Additive Manufactured Large Zr-Based Bulk Metallic Glass Composites with Desired Deformation Ability and Corrosion Resistance
Abstract
1. Introduction
2. Experiment
2.1. Materials Preparation
2.2. Mechanical Properties Testing
2.3. Electrochemical Corrosion Testing
3. Results
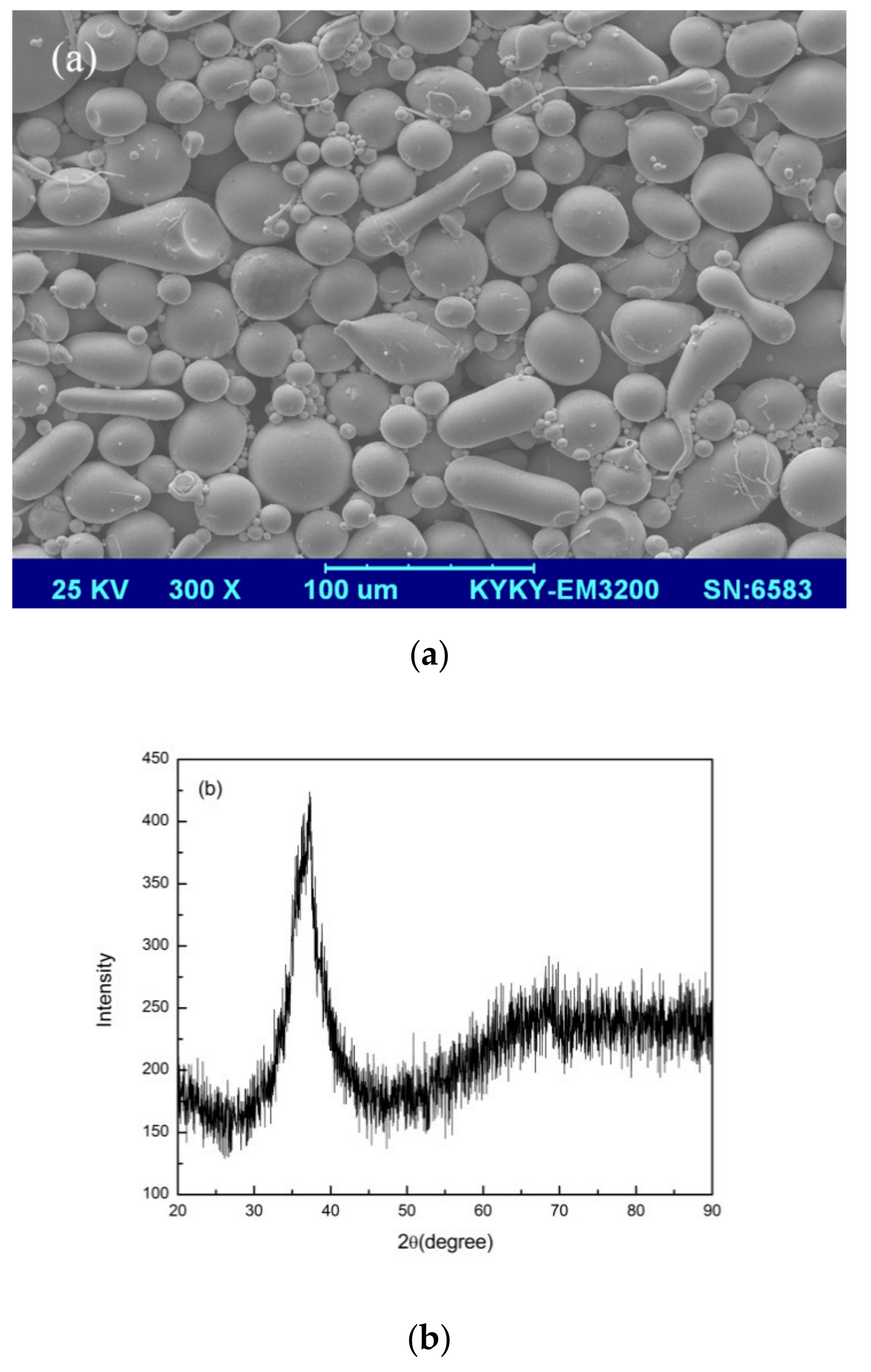
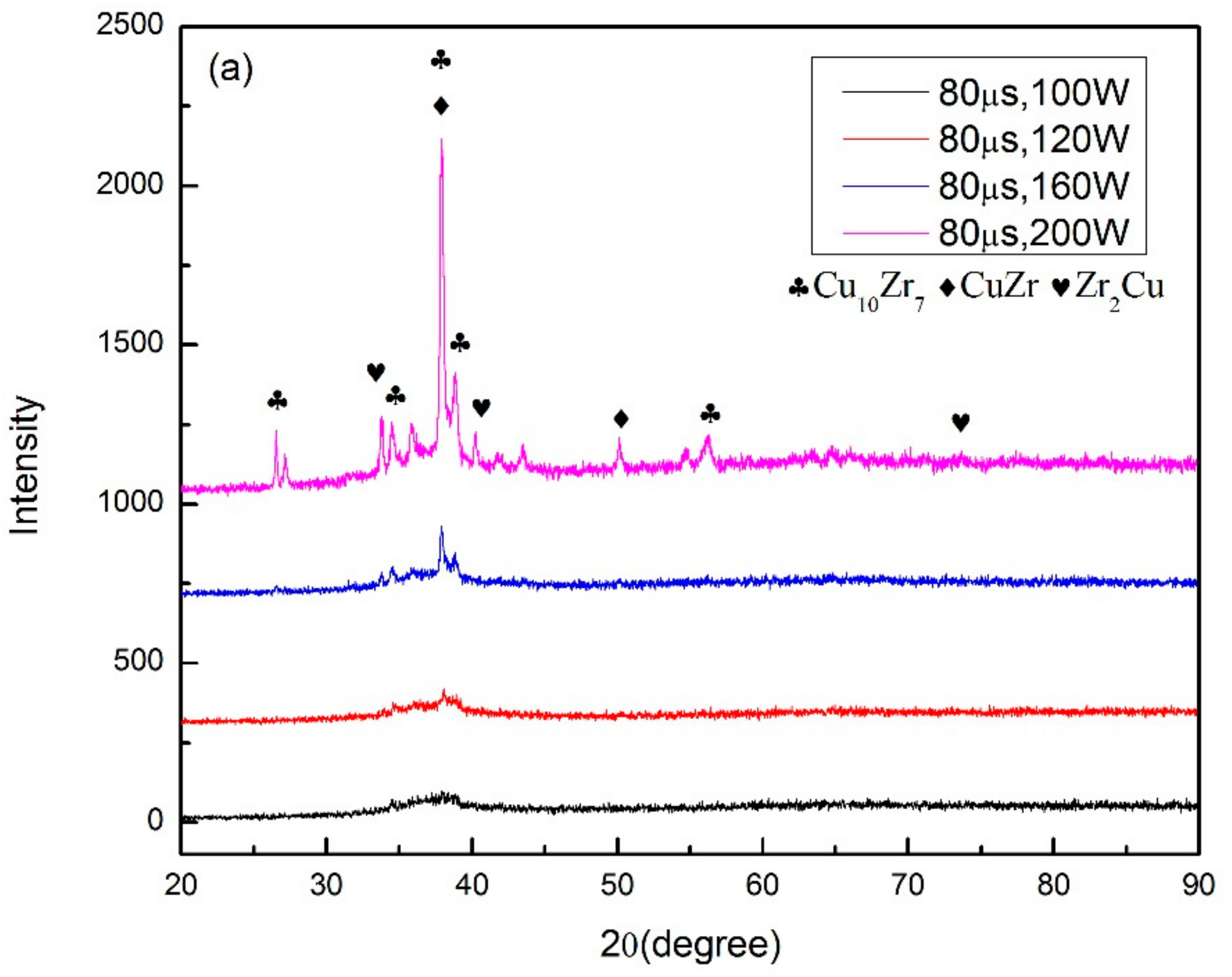
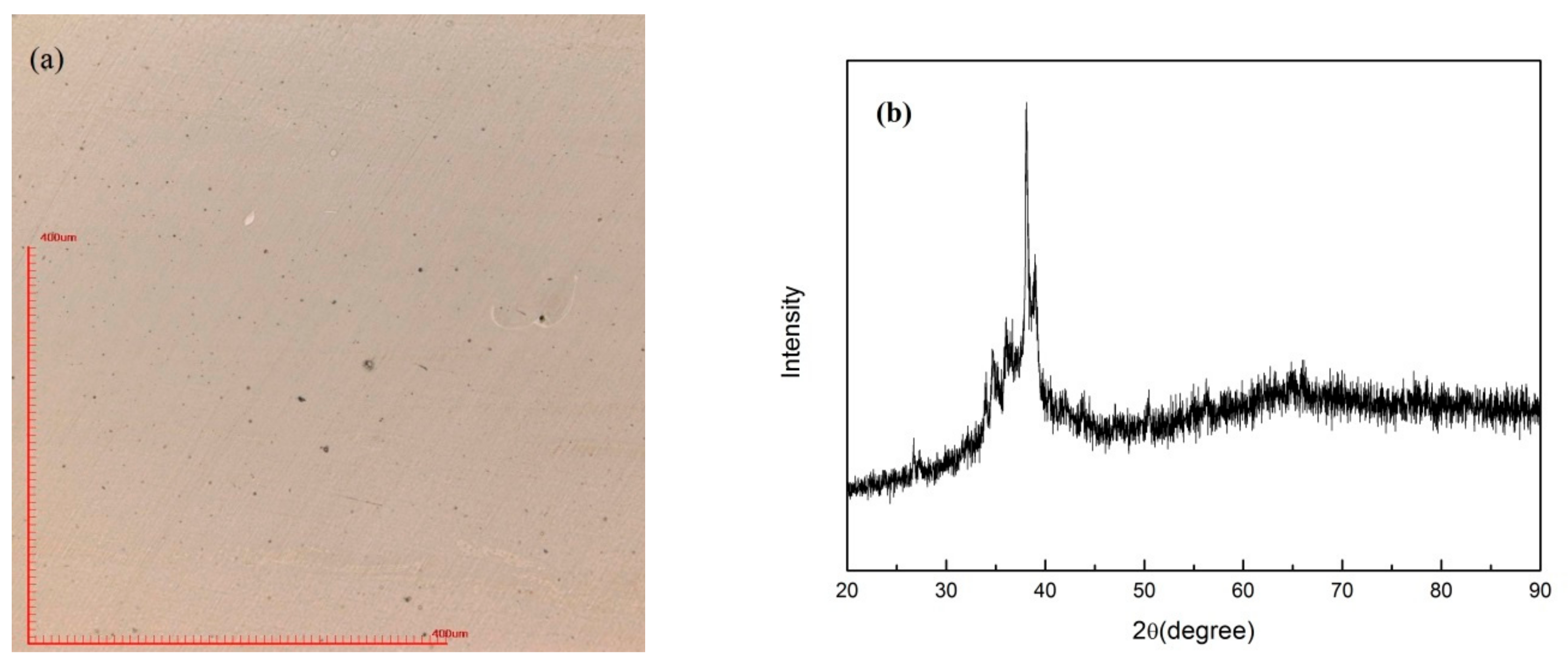
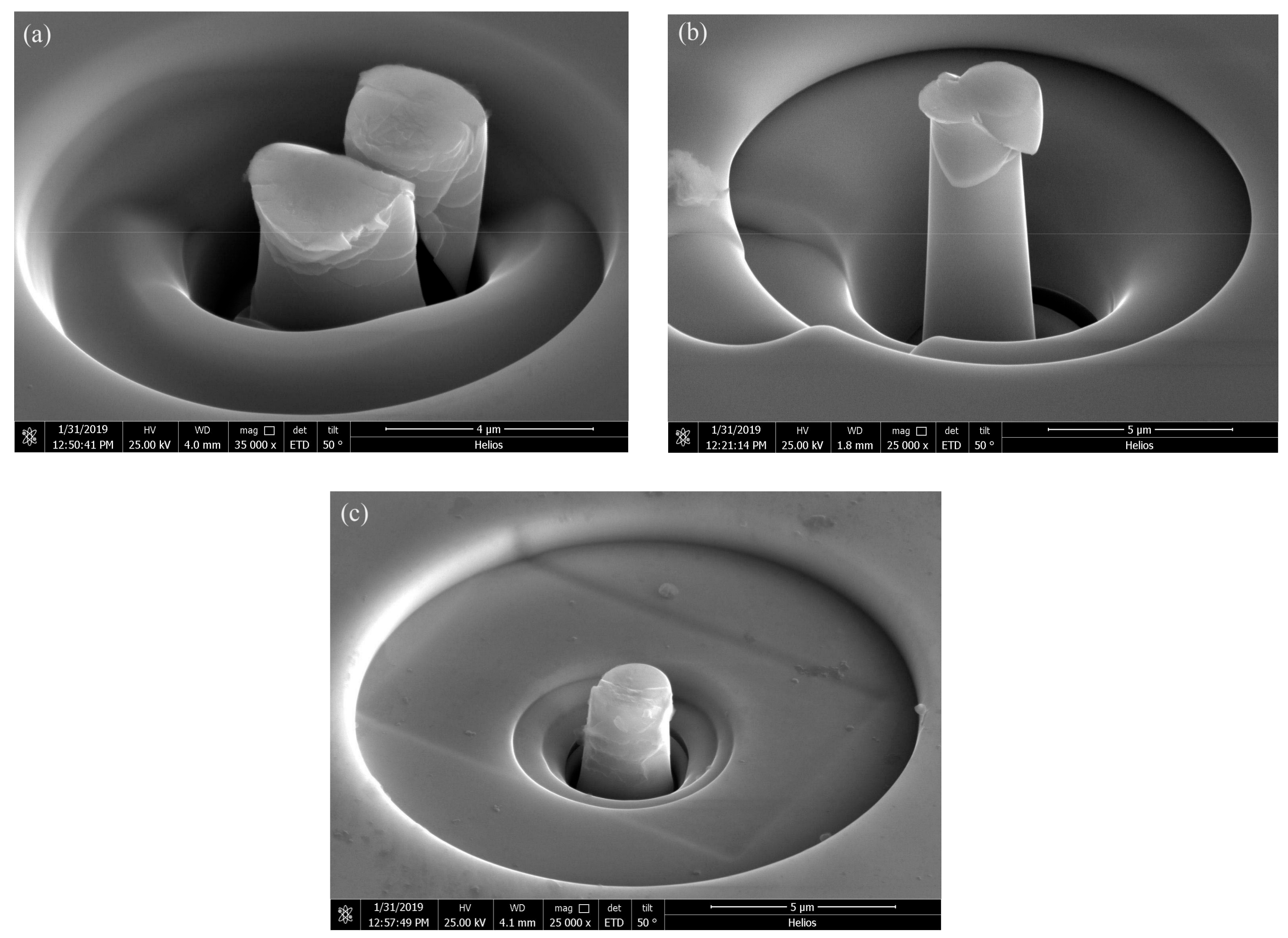
3.1. Selective Laser Melting Processing
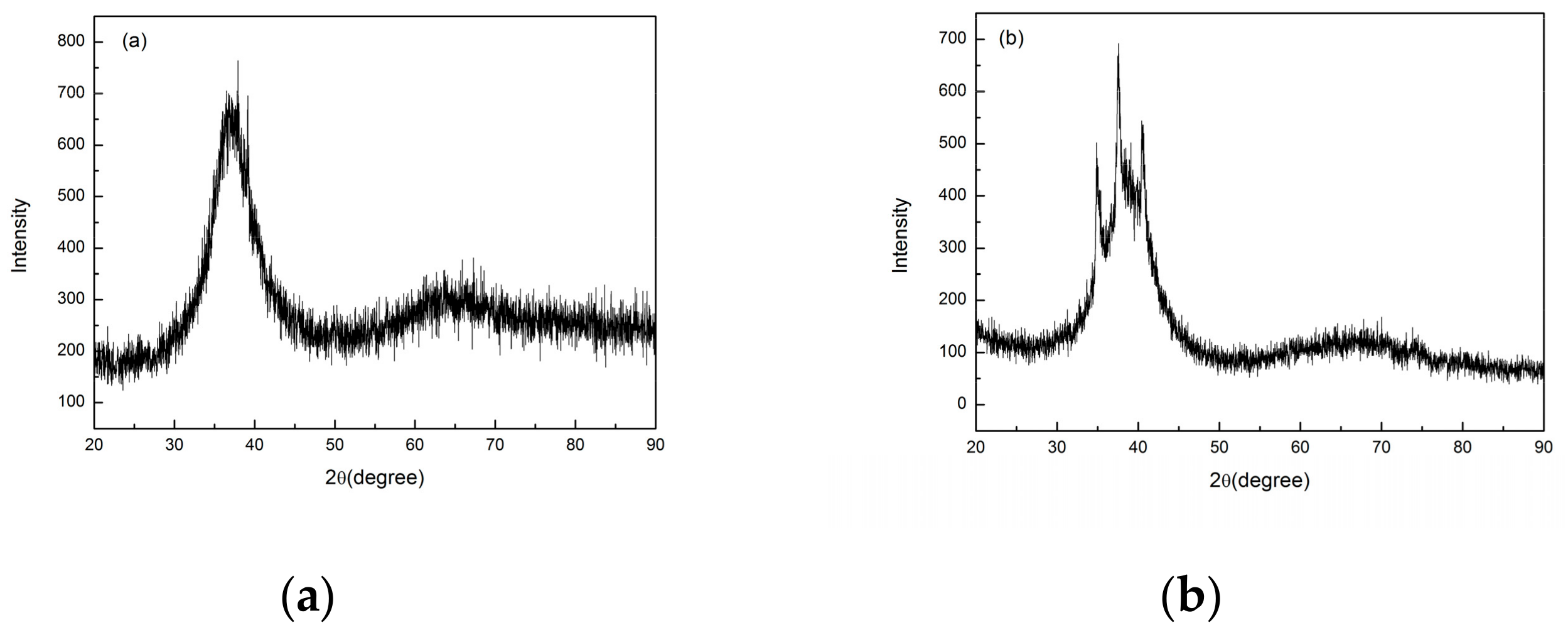
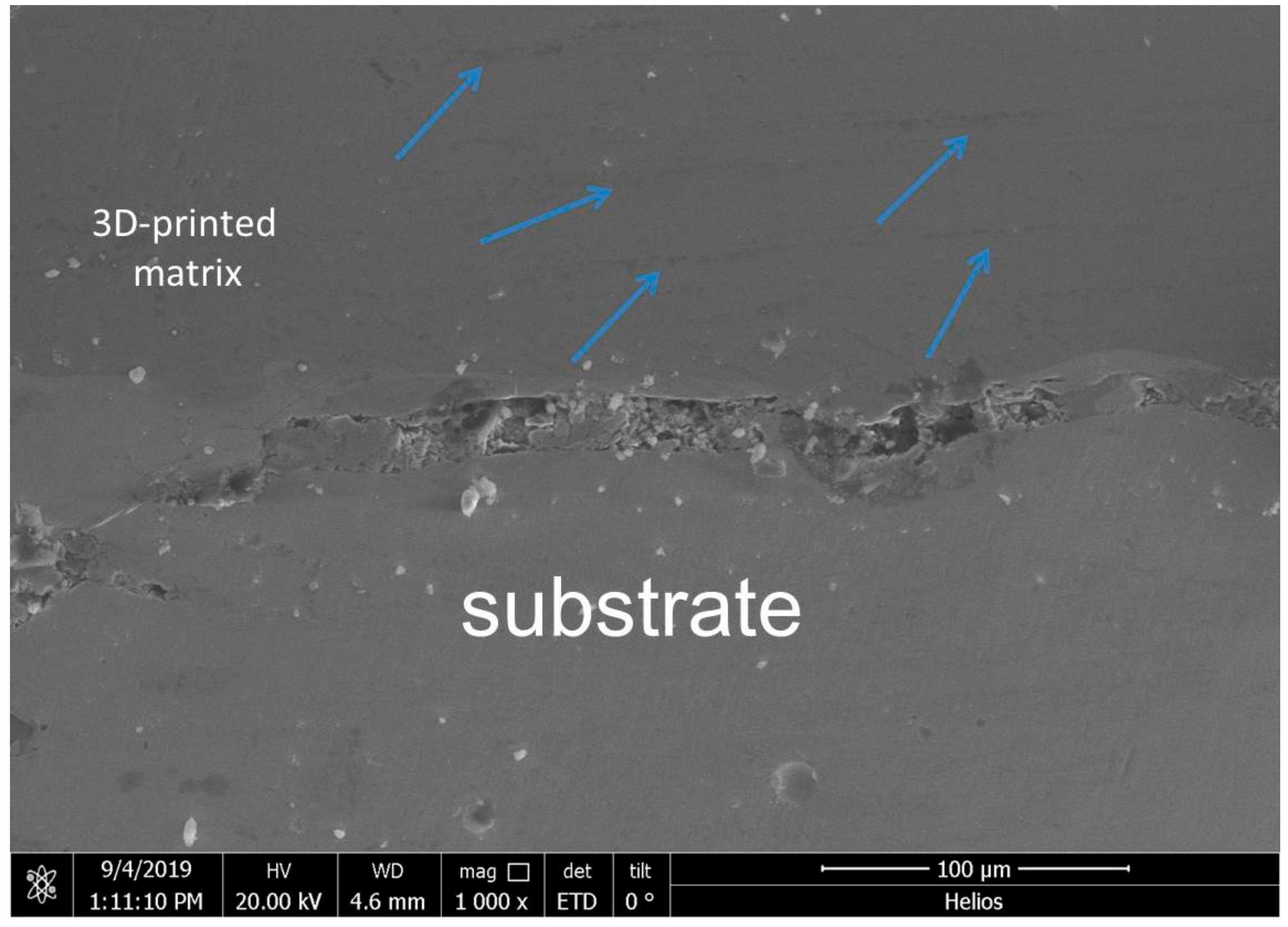
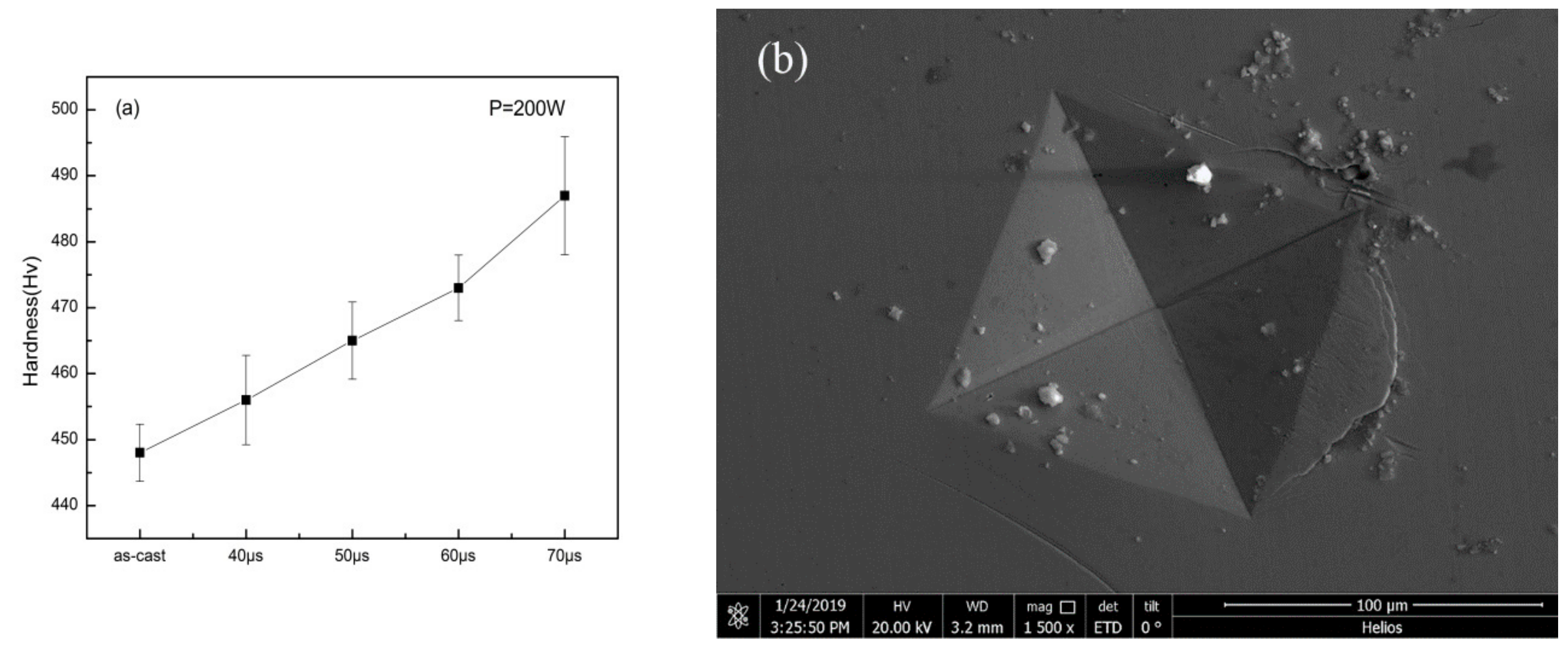
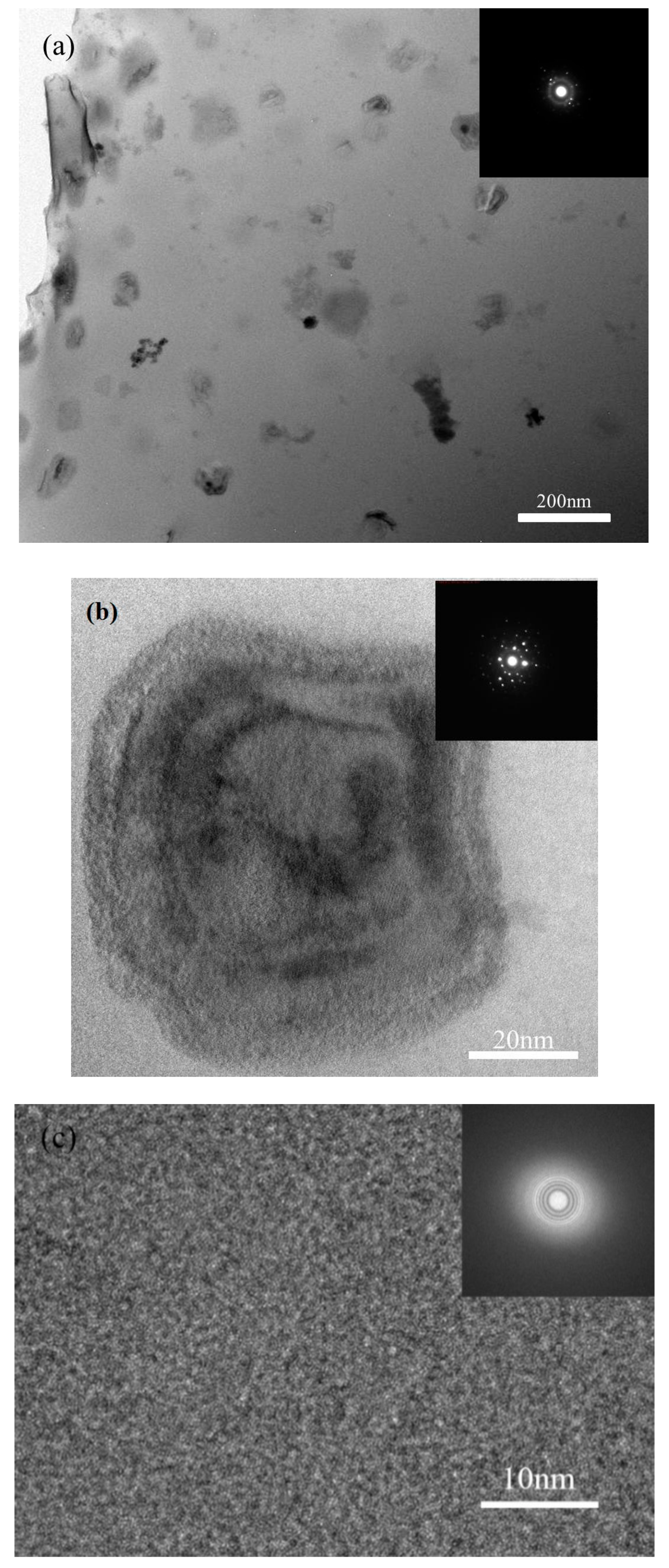
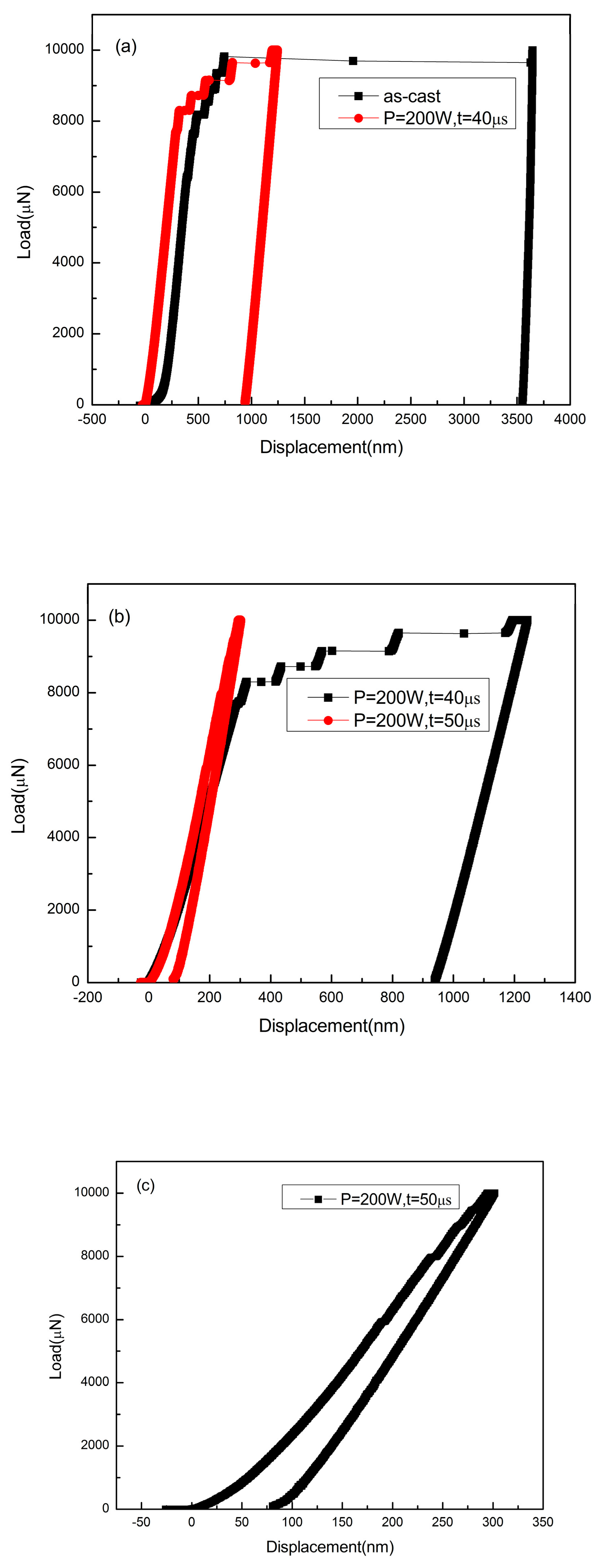
3.2. Mechanical Properties
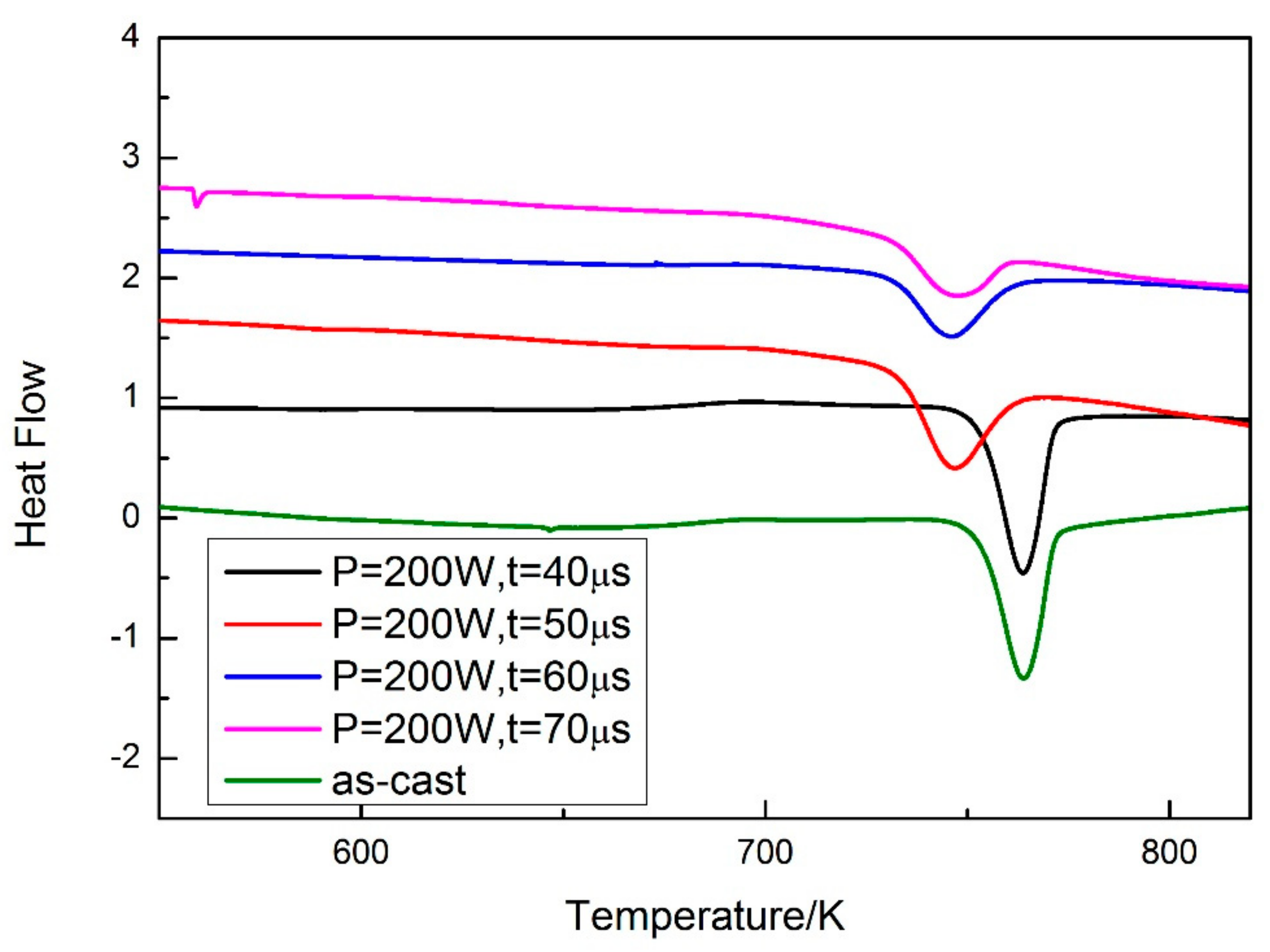
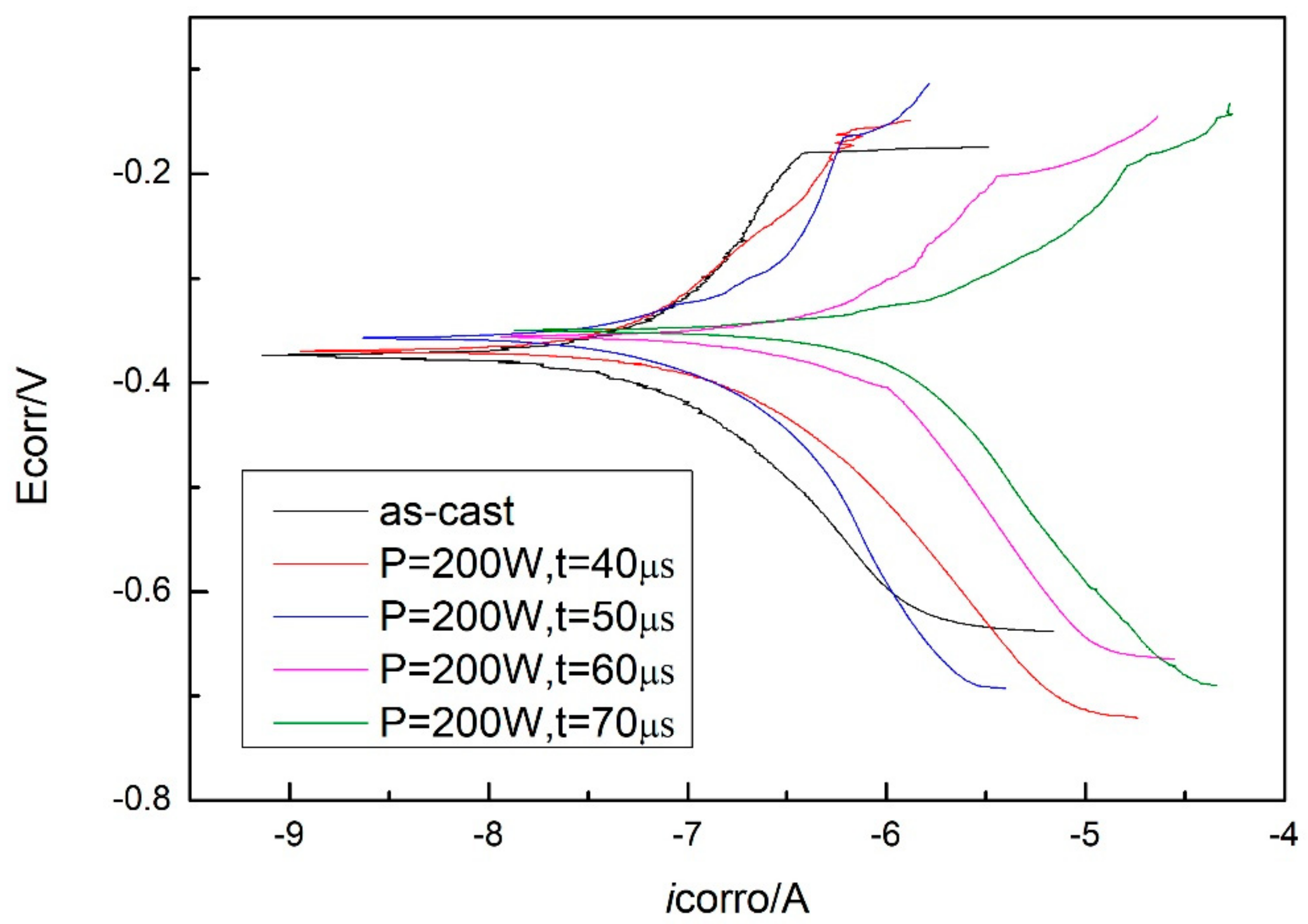
3.3. Corrosion Resistance Measurement
4. Discussion
4.1. Laser Parameter Study
4.2. Hardness and Micro-Compression
4.3. Corrosion Behavior
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Suryanarayana, C.; Inoue, A. Bulk Metallic Glasses; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Eckert, J.; Das, J.; Pauly, S.; Duhamel, C. Mechanical properties of bulk metallic glasses and composites. J. Mater. Res. 2007, 22, 285–301. [Google Scholar] [CrossRef]
- Gebert, A.; Buchholz, K.; Leonhard, A.; Mummert, K.; Eckert, J.; Schultz, L. Investigations on the electrochemical behavior of Zr-based bulk metallic glasses. Mater. Sci. Eng. A. 1999, 267, 294–300. [Google Scholar] [CrossRef]
- Zhang, H.W.; Subhash, G.; Jing, X.N.; Kecskes, L.J.; Dowding, R.J. Evaluation of hardness–yield strength relationships for bulk metallic glasses. Philos. Mag. Lett. 2006, 86, 333–345. [Google Scholar] [CrossRef]
- Wang, W.H. The elastic properties, elastic models and elastic perspectives of metallic glasses. Prog. Mater. Sci. 2012, 57, 487–656. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization of metallic supercooled liquid and bulk amorphous alloys. Acta Mater. 2000, 48, 279–306. [Google Scholar] [CrossRef]
- Yu, P.; Bai, H.; Tang, M.; Wang, W. Excellent glass-forming ability in simple Cu50Zr50-based alloys. J. Non-Crystalline Solids 2005, 351, 1328–1332. [Google Scholar] [CrossRef]
- Inoue, A.; Shen, B.; Koshiba, H.; Kato, H.; Yavari, A.R. Cobalt-based bulk glassy alloy with ultrahigh strength and soft magnetic properties. Nat. Mater. 2003, 2, 661–663. [Google Scholar] [CrossRef]
- Li, Z.; Wang, A.; Chang, C.; Wang, Y.; Dong, B.; Zhou, S. FeSiBPNbCu alloys with high glass-forming ability and good soft magnetic properties. Intermetallic 2014, 54, 225–231. [Google Scholar] [CrossRef]
- Peker, A.; Johnson, W.L. A highly processable metallic glass: Zr41.2Ti13.8Cu12.5Ni10.0Be22.5. Appl. Phys. Lett. 1993, 63, 2342–2344. [Google Scholar] [CrossRef]
- Kim, J.T.; Hong, S.H.; Bian, X.; Gokuldoss, P.K.; Song, K.; Eckert, J.; Park, J.M.; Kim, K.B. Effect of boron addition on thermal and mechanical properties of Co-Cr-Mo-C-(B) glass-forming alloys. Intermetallic 2018, 99, 1–7. [Google Scholar] [CrossRef]
- Inoue, A. Stabilization and high strain-rate superplasticity of metallic supercooled liquid. Mater. Sci. Eng. 1999, 267, 171–183. [Google Scholar] [CrossRef]
- Xi, X.K.; Zhao, D.Q.; Pan, M.X.; Wang, W.H.; Wu, Y.; Lewandowski, J.J. Fracture of brittle metallic glasses: Brittleness or plasticity. Phys. Rev. Lett. 2005, 94, 125510. [Google Scholar] [CrossRef]
- Rombouts, M.; Kruth, J.P.; Froyen, L.; Mercelis, P. Fundamentals of selective laser melting of alloyed steel powders. CIRP Ann. 2006, 55, 187–192. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Rombouts, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2004, 149, 616–622. [Google Scholar] [CrossRef]
- Katakam, S.; Hwang, J.Y.; Paital, S.; Banerjee, R.; Vora, H.; Dahotre, N.B. In Situ Laser Synthesis of Fe-Based Amorphous Matrix Composite Coating on Structural Steel. Met. Mater. Trans. A 2012, 43, 4957–4966. [Google Scholar] [CrossRef]
- Audebert, F.; Colaco, R.; Vilar, R.; Sirkin, H. Production of glassy metallic layers by laser surface treatment. Scr. Mater. 2003, 48, 281–286. [Google Scholar] [CrossRef]
- Jung, H.Y.; Choi, S.J.; Prashanth, K.G.; Stoica, M.; Scudino, S.; Yi, S.; Kühn, U.; Kim, D.H.; Kim, K.B.; Eckert, J. Fabrication of Fe-based bulk metallic glass by selective laser melting: A parameter study. Mater. Des. 2015, 86, 703–708. [Google Scholar] [CrossRef]
- Li, X.; Roberts, M.; O’Keeffe, S.; Sercombe, T. Selective laser melting of Zr-based bulk metallic glasses: Processing, microstructure and mechanical properties. Mater. Des. 2016, 112, 217–226. [Google Scholar] [CrossRef]
- Li, X.; Roberts, M.; Liu, Y.; Kang, C.; Huang, H.; Sercombe, T. Effect of substrate temperature on the interface bond between support and substrate during selective laser melting of Al–Ni–Y–Co–La metallic glass. Mater. Des. 2015, 65, 1–6. [Google Scholar] [CrossRef]
- Ouyang, D.; Li, N.; Xing, W.; Zhang, J.; Liu, L. 3D printing of crack-free high strength Zr-based bulk metallic glass composite by selective laser melting. Intermetallic 2017, 90, 128–134. [Google Scholar] [CrossRef]
- Li, N.; Zhang, J.; Xing, W.; Ouyang, D.; Liu, L. 3D printing of Fe-based bulk metallic glass composites with combined high strength and fracture toughness. Mater. Des. 2018, 143, 285–296. [Google Scholar] [CrossRef]
- Schuh, C.A.; Lund, A.C. Atomistic basis for the plastic yield criterion of metallic glass. Nat. Mater. 2003, 2, 449–452. [Google Scholar] [CrossRef]
- Argon, A.S. Plastic deformation in metallic glasses. Acta Metal. 1978, 27, 47–58. [Google Scholar] [CrossRef]
- Ma, E. Controlling plastic instability. Nat. Mater. 2003, 2, 7–8. [Google Scholar] [CrossRef]
- Pan, J.; Liu, L.; Chan, K. Enhanced plasticity by phase separation in CuZrAl bulk metallic glass with micro-addition of Fe. Scr. Mater. 2009, 60, 822–825. [Google Scholar] [CrossRef]
- Kim, K.; Das, J.; Lee, M.; Yi, S.; Fleury, É.; Zhang, Z.; Wang, W.; Eckert, J. Propagation of shear bands in a Cu47.5Zr47.5Al5 bulk metallic glass. J. Mater. Res. 2008, 23, 6–12. [Google Scholar] [CrossRef]
- Mahbooba, Z.; Thorsson, L.; Unosson, M.; Skoglund, P.; West, H.; Horn, T.; Rock, C.; Vogli, E.; Harrysson, O. Additive manufacturing of an iron-based bulk metallic glass larger than the critical casting thickness. Appl. Mater. Today 2018, 11, 264–269. [Google Scholar] [CrossRef]
- Mondal, K.; Ohkubo, T.; Toyama, T.; Nagai, Y.; Hasegawa, M.; Hono, K. The effect of nanocrystallization and free volume on the room temperature plasticity of Zr-based bulk metallic glasses. Acta Mater. 2008, 56, 5329–5339. [Google Scholar] [CrossRef]
- Jin, K.; Löffler, J.F. Bulk metallic glass formation in Zr–Cu–Fe–Al alloys. Appl. Phys. Lett. 2005, 86, 241909. [Google Scholar] [CrossRef]
- Link, S.; Burda, C.; Mohamed, M.B.; Nikoobakht, B.; El-Sayed, M.A. Laser Photothermal Melting and Fragmentation of Gold Nanorods: Energy and Laser Pulse-Width Dependence. J. Phys. Chem. A 1999, 103, 1165–1170. [Google Scholar] [CrossRef]
- Hashimoto, K.; Osada, K.; Masumoto, T.; Shimodaira, S. Characteristics of passivity of extremely corrosion-resistant amorphous iron alloys. Corros. Sci. 1976, 16, 71–76. [Google Scholar] [CrossRef]
- Poorhaudari, K.; Patchett, B.M.; Ivey, D.G. Estimation of cooling rate in the welding of plates with intermediate thickness. Weld. J. 2005, 84, 149–154. [Google Scholar]
- Yang, G.; Lin, X.; Liu, F.; Hu, Q.; Ma, L.; Li, J.; Huang, W. Laser solid forming Zr-based bulk metallic glass. Intermet. 2012, 22, 110–115. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Wang, D. A study on the residual stress during selective laser melting (SLM) of metallic powder. Int. J. Adv. Manuf. Technol. 2016, 87, 647–656. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, S.; Gan, Y.; Huang, T.; Yang, C.; Junjie, L.; Lin, J. Study on the microstructure, mechanical property and residual stress of SLM Inconel-718 alloy manufactured by differing island scanning strategy. Opt. Laser Technol. 2015, 75, 197–206. [Google Scholar] [CrossRef]
- Kumar, G.; Ohkubo, T.; Mukai, T.; Hono, K. Plasticity and microstructure of Zr–Cu–Al bulk metallic glasses. Scr. Mater. 2007, 57, 173–176. [Google Scholar] [CrossRef]
- Shiomi, M.; Osakada, K.; Nakamura, K.; Yamashita, T.; Abe, F. Residual Stress within Metallic Model Made by Selective Laser Melting Process. CIRP Ann. 2004, 53, 195–198. [Google Scholar] [CrossRef]
- Kim, K.B.; Das, J.; Baier, F.; Tang, M.B.; Wang, W.H.; Eckert, J. Heterogeneity of a Cu47.5Zr47.5Al5 bulk metallic glass. Appl. Phys. Lett. 2006, 88, 51911. [Google Scholar] [CrossRef]
- Hajlaoui, K.; Yavari, A.; Doisneau, B.; Lemoulec, A.; Vaughan, G.; Greer, A.; Inoue, A.; Zhang, W.; Kvick, Å. Shear delocalization and crack blunting of a metallic glass containing nanoparticles: In situ deformation in TEM analysis. Scr. Mater. 2006, 54, 1829–1834. [Google Scholar] [CrossRef]
- Hsiao, Z.; Fu, C.; Tsai, P.; Jang, J.S.; Jian, S.; Huang, J. Effect of Nano-Crystallization on the Mechanical Properties of the (Zr53Cu30Ni9Al8)99.5Si0.5 Bulk Metallic Glass. Mater. Sci. Forum 2010, 638, 2933–2937. [Google Scholar] [CrossRef]
- Jiang, W.H.; Jiang, F.; Green, B.A.; Liu, F.X.; Liaw, P.K.; Choo, H.; Qiu, K.Q. Electrochemical corrosion behavior of a Zr-based bulk-metallic glass. Appl. Phys. Lett. 2007, 91, 041904. [Google Scholar] [CrossRef]
- Gebert, A.; Mudali, U.K.; Eckert, J.; Schultz, L. Electrochemical Reactivity of Zirconium-Based Bulk Metallic Glasses. Mater. Res. Soc. Symp. Proc. 2004, 806, 369. [Google Scholar] [CrossRef]
- Peter, W.; Buchanan, R.; Liu, C.T.; Liaw, P.; Morrison, M.; Horton, J.; Carmichael, C.; Wright, J. Localized corrosion behavior of a zirconium-based bulk metallic glass relative to its crystalline state. Intermet. 2002, 10, 1157–1162. [Google Scholar] [CrossRef]
- Atashin, S.; Pakshir, M.; Yazdani, A. Synergistic investigation into the marine parameters’ effect on the corrosion rate of AISI 316 stainless steel. Mater. Des. 2011, 32, 1315–1324. [Google Scholar] [CrossRef]
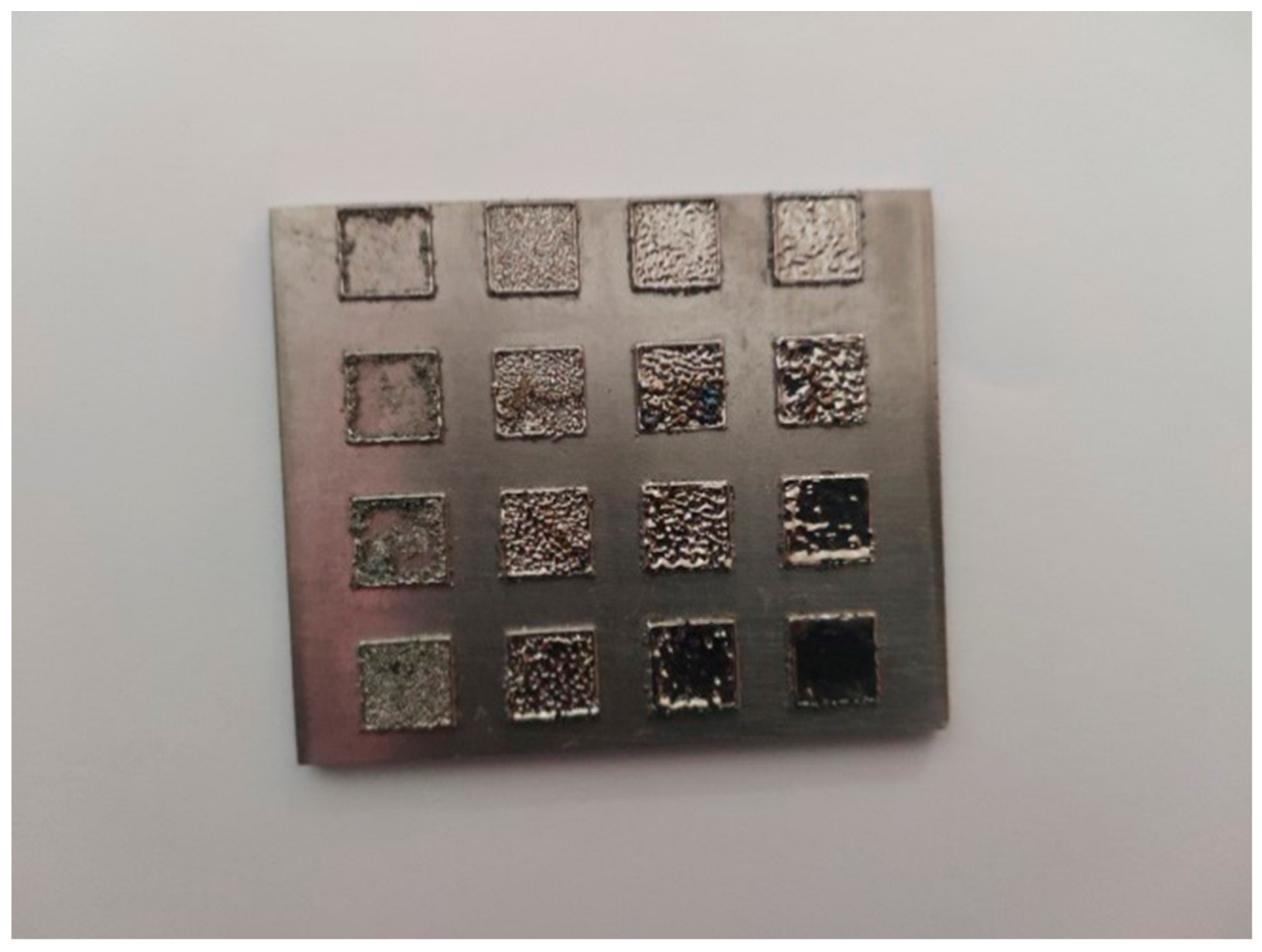



| Laser Parameters | |||
|---|---|---|---|
| 100 W, 20 μs | 100 W, 40 μs | 100 W, 60 μs | 100 W, 80 μs |
| 120 W, 20 μs | 120 W, 40 μs | 120 W, 60 μs | 120 W, 80 μs |
| 160 W, 20 μs | 160 W, 40 μs | 160 W, 60 μs | 160 W, 80 μs |
| 200 W, 20 μs | 200 W, 40 μs | 200 W, 60 μs | 200 W, 80 μs |
| Laser Parameters | ||||
|---|---|---|---|---|
| 100 W, 30 μs | 100 W, 40 μs | 100 W, 60 μs | 100 W, 80 μs | 100 W, 100 μs |
| 120 W, 30 μs | 120 W, 40 μs | 120 W, 60 μs | 120 W, 80 μs | 120 W, 100 μs |
| 160 W, 30 μs | 160 W, 40 μs | 160 W, 60 μs | 160 W, 80 μs | 160 W, 100 μs |
| 180 W, 20 μs | 180 W, 30 μs | 180 W, 40 μs | 180 W, 50 μs | 180 W, 60 μs |
| 200 W, 20 μs | 200 W, 30 μs | 200 W, 40 μs | 200 W, 50 μs | 200 W, 60 μs |
| As-Cast | 200 W, 40 μs | 200 W, 50 μs | 200 W, 60 μs | 200 W, 70 μs | |
|---|---|---|---|---|---|
| △H (J/g) | −49.2 | −47.7 | −40.0 | −29.3 | −22.7 |
| - | 96.95% | 81.3% | 59.55% | 46.14% |
| As-Cast | 200 W, 40 μs | 200 W, 50 μs | 200 W, 60 μs | 200 W, 70 μs | |
|---|---|---|---|---|---|
| Ecorro (V) | −0.373 | −0.370 | −0.366 | −0.359 | −0.351 |
| Icorro (A) | −9.77e−8 | −1.65e−7 | −2.79e−7 | −6.79e−7 | −1.87e−6 |
| CPR (μm) | 0.11 | 0.18 | 0.31 | 0.76 | 2.09 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.; Xing, L.; Jiang, Y.; Li, R.; Lu, C.; Zeng, R.; Luo, J.; Zhang, P.; Liu, W. Additive Manufactured Large Zr-Based Bulk Metallic Glass Composites with Desired Deformation Ability and Corrosion Resistance. Materials 2020, 13, 597. https://doi.org/10.3390/ma13030597
Luo Y, Xing L, Jiang Y, Li R, Lu C, Zeng R, Luo J, Zhang P, Liu W. Additive Manufactured Large Zr-Based Bulk Metallic Glass Composites with Desired Deformation Ability and Corrosion Resistance. Materials. 2020; 13(3):597. https://doi.org/10.3390/ma13030597
Chicago/Turabian StyleLuo, Yu, Leilei Xing, Yidong Jiang, Ruiwen Li, Chao Lu, Rongguang Zeng, Jinru Luo, Pengcheng Zhang, and Wei Liu. 2020. "Additive Manufactured Large Zr-Based Bulk Metallic Glass Composites with Desired Deformation Ability and Corrosion Resistance" Materials 13, no. 3: 597. https://doi.org/10.3390/ma13030597
APA StyleLuo, Y., Xing, L., Jiang, Y., Li, R., Lu, C., Zeng, R., Luo, J., Zhang, P., & Liu, W. (2020). Additive Manufactured Large Zr-Based Bulk Metallic Glass Composites with Desired Deformation Ability and Corrosion Resistance. Materials, 13(3), 597. https://doi.org/10.3390/ma13030597




