 195606. [Google Scholar] [CrossRef]
- Reich, S.; Göttlicher, J.; Letzel, A.; Gökce, B.; Barcikowski, S.; dos Santos Rolo, T.; Baumbach, T.; Plech, A. X-ray spectroscopic and stroboscopic analysis of pulsed-laser ablation of Zn and its oxidation. Appl. Phys. A 2018, 124, 71. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Preparation of zinc oxide nanorods using pulsed laser ablation in water media at high temperature. J. Colloid Interface Sci. 2006, 300, 612–615. [Google Scholar] [CrossRef]
- Kulinich, S.A.; Kondo, T.; Shimizu, Y.; Ito, T. Pressure effect on ZnO nanoparticles prepared via laser ablation in water. J. Appl. Phys. 2013, 113, 033509. [Google Scholar] [CrossRef]
- Goto, T.; Honda, M.; Kulinich, S.A.; Shimizu, Y.; Ito, T. Defects in ZnO nanoparticles laser-ablated in water-ethanol mixture at different pressures. Jpn. J. Appl. Phys. 2015, 54, 070305. [Google Scholar] [CrossRef]
- Cho, J.M.; Song, J.K.; Park, S.M. Characterization of ZnO nanoparticles grown by laser ablation of a Zn target in neat water. Bull. Korean Chem. Soc. 2009, 30, 1616–1618. [Google Scholar] [CrossRef][Green Version]
- Kim, K.K.; Kim, D.; Kim, S.K.; Park, S.M.; Song, J.K. Formation of ZnO nanoparticles by laser ablation in neat water. Chem. Phys. Lett. 2011, 511, 116–120. [Google Scholar] [CrossRef]
- Dorranian, D.; Solati, E.; Dejam, L. Photoluminescence of ZnO nanoparticles generated by laser ablation in deionized water. Appl. Phys. A 2012, 109, 307–314. [Google Scholar] [CrossRef]
- Zamiri, R.; Zakaria, A.; Ahangar, H.A.; Darroudi, M.; Zak, A.K.; Drummen, G.P.C. Aqueous starch as a stabilizer in zinc oxide nanoparticle synthesis via laser ablation. J. Alloys Compd. 2012, 516, 41–48. [Google Scholar] [CrossRef]
- Usui, H.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. Photoluminescence of ZnO nanoparticles prepared by laser ablation in different surfactant solutions. J. Phys. Chem. B 2005, 109, 120–124. [Google Scholar] [CrossRef]
- Kawabata, K.; Nanai, Y.; Kimura, S.; Okuno, T. Fabrication of ZnO nanoparticles by laser ablation of sintered ZnO in aqueous solution. Appl. Phys. A 2012, 107, 213–220. [Google Scholar] [CrossRef]
- He, C.; Sasaki, T.; Usui, H.; Shimizu, Y.; Koshiza, N. Fabrication of ZnO nanoparticles by pulsed laser ablation in aqueous media and pH-dependent particle size: An approach to study the mechanism of enhanced green photoluminescence. J. Photochem. Photobiol. A 2007, 191, 66–73. [Google Scholar] [CrossRef]
- Desarkar, H.S.; Kumbhakar, P.; Mitra, A.K. One-step synthesis of Zn/ZnO hollow nanoparticles by the laser ablation in liquid technique. Laser Phys. Lett. 2013, 10, 055903. [Google Scholar] [CrossRef]
- Niu, K.Y.; Yang, J.; Kulinich, S.A.; Sun, J.; Du, X.W. Hollow nanoparticles of metal oxides and sulfides: Fast preparation via laser ablation in liquid. Langmuir 2010, 26, 16652–16657. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Goto, T.; Owashi, T.; Rozhin, A.G.; Yamaguchi, S.; Ito, T.; Kulinich, S.A. ZnO nanorods prepared via ablation of Zn with millisecond laser in liquid media. Phys. Chem. Chem. Phys. 2016, 18, 23628–23637. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Sato, Y.; Kinoshita, M.; Shankar, P.; Mintcheva, N.; Honda, M.; Iwamori, S.; Kulinich, S.A. Room temperature ethanol sensor based on ZnO prepared via laser ablation in water. Jpn. J. Appl. Phys. 2017, 56, 080304. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-ablated ZnO nanoparticles and their photocatalytic activity towards organic pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Abbas, K.N.; Bidin, N. Morphological driven photocatalytic activity of ZnO nanostructures. Appl. Surf. Sci. 2017, 394, 498–508. [Google Scholar] [CrossRef]
- Kubiak, A.; Siwinska-Ciesielczyk, K.; Jesionowski, T. Titania-based hybrid materials with ZnO, ZrO2 and MoS2: A review. Materials 2018, 11, 2295. [Google Scholar] [CrossRef]
- Lee, B.-H.; Nakayama, T.; Tokoi, Y.; Suzuki, T.; Niihara, K. Synthesis of CeO2/TiO2 nanoparticles by laser ablation of Ti target in cerium (III) nitrate hexahydrate (Ce(NO3)3·6H2O) aqueous solution. J. Alloys Compd. 2011, 509, 1231–1235. [Google Scholar] [CrossRef]
- Gondal, M.A.; Ilyas, A.M.; Fasasi, T.A.; Dastageer, M.A.; Seddigi, Z.S.; Qahtan, T.F.; Faiz, M.; Khattak, G.D. Synthesis of green TiO2/ZnO/CdS hybrid nano-catalyst for efficient light harvesting using an elegant pulsed laser ablation in liquids method. Appl. Surf. Sci. 2015, 357, 2217–2222. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, J.; Zhu, J.; Fan, L.; Chen, H.; He, H.; Wang, Q. Visible light photocatalytic performance of laser-modified TiO2/SnO2 powders decorated with SiC nanocrystals. Ceram. Int. 2019, 45, 12449–12454. [Google Scholar] [CrossRef]
- Gondal, M.A.; Ilyas, A.M.; Baig, U. Pulsed laser ablation in liquid synthesis of ZnO/TiO2 nanocomposite catalyst with enhanced photovoltaic and photocatalytic performance. Ceram. Int. 2016, 42, 13151–13160. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation, Physical Electronics Division: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Zhang, X.; Qin, J.; Xue, Y.; Yu, P.; Zhang, B.; Wang, L.; Liu, R. Effect of aspect ratio and surface defects on the photocatalytic activity of ZnO nanorods. Sci. Rep. 2014, 4, 4596. [Google Scholar] [CrossRef] [PubMed]





| Sample Notation | Laser Conditions | |||||
|---|---|---|---|---|---|---|
| Pulse Peak Power (kW) | Pulse Width (ms) | Pulse Energy (J/pulse) | Time (min) | Liquid Medium | Plate | |
| TiO2-1 | 1.0 | 2.0 | 2.0 | 30 | Water | Ti |
| ZnO-1 | 1.0 | 2.0 | 2.0 | 30 | Water | Zn |
| ZnO/TiO2-1 | 1.0 | 2.0 | 2.0 | 30 + 30 | ZnO colloid * | Zn/Ti |
| TiO2/ZnO-1 | 1.0 | 2.0 | 2.0 | 30 + 30 | TiO2 colloid * | Ti/Zn |
| TiO2-2 | 5.0 | 1.0 | 5.0 | 30 | Water | Ti |
| ZnO-2 | 5.0 | 1.0 | 5.0 | 30 | Water | Zn |
| ZnO/TiO2-2 | 5.0 | 1.0 | 5.0 | 30 + 30 | ZnO colloid * | Zn/Ti |
| TiO2/ZnO-2 | 5.0 | 1.0 | 5.0 | 30 + 30 | TiO2 colloid * | Ti/Zn |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mintcheva, N.; Yamaguchi, S.; Kulinich, S.A. Hybrid TiO2-ZnO Nanomaterials Prepared Using Laser Ablation in Liquid. Materials 2020, 13, 719. https://doi.org/10.3390/ma13030719
Mintcheva N, Yamaguchi S, Kulinich SA. Hybrid TiO2-ZnO Nanomaterials Prepared Using Laser Ablation in Liquid. Materials. 2020; 13(3):719. https://doi.org/10.3390/ma13030719
Chicago/Turabian StyleMintcheva, Neli, Shigeru Yamaguchi, and Sergei A. Kulinich. 2020. "Hybrid TiO2-ZnO Nanomaterials Prepared Using Laser Ablation in Liquid" Materials 13, no. 3: 719. https://doi.org/10.3390/ma13030719
APA StyleMintcheva, N., Yamaguchi, S., & Kulinich, S. A. (2020). Hybrid TiO2-ZnO Nanomaterials Prepared Using Laser Ablation in Liquid. Materials, 13(3), 719. https://doi.org/10.3390/ma13030719







