Effective Utilization of Waste Glass as Cementitious Powder and Construction Sand in Mortar
Abstract
1. Introduction
2. Materials and Experimental Methods
2.1. Raw Materials
2.1.1. Cement
2.1.2. Waste Glass Powder (WGP)
2.1.3. Waste Glass Sand (WGS)
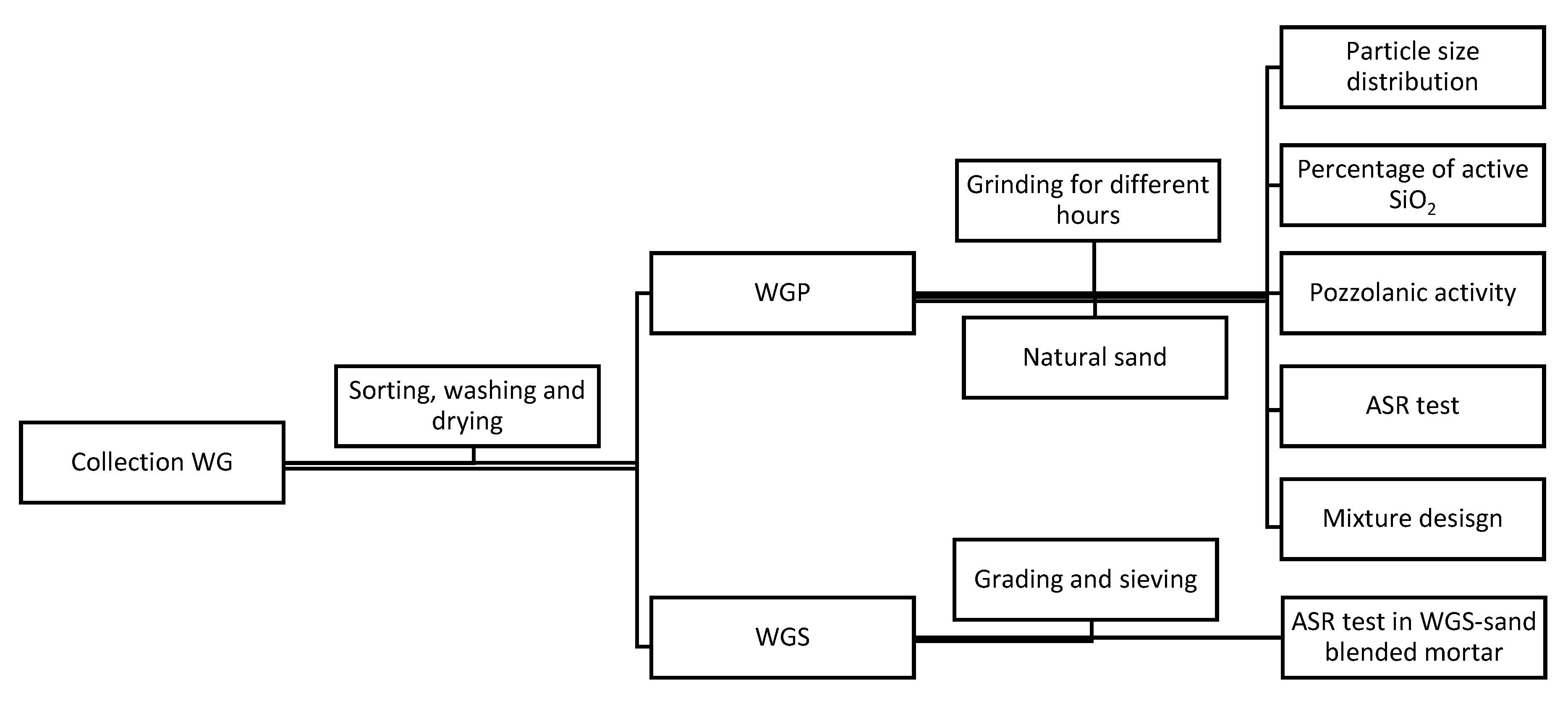
2.2. Research Framework
2.3. Experimental Methods
2.3.1. Alkali–Silica Reaction (ASR) Test
- When ∑16 ≤ 0.10%, in most cases, it can be judged that there is no potential ASR hazard;
- When ∑16 ≥ 0.20%, it can be determined that there is a potential ASR hazard;
- When ∑16 is between 0.10% and 0.20%, the potential ASR hazard cannot be finally determined, while supplementary information should be supplied.
2.3.2. Pozzolanic Activity Test
SiO2 Activity Rate (Ka)
Activity Rate Index (Kc)
2.3.3. Taguchi Design for WGP Blended Mortars
2.3.4. Thermogravimetric Analysis-Derivative Thermogravimetry (TGA-DTG) Test
3. Results and Discussions
3.1. Pozzolanic Activity Rate
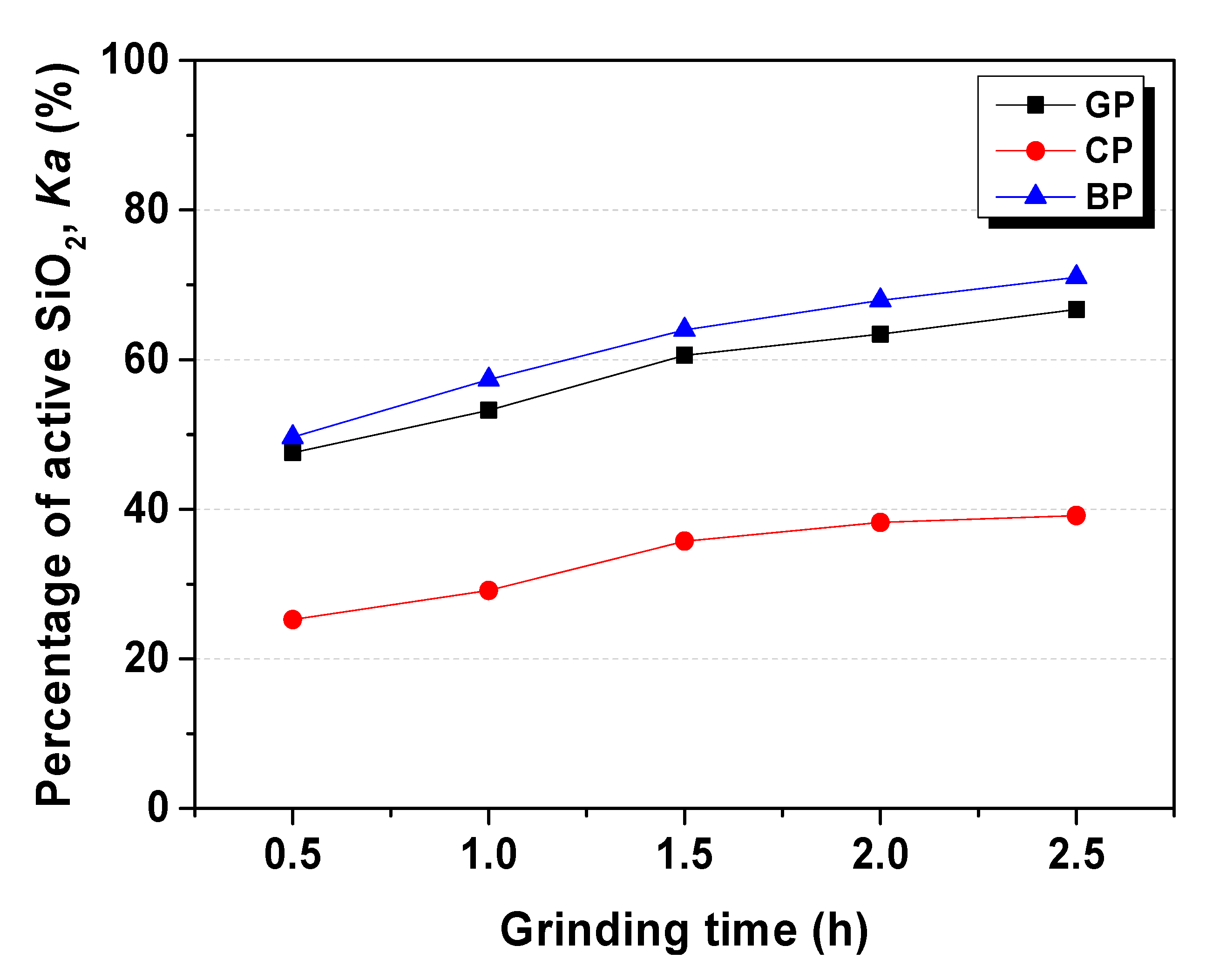
3.1.1. Percentage of Active SiO2 (Ka) in WGP
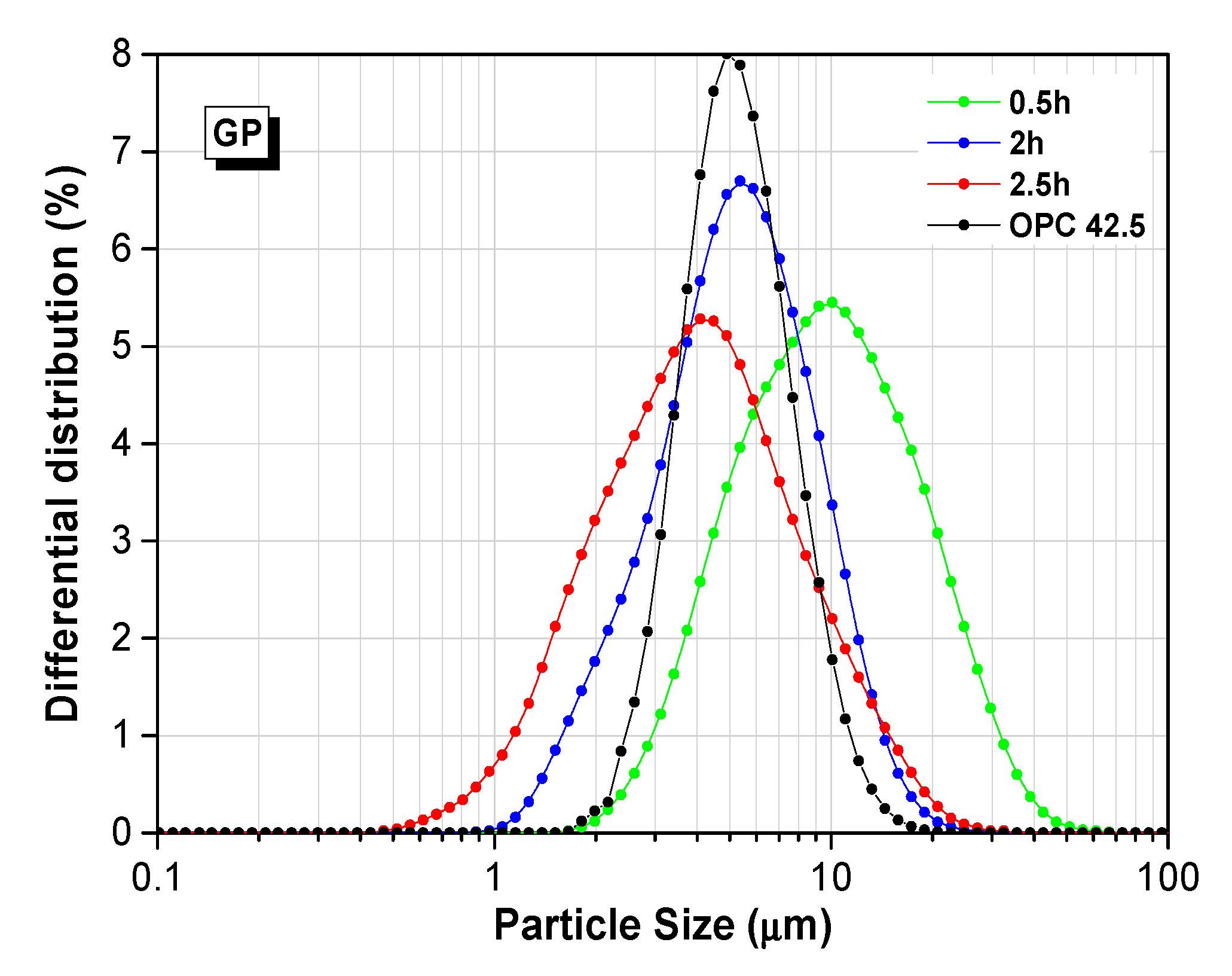
3.1.2. Particle Size Distribution of Three WGPs
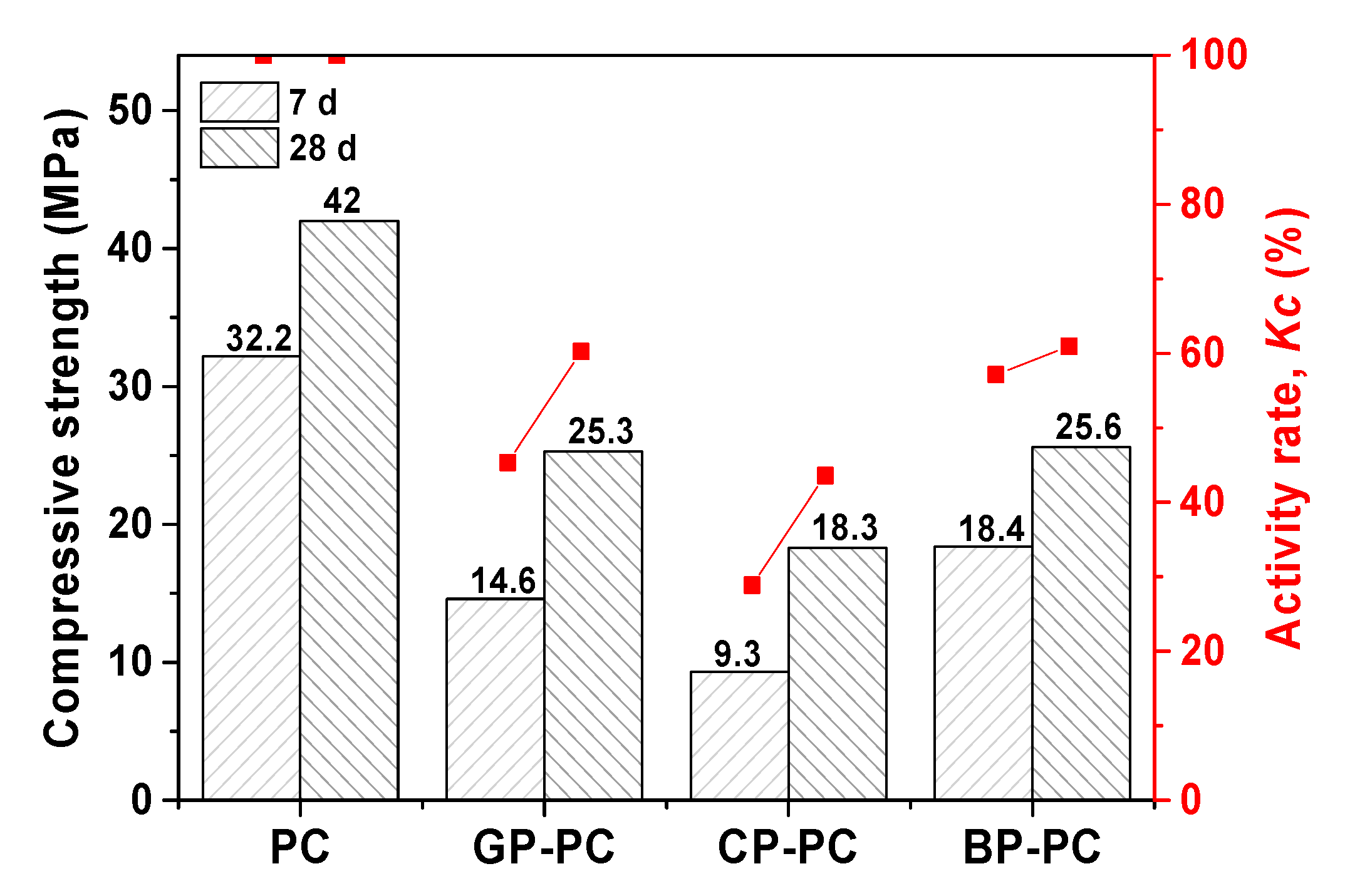
3.1.3. Pozzolanic Activity Rate of WGP (Kc)
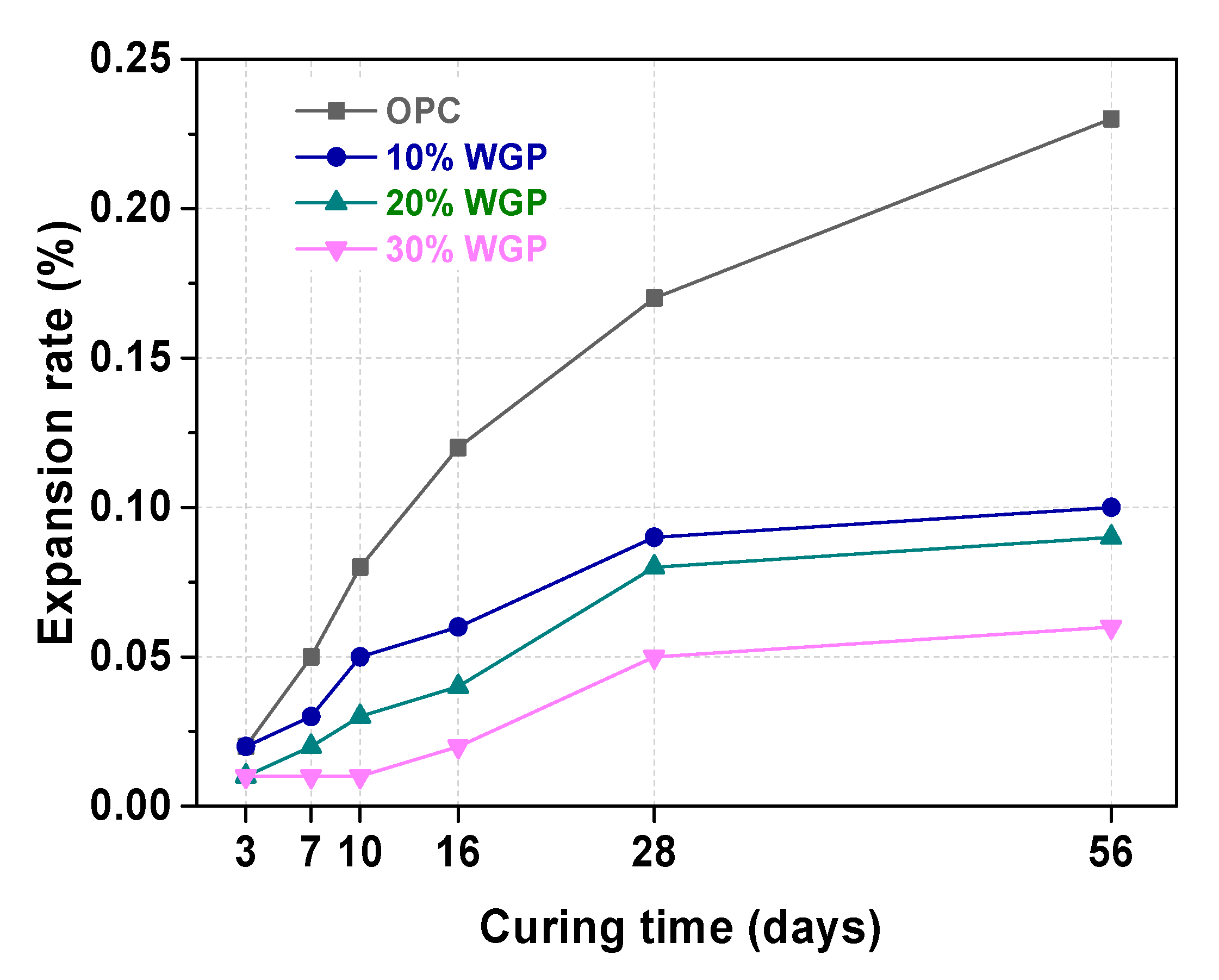
3.2. ASR Test of WGP-Cement Mortar
3.3. Taguchi Test
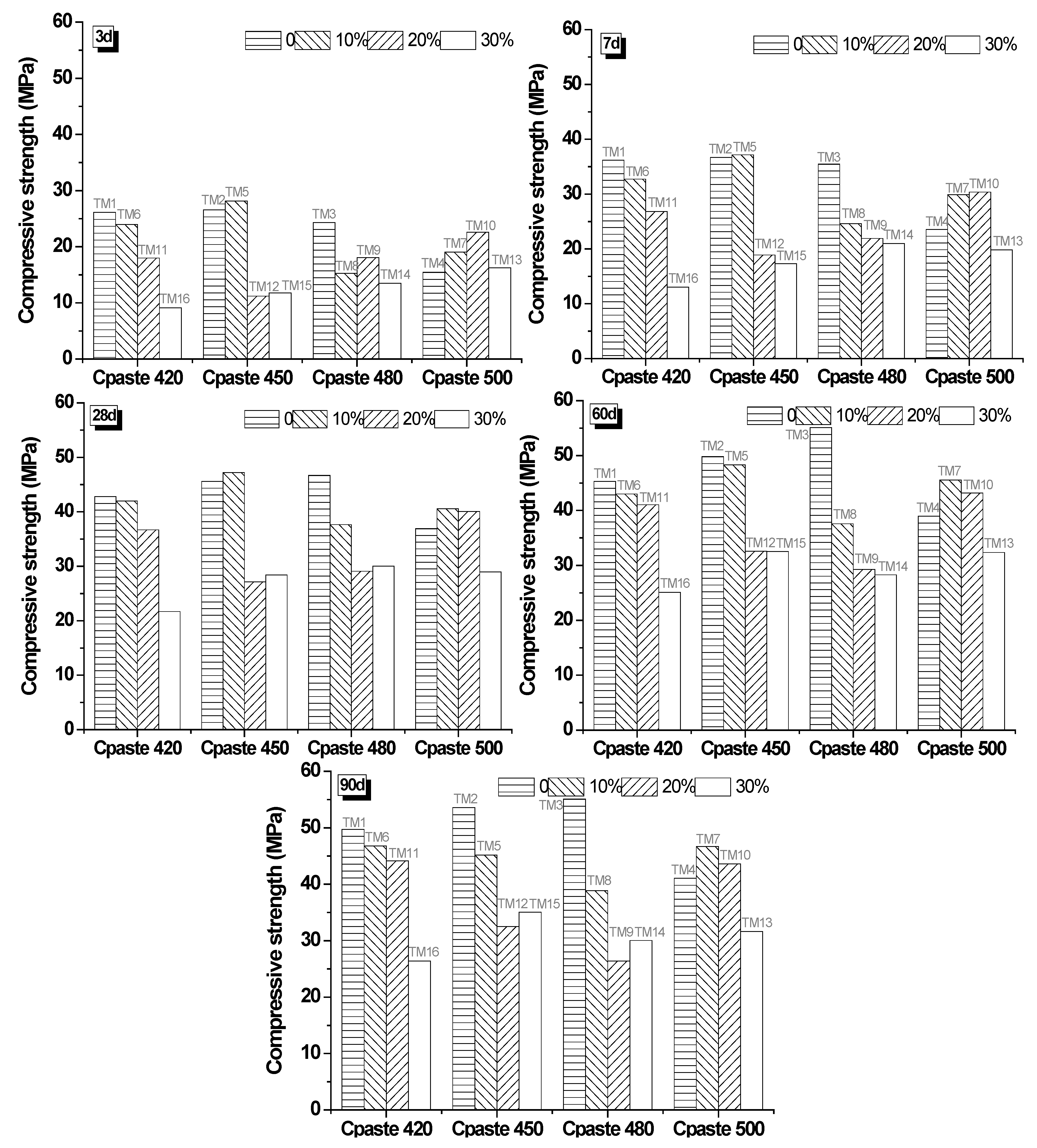
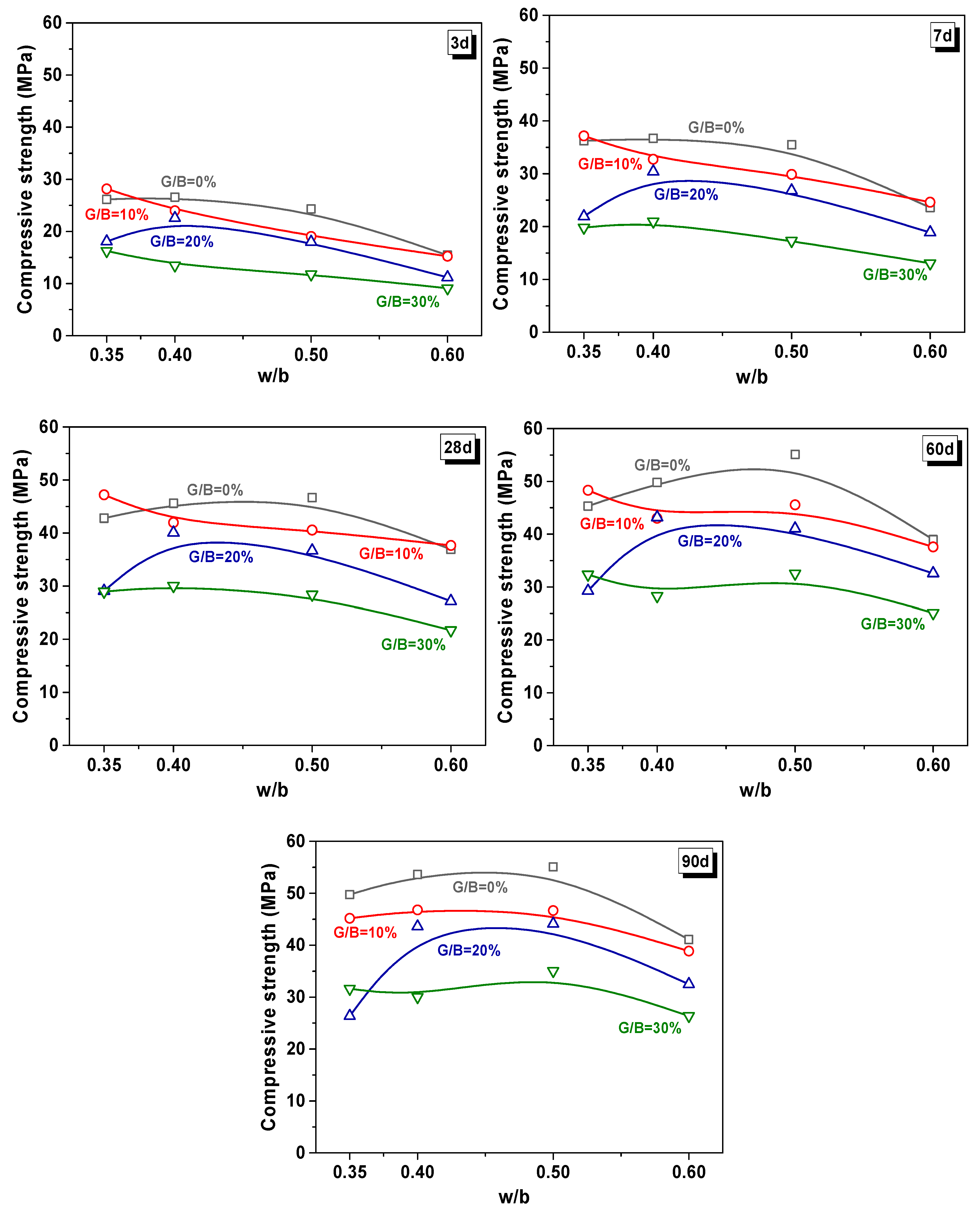
3.3.1. Compressive Strength of Mixtures
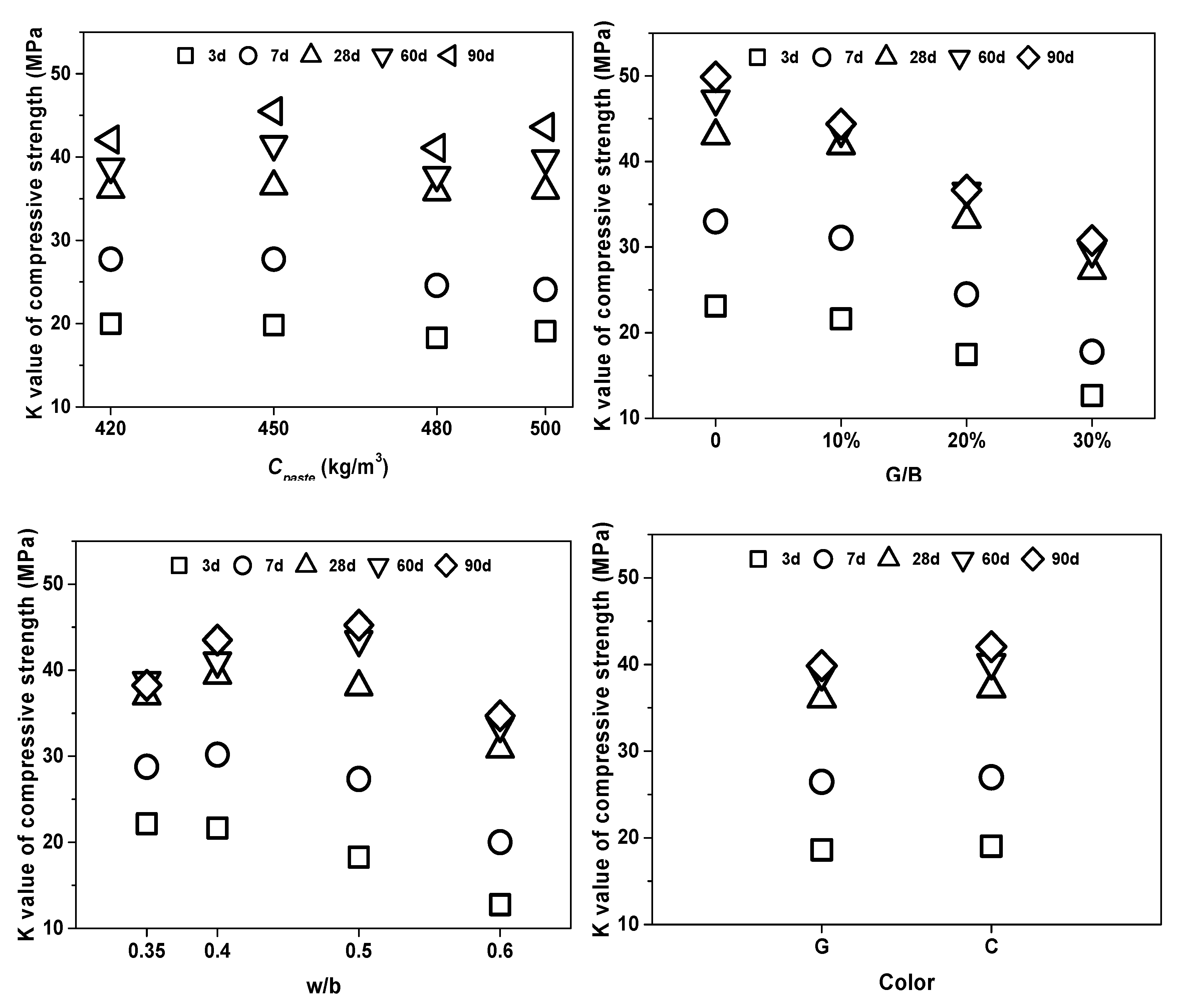
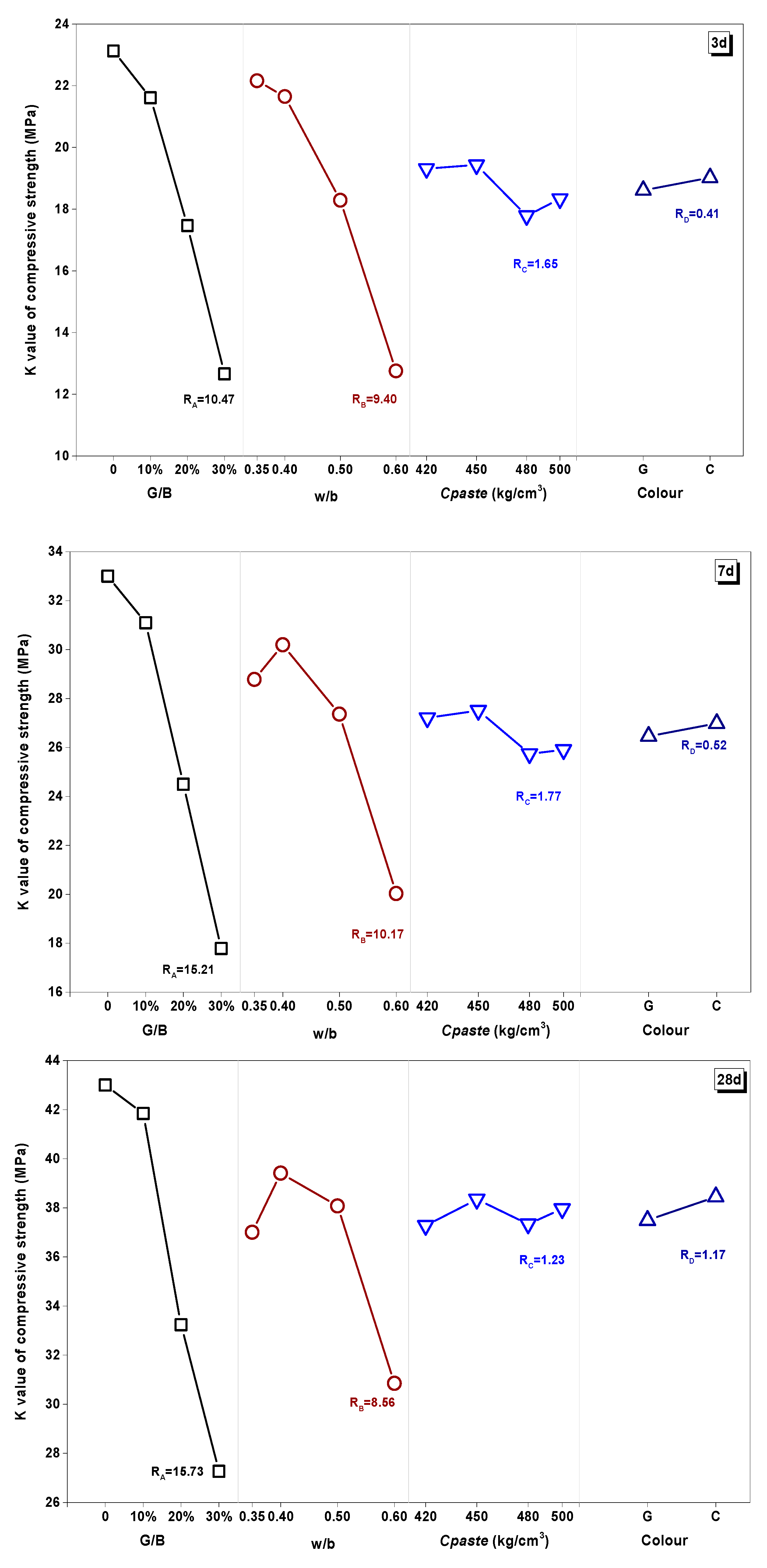
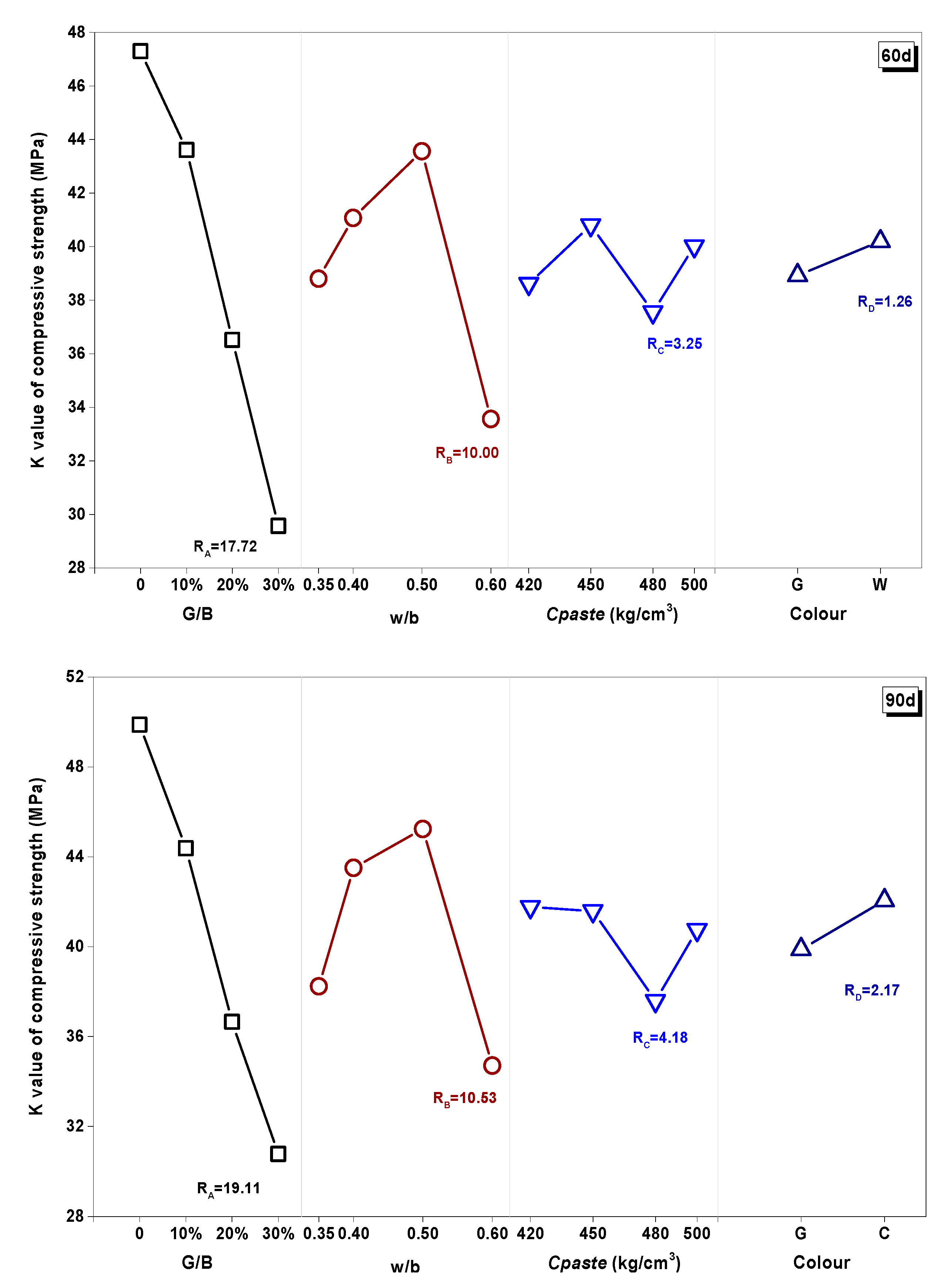
3.3.2. K Value and R Value Analysis
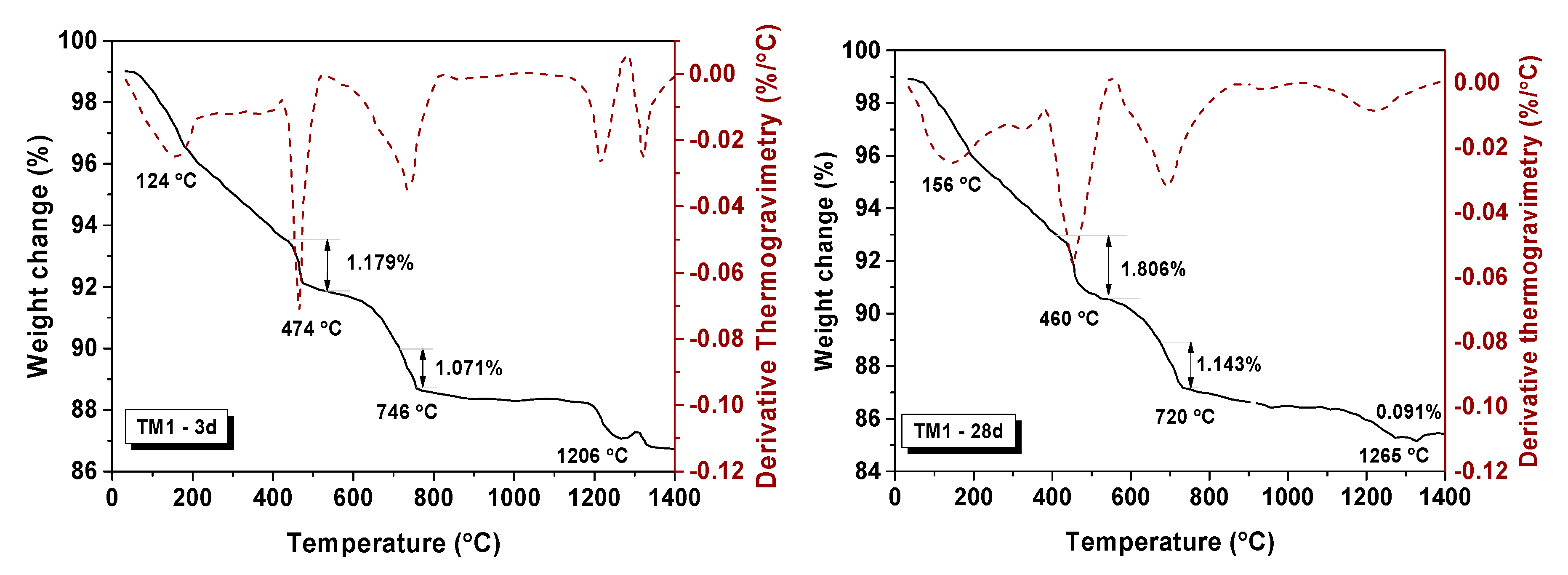
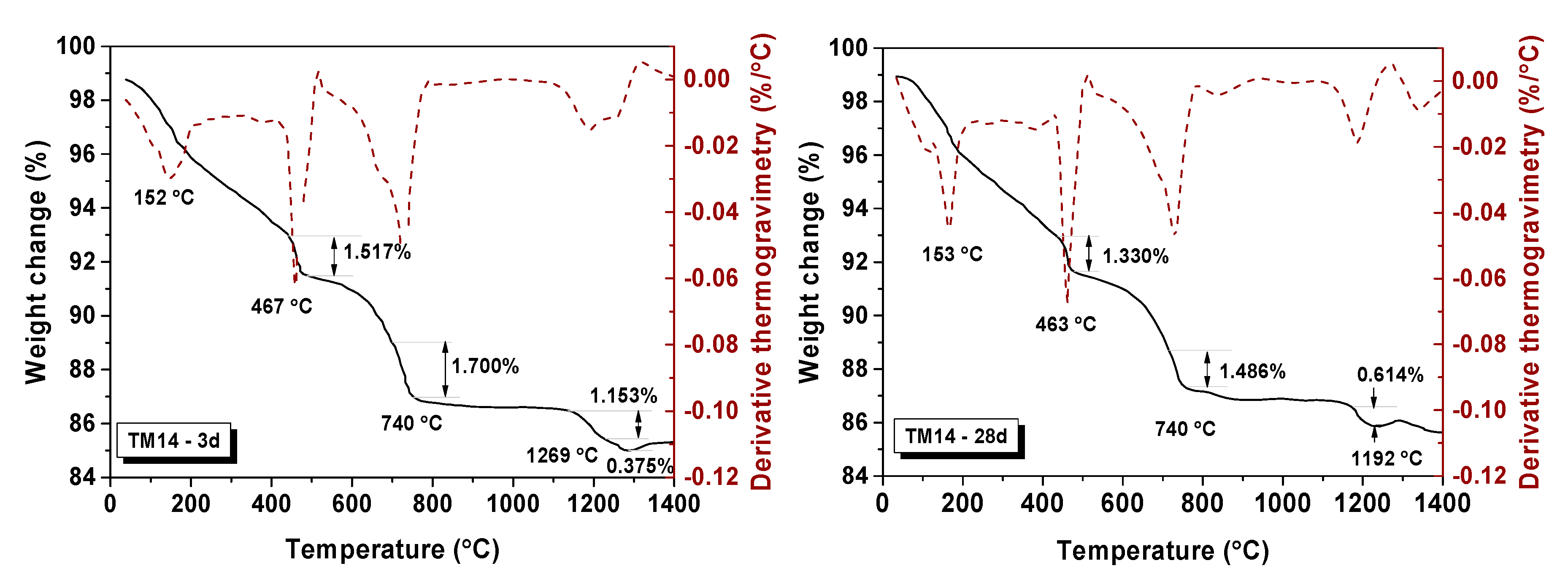
3.3.3. Thermogravimetric Analysis (TGA-DTG)
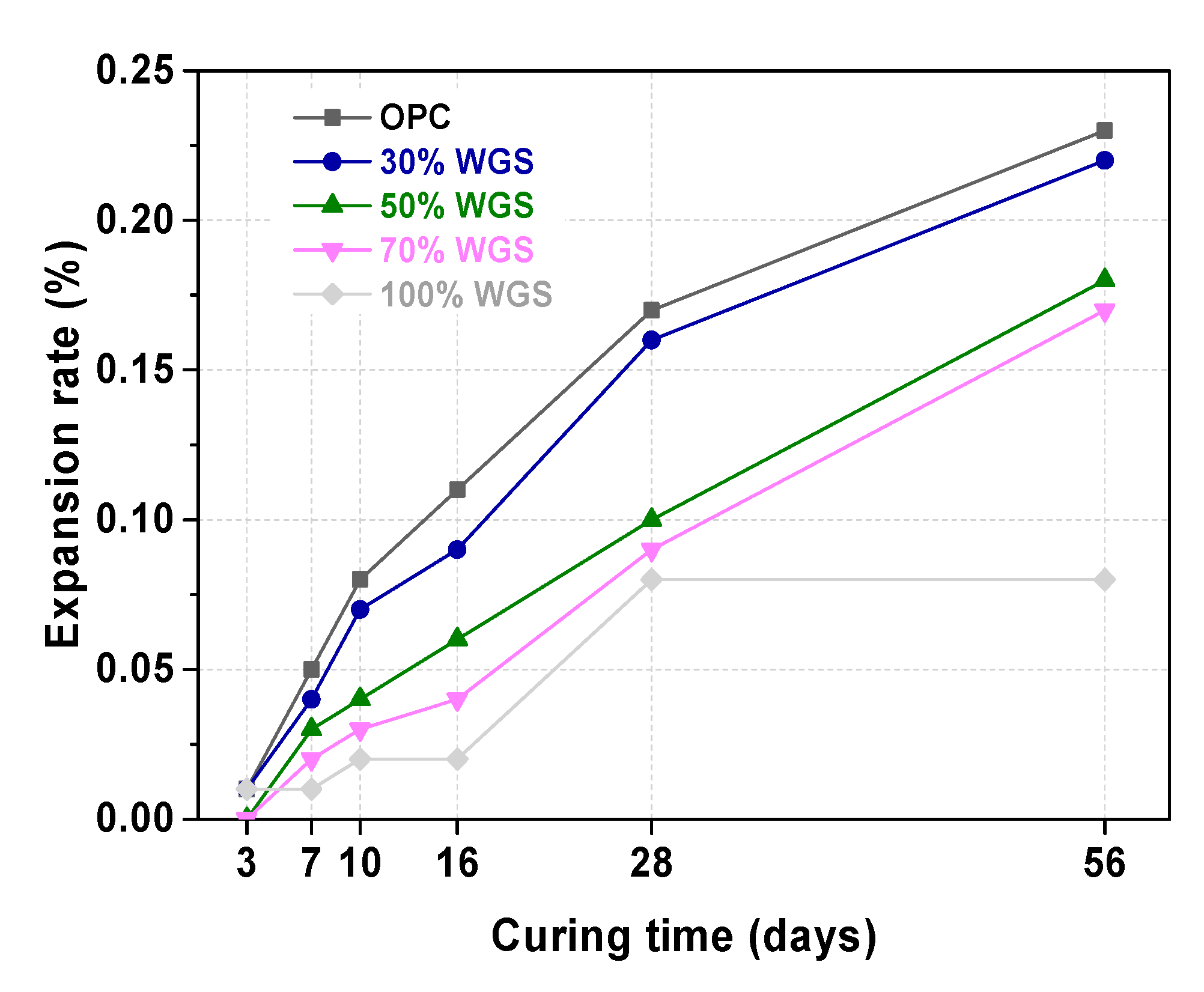
3.4. ASR Test of WGS-Sand Mortar
4. Conclusions
- Waste glass could be effectively used as construction materials. The ground glass powder could be used as supplementary cementitious materials. The remaining glass cullet could be used as standard sand.
- Grinding time played a dominant role in percentage of active SiO2 (Ka) and particle size distribution of WGP. In terms of green glass powder, 0.5 h of grinding was enough to achieve a suitable particle size distribution to replace PC in mortar.
- The ASR test results of WGP-blended samples with different substituted rates were encouraging. There was no potential ASR risk presented.
- The effect of the water/binder (w/b) ratio, cementitious material dosage (Cpaste), replacement rate (G/B), and color of glass powder had different impact proportions on the properties of WGP-blended mortars. The effect of Cpaste and w/b had a notable difference between the earlier and long-term curing ages. Therefore, in terms of practical application, the optimum design for WGP substituted samples should take account to the influence of curing age.
- Optimal mixture design could not be addressed from this contribution, owing to the decrease of compressive strength. However, future studies should focus on the optimal mixture design depending on different compressive strength levels.
- After being graded into standard sand, the WGS could substitute construction sand in cement mortar without an ASR expansion risk.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ASR | Alkali Silica Reaction |
| BP | Brown Powder |
| CP | Colorless Powder |
| CSH | Calcium Silicate Hydrate |
| GP | Green Powder |
| Ka | Percentage of Active SiO2 |
| Kc | Activity Rate of Waste Glass Powder |
| OPC | Ordinary Portland Cement |
| SEM | Scanning Electron Microscopy |
| WG | Waste Glass |
| WGP | Waste Glass Powder |
| WGS | Waste Glass Sand |
| XRF | X-Ray Fluorescence |
| XRD | X-Ray Diffraction |
References
- Aitcin, P.C. Cements of Yesterday and Today: Concrete of Tomorrow. Cem. Concr. Res. 2000, 30, 1349–1359. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Lura, P.; Müller, H.S.; Han, S.; Zhao, T. Application of Neutron Imaging to Investigate Fundamental Aspects of Durability of Cement-Based Materials: A Review. Cem. Concr. Res. 2018, 108, 152–166. [Google Scholar] [CrossRef]
- Biernacki, J.J.; Bullard, J.W.; Sant, G.; Brown, K.; Glasser, F.P.; Jones, S.; Ley, T.; Livingston, R.; Nicoleau, L.; Olek, J.; et al. Cements in the 21st Century: Challenges, Perspectives, and Opportunities. J. Am. Ceram. Soc. 2017, 100, 2746–2773. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Li, H.; Ren, Q. Performance and Itz of Pervious Concrete Modified by Vinyl Acetate and Ethylene Copolymer Dispersible Powder. Constr. Build. Mater. 2020, 235, 117532. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.H.; Vogel, M.; Müller, H.S.; Zhao, T. Influence of Freeze-Thaw Cycles on Capillary Absorption and Chloride Penetration into Concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Bao, J.; Li, S.; Zhang, P.; Ding, X.; Xue, S.; Cui, Y.; Zhao, T. Influence of the Incorporation of Recycled Coarse Aggregate on Water Absorption and Chloride Penetration into Concrete. Constr. Build. Mater. 2020, 239, 117845. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Zhang, P.; Li, D.; Qiao, Y.; Zhang, S.; Sun, C.; Zhao, T. Effect of Air Entrainment on the Mechanical Properties, Chloride Migration, and Microstructure of Ordinary Concrete and Fly Ash Concrete. J. Mater. Civ. Eng. 2018, 30, 04018265. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-Activated Materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Zhang, Z.; Wu, Q.; Du, J. Partial Replacement of Metakaolin with Thermally Treated Rice Husk Ash in Metakaolin-Based Geopolymer. Constr. Build. Mater. 2019, 221, 527–538. [Google Scholar] [CrossRef]
- Kyaw Oo D’Amore, G.; Caniato, M.; Travan, A.; Turco, G.; Marsich, L.; Ferluga, A.; Schmid, C. Innovative Thermal and Acoustic Insulation Foam from Recycled Waste Glass Powder. J. Clean. Prod. 2017, 165, 1306–1315. [Google Scholar] [CrossRef]
- Chandra Paul, S.; Šavija, B.; Babafemi, A.J. A Comprehensive Review on Mechanical and Durability Properties of Cement-Based Materials Containing Waste Recycled Glass. J. Clean. Prod. 2018, 198, 891–906. [Google Scholar] [CrossRef]
- Butler, J.H.; Hooper, P. Chapter 11—Glass Waste. In Waste; Letcher, T.M., Vallero, D.A., Eds.; Academic Press: Boston, MA, USA, 2011; pp. 151–165. [Google Scholar]
- Zimmer, A.; Bragança, S.R. A Review of Waste Glass as a Raw Material for Whitewares. J. Environ. Manag. 2019, 244, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Polley, C.; Cramer, S.M.; de la Cruz, R.V. Potential for Using Waste Glass in Portland Cement Concrete. J. Mater. Civ. Eng. 1998, 10, 210–219. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on Concrete Containing Ground Waste Glass. Cem. Concr. Res. 2000, 30, 91–100. [Google Scholar] [CrossRef]
- Topcu, I.B.; Canbaz, M. Properties of Concrete Containing Waste Glass. Cem. Concr. Res. 2004, 34, 267–274. [Google Scholar] [CrossRef]
- Taha, B.; Nounu, G. Properties of Concrete Contains Mixed Colour Waste Recycled Glass as Sand and Cement Replacement. Constr. Build. Mater. 2008, 22, 713–720. [Google Scholar] [CrossRef]
- Jani, Y.; Hogland, W. Waste Glass in the Production of Cement and Concrete—A Review. J. Environ. Chem. Eng. 2014, 2, 1767–1775. [Google Scholar] [CrossRef]
- Saccani, A.; Bignozzi, M.C. Asr Expansion Behavior of Recycled Glass Fine Aggregates in Concrete. Cem. Concr. Res. 2010, 40, 531–536. [Google Scholar] [CrossRef]
- Johnston, C.D. Waste Glass as Coarse Aggregate for Concrete. J. Test. Eval. 1974, 2, 344–350. [Google Scholar]
- Meyer, C.N.E.; Andela, C. Concrete with Waste Glass as Aggregate. In Proceedings of the International Symposium Concrete Technology Unit of ASCE and University of Dundee, Dundee, UK, 19–20 March 2001. [Google Scholar]
- Ducman, V.; Mladenovič, A.; Šuput, J.S. Lightweight Aggregate Based on Waste Glass and Its Alkali–Silica Reactivity. Cem. Concr. Res. 2002, 32, 223–226. [Google Scholar] [CrossRef]
- Park, S.B.; Lee, B.C.; Kim, J.H. Studies on Mechanical Properties of Concrete Containing Waste Glass Aggregate. Cem. Concr. Res. 2004, 34, 2181–2189. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Beglarigale, A.; Yazıcı, H.; Ramyar, K. Transport Properties and Freeze-Thaw Resistance of Mortar Mixtures Containing Recycled Concrete and Glass Aggregates. Eur. J. Environ. Civ. Eng. 2019, 23, 53–69. [Google Scholar] [CrossRef]
- Islam, G.; Sadiqul, M.; Rahman, M.H.; Kazi, N. Waste Glass Powder as Partial Replacement of Cement for Sustainable Concrete Practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Shi, C.; Wu, Y.; Riefler, C.; Wang, H. Characteristics and Pozzolanic Reactivity of Glass Powders. Cem. Concr. Res. 2005, 35, 987–993. [Google Scholar] [CrossRef]
- Shayan, A.; Xu, A. Performance of Glass Powder as a Pozzolanic Material in Concrete: A Field Trial on Concrete Slabs. Cem. Concr. Res. 2006, 36, 457–468. [Google Scholar] [CrossRef]
- Matos, A.M.; Sousa-Coutinho, J. Durability of Mortar Using Waste Glass Powder as Cement Replacement. Constr. Build. Mater. 2012, 36, 205–215. [Google Scholar] [CrossRef]
- Omran, A.; Tagnit-Hamou, A. Performance of Glass-Powder Concrete in Field Applications. Constr. Build. Mater. 2016, 109, 84–95. [Google Scholar] [CrossRef]
- Pascual, A.B.; Tognonvi, M.T.; Tagnit-Hamou, A. Waste Glass Powder-Based Alkali-Activated Mortar. Int. J. Res. Eng. Technol. 2014, 3, 15–19. [Google Scholar]
- Redden, R.; Neithalath, N. Microstructure, Strength, and Moisture Stability of Alkali Activated Glass Powder-Based Binders. Cem. Concr. Compos. 2014, 45, 46–56. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, C.; Zhang, Z.; Li, N. An Overview on the Reuse of Waste Glasses in Alkali-Activated Materials. Resour. Conserv. Recycl. 2019, 144, 297–309. [Google Scholar] [CrossRef]
- Lu, J.-X.; Poon, C.S. Use of Waste Glass in Alkali Activated Cement Mortar. Constr. Build. Mater. 2018, 160, 399–407. [Google Scholar] [CrossRef]
- Xuan, D.; Tang, P.; Poon, C.S. Mswiba-Based Cellular Alkali-Activated Concrete Incorporating Waste Glass Powder. Cem. Concr. Compos. 2019, 95, 128–136. [Google Scholar] [CrossRef]
- ASTM, C. 1260-07. In Standard Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method), Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2007. [Google Scholar]
- Köksal, F.; Altun, F.; Yiğit, İ.; Şahin, Y. Combined Effect of Silica Fume and Steel Fiber on the Mechanical Properties of High Strength Concretes. Constr. Build. Mater. 2008, 22, 1874–1880. [Google Scholar] [CrossRef]
- Panjehpour, M.; Ali, A.A.A.; Demirboga, R. A Review for Characterization of Silica Fume and Its Effects on Concrete Properties. Int. J. Sustain. Constr. Eng. Technol. 2011, 2. [Google Scholar]
- General Administration of Quality Supervision, Inspection and Quarantine; Standardization Administration Committee. Sand for Construction. In GB/T 14684—2011; China Standard Press: Beijing, China, 2011. [Google Scholar]
- Chen, J.-L.; Zhou, W.; Niu, W.; Zhang, C.; Li, F. Explanation About Revision of Sand for Construction (Gb/T 14684-2011). Archit. Technol. 2012, 5. [Google Scholar]
- Agarwal, S.K. Pozzolanic Activity of Various Siliceous Materials. Cem. Concr. Res. 2006, 36, 1735–1739. [Google Scholar] [CrossRef]
- Taguchi, G. Quality Engineering (Taguchi Methods) for the Development of Electronic Circuit Technology. IEEE Trans. Reliab. 1995, 44, 225–229. [Google Scholar] [CrossRef]
- Pereira-de-Oliveira, L.A.; Castro-Gomes, J.P.; Santos, P.M.S. The Potential Pozzolanic Activity of Glass and Red-Clay Ceramic Waste as Cement Mortars Components. Constr. Build. Mater. 2012, 31, 197–203. [Google Scholar] [CrossRef]
- Li, N.; Shi, C.; Wang, Q.; Zhang, Z.; Ou, Z. Composition Design and Performance of Alkali-Activated Cements. Mater. Struct. 2017, 50, 1–11. [Google Scholar] [CrossRef]
- Avet, F.; Scrivener, K. Investigation of the Calcined Kaolinite Content on the Hydration of Limestone Calcined Clay Cement (Lc3). Cem. Concr. Res. 2018, 107, 124–135. [Google Scholar] [CrossRef]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; Crc Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Long, S.; Liu, Y.; Zhang, F. Detection and Analysis of Cement Particle Size Distribution in Large Cement Companies in China. Cem. (Chin. J.) 2010, 10, 20–24. [Google Scholar]
- Alomayri, T. The Microstructural and Mechanical Properties of Geopolymer Composites Containing Glass Microfibres. Ceram. Int. 2017, 43, 4576–4582. [Google Scholar] [CrossRef]
- Afshinnia, K.; Rangaraju, P.R. Influence of Fineness of Ground Recycled Glass on Mitigation of Alkali–Silica Reaction in Mortars. Constr. Build. Mater. 2015, 81, 257–267. [Google Scholar] [CrossRef]
- Thomas, M. The Effect of Supplementary Cementing Materials on Alkali-Silica Reaction: A Review. Cem. Concr. Res. 2011, 41, 1224–1231. [Google Scholar] [CrossRef]
- Al-Zubaid, A.B.; Shabeeb, K.M.; Ali, A.I. Study the Effect of Recycled Glass on the Mechanical Properties of Green Concrete. Energy Procedia 2017, 119, 680–692. [Google Scholar] [CrossRef]
- Wang, Z.; Shi, C.; Song, J. Effect of Glass Powder on Chloride Ion Transport and Alkali-Aggregate Reaction Expansion of Lightweight Aggregate Concrete. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 312–317. [Google Scholar] [CrossRef]
- Urhan, S. Alkali Silica and Pozzolanic Reactions in Concrete. Part 1: Interpretation of Published Results and an Hypothesis Concerning the Mechanism. Cem. Concr. Res. 1987, 17, 141–152. [Google Scholar] [CrossRef]
- Islam, G.M.S.; Islam, M.M.; Akter, A.; Islam, M.S. Green Construction Materials-Bangladesh Perspective. In Proceedings of the International Conference on Mechanical Engineering and Renewable Energy 2011, Chittagong, Bangladesh, 22–24 December 2011. [Google Scholar]
- Liu, G.; Florea, M.V.A.; Brouwers, H.J.H. The Hydration and Microstructure Characteristics of Cement Pastes with High Volume Organic-Contaminated Waste Glass Powder. Constr. Build. Mater. 2018, 187, 1177–1189. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Zhang, P.; Ma, Y.; Zhao, T.; Wang, H.; Zhang, Z. Water Absorption and Chloride Diffusivity of Concrete under the Coupling Effect of Uniaxial Compressive Load and Freeze–Thaw Cycles. Constr. Build. Mater. 2019, 209, 566–576. [Google Scholar] [CrossRef]
- Dewing, E.W.; Richardson, F.D. Decomposition Equilibria for Calcium and Magnesium Sulphates. Trans. Faraday Soc. 1959, 55, 611–615. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Patel, H.H.; Pratt, P.L.; Parrott, L.J. Analysis of Phases in Cement Paste Using Backscattered Electron Images, Methanol Adsorption and Thermogravimetric Analysis. MRS Proc. 1986, 85, 67. [Google Scholar] [CrossRef]
- Pane, I.; Hansen, W. Investigation of Blended Cement Hydration by Isothermal Calorimetry and Thermal Analysis. Cem. Concr. Res. 2005, 35, 1155–1164. [Google Scholar] [CrossRef]
- Xue, S.; Zhang, P.; Bao, J.; He, L.; Hu, Y.; Yang, S. Comparison of Mercury Intrusion Porosimetry and Multi-Scale X-Ray Ct on Characterizing the Microstructure of Heat-Treated Cement Mortar. Mater. Charact. 2019, 160, 110085. [Google Scholar] [CrossRef]
- Lu, L.N.; Yu, S.M.; He, Y.J.; Hu, S.G. Dehydration Process of Calcium Silicate Hydrate During Heating in the Air. Adv. Mater. Res. 2011, 287–290, 785–788. [Google Scholar] [CrossRef]
- Tajuelo Rodriguez, E.; Garbev, K.; Merz, D.; Black, L.; Richardson, I.G. Thermal Stability of C-S-H Phases and Applicability of Richardson and Groves’ and Richardson C-(a)-S-H(I) Models to Synthetic C-S-H. Cem. Concr. Res. 2017, 93, 45–56. [Google Scholar] [CrossRef]
- Chatterji, A.K.; Rawat, R.S. Hydration of Portland Cement. Nature 1965, 207, 713–715. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Nonat, A. Hydration of Cementitious Materials, Present and Future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.E.M.A.; Aboshama, A.Y. Utilization of Waste Glass Powder in the Production of Cement and Concrete. Constr. Build. Mater. 2016, 124, 866–877. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Properties of High Volume Glass Powder Concrete. Cem. Concr. Compos. 2017, 75, 22–29. [Google Scholar] [CrossRef]
- Shi, C.; Zheng, K. A Review on the Use of Waste Glasses in the Production of Cement and Concrete. Resour. Conserv. Recycl. 2007, 52, 234–247. [Google Scholar] [CrossRef]
- Gorospe, K.; Booya, E.; Ghaednia, H.; Das, S. Effect of Various Glass Aggregates on the Shrinkage and Expansion of Cement Mortar. Constr. Build. Mater. 2019, 210, 301–311. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Concrete with Recycled Glass as Fine Aggregates. ACI Mater. J. 2014, 111, 47–57. [Google Scholar]
- Du, H.; Tan, K.H. Effect of Particle Size on Alkali–Silica Reaction in Recycled Glass Mortars. Constr. Build. Mater. 2014, 66, 275–285. [Google Scholar] [CrossRef]


| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | TiO2 | Na2O | K2O | SO3 | LoI |
| Type 42.5 | 20.08 | 5.09 | 2.92 | 61.71 | 1.57 | 0.35 | 0.70 | 0.36 | 1.99 | 5.23 |
| Type 52.5 | 20.87 | 4.87 | 3.59 | 64.47 | 2.13 | 0.11 | 0.65 | 2.52 | 0.77 | |
| Material | Setting Time (h) | Compressive Strength (MPa) | Tensile Strength (MPa) | Specific Surface Area (m2/kg) | ||||||
| Initial Setting | Final Setting | 3 d | 28 d | 3 d | 28 d | |||||
| Type 42.5 | 161 | 217 | 28.2 | 41.4 | 5.4 | 8.2 | 350 | |||
| Type 52.5 | 127 | 128 | 38.4 | 7.3 | 368.9 | |||||
| Waste Glass Powder | SiO2 | Na2O | K2O | CaO | MgO | Al2O3 | Fe2O3 |
|---|---|---|---|---|---|---|---|
| GP | 65.97 | 11.08 | 0.35 | 11.85 | 1.11 | 3.33 | 0.62 |
| CP | 68.43 | 10.97 | 0.32 | 11.79 | 0.91 | 2.21 | 0.17 |
| BP | 70.69 | 10.22 | 1.86 | 10.79 | 1.32 | 3.63 | 0.52 |
| Sieve Size (mm) | 4.75–2.36 | 2.36–1.18 | 1.18–0.6 | 0.6–0.3 | 0.3–0.15 |
|---|---|---|---|---|---|
| Cumulative percentage (%) | 10% | 25% | 25% | 25% | 15% |
| Weight (g) | 99 | 247.5 | 2475 | 247.5 | 148.5 |
| No. | Portland Cement (PC) | WGP | WGS | Standard Sand | w/b | WGP/PC | WGS/Sand |
|---|---|---|---|---|---|---|---|
| OPC | 440 | 0 | 990 | 0.47 | 0 | ||
| 10% WGP | 396 | 44 | 990 | 0.47 | 0.1 | ||
| 20% WGP | 352 | 88 | 990 | 0.47 | 0.2 | ||
| 30% WGP | 308 | 132 | 990 | 0.47 | 0.3 | ||
| 30% WGS | 440 | 297 | 693 | 0.47 | 0.3 | ||
| 50% WGS | 440 | 495 | 495 | 0.47 | 0.5 | ||
| 70% WGS | 440 | 693 | 297 | 0.47 | 0.7 | ||
| 100% WGS | 440 | 990 | 0 | 0.47 | 1.0 |
| No. | Factor | Level 1 | Level 2 | Level 3 | Level 4 |
|---|---|---|---|---|---|
| A | G/B | 0 | 10% | 20% | 30% |
| B | w/b | 0.35 | 0.40 | 0.50 | 0.60 |
| C | Cpaste (kg/m3) | 420 | 450 | 480 | 500 |
| D | Color | Green | White | Green | Green |
| No. | A | B | C | D | Design of Mixture | G/B | w/b | Cpaste (kg/m3) | Color |
|---|---|---|---|---|---|---|---|---|---|
| TM1 | 1 | 1 | 1 | 1 | A1B1C1D1 | 0 | 0.35 | 420 | - |
| TM2 | 1 | 2 | 2 | 2 | A1B2C2D2 | 0 | 0.4 | 450 | - |
| TM3 | 1 | 3 | 3 | 3 | A1B3C3D3 | 0 | 0.5 | 480 | - |
| TM4 | 1 | 4 | 4 | 4 | A1B4C4D4 | 0 | 0.6 | 500 | - |
| TM5 | 2 | 1 | 2 | 3 | A2B1C2D3 | 10% | 0.35 | 450 | G |
| TM6 | 2 | 2 | 1 | 4 | A2B2C1D4 | 10% | 0.4 | 420 | G |
| TM7 | 2 | 3 | 4 | 1 | A2B3C4D1 | 10% | 0.5 | 500 | G |
| TM8 | 2 | 4 | 3 | 2 | A2B4C3D2 | 10% | 0.6 | 480 | W |
| TM9 | 3 | 1 | 3 | 4 | A3B1C3D4 | 20% | 0.35 | 480 | G |
| TM10 | 3 | 2 | 4 | 3 | A3B2C4D3 | 20% | 0.4 | 500 | G |
| TM11 | 3 | 3 | 1 | 2 | A3B3C1D2 | 20% | 0.5 | 420 | W |
| TM12 | 3 | 4 | 2 | 1 | A3B4C2D1 | 20% | 0.6 | 450 | G |
| TM13 | 4 | 1 | 4 | 2 | A4B1C4D2 | 30% | 0.35 | 500 | W |
| TM14 | 4 | 2 | 3 | 1 | A4B2C3D1 | 30% | 0.4 | 480 | G |
| TM15 | 4 | 3 | 2 | 4 | A4B3C2D4 | 30% | 0.5 | 450 | G |
| TM16 | 4 | 4 | 1 | 3 | A4B4C1D3 | 30% | 0.6 | 420 | G |
| Milling Time | 0.5 h | 1 h | 1.5 h | 2 h | 2.5 h |
|---|---|---|---|---|---|
| m(H2SiO3) (g) | 0.201 | 0.225 | 0.256 | 0.268 | 0.282 |
| m(active SiO2) (g) | 0.156 | 0.175 | 0.199 | 0.209 | 0.220 |
| m(total SiO2) (g) | 0.329 | ||||
| GP-Ka | 47.6% | 53.2% | 60.6% | 63.4% | 66.7% |
| Milling Time | 0.5 h | 1 h | 1.5 h | 2 h | 2.5 h |
|---|---|---|---|---|---|
| m(H2SiO3) (g) | 0.111 | 0.128 | 0.157 | 0.168 | 0.172 |
| m(active SiO2) (g) | 0.086 | 0.100 | 0.122 | 0.131 | 0.134 |
| m(total SiO2) (g) | 0.342 | ||||
| WP-Ka | 25.3% | 29.1% | 35.7% | 38.2% | 39.2% |
| Milling Time | 0.5 h | 1 h | 1.5 h | 2 h | 2.5 h |
|---|---|---|---|---|---|
| m(H2SiO3) (g) | 0.225 | 0.260 | 0.290 | 0.308 | 0.322 |
| m(active SiO2) (g) | 0.175 | 0.202 | 0.225 | 0.239 | 0.250 |
| m(total SiO2) (g) | 0.353 | ||||
| WP-Ka | 49.6% | 57.3% | 63.9% | 67.9% | 71.0% |
| Samples | Weight Change (%) | Decomposition of Relative Molar (mol) | Total Molar Amount of Ca(OH)2 (mol) | ||
|---|---|---|---|---|---|
| Ca(OH)2 | CaCO3 | Ca(OH)2 | CaCO3 | ||
| TM1-3d | 1.179 | 1.071 | 0.016 | 0.011 | 0.027 |
| TM1-28d | 1.806 | 1.143 | 0.024 | 0.011 | 0.035 |
| TM14-3d | 1.517 | 1.700 | 0.021 | 0.017 | 0.038 |
| TM14-28d | 1.330 | 1.486 | 0.018 | 0.015 | 0.033 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Cao, Y.; Zhang, P.; Ma, Y. Effective Utilization of Waste Glass as Cementitious Powder and Construction Sand in Mortar. Materials 2020, 13, 707. https://doi.org/10.3390/ma13030707
Wang Y, Cao Y, Zhang P, Ma Y. Effective Utilization of Waste Glass as Cementitious Powder and Construction Sand in Mortar. Materials. 2020; 13(3):707. https://doi.org/10.3390/ma13030707
Chicago/Turabian StyleWang, Yanru, Yubin Cao, Peng Zhang, and Yuwei Ma. 2020. "Effective Utilization of Waste Glass as Cementitious Powder and Construction Sand in Mortar" Materials 13, no. 3: 707. https://doi.org/10.3390/ma13030707
APA StyleWang, Y., Cao, Y., Zhang, P., & Ma, Y. (2020). Effective Utilization of Waste Glass as Cementitious Powder and Construction Sand in Mortar. Materials, 13(3), 707. https://doi.org/10.3390/ma13030707





