Ecological Cutting Fluids
Abstract
1. Introduction
2. Aqueous Solutions of Anionic Surfactants as SWF Bases
2.1. Adsorption at the Interface: Solid-Surfactant Solution
2.2. Alkyl Sulfates and Alkyl Ether Sulfates
2.3. Sulfosuccinate Derivatives Produced on the Basis of Polyoxyalkylates of 2-Ethylhexyl Alcohol
2.4. Sodium Sarcosinates
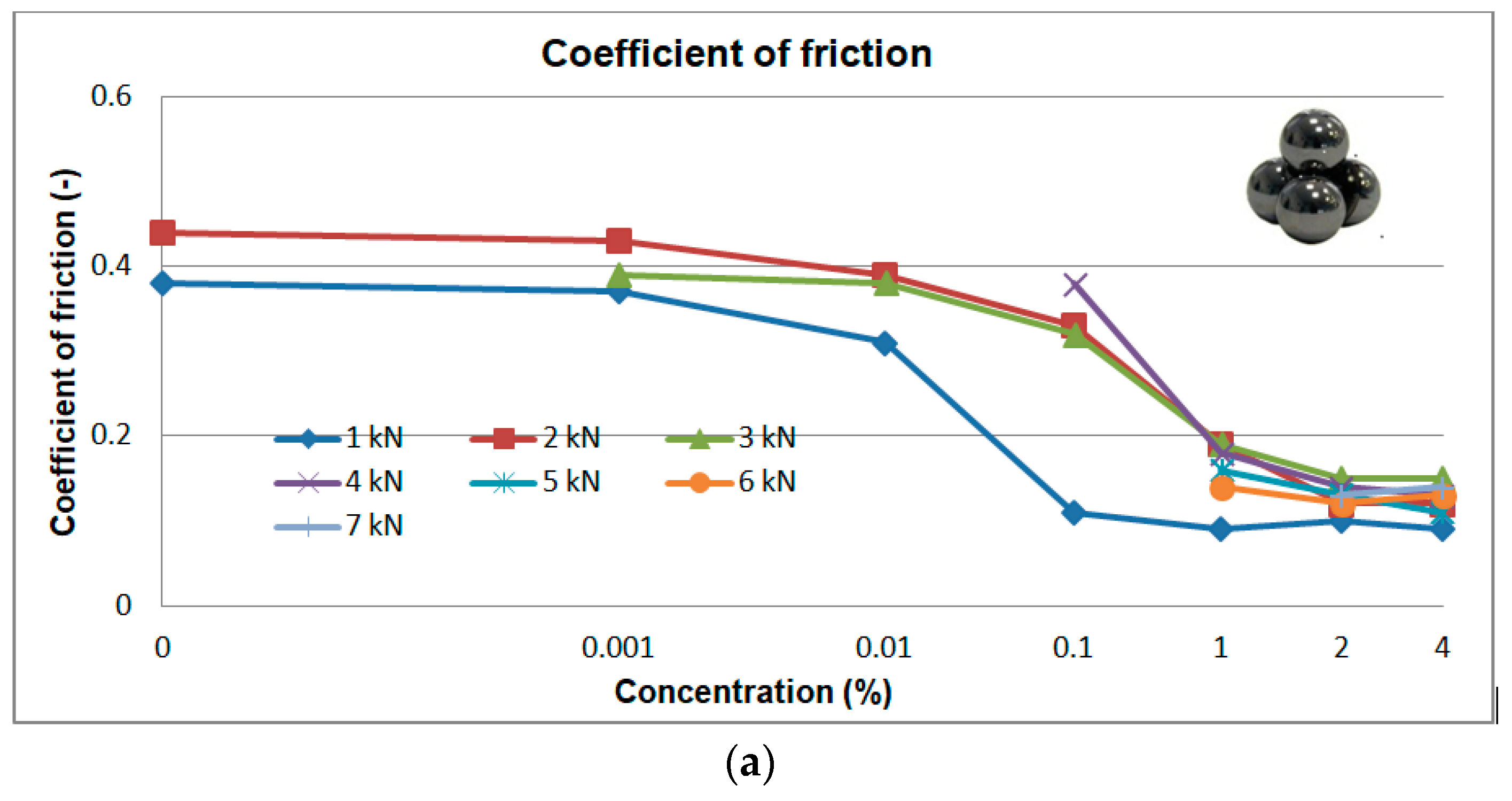
3. Experimental Results
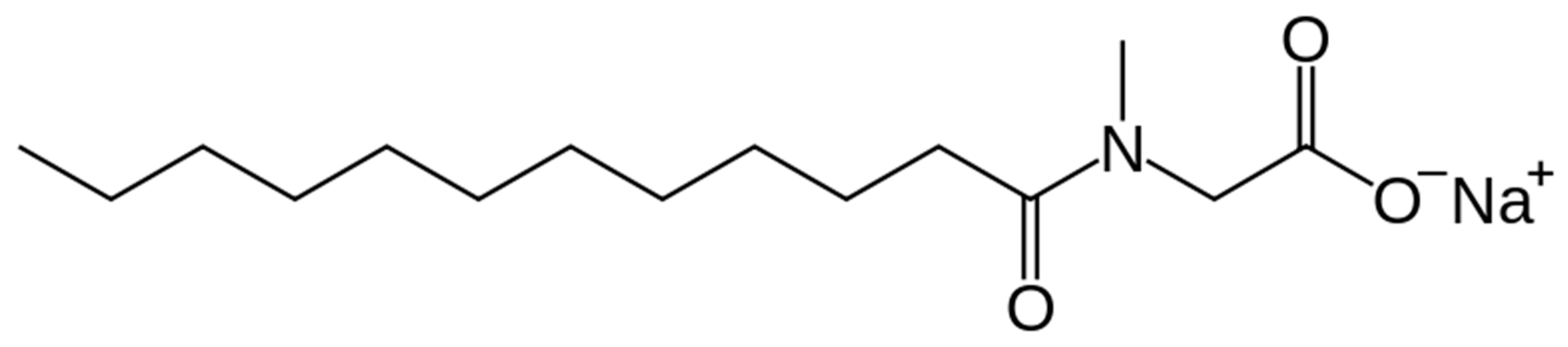
3.1. Physicochemical Properties of Aqueous Solutions of Sodium Lauroyl Sarcosinate as SWF Bases
- Environmental safety and human health in the workplace,
- Stability and biodegradability,
- Biocidal action on microorganisms,
- High surface activity in a wide interval.
3.1.1. Stability
3.1.2. Surface Activity
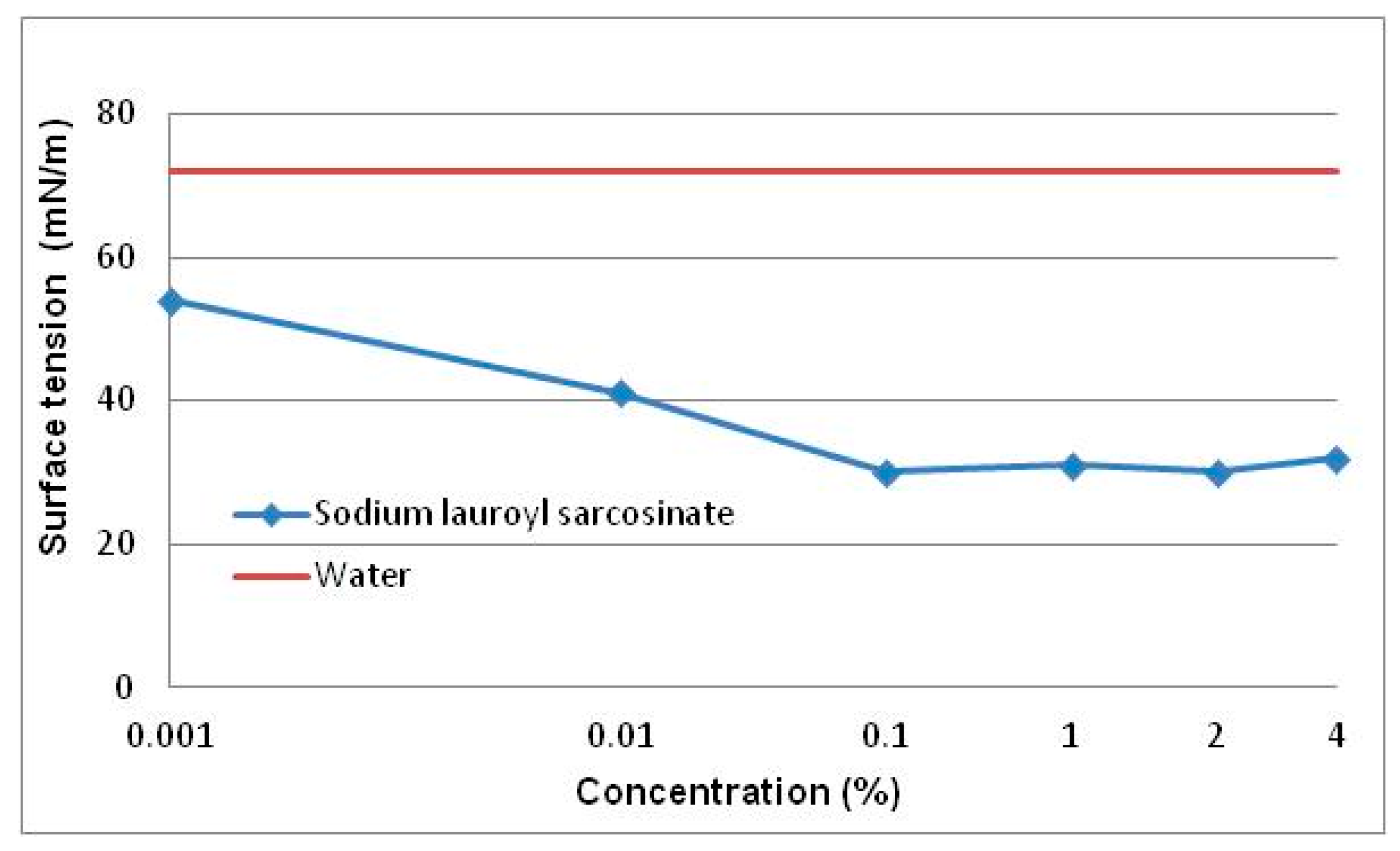
3.1.3. Surface Tension
3.1.4. Surface Wettability
3.1.5. Kinematic Viscosity (ν)
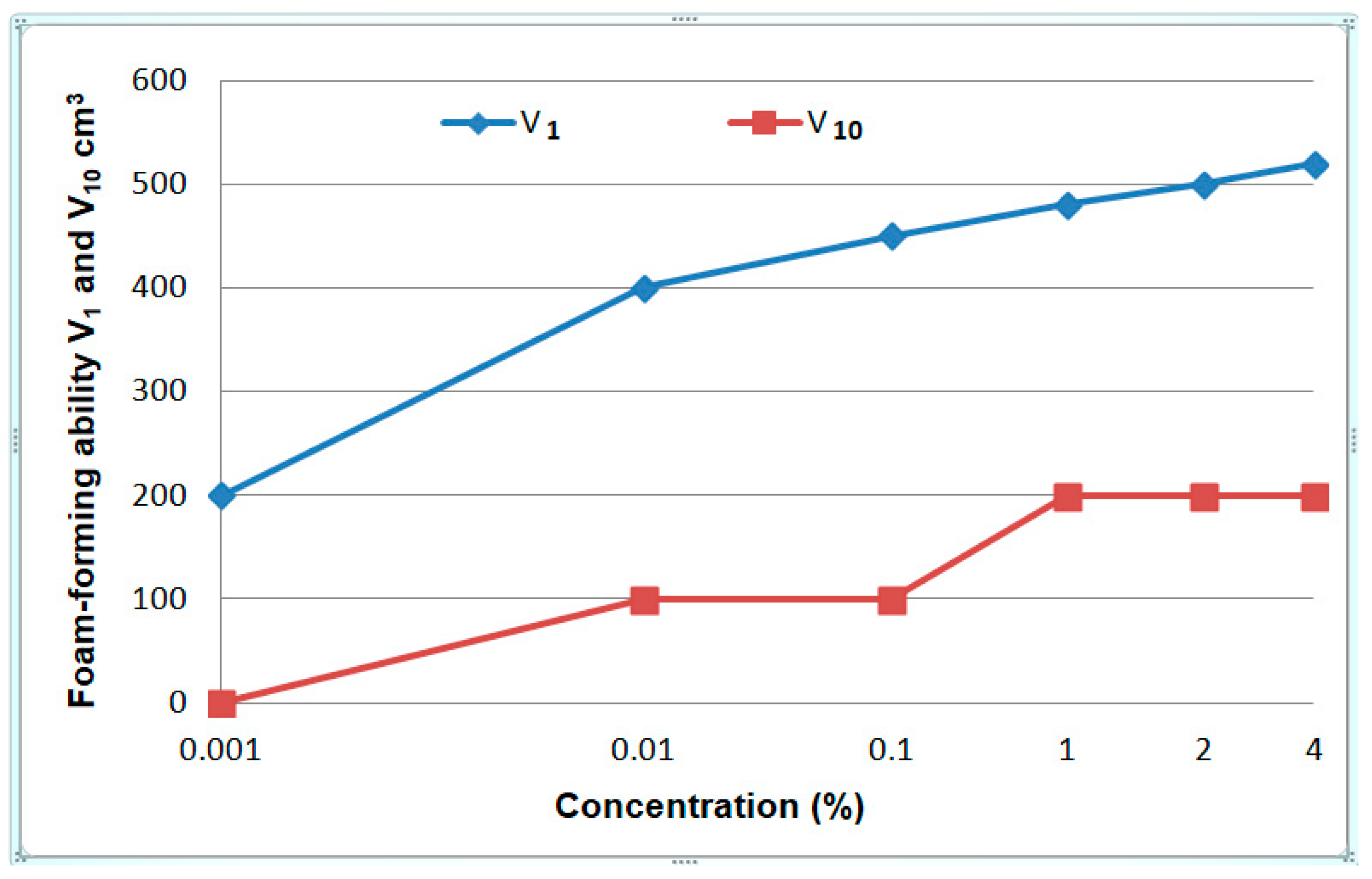
3.1.6. Foamability
3.1.7. Corrosion
3.2. Tribological Properties of Aqueous Solutions
3.2.1. Tests at Constant Load (1, 2, 3, 4, 5, 6, 7 kN)
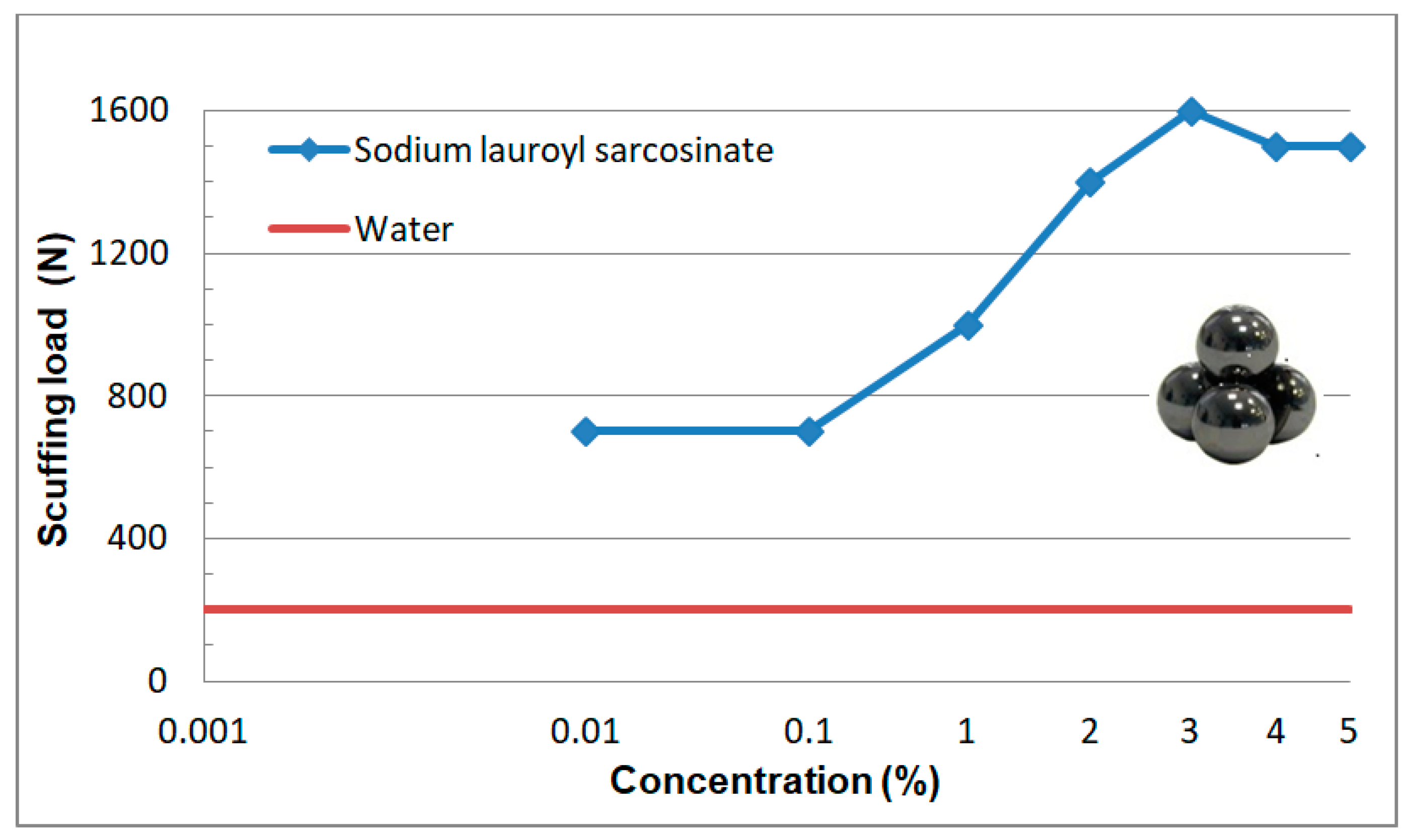
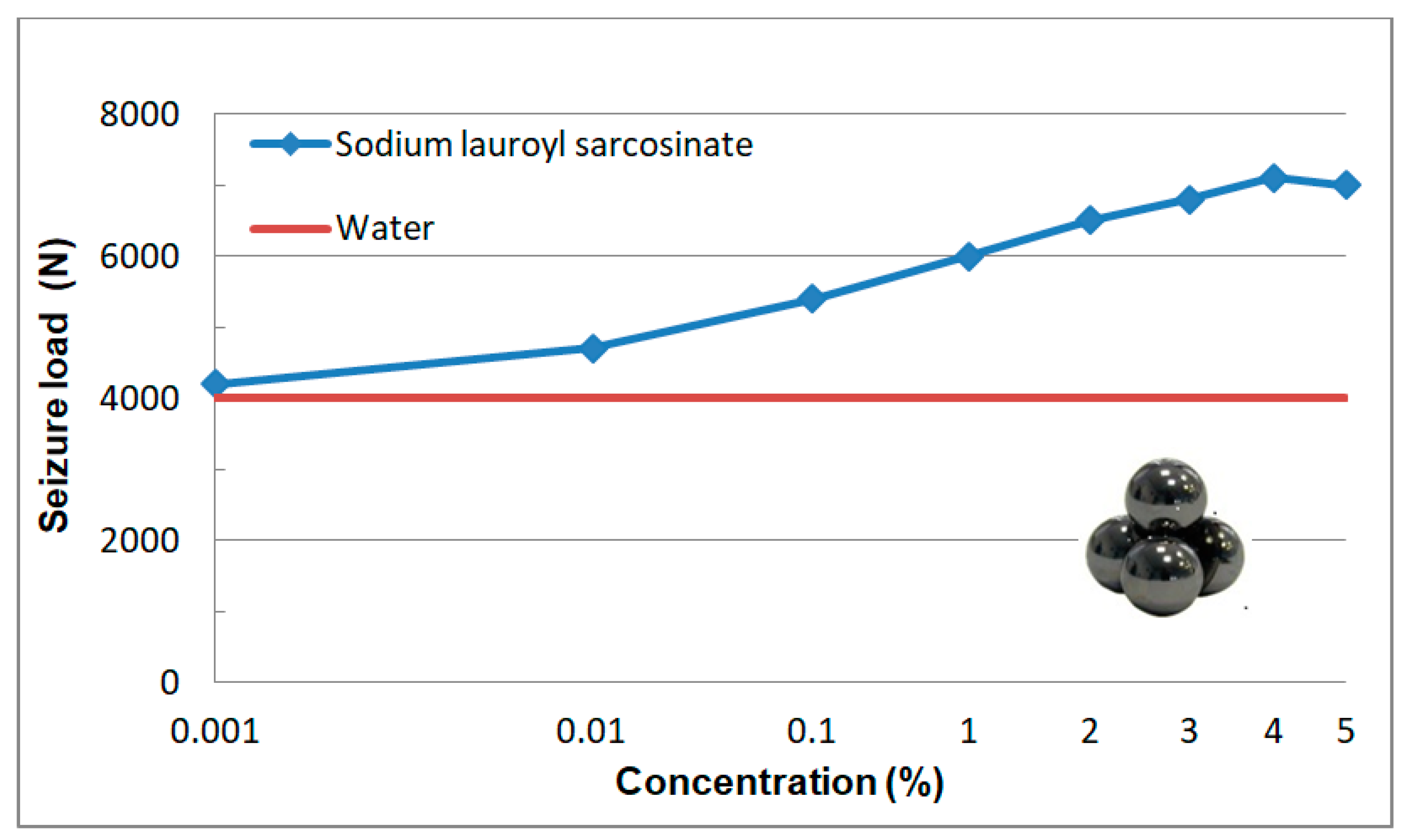
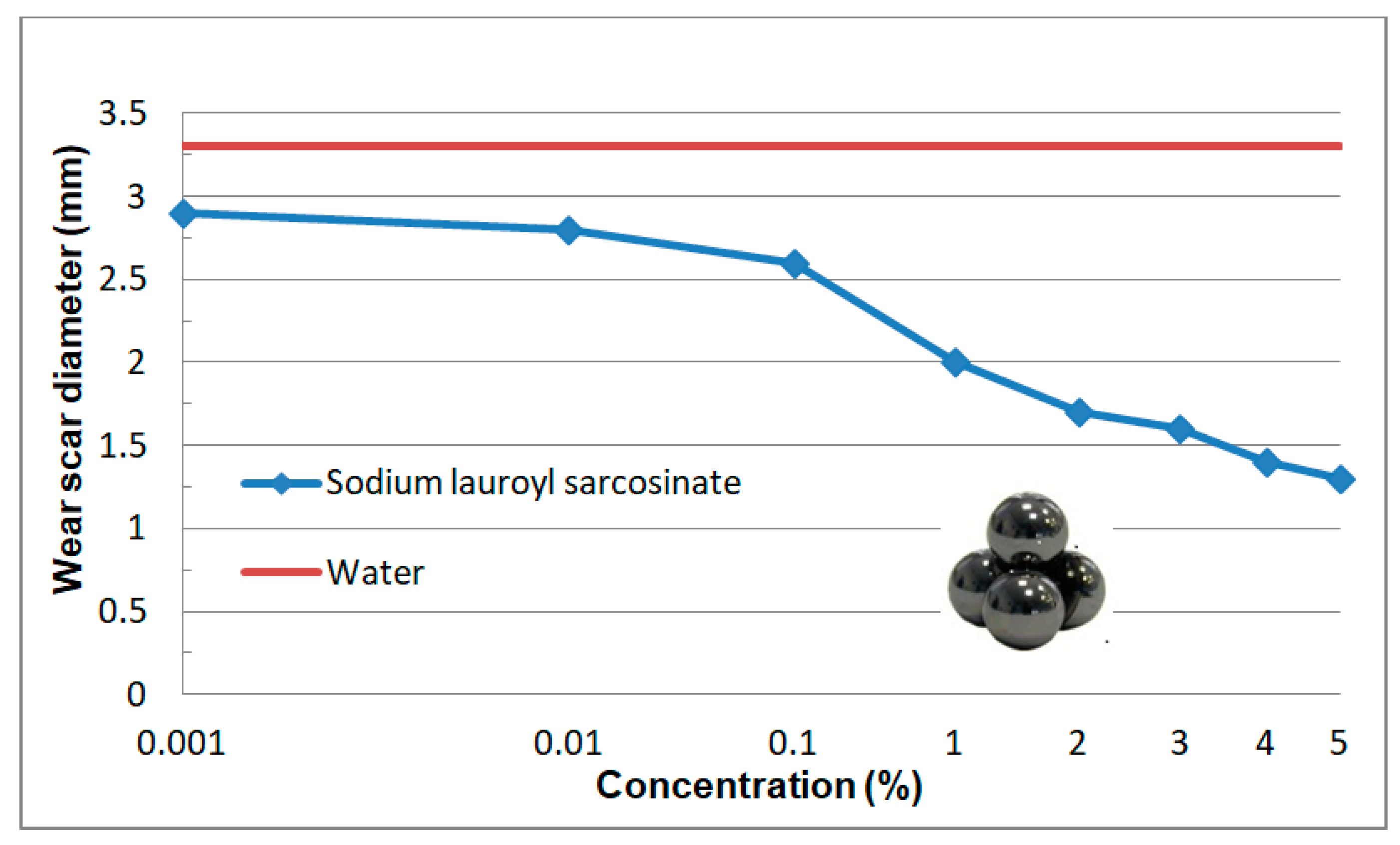
3.2.2. Tests at Linearly Increasing Load
3.2.3. Scuffing Load (Pt)
3.2.4. Seizure Load (Poz)
3.2.5. Wear Scar Diameter (doz)
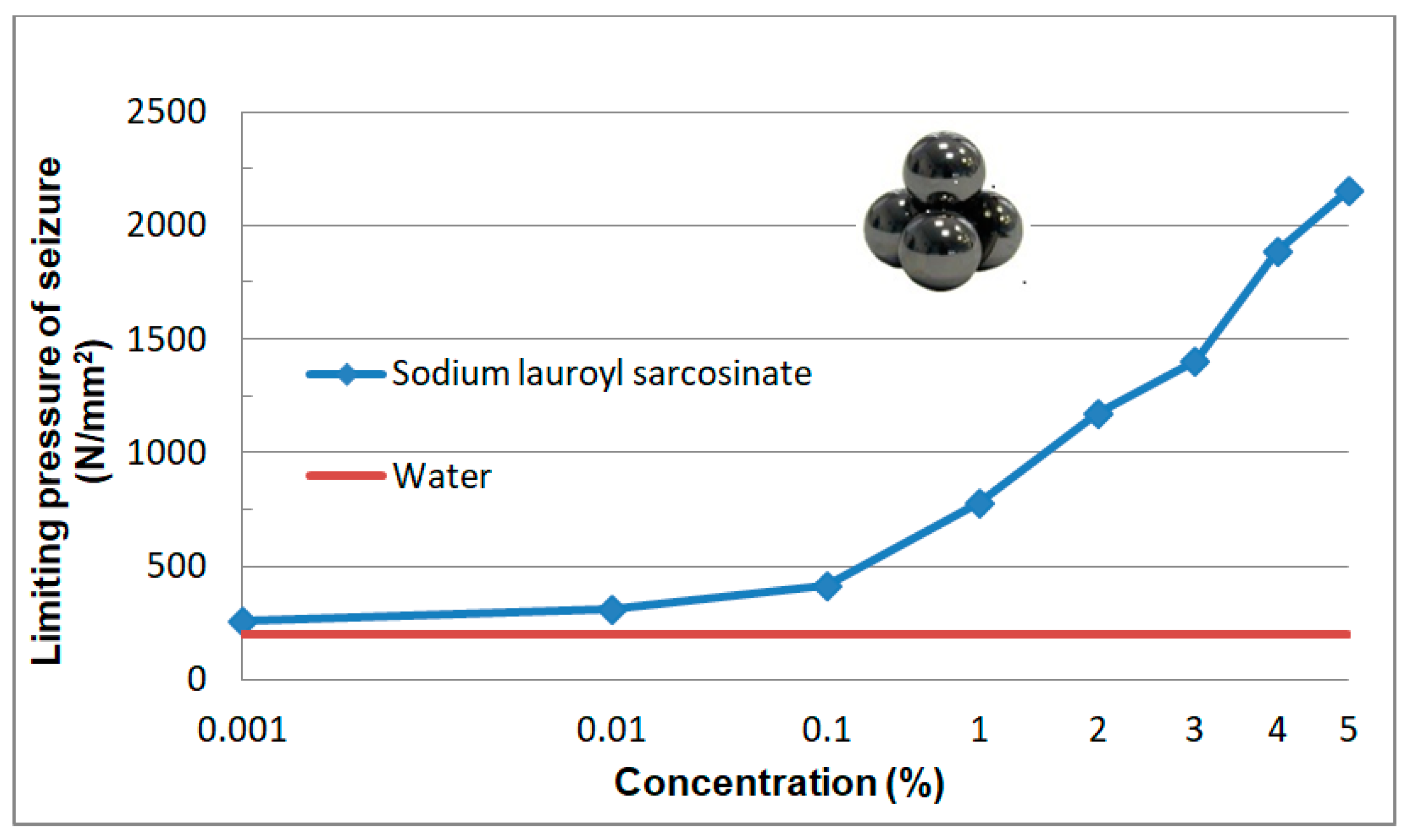
3.2.6. Limiting Pressure of Seizure poz
4. Surfactant Working Fluids (SWFs) Friction Mechanisms
5. Stand and Operational Tests
5.1. The Composition of a Surfactant Working Fluid (SWF)
- Water with hardness of 10°n (in German degrees) prepared according to the procedure described in the PN-92/M 55789 standard. The hardness of this kind of water is close to that of commonly used tap water in Poland,
- Sodium lauroyl sarcosinate (2% concentration),
- Antifoam additive (Silfoam SE 47-silicone emulsion, 0.05% concentration),
- Biocide (Euxyl K120—mixture of methylchloroisothiazolinone and methylisothiazolinone—0.1% concentration).
5.2. Physicochemical and Tribological Properties
5.3. Test Stand Studies
- Tool life of sintered carbide cutting edges at straight turning of standard steel C45.
- Tool life of a cutting edge and cutting resistance using the WBS device.
5.4. Tool Life of Sintered Carbide Cutting Edges at Straight Turning of Standard Steel C45
- In the case of turning using cutting edges in the initial phase of wear (VB up to 0.05 mm) the Ra values were 1.8 µm for the original fluid and 1.5 μm for the reference fluid,
- In the case of turning with cutting edges with a new degree of wear (0.05 < VB ≤ 0.30 mm) the Ra values were 2.3 μm for the original fluid and 2.0 μm for the reference fluid.
- All fluids displayed good cooling and lubricating properties. No significant differences were observed compared to commercial fluids,
- No excessive tool wear or deterioration of quality of the worked surfaces were observed,
- No corrosion occurred on surfaces of the elements worked or machine parts.
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- 51385 DIN (2013) Lubricants—Processing Fluids for Forming and Machining of Materials—Terms, Beuth-Verlag; German Institute for Standardization: Berlin, Germany, 2013.
- Sułek, M.; Bocho-Janiszewska, A. The Effect of Metal 8-Hydroxyquinolinates as Lubricant Additives on the Friction Process. Tribol. Lett. 2003, 15, 301–307. [Google Scholar] [CrossRef]
- Wasilewski, T.; Sulek, M.W. Paraffin oil solutions of the mixture of sorbitan monolaurate–ethoxylated sorbitan monolaurate as lubricants. Wear 2006, 261, 230–234. [Google Scholar] [CrossRef]
- Sulek, M.W.; Wasilewski, T. Tribological properties of aqueous solutions of alkyl polyglucosides. Wear 2006, 260, 193–204. [Google Scholar] [CrossRef]
- Sulek, M.; Bocho-Janiszewska, A. The effect of ethoxylated esters on the lubricating properties of their aqueous solutions. Tribol. Lett. 2006, 24, 187–194. [Google Scholar] [CrossRef]
- Sulek, M.; Bak-Sowinska, A. Aqueous Solutions of Surfactants in Materials Engineering of Tribological Systems. In Surfactants in Tribology, Volume 4; Informa UK Limited: Colchester, UK, 2014; pp. 239–258. [Google Scholar]
- Sulek, M.W.; Wasilewski, T.; Kurzydłowski, K.J. The Effect of Concentration on Lubricating Properties of Aqueous Solutions of Sodium Lauryl Sulfate and Ethoxylated Sodium Lauryl Sulfate. Tribol. Lett. 2010, 40, 337–345. [Google Scholar] [CrossRef]
- Sułek, M.W.; Bąk-Sowińska, A. 10. Chapter in the Monograph “Surfactants in Tribology”, “Aqueous Solution of Surfactants in Materials Engineering of Tribological Systems”; Biresaw, G., Mittal, K.L., Eds.; CRC Press: Boca Raton, FL, USA, 2014; Volume 4, pp. 239–258. [Google Scholar]
- Przondo, J.; Sułek, M.W.; Sas, W. Concentrate for the Preparation of Cutting Fluids and Non-Emulsion Hydraulic Fluids. Patent No. 208,951, 30 June 2011. [Google Scholar]
- Pernak, J.; Walkiewicz, F.; Sułek, M.W.; Wasilewski, T. Multifunctional Grease Containing Ionic Liquids. Patent No. 215,892, 10 April 2013. [Google Scholar]
- Sułek, M.W.; Bąk, A.; Wasilewski, T.; Wachowicz, J.; Pytlik, A.J. Flame-Retardant Water-Based Hydraulic Fluid. Patent No. 218,550, 5 March 2014. [Google Scholar]
- Sułek, M.W.; Bąk, A.; Wasilewski, T.; Wachowicz, J.; Pytlik, A.J. Flame-Retardant Water-Based Hydraulic Fluid. Patent No. 218,551, 5 March 2014. [Google Scholar]
- Sułek, M.W.; Bąk, A.; Wasilewski, T.; Wachowicz, J.; Pytlik, A.J. Flame-Retardant Water-Based Hydraulic Fluid. Patent No. 218,566, 25 February 2014. [Google Scholar]
- Sułek, M.W.; Wasilewski, T.; Piotrowska, U.; Seweryn, A. Cooling—Lubricating Liquid for Metalworking. Patent No. 225,734, 2 December 2016. [Google Scholar]
- Sułek, M.W.; Wasilewski, T.; Sas, W.; Piotrowska, U. Cooling and Lubricating Liquid for Metal Processing. Patent No. 221,760, 17 June 2015. [Google Scholar]
- Briscoe, W.H. Aqueous boundary lubrication: Molecular mechanisms, design strategy, and terra incognita. Curr. Opin. Colloid Interface Sci. 2017, 27, 1–8. [Google Scholar] [CrossRef]
- Ratoi, M.; Spikes, H.A. Lubricating Properties of Aqueous Surfactant Solutions. Tribol. Trans. 1999, 42, 479–486. [Google Scholar] [CrossRef]
- Sorkin, R.; Kampf, N.; Dror, Y.; Shimoni, E.; Klein, J. Origins of extreme boundary lubrication by phosphatidylcholine liposomes. Biomaterials 2013, 34, 5465–5475. [Google Scholar] [CrossRef]
- Botan, A.; Joly, L.; Fillot, N.; Loison, C. Mixed Mechanism of Lubrication by Lipid Bilayer Stacks. Langmuir 2015, 31, 12197–12202. [Google Scholar] [CrossRef]
- Briscoe, W.H.; Titmuss, S.; Tiberg, F.; Thomas, R.K.; McGillivray, D.J.; Klein, J. Boundary lubrication under water. Nat. Cell Biol. 2006, 444, 191–194. [Google Scholar] [CrossRef]
- Benedicto, E.; Rubio, E.M.; Carou, D.; Santacruz, C. The Role of Surfactant Structure on the Development of a Sustainable and Effective Cutting Fluid for Machining Titanium Alloys. Metals 2020, 10, 1388. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, C.; Liu, S. Progress in experimental study of aqueous lubrication. Chin. Sci. Bull. 2012, 57, 2062–2069. [Google Scholar] [CrossRef]
- Chen, M.; Briscoe, W.H.; Armes, S.P.; Klein, J. Lubrication at Physiological Pressures by Polyzwitterionic Brushes. Science 2009, 323, 1698–1701. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Amann, T.; Kailer, A.; Rühe, J. Macroscopic Friction Studies of Alkylglucopyranosides as Additives for Water-Based Lubricants. Lubricants 2020, 8, 11. [Google Scholar] [CrossRef]
- Han, T.; Zhang, C.; Luo, J. Macroscale Superlubricity Enabled by Hydrated Alkali Metal Ions. Langmuir 2018, 34, 11281–11291. [Google Scholar] [CrossRef] [PubMed]
- Klein, J. Hydration lubrication. Friction 2013, 1, 1–23. [Google Scholar] [CrossRef]
- Gaisinskaya, A.; Ma, L.; Silbert, G.; Sorkin, R.; Tairy, O.; Goldberg, R.; Kampf, N.; Klein, J. Hydration lubrication: Exploring a new paradigm. Faraday Discuss. 2012, 156, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Bajani, D.; Gharai, D.; Dey, J. A comparison of the self-assembly behaviour of sodium N-lauroyl sarcosinate and sodium N-lauroyl glycinate surfactants in aqueous and aqueo-organic media. J. Colloid Interface Sci. 2018, 529, 314–324. [Google Scholar] [CrossRef]
- Sułek, M.W.; Przepiórka, J.; Kulczycki, A.; Hreczuch, W. The Effect of Surfactants with Steric Hindrance on the Physicochemical and Tribological Properties of Metalworking FluidS. Tribology 2020, 290, 75–84. [Google Scholar] [CrossRef]
- Boschkova, K.; Elvesjö, J.; Kronberg, B. Frictional properties of lyotropic liquid crystalline mesophases at surfaces. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 67–77. [Google Scholar] [CrossRef]
- Sułek, M.W.; Hreczuch, W.; Przepiórka, J.; Adach, A. Solutions of Water Sterically Specific Surfactants as Model Ecological Cutting Fluids. Tribology 2018, 271, 87–95. [Google Scholar] [CrossRef]
- Paria, S.; Manohar, C.; Khilar, K.C. Effect of cationic surfactant on the adsorption characteristics of anionic surfactant on cellulose surface. Colloids Surf. A 2004, 232, 139–142. [Google Scholar] [CrossRef]
- National Center for Research and Development (Poland). PBS1/A5/28/2012-2016, Research on the Synthesis and Use of 2-Ethylhexanol Oxyalkylates as Innovative, Sterically Specific Surfactants; National Center for Research and Development (Poland); Unpublished work.
- Agafonkina, M.O.; Semiletov, A.M.; Kuznetsov, Y.I. Adsorption of sodium oleyl sarcosinate on zinc and its passivating action in a neutral aqueous solution. Prot. Met. Phys. Chem. Surf. 2018, 54, 1284–1291. [Google Scholar] [CrossRef]
- Brinksmeier, E.D.; Huesmann-Cordes, A.G.; Herrmann, C. Metalworking fluids—Mechanisms and performance, CIRP Annals—Manufacturing. Technology 2015, 64, 605–628. [Google Scholar]
- Agafonkina, M.O.; Kuznetsov, Y.I.; Andreeva, N.P. Inhibitor properties of carboxylates and their adsorption on copper from aqueous solutions. Russ. J. Phys. Chem. A 2015, 89, 1070–1076. [Google Scholar] [CrossRef]
- Patra, N.; Ray, D.; Aswal, V.K.; Ghosh, S. Exploring Physicochemical Interactions of Different Salts with SodiumN-Dodecanoyl Sarcosinate in Aqueous Solution. ACS Omega 2018, 3, 9256–9266. [Google Scholar] [CrossRef]
- PN-C-04055:1985 Petroleum Products—Determination of Resistance of Oils to Foaming; The Polish Committee for Standardization: Warszawa, Poland, 1985.
- PN-92 M-55789 (Polish Version) Research on the Corrosive Effect of Technological Liquids on Iron Alloys; The Polish Committee for Standardization: Warszawa, Poland, 1992.
- Piekoszewski, W.; Szczerek, M.; Tuszynski, W. The action of lubricants under extreme pressure conditions in a modified four-ball tester. Wear 2001, 249, 188–193. [Google Scholar] [CrossRef]
- Lanigan, R.S. Final Report on the Safety Assessment of Cocoyl Sarcosine, Lauroyl Sarcosine, Myristoyl Sarcosine, Oleoyl Sarcosine, Stearoyl Sarcosine, Sodium Cocoyl Sarcosinate, Sodium Lauroyl Sarcosinate, Sodium Myristoyl Sarcosinate, Ammonium Cocoyl Sarcosinate, and Ammonium Lauroyl Sarcosinate. Int. J. Toxicol. 2001, 20, 1–14. [Google Scholar]
- Final Amended Report. Amended Safety Assessment of Fatty Acyl Sarcosines and Sarcosinate Salts as Used in Cosmetics; Cosmetic Ingredient Review: Washington DC, DC, USA, 2016; pp. 1–19. Available online: https://www.cir-safety.org/sites/default/files/sarcos092016FAR.pdf (accessed on 19 December 2020).
- Schwarz, M.; Dado, M.; Hnilica, R.; Veverková, D. Environmental and Health Aspects of Metalworking Fluid Use. Pol. J. Environ. Stud. 2015, 24, 37–45. [Google Scholar]
- Zhu, B.-Y. Statistical mechanics approach to the general isotherm equation for adsorption of surfactant at the solid/liquid interface. J. Chem. Soc. Faraday Trans. 1992, 88, 611. [Google Scholar] [CrossRef]
- Klein, J.; Raviv, U.; Perkin, S.; Kampf, N.; Chai, L.; Giasson, S. Fluidity of water and of hydrated ions confined between solid surfaces to molecularly thin films. J. Phys. Condens. Matter 2004, 16, S5437–S5448. [Google Scholar] [CrossRef]
- PN-EN 10083-8: 2008 Advanced Quenched and Tempered Steels-Properties and Benefits of Their Application; The Polish Committee for Standardization: Warszawa, Poland, 2008.
- PN-ISO 3685 (Polish Version) Testing the Durability of Point Turning Knives; The Polish Committee for Standardization: Warszawa, Poland, 1996.
- Donose, B.C.; Vakarelski, I.U.; Higashitani, K. Silica Surfaces Lubrication by Hydrated Cations Adsorption from Electrolyte Solutions. Langmuir 2005, 21, 1834–1839. [Google Scholar] [CrossRef] [PubMed]






| Degree of Rusting | Description | Stained Area (%) |
|---|---|---|
| 0 | no corrosion | no traces of corrosion |
| 1 | traces of corrosion | maximum 3 rust spots of up to 1 mm in diameter |
| 2 | light corrosion | not more than 1%, but spots larger than for 1 |
| 3 | moderate corrosion | above 1% up to 5% |
| 4 | significant corrosion | above 5% up to 20% |
| 5 | strong corrosion | above 20% up to 50% |
| 6 | very strong corrosion | above 50% |
| Kind of Fluid | Physicochemical Properties | |||||
|---|---|---|---|---|---|---|
| б (mN/m) | θ (deg) | ν (mm2/s) | Foamability (mL) | pH (-) | Corrosivity | |
| Water 10°n | 72.4 | 81 | 1.01 | 0 | 7.4 | F3/6 |
| Commercial fluid | 35 | 42 | 0.99 | 0 | 9.6 | F3/0 |
| 2% SLS solutions with additives | 31 | 45 | 1.10 | 0 | 10 | F3/0 |
| Kind of Fluid | Tests at Constant Load T-02—2 kN | Tests at Increasing Load—T-02 | ||||
|---|---|---|---|---|---|---|
| μ (-) | d (mm) | Pt (N) | Poz (N) | poz (N/mm2) | doz (mm) | |
| Water | 0.47 | 1.8 | 200 | 4000 | 200 | 3.3 |
| Commercial fluid | 0.12 | 1.1 | 1000 | 3900 | 300 | 2.6 |
| 2% SLS solutions with additives | 0.13 | 1.0 | 1300 | 6400 | 1200 | 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sułek, M.W.; Bąk-Sowińska, A.; Przepiórka, J. Ecological Cutting Fluids. Materials 2020, 13, 5812. https://doi.org/10.3390/ma13245812
Sułek MW, Bąk-Sowińska A, Przepiórka J. Ecological Cutting Fluids. Materials. 2020; 13(24):5812. https://doi.org/10.3390/ma13245812
Chicago/Turabian StyleSułek, Marian Włodzimierz, Anna Bąk-Sowińska, and Jacek Przepiórka. 2020. "Ecological Cutting Fluids" Materials 13, no. 24: 5812. https://doi.org/10.3390/ma13245812
APA StyleSułek, M. W., Bąk-Sowińska, A., & Przepiórka, J. (2020). Ecological Cutting Fluids. Materials, 13(24), 5812. https://doi.org/10.3390/ma13245812





