Platelet-Rich Plasma in Maxillary Sinus Augmentation: Systematic Review
Abstract
1. Introduction
Rationale
2. Objectives
- P = Patients requiring unilateral or bilateral maxillary sinus augmentation
- I = Sole use of platelet concentrates / PRF + grafting materials
- C = Grafting materials or nothing
- O = Newly formed bone, augmented bone height, implant stability and implant survival
3. Methods
3.1. Eligibility Criteria
- Patients in need of unilateral or bilateral sinus augmentation before implant dental placement.
- Randomized Clinical Trials (RCT), Controlled Clinical Trials (CCT), and comparative studies assessing histological, histomorphometric, clinical, and radiographic outcomes on the additional effects of PRF in sinus augmentation versus the non-use of PRF.
- Specified follow-up period.
3.2. Information Sources
3.3. Search Strategy
3.4. Study Selection
3.5. Data Collection Process and Items
3.6. Outcomes and Summary Measures
3.7. Risk of Bias in Individual Studies
3.8. Synthesis of Results
4. Results
4.1. Study Selection
4.2. Study Characteristics
4.2.1. Sole Use of Platelet Concentrates in Sinus Floor Augmentation:
4.2.2. PRF + Grafting Biomaterials in Sinus Floor Augmentation:
4.3. Risk of Bias within Studies
4.4. Results of Individual Studies
4.5. Synthesis of Results
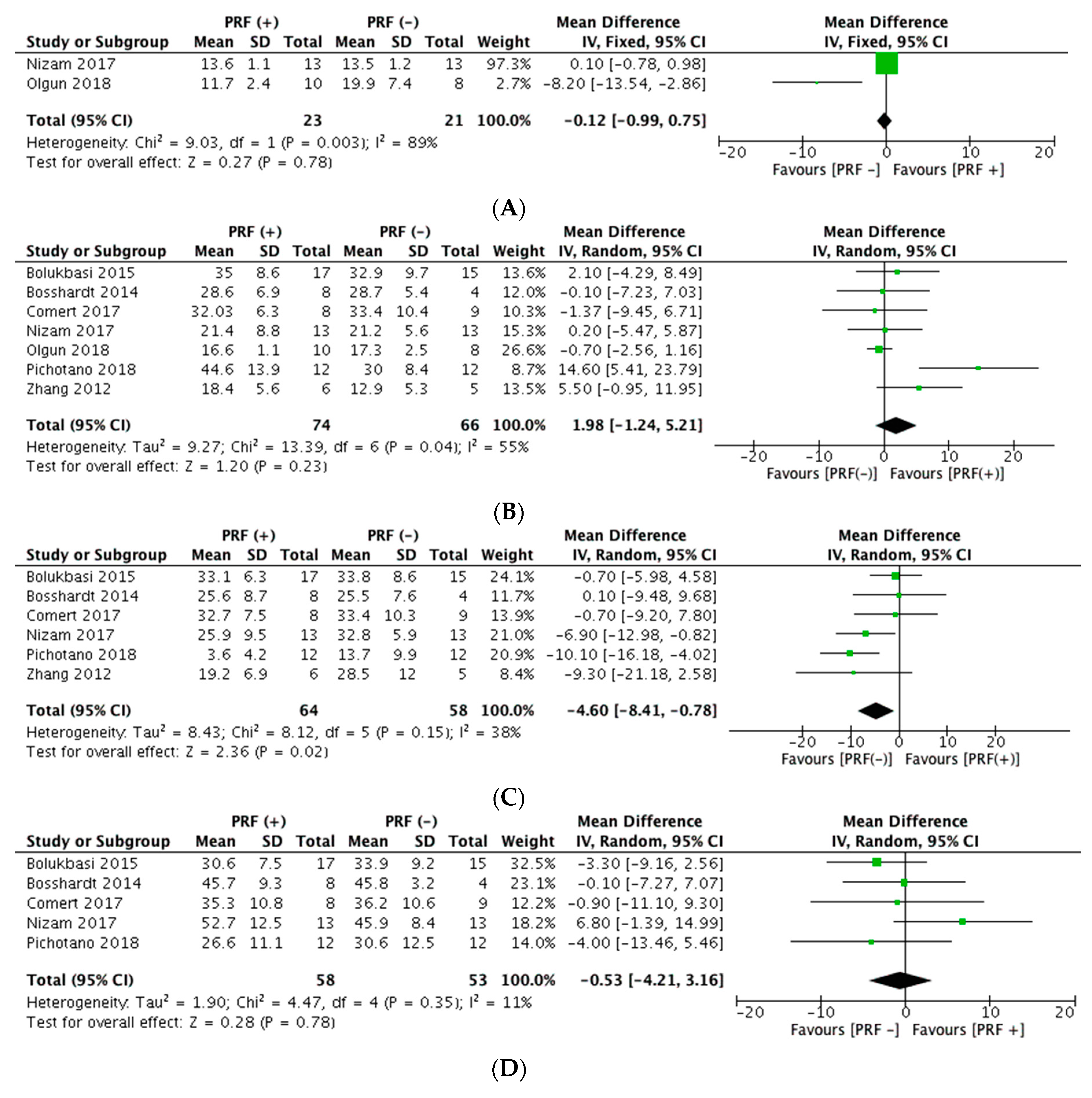
4.5.1. Sole Use of Platelet Concentrates in Sinus Floor Augmentation
4.5.2. PRF + Grafting Biomaterials in Sinus Floor Augmentation
5. Discussion
5.1. Summary of Evidence
5.2. Limitations
5.3. Innovation and Challenges
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Briceño, J.F.; Estrada, J.H. Maxillary Sinus Augmentation: Anatomic and Clinic Considerations. Literature Review. Univ. Odontol. 2012, 31, 27–55. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Felice, P.; Karatzopoulos, G.; Worthington, H.V.; Coulthard, P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants - a Cochrane systematic review. Eur. J. Oral Implantol. 2009, 2, 167–184. [Google Scholar] [PubMed]
- Monje, A.; Urban, I.; Miron, R.; Caballe-Serrano, J.; Buser, D.; Wang, H. Morphologic patterns of the atrophic posterior maxilla and clinical implications for bone regenerative therapy. Int. J. Periodontics Restorative Den. 2017, 37, e279–e289. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tan, W.C.; Lang, N.P.; Zwahlen, M.; Pjetursson, B.E. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Part II: transalveolar technique. J. Clin. Periodontol. 2008, 35, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Zijderveld, S.A.; Van den Bergh, J.P.; Schulten, E.A.; Bruggenkate, C.M. Anatomical and surgical findings and complications in 100 consecutive maxillary sinus floor elevation procedures. J. Oral Maxillofac. Surg. 2008, 66, 1426–1438. [Google Scholar] [CrossRef] [PubMed]
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar]
- Tatum, H., Jr. Maxillary and sinus implant reconstructions. Dent. Clin. North Am. 1986, 30, 207–229. [Google Scholar]
- Pjetursson, B.E.; Tan, W.C.; Zwahlen, M.; Lang, N.P. A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation: part I: lateral approach. J. Clin. Periodontol. 2008, 35, 216–240. [Google Scholar] [CrossRef]
- Thoma, D.S.; Cha, J.K.; Jung, U.W. Treatment concepts for the posterior maxilla and mandible: short implants versus long implants in augmented bone. J. Periodontal Implant Sci. 2017, 47, 2–12. [Google Scholar] [CrossRef]
- Mohan, N.; Wolf, J.; Dym, H. Maxillary sinus augmentation. Dent. Clin. North Am. 2015, 59, 375–388. [Google Scholar] [CrossRef]
- Al-Moraissi, E.A.; Altairi, N.H.; Abotaleb, B.; Al-Iryani, G.; Halboub, E.; Alakhali, M.S. What is the most effective rehabilitation method for posterior maxillas with 4 to 8 mm of residual alveolar bone height below the maxillary sinus with implant-supported prostheses? A frequentist network meta-analysis. J. Oral Maxillofac. Surg. 2019, 77, 70.e1–70.e33. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Pippi, R. Intrasinus bone gain with the osteotome sinus floor elevation technique: a review of the literature. Int. J. Oral Maxillofac. Implants 2018, 33, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Carreño-Carreño, J.; Aguilar-Salvatierra, A.; Gómez-Moreno, G.; García-Carreño, E.M.; Menéndez López-Mateos, M.L.; Perrotti, V.; Piattelli, A.; Calvo-Guirado, J.L.; Menéndez-Núñez, M. Update of surgical techniques for maxillary sinus augmentation: a systematic literature review. Implant Dent. 2016, 25, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Cricchio, G.; Hallman, M.; Jungner, M.; Rasmusson, L.; Sennerby, L. Sinus floor elevation procedures to enable implant placement and integration: techniques, biological aspects and clinical outcomes. Periodontology 2000 2017, 73, 103–120. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.B. The osteotome technique: part 3–less invasive methods of elevating the sinus floor. Compendium 1994, 15, 698, 702–704. [Google Scholar] [PubMed]
- Ting, M.; Rice, J.G.; Braid, S.M.; Lee, C.Y.; Suzuki, J.B. Maxillary sinus augmentation for dental implant rehabilitation of the edentulous ridge: a comprehensive overview of systematic reviews. Implant Dent. 2017, 26, 438–464. [Google Scholar] [CrossRef]
- Lundgren, S.; Andersson, S; Gualini, F; Sennerby, L. Bone reformation with sinus membrane elevation: a new surgical technique for maxillary sinus floor augmentation. Clin Implant Dent Relat Res. 2004, 6, 165–173. [Google Scholar] [CrossRef]
- Duan, D.H.; Fu, J.H.; Qi, W.; Du, Y.; Pan, J.; Wang, H.L. Graft-free maxillary sinus floor elevation: A systematic review and meta-analysis. J. Periodontol. 2017, 88, 550–564. [Google Scholar] [CrossRef]
- Starch-Jensen, T.; Schou, S. Maxillary sinus membrane elevation with simultaneous installation of implants without the use of a graft material: a systematic review. Implant Dent. 2017, 26, 621–633. [Google Scholar] [CrossRef]
- Moraschini, V.; Uzeda, M.G.; Sartoretto, S.C.; Calasans-Maia, M.D. Maxillary sinus floor elevation with simultaneous implant placement without grafting materials: a systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 636–647. [Google Scholar] [CrossRef]
- Danesh-Sani, S.A.; Loomer, P.M.; Wallace, S.S. A comprehensive clinical review of maxillary sinus floor elevation: anatomy, techniques, biomaterials and complications. Br. J. Oral Maxillofac. Surg. 2016, 54, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Jensen, J.D. Maxillary sinus floor augmentation: a review of selected treatment modalities. J. Oral Maxillofac. Res. 2017, 8, e3. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.I.; Kim, S.G.; Kim, Y.K.; Oh, J.S.; Jeong, M.A.; Park, J.J. Clinical study of graft materials using autogenous teeth in maxillary sinus augmentation. Implant Dent. 2011, 20, 471–475. [Google Scholar] [CrossRef] [PubMed]
- Roselló-LLabres, X.; Camps, A.R.; Jané-Salas, E.; Albuquerque, R.; Velasco-Ortega, E.; López-López, J. Graft materials in oral surgery: revision. J. Biomim. Biomater. Tissue Eng. 2014, 19, 124. [Google Scholar] [CrossRef]
- Gual-Vaqués, P.; Polis-Yanes, C.; Estrugo-Devesa, A.; Ayuso-Montero, R.; Marí-Roig, A.; López-López, J. Autogenous teeth used for bone grafting: a systematic review. Med. Oral Patol Oral Cir. Bucal. 2018, 23, e112–e119. [Google Scholar] [CrossRef] [PubMed]
- Clavero, J.; Lundgren, S. Ramus or chin grafts for maxillary sinus inlay and local onlay augmentation: comparison of donor site morbidity and complications. Clin. Implant Dent. Relat. Res. 2003, 5, 154–160. [Google Scholar] [CrossRef]
- Shanbhag, S.; Shanbhag, V.; Stavropoulos, A. Volume changes of maxillary sinus augmentation over time: a systematic review. Int. J. Oral Maxillofac. Implants 2014, 29, 881–892. [Google Scholar] [CrossRef]
- Nkenke, E.; Neukam, F. Autogenous bone harvesting and grafting in advanced jaw resorption: morbidity, resorption and implant survival. Eur. J. Oral. Implantol. 2014, 7 (Suppl. S2), S203–S217. [Google Scholar]
- Jensen, T.; Schou, S.; Stavropoulos, A.; Terheyden, H.; Holmstrup, P. Maxillary sinus floor augmentation with Bio-Oss or Bio-Oss mixed with autogenous bone as graft in animals: a systematic review. Int. J. Oral Maxillofac. Surg. 2012, 41, 114–120. [Google Scholar] [CrossRef]
- Aludden, H.C.; Mordenfeld, A.; Hallman, M.; Dahlin, C.; Jensen, T. Lateral ridge augmentation with Bio-Oss alone or Bio-Oss mixed with particulate autogenous bone graft: a systematic review. Int. J. Oral Maxillofac. Surg. 2017, 46, 1030–1038. [Google Scholar] [CrossRef]
- Esposito, M.; Felice, P.; Worthington, H.V. Interventions for replacing missing teeth: augmentation procedures of the maxillary sinus. Cochrane Database Syst. Rev. 2014, 13, CD008397. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Misch, C.; Lin, G.H.; Iacono, V.J.; Wang, H.L. Bone augmentation of the edentulous maxilla for implant placement: a systematic review. Int. J. Oral Maxillofac. Implants 2016, 31, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Danesh-Sani, S.A.; Engebretson, S.P.; Janal, M.N. Histomorphometric results of different grafting materials and effect of healing time on bone maturation after sinus floor augmentation: a systematic review and meta-analysis. J. Periodontal Res. 2017, 52, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Starch-Jensen, T.; Aludden, H.; Hallman, M.; Dahlin, C.; Christensen, A.E.; Mordenfeld, A. A systematic review and meta-analysis of long-term studies (five or more years) assessing maxillary sinus floor augmentation. Int. J. Oral Maxillofac. Surg. 2017, 22, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Whitman, D.H.; Berry, R.L.; Green, D.M. Platelet gel: an autologous alternative to fibrin glue with applications in oral maxillofacial surgery. J Oral Maxillofac. Surg. 1997, 55, 1294–1299. [Google Scholar] [CrossRef]
- Dohan, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Schliephake, H.; Sicilia, A.; Nawas, B.A.; Donos, N.; Gruber, R.; Jepsen, S.; Milinkovic, I.; Mombelli, A.; Navarro, J.M.; Quirynen, M.; et al. Drugs and diseases: summary and consensus statements of group 1. The 5th EAO consensus conference 2018. Clin. Oral Implants Res. 2018, 29, 93–99. [Google Scholar] [CrossRef]
- Chieruzzi, M.; Pagano, S.; Moretti, S.; Pinna, R.; Milia, E.; Torre, L.; Eramo, S. Nanomateriasl for tissue engineering in dentistry. Nanomaterials 2016, 6, 134. [Google Scholar] [CrossRef]
- Strauss, F.J.; Stähli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implants therapy: a systematic review. Clin. Oral Implants Res. 2018, 29, 6–19. [Google Scholar] [CrossRef]
- Liu, R.; Yan, M.; Chen, S.; Huang, W.; Wu, D.; Chen, J. Effectiveness of platelet-rich fibrin as an adjunctive material to bone graft in maxillary sinus augmentation: a meta-analysis of randomized controlled trails. Biomed. Res. Int. 2019, 2019, 1–10. [Google Scholar] [CrossRef]
- Nizam, N.; Eren, G.; Akcalı, A.; Donos, N. Maxillary sinus augmentation with leukocyte and platelet-rich fibrin and deproteinized bovine bone mineral: a split-mouth histological and histomorphometric study. Clin. Oral Implants Res. 2018, 29, 67–75. [Google Scholar] [CrossRef]
- Pichotano, E.C.; de Molon, R.S.; de Souza, R.V.; Austin, R.S.; Marcantonio, E.; Zandim-Barcelos, D.L. Evaluation of L-PRF combined with deproteinized bovine bone mineral for early implant placement after maxillary sinus augmentation: a randomized clinical trial. Clin. Implant Dent. Relat. Res. 2019, 21, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Roldán, C.J.; Jepsen, S.; Schmidt, C.; Knuppel, H.; Rueger, D.C.; Acil, Y.; Terheyden, H. Sinus floor augmentation with simultaneous placement of dental implants in the presence of platelet-rich plasma or recombinant human bone morphogenetic protein-7. Clin. Oral Impl. Res. 2004, 15, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.M.; Lee, C.U.; Son, J.S.; Oh, J.H.; Fang, Y.; Choi, B.H. Simultaneous sinus lift and implantation using platelet-rich fibrin as sole grafting material. J. Craniomaxillofac. Surg. 2014, 42, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Olgun, E.; Ozkan, S.Y.; Atmaca, H.T.; Yalim, M.; Hendek, M.K. Comparison of the clinical, radiographic, and histological effects of titanium-prepared platelet rich fibrin to allograft materials in sinus-lifting procedures. J. Investig. Clin. Dent. 2018, 9, e12347. [Google Scholar] [CrossRef]
- Kanayama, T.; Horii, K.; Senga, Y.; Shibuya, Y. Crestal approach to sinus floor elevation for atrophic maxilla using platelet-rich fibrin as the only grafting material: a 1-Year prospective study. Implant Dent. 2016, 25, 32–38. [Google Scholar] [CrossRef]
- Tajima, N.; Ohba, S.; Sawase, T.; Asahina, I. Evaluation of sinus floor augmentation with simultaneous implant placement using platelet-rich fibrin as sole grafting material. Int. J. Oral Maxillofac. Implants 2013, 28, 77–83. [Google Scholar] [CrossRef]
- Ocak, H.; Kutuk, N.; Demetoglu, U.; Balcioglu, E.; Ozdamar, S.; Alkan, A. Comparison bovine bone-autogenic bone misxture versus platelet-rich fibrin for maxillary sinus grafting: histological and histomorphological study. J. Oral Implantol. 2017, 43, 194–201. [Google Scholar] [CrossRef]
- Aoki, N.; Kanayama, T.; Maeda, M.; Horii, K.; Miyamoto, H.; Wada, K.; Ojima, Y.; Tsuchimochi, T.; Shibuya, Y. Sinus augmentation by platelet-rich fibrin alone: a report of two cases with histological examinations. Case Rep. Dent. 2016, 2016, 2654645. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Guo, T.; Ding, X.; Wanqi, Y.; Zhao, J.; Zhou, Y. The endoscopically assisted transcrestal sinus floor elevation with platelet-rich fibrin at an immediate implantation of periapical lesion site: A case report. Medicine (Baltimore) 2019, 98, e16251. [Google Scholar] [CrossRef]
- Zhao, J.H.; Chung, H.T.; Yu, C.C. Clinical application of platelet-rich fibrin as the sole grafting material in maxillary sinus augmentation. J. Formos Med. Assoc. 2015, 114, 779–780. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Prado, R.; Orive, G. A lateral approach for sinus elevation using PRGF technology. Clin. Implant Den. Relat. Res. 2009, 11, 23–31. [Google Scholar] [CrossRef]
- Anitua, E.; Flores, J.; Alkhraisat, M.H. Transcrestal sinus lift using platelet concentrates in association to short implant placement: a retrospective study of augmented bone height remodeling. Clin. Implant Den. Relat. Res. 2016, 18, 993–1002. [Google Scholar] [CrossRef] [PubMed]
- Barbu, H.M.; Andreescu, C.F.; Comaneanu, M.R.; Referendaru, D.; Mijiritsky, E. Maxillary sinus floor augmentation to enable one-stage implant placement by using bovine bone substitute and platelet-rich fibrin. BioMed. Res. Int. 2018, 2018, 6562958. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Scacco, S.; Inchingolo, A.D.; Dipalma, G.; Vermesan, D.; Abbinante, A.; Cagiano, R. Trial with platelet-rich fibrin and Bio-Oss used as grafting materials in the treatment of the severe maxillar bone atrophy: Clinical and radiological evaluations. EuR. Rev. Med. Pharmacol Sci. 2010, 14, 1075–1084. [Google Scholar] [PubMed]
- Khouly, I.; Pardiñas-López, S.; Aliaga, I.; Froum, S. Long-term implant survival after 100 maxillary sinus augmentations using plasma rich in growth factors. Implant. Dent. 2017, 26, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M. Direct maxillary sinus floor augmentation for simultaneous dental implant placement. Ann. Maxillofac. Surg. 2018, 8, 188–192. [Google Scholar] [CrossRef]
- Mazor, Z.; Horowitz, R.A.; Del Corso, M.; Prasad, H.S.; Rohrer, M.D.; Ehrenfest, D. Sinus floor augmentation with simultaneous implant placement using Choukroun’s platelet-rich fibrin as the sole mrafting Material: A radiologic and histologic study at 6 months. J. Periodontol. 2009, 80, 2056–2064. [Google Scholar] [CrossRef]
- Öncü, E.; Kaymaz, E. Assessment of the effectiveness of platelet rich fibrin in the treatment of schneiderian membrane perforation. Clin. Implant Dent. Relat. Res. 2017, 19, 1009–1014. [Google Scholar] [CrossRef]
- Simonpieri, A.; Choukroun, J.; Del Corso, M.; Sammartino, G.; Ehrenfest, D. Simultaneous sinus-lift and implantation using microthreaded implants and leukocyte- and platelet-rich fibrin as sole grafting material: A six-year experience. Implant Dent. 2011, 20, 2–12. [Google Scholar] [CrossRef]
- Taschieri, S.; Del Fabbro, M. Postextraction osteotome sinus floor elevation technique using plasma-rich growth factors. Implant Dent. 2011, 20, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Badr, M.; Oliver, R.; Pemberton, P.; Coulthard, P. Platelet-rich plasma in grafted maxillae: growth factor quantification and dynamic histomorphometric evaluation. Implant Dent. 2016, 25, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Khairy, N.M.; Shendy, E.E.; Askar, N.A.; El-Rouby, D.H. Effect of platelet rich plasma on bone regeneration in maxillary sinus augmentation (randomized clinical trial). Int. J. Oral Maxillofac. Surg. 2013, 42, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Maeda, M.; Kurata, M.; Hirose, M.; Ojima, Y.; Wada, K.; Shibuya, Y. Sinus floor elevation with platelet-rich fibrin alone: A clinical retrospective study of 1-7 years. J. Clin. Exp. Dent. 2018, 10, e984–e991. [Google Scholar] [CrossRef]
- Gülşen, U.; Dereci, O. Evaluation of new bone formation in sinus floor augmentation with injectable platelet-rich fibrin-soaked collagen plug: A pilot study. Implant Dentistry 2019, 28, 220–225. [Google Scholar] [CrossRef]
- Diss, A.; Dohan, D.M.; Mouhyi, J.; Mahler, P. Osteotome sinus floor elevation using Choukroun’s platelet-rich fibrin as grafting material: A 1-year prospective pilot study with microthreaded implants. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 572–579. [Google Scholar] [CrossRef]
- Molemans, B.; Cortellini, S.; Jacobs, R.; Teughels, W.; Pinto, Quirynen, M. Simultaneous sinus floor elevation and implant placement using leukocyte- and platelet-rich fibrin as a sole graft material. Int. J. Oral Maxillofac. Implants 2019, 34, 1195–1201. [Google Scholar] [CrossRef]
- Toffler, M.; Toscano, N.; Holtzclaw, D. Osteotome-mediated sinus floor elevation using only platelet-rich fibrin: an early report on 110 patients. Implant Dent. 2010, 19, 447–456. [Google Scholar] [CrossRef]
- Bolukbasi, N.; Ersanli, S.; Keklikoglu, N.; Basegmez, C.; Ozdemir, T. Sinus augmentation with platelet-rich fibrin in combination with bovine bone graft versus bovine bone graft in combination with collagen membrane. J. Oral Implantol. 2015, 41, 586–595. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol, Oral Radiol Endodon. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Bornstein, M.M.; Carrel, J.P.; Buser, D.; Bernard, J.P. Maxillary sinus grafting with a synthetic, nanocrystalline hydroxyapatite-silica gel in humans: histologic and histomorphometric results. Int. J. Periodontics Restorative Dent. 2014, 34, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Cömert, K.S.; Güngörmüş, M.; Parlak, S.N. Histologic and histomorphometric assessment of sinus-floor augmentation with beta-tricalcium phosphate alone or in combination with pure-platelet-rich plasma or platelet-rich fibrin: a randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 959–967. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Corbella, S.; Ceresoli, V.; Ceci, C.; Taschieri, S. Plasma rich in growth factors improves patients’ postoperative quality of life in maxillary sinus floor augmentation: preliminary results of a randomized clinical study. Clin. Implant Dent. Relat. Res. 2015, 17, 708–716. [Google Scholar] [CrossRef] [PubMed]
- Gassling, V.; Nicolai, P.; Braesen, J.H.; Will, M.; Gierloff, M.; Behrens, E.; Açil, Y.; Wiltfang, J. Comparison of two different absorbable membranes for the coverage of lateral osteotomy sites in maxillary sinus augmentation: a preliminary study. J. Cranio-maxillofac. Surg. 2013, 41, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Gurler, G.; Delilbasi, C. Effects of leukocyte-platelet rich fibrin (L-PRF) on post-operative complications of direct sinus lifting. Minerva Stomatol. 2016, 65, 207–212. [Google Scholar] [PubMed]
- Tatullo, M.; Marrelli, M.; Cassetta, M.; Pacifici, A.; Stefanelli, L.; Scacco, S.; Dipalma, G.; Pacifici, L.; Inchingolo, F. Platelet rich fibrin (P.R.F.) in reconstructive surgery of atrophied maxillary bones: Clinical and histological evaluations. Int. J. Med. Sci. 2012, 9, 872–880. [Google Scholar] [CrossRef]
- Zhang, Y.; Tangl, S.; Huber, C.D.; Lin, Y.; Qiu, L.; Rausch-Fan, X. Effects of Choukroun’s platelet-rich fibrin on bone regeneration in combination with deproteinized bovine bone mineral in maxillary sinus augmentation: A histological and histomorphometric study. J. Craniomaxillofac Surg. 2012, 40, 321–328. [Google Scholar] [CrossRef]
- Ali, S.; Bakry, S.A.; Abd-Elhakam, H. Platelet rich fibrin in maxillary sinus augmentation: a systematic review. J. Oral Implantol. 2015, 41, 746–753. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clinical Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef]
- Dragonas, P.; Katsaros, T.; Avila-Ortiz, G.; Chambrone, L.; Schiavo, J.H.; Palaiologou, A. Effects of leukocyte–platelet-rich fibrin (L-PRF) in different intraoral bone grafting procedures: a systematic review. Int. J. Oral Maxillofac. Surg. 2019, 48, 250–262. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): a second generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
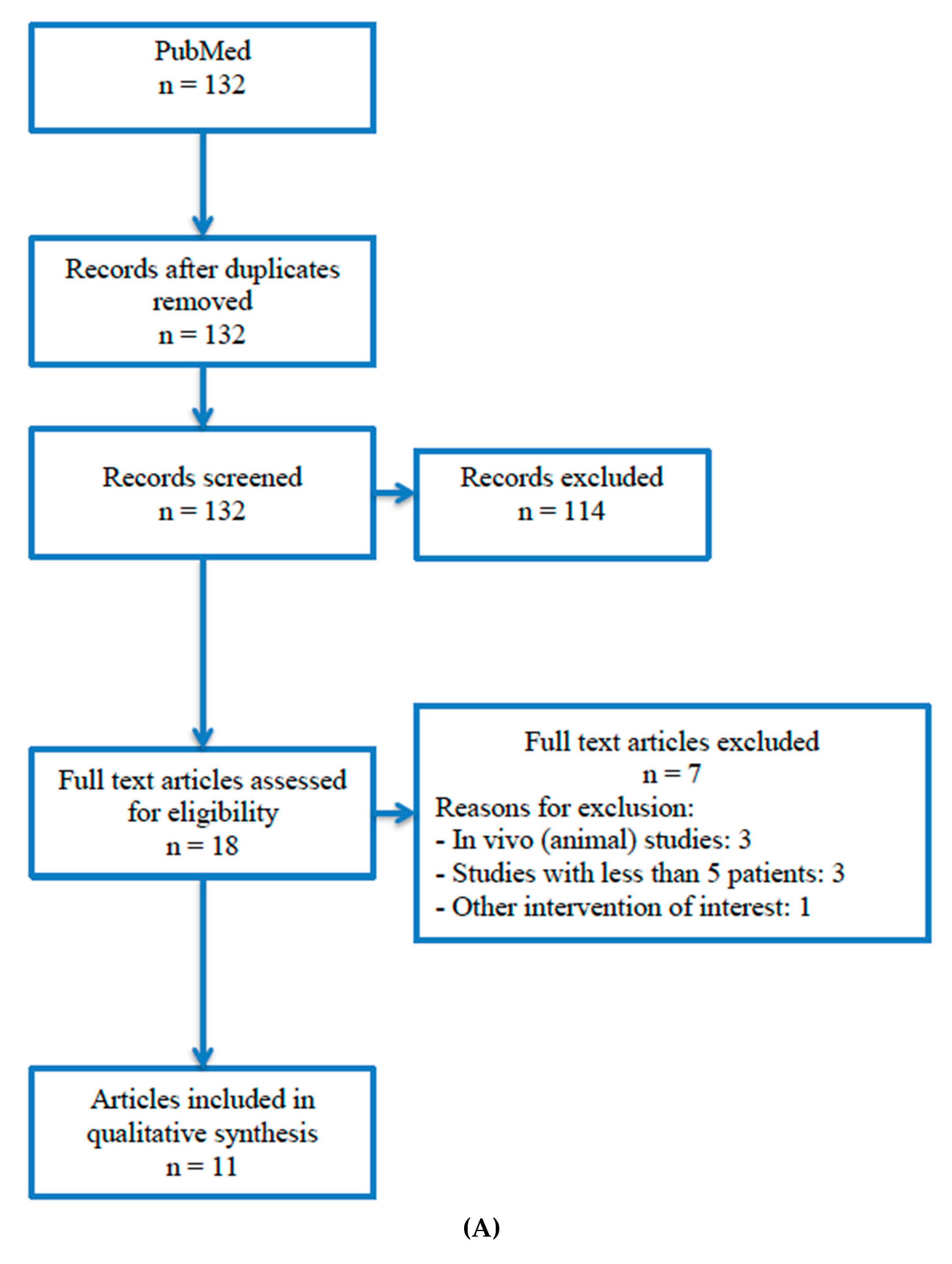
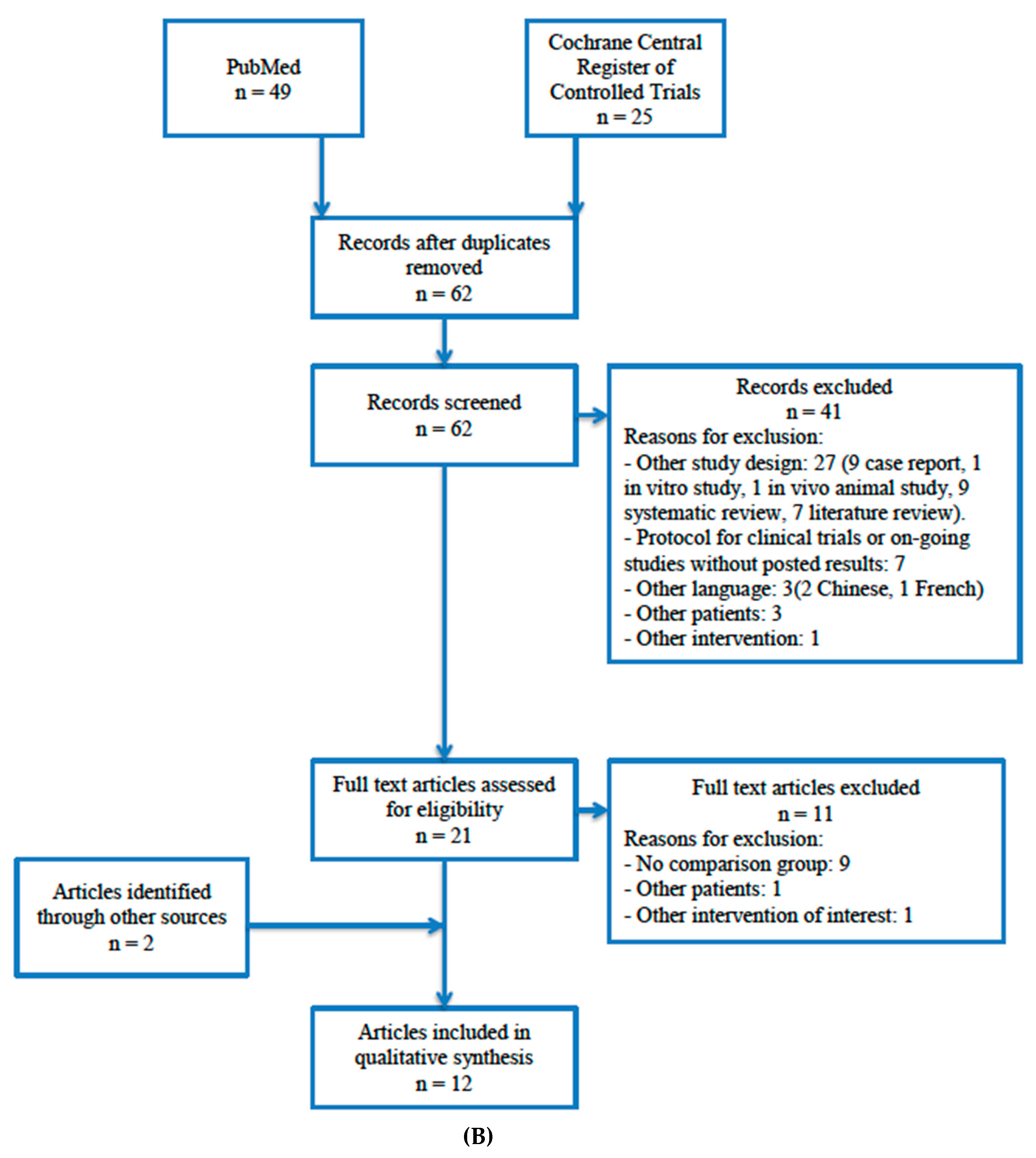
| Author, Year Country | Design Study Period Follow-Up | Nº Patients | Gender Age | Nº Sinus | Sinus Lift Complications | Implant Surgical Stage | Nº Implants | Platelet Concentrate | Outcome Measures |
|---|---|---|---|---|---|---|---|---|---|
| Anitua et al, 2016 Spain [53] | Retrospective Study 3 years | 26 | F: 14 M: 12 55 ± 7y | NR | NR | Immediate placement | 41 | PRGF PRGF + bone graft in 7 implants | Radiographic assessment (mean ±sd) * Bone height (1year): 8.2 ± 1.2 mm Bone height (3 years): 8.8 ± 1.4 mm Bone gain (1 year): 3.6 ± 1.8 mm Bone gain (3 years): 4.3 ± 2.0mm |
| Aoki et al, 2018 Japan [64] | Retrospective Study 2010–2015 Χ = 3.43 years (1–7 y) | 34 | F: 17 M: 17 57.6y | NR | NR | Immediate placement | 71 | PRF | Clinical assessment Implant survival: 85.5% |
| Diss et al, 2008 France [66] | Prospective Study 2004–2005 1 year | 20 | F: 14 M: 6 54.8 ± 11.1y | NR | 4 (Sinus membrane perforation) | Immediate placement | 35 | PRF | Radiographic assessment (mean ±sd) Bone gain: 3.2 ± 1.5mm (0.1–5.8 mm) Clinical assessment Implant survival: 97.1% |
| Gulsen et al, 2019 Turkey [65] | Retrospective Study 2015–2018 6 months | 12 | F: 7 M: 5 55.7 ± 8.3 | NR | 1 (Sinus membrane perforation) | Immediate placement | 18 | i-PRF soaked collagen | Radiographic assessment (mean ±sd) Bone height: 11.6 ± 1.6mm (8–14 mm) Bone gain: 6.3 ± 1.3mm (4.2–8.5 mm) Clinical assessment Implant survival: 100% |
| Kanayama et al, 2016 Japan [46] | Prospective Study 2011–2013 1 year | 27 | F: 15 M: 12 54.2y (29-74y) | NR | 0 | Immediate placement | 39 | PRF | Radiographic assessment (mean ±sd) Bone gain: SA implant: 4.4 ± 1.7 mm (p < 0.001) HA implant: 4.0 ± 1.6 mm Clinical assessment Implant survival: 100% |
| Mazor et al, 2009 Israel [58] | Case series 2007–2008 6 months | 20 | F: 14 M: 6 54.1 ± 5.2y | 25 | 0 | Immediate placement | 41 | L-PRF | Radiographic assessment (mean ±sd) Bone gain: 10.1 ± 0.9 mm (7–13 mm) Histomorphometric assessment (mean ±sd) Newly formed bone: 33% ± 5% Clinical assessment Implant survival: 100% |
| Molemans et al, 2019 Belgium [67] | Prospective Study 2015–2016 6 months | 26 | F: 12 M: 14 55y (38–78) | 28 | NR | Immediate placement | 29 | L-PRF | Radiographic assessment (mean ±sd) Bone gain: TSFE: 3.4 ± 1.2 mm // LSFE: 5.4 ± 1.5 mm Clinical assessment Implant survival: 93.1% (2 implants were not osseointegrated and were removed) |
| Simonpieri et al, 2011 France [60] | Case series 2003–2008 6 years | 20 | F: 12 M: 8 59.8 ± 11.1y | 23 | 3 (Sinus membrane perforation) | Immediate placement | 52 | L-PRF | Radiographic assessment (mean ±sd) Bone gain: 10.4 ± 1.2 mm (8.5–12 mm) Clinical assessment Implant survival: 100% |
| Tajima et al, 2013 Japan [47] | Prospective Study 2009–2011 6 months | 6 | F: 6 67.8y (53–82y) | 9 | 0 | Immediate placement | 17 | PRF | Radiographic assessment (mean ±sd) Bone height: 11.8 ± 1.7 mm (9.1–14.1 mm) Bone gain: 7.5mm Bone density: 323 ± 156.2 HU (185–713) Bone volume: 0.7 ± 0.3 mL |
| Toffler et al, 2010 USA [68] | Prospective Study 2008–2010 11 months | 110 | F: 70 M: 40 58.4 y (34–90 y) | 138 | 5 (Sinus membrane perforation) | Immediate placement | 138 | PRF | Radiographic assessment (mean ±sd) Bone gain: 3.4 mm (3.5–5 mm) Clinical assessment Implant survival: 96.4% |
| Author, Year Country | Design/ Study Period Follow Up | Nº Patients | Nº Sinuses | Sinus Lift Complications | Implant Surgery | Nº Implants | Intervention Group (I) | Control Group (C) |
|---|---|---|---|---|---|---|---|---|
| Bolukbasi et al, 2015 Turkey [69] | Retrospective Study 2008–2012 2 years | 25 | I: 17 C: 15 | I: 0 C: 0 | 6 months after sinus lifting | I: 34 C: 32 | Bovine bone graft material (Bio-Oss) + PRF mixture | Bovine bone graft material (Bio-Oss) + collagen membrane (Bio-Gide) |
| Bosshardt et al, 2014 Switzerland [71] | Controlled Trial 7–11 months | 8 I: 5 C: 3 | 12 I: 8 C: 4 | I: 0 C: 0 | 7-11 months after sinus lifting | 16 | Synthetic nanocrystalline hydroxyapatite embedded in highly porous silica gel matrix (NanoBone) + PRF | Synthetic nanocrystalline hydroxyapatite embedded in highly porous silica gel matrix (NanoBone) + collagen membrane (BioGide) |
| Choukroun et al, 2006 France [70] | Retrospective study 2001–2003 8 months | 9 | I: 6 C: 3 | I: 1 (Perforation of the sinus membrane) C: 0 | I: 4 months after sinus lifting C: 8 months after sinus lifting | NR | Freeze-dried bone allograft + PRF | Freeze-dried bone allograft |
| Comert et al, 2017 Turkey [72] | RCT 2012–2013 6 months | 26 Group A: 9 Group B: 8 Control: 9 | NR | Group A: 1 Group B: 2 Control: 2 (Perforation of the sinus membrane) | 6 months after sinus lifting | NR | Group A: P-PRP mixed β- TCP Group B: PRF mixed β- TCP | β- TCP |
| Del Fabbro et al 2013, Italy [73] | RCT 2011–2012 1 week | 30 I: 15 C: 15 | NR | I: 1 C: 2 (Perforation of the sinus membrane) | 6-8 months after sinus lifting | NR | Deproteinized bovine bone matrix (Bio-Oss) + PRGF-Endoret | Deproteinized bovine bone matrix (Bio-Oss) |
| Gassling et al, 2013 Germany [74] | Split mouth RCT 2010–2011 5 months | 6 | I: 6 C: 6 | NR | 5 months after sinus lifting | 32 | Autologous bone and bone-substitute material (Bio-Oss) mixed in 1:1 ratio + PRF | Autologous bone and bone-substitute material (Bio-Oss) mixed in 1:1 ratio + collagen membrane (Bio-Gide) |
| Gurler et al. 2016 Turkey [75] | RCT 2 weeks | 28 (4 excluded from analyses) I: 12 C: 12 | 28 I: 12 C: 12 | 4 (2 postoperative maxillary sinusitis and 2 perforation of the sinus membrane) | NR | NR | Allogenous freeze dried corticocancellous bone chips (MinerOss) + L-PRF | Allogenous freeze dried corticocancellous bone chips (MinerOss) |
| Nizam et al, 2017 Turkey [41] | Split mouth RCT 2013-2015 6 months | 13 | 26 I: 13 C: 13 | I: 0 C: 0 | 6 months after sinus lifting | I: 30 C: 28 | Deproteinized bovine bone mineral (BioOss) + L-PRF | Deproteinized bovine bone mineral (BioOss) |
| Olgun et al, 2018 Turkey [45] | RCT 2013–2014 9 months | 18 | 18 I: 10 C: 8 | I: 0 C: 0 | I: 4 months after sinus lifting C: 6 months after sinus lifting | 37 | Titanium-PRF | Allograft (CTBA Allograft) |
| Pichotano et al, 2018 Brazil [42] | Split mouth RCT 2014–2015 8 months | 12 | 22 I: 12 C: 12 | I: 0 C: 0 | I: 4 months after sinus lifting C: 8 months after sinus lifting | I: 19 C: 19 | Demineralized bovine bone mineral (BioOss) + L-PRF | Demineralized bovine bone mineral (BioOss) |
| Tatullo et al, 2012 Italy [76] | RCT 150 days | 60 I: 30 C: 18 Split mouth: 12 | 72 | I: 0 C: 0 | Early (106 days after sinus lift): 20 Intermediate (120 days after sinus lift): 20 Late (150 days after sinus lift): 20 | 240 | Deproteinized bovine bone mineral (BioOss) + PRF | Deproteinized bovine bone mineral (BioOss) |
| Zhang et al, 2012 China [77] | RCT 6 months | 11 | 11 I: 6 C: 5 | NR | 6 months after sinus lifting | I: 6 C: 5 | Deproteinized bovine bone mineral (BioOss) + PRF | Deproteinized bovine bone mineral (BioOss) |
| Randomized Clinical Trials | Random Sequence Generation | Allocation Concealment | Blinding of Participants, Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias | |
|---|---|---|---|---|---|---|---|---|
| Comert et al [72] | ? | ? | - | + | + | + | + | |
| Del Fabbro et al [73] | + | ? | ? | − | + | + | + | |
| Gassling et al [74] | ? | + | ? | + | + | + | + | |
| Gurler et al [75] | + | ? | - | + | − | − | + | + |
| Nizam et al [41] | + | ? | - | + | ? | + | + | |
| Olgun et al [45] | + | ? | - | ? | + | + | + | |
| Pichotano et al [42] | + | + | + | + | + | + | + | |
| Tatullo et al [76] | + | ? | − | ? | + | + | + | |
| Zhang et al [77] | ? | ? | - | ? | + | + | + | |
| Author, Year Country | Outcome Measures | |||
|---|---|---|---|---|
| Bolukbasi et al, 2015 Turkey [69] | Radiographic assessment (T0 − T5) (mean ± standard deviation) Relationship between sinus-graft height and the implant: BL/IL ratio a: Intervention group: 1.36 ± 0.04 // Control group: 1.36 ± 0.03 During study period, intervention group showed less change in BL/IL values than control group (p = 0.022) Change in the height of grafted sinus: GSH/OSH ratio b: Intervention group: 4.49 ± 0.29 // Control group: 4.14 ± 0.20 (p = 0.093) Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Intervention group: 35 ± 8.6 // Control group: 32.9 ± 9.7 (p = 0.61) Connective tissue: Intervention group: 30.6 ± 7.5 // Control group: 33.9 ± 9.2 (p = 0.34) Biomaterial remnants: Intervention group: 33.1 ± 6.3 // Control group: 33.8 ± 8.6 (p = 0.87) | |||
| Bosshardt et al, 2014 Switzerland [71] | Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Intervention group (PRF): 28.6 ± 6.9 // Control group: 28.7 ± 5.4 Residual bone substitute material: Intervention group (PRF): 25.6 ± 8.7 // Control group: 25.5 ± 7.6 Soft Tissue: Intervention group (PRF): 45.7 ± 9.3 // Control group: 45.8 ± 3.2 No statistically significant differences | |||
| Choukroun et al, 2006 France [70] | Histomorphometric assessment (%) New bone formation: FDBA + PRF after 4 months: 65% (mean: 20.9% range: 18.6–30.3%) FDBA after 8 months: 69% (mean: 20.3% range: 18–23.7%) | |||
| Comert et al, 2017 Turkey [72] | Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Group A (P-PRP + β-TCP): 34.83 ± 10.1 // Group B (PRF + β-TCP): 32.03 ± 6.3 // Control group (β-TCP): 33.40 ± 10.4 (p = 0.83) Residual graft particle area: Group A (P-PRP + β-TCP): 28.98 ± 7.9 // Group B (PRF + β-TCP): 32.66 ± 7.5 // Control group (β-TCP): 33.39 ± 10.3 (p = 0.69) Soft (fibrous) tissue area: Group A (P-PRP + β-TCP): 36.19 ± 13.9 // Group B (PRF + β-TCP): 35.31 ± 10.8 // Control group (β-TCP): 36.21 ± 10.6 (p = 0.98) | |||
| Del Fabbro et al, 2013 Italy [73] | Quality of life in the postoperative treatment Pain (VAS Score): The use of PRGF resulted in significant reduction of the perceived pain during the second and third postoperative day as compared to the control group. From day 4, the mean VAS scores of the two groups were similar. Patients in the PRGF group reported consistently less swelling, less hematoma and less discomfort regarding chewing and speaking throughout the evaluation period. Bleeding was significantly lower in the first 2 days in the PRGF group (p = 0.01) | |||
| Gassling et al, 2013 Germany [74] | Histomorphometric assessment (%) New (vital) bone formation: Intervention group (PRF): mean: 17.0% range: 7.8–27.8% // Control group (collagen membrane): mean: 17.2% range: 8.5–24.2% Residual bone-substitute material: Intervention group (PRF): mean: 15.9% range: 0.9–33.4% // Control group (collagen membrane): mean: 17.3% range: 0.7–33.5% | |||
| Gurler et al, 2016 Turkey [75] | Postoperative complications of direct sinus lifting Gradual improvements in the L-PRF group regarding postoperative pain, swelling, sleeping, eating, phonetics, activities of daily living and missed work days, but not statistically significant (p > 0.05) Wound healing in both groups was uneventful Healing index scores of the L-PRF group were higher than the control group on the 7th (4.2 ± 0.9 vs. 3.6 ± 0.7) and 14th (4.7 ± 0.4 vs. 4.4 ± 0.5) days, but not statistically significant (p = 0.13, p = 0.19; respectively) | |||
| Nizam et al, 2017 Turkey [41] | Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Intervention group (L-PRF): 21.4 ± 8.8 // Control group: 21.2 ± 5.6 (p = 0.96) Residual bone graft: Intervention group (L-PRF): 25.9 ± 9.5 // Control group: 32.8 ± 5.9 (p = 0.06) Bone graft in contact with newly formed bone: Intervention group (L-PRF): 47.3 ± 12.3 // Control group: 54.0 ± 8.4 (p = 0.16) Soft tissue component: Intervention group (L-PRF): 52.7 ± 12.5 // Control group: 45.9 ± 8.4 (p = 0.16) Radiographic assessment (mm) (mean ± standard deviation) Augmented bone height: Intervention group (L-PRF): 13.6 ± 1.1 // Control group: 13.5 ± 1.2 (p = 0.88) Implant survival (after 1 year of follow-up) 100% in both groups | |||
| Olgun et al, 2018 Turkey [45] | Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Intervention group (T-PRF): 16.6 ± 1.1 // Control group: 17.3 ± 2.5 (p = 0.85) Cancellous bone ratio: Intervention group (T-PRF): 24.0 ± 1.5 // Control group: 22.7 ± 2.6 (p = 0.74) Radiographic assessment (mean ± standard deviation or median (IQR)) Bone height (mm): Intervention group (T-PRF): 11.7 ± 2.4 // Control group: 19.9 ± 7.4 (p = 0.05) Bone volume (mm3): Intervention group (T-PRF): 172.7 (82.6) // Control group: 264.6 (70.2) (p = 0.001) Bone density (hu): Intervention group (T-PRF): 86.7 (43.6) // Control group: 160.8 (63.6) (p = 0.001) Control group had 53% better volume, 86% better density and 69% better height compared to T-PRF group. Clinical assessment (mean ± standard deviation) Implant stability (Implant stability quotient): Intervention group (T-PRF): 68.5 ± 8.9 // Control group: 66.4 ± 8.3 (p = 0.61) | |||
| Pichotano et al, 2018 Brazil [42] | Radiographic assessment Graft volume dimensional changes (mean graft reduction between T1 − T2c) (mean ± standard deviation) Cm3= Intervention group (L-PRF): 0.58 ± 0.26 // Control group: 0.55 ± 0.34 (p = 0.78) % = Intervention group (L-PRF): 33.1 ± 10.7 // Control group: 36.7 ± 15.8 (p = 0.47) Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Intervention group (L-PRF): 44.6 ± 13.9 // Control group: 30.0 ± 8.4 (p = 0.008) Residual graft material: Intervention group (L-PRF): 3.6 ± 4.2 // Control group: 13.7 ± 9.9 (p = 0.01) Soft (fibrous) tissue: Intervention group (L-PRF): 26.6 ± 11.1 // Control group: 30.6 ± 12.5 (p = 0.38) Clinical assessment Implant stability (Implant stability quotient) (mean ± standard deviation) After implant placement: Intervention group (L-PRF): 60.9 ± 9.3 // Control group: 75.1 ± 5.7 (p < 0.001) At implant loading: Intervention group (L-PRF): 76.1 ± 5.9 // Control group: 75.7 ± 6.1 (p = 0.99) Implant survival (after 1 year of follow-up): 100% in both groups | |||
| Tatullo et al, 2012 Italy [76] | Histomorphometric assessment (%) | |||
| Early protocol | Intermediate protocol | Late protocol | ||
| Medullary spaces | I (PRF): 70.2 // C: 68.4 | I (PRF): 70.0 // C: 68.2 | I (PRF): 61.4 // C: 58.2 | |
| Osteoid borders | I (PRF): 7.01 // C: 5.12 | I (PRF): 3.84 // C: 3.12 | I (PRF): 3.5 // C: 2.9 | |
| Trabecular bone | I (PRF): 22.8 // C: 26.4 | I (PRF): 26.2 // C: 28.7 | I (PRF): 37.1 // C: 38.9 | |
| Clinical assessment Implant stability (Implant stability quotient): No statistically significant differences were found between groups in each of the protocols performed Implant survival (after 36 ± 10 months of follow-up): 100% in both groups | ||||
| Zhang et al, 2012 China [77] | Histomorphometric assessment (%) (mean ± standard deviation) New bone formation: Intervention group (PRF): 18.4 ± 5.6 // Control group: 12.9 ± 5.3 (p = 0.14) Residual bone substitute (BioOss): Intervention group (PRF): 19.2 ± 6.9 // Control group: 28.5 ± 12.0 (p = 0.14) Bone-to-bone substitute contact: Intervention group (PRF): 21.5 ± 14.6 // Control group: 18.6 ± 5.4 (p > 0.05) | |||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Mejia, H.; Estrugo-Devesa, A.; Saka-Herrán, C.; Ayuso-Montero, R.; López-López, J.; Velasco-Ortega, E. Platelet-Rich Plasma in Maxillary Sinus Augmentation: Systematic Review. Materials 2020, 13, 622. https://doi.org/10.3390/ma13030622
Ortega-Mejia H, Estrugo-Devesa A, Saka-Herrán C, Ayuso-Montero R, López-López J, Velasco-Ortega E. Platelet-Rich Plasma in Maxillary Sinus Augmentation: Systematic Review. Materials. 2020; 13(3):622. https://doi.org/10.3390/ma13030622
Chicago/Turabian StyleOrtega-Mejia, Holmes, Albert Estrugo-Devesa, Constanza Saka-Herrán, Raúl Ayuso-Montero, José López-López, and Eugenio Velasco-Ortega. 2020. "Platelet-Rich Plasma in Maxillary Sinus Augmentation: Systematic Review" Materials 13, no. 3: 622. https://doi.org/10.3390/ma13030622
APA StyleOrtega-Mejia, H., Estrugo-Devesa, A., Saka-Herrán, C., Ayuso-Montero, R., López-López, J., & Velasco-Ortega, E. (2020). Platelet-Rich Plasma in Maxillary Sinus Augmentation: Systematic Review. Materials, 13(3), 622. https://doi.org/10.3390/ma13030622








