Effect of Reactive SPS on the Microstructure and Properties of a Dual-Phase Ni-Al Intermetallic Compound and Ni-Al-TiB2 Composite
Abstract
1. Introduction
2. Research Procedure
- dw—the depth of the wear scar;
- t—test duration.
- V—the volume of material worn;
- N—the load applied;
- l—the sliding distance.
- ∆m = mo − mk;
- mo—the initial mass of the sample (before the friction test);
- mk—mass of the sample after the friction test.
3. Results and Discussion
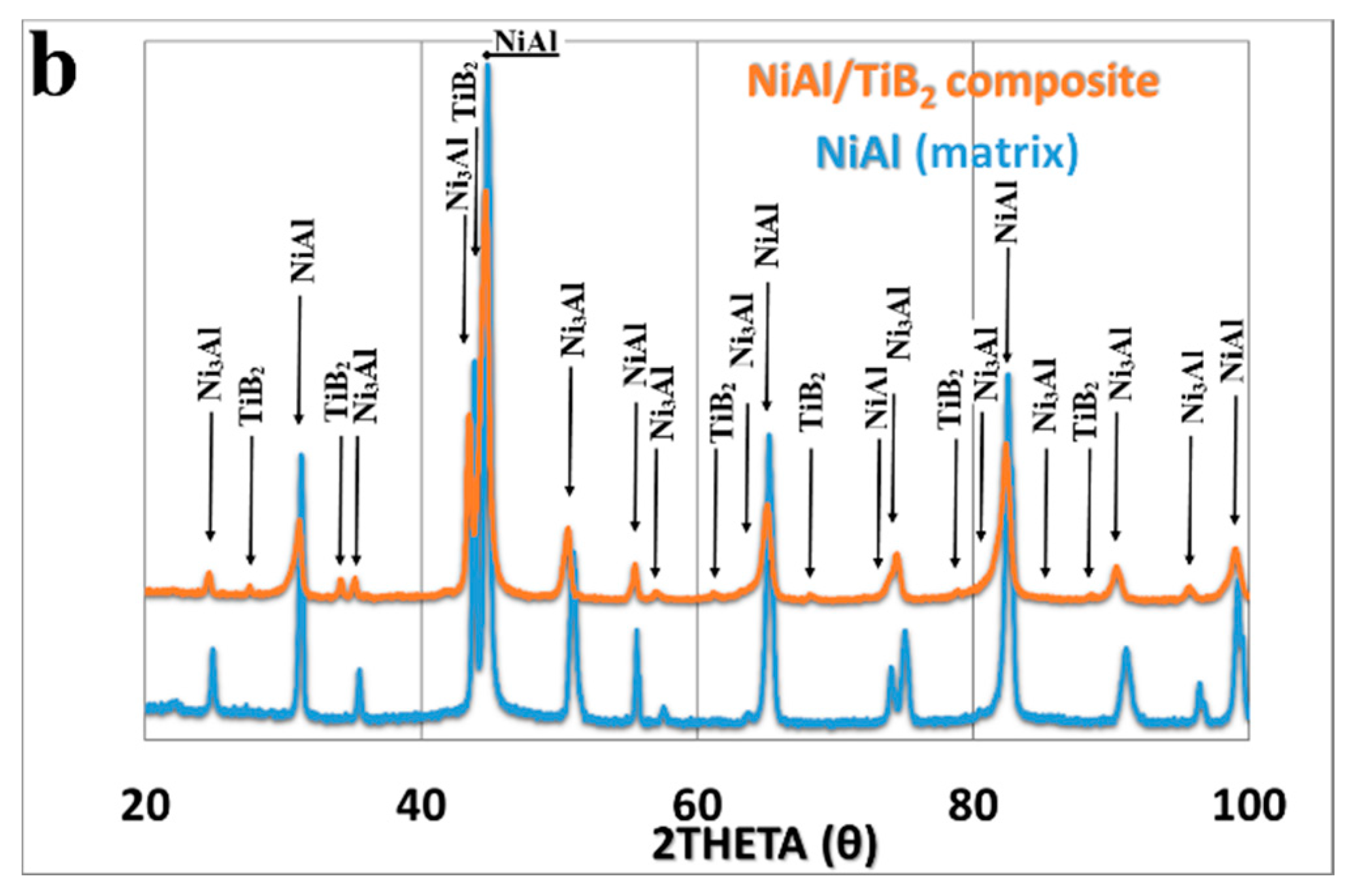
3.1. The SPS Process and Phase Identification
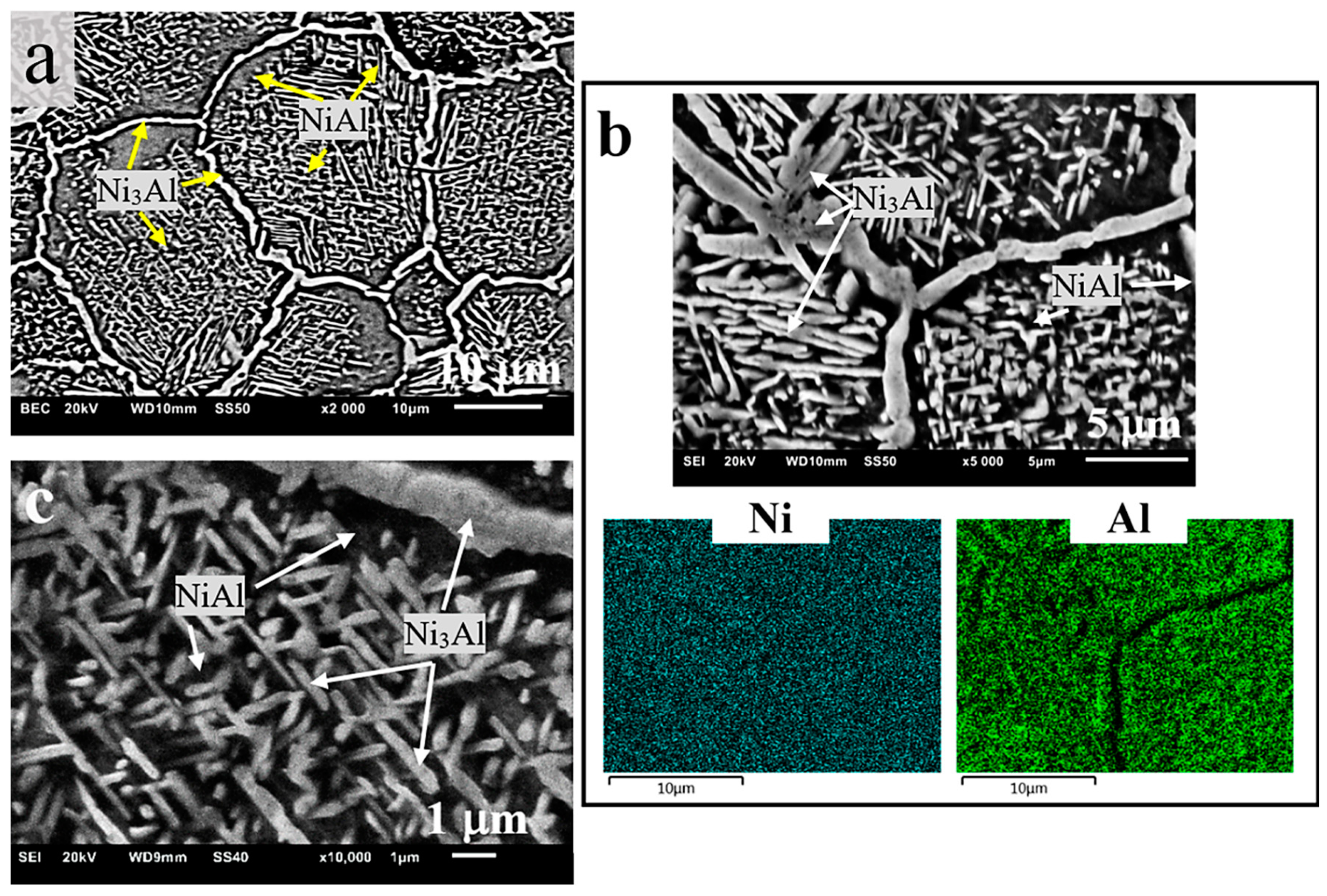
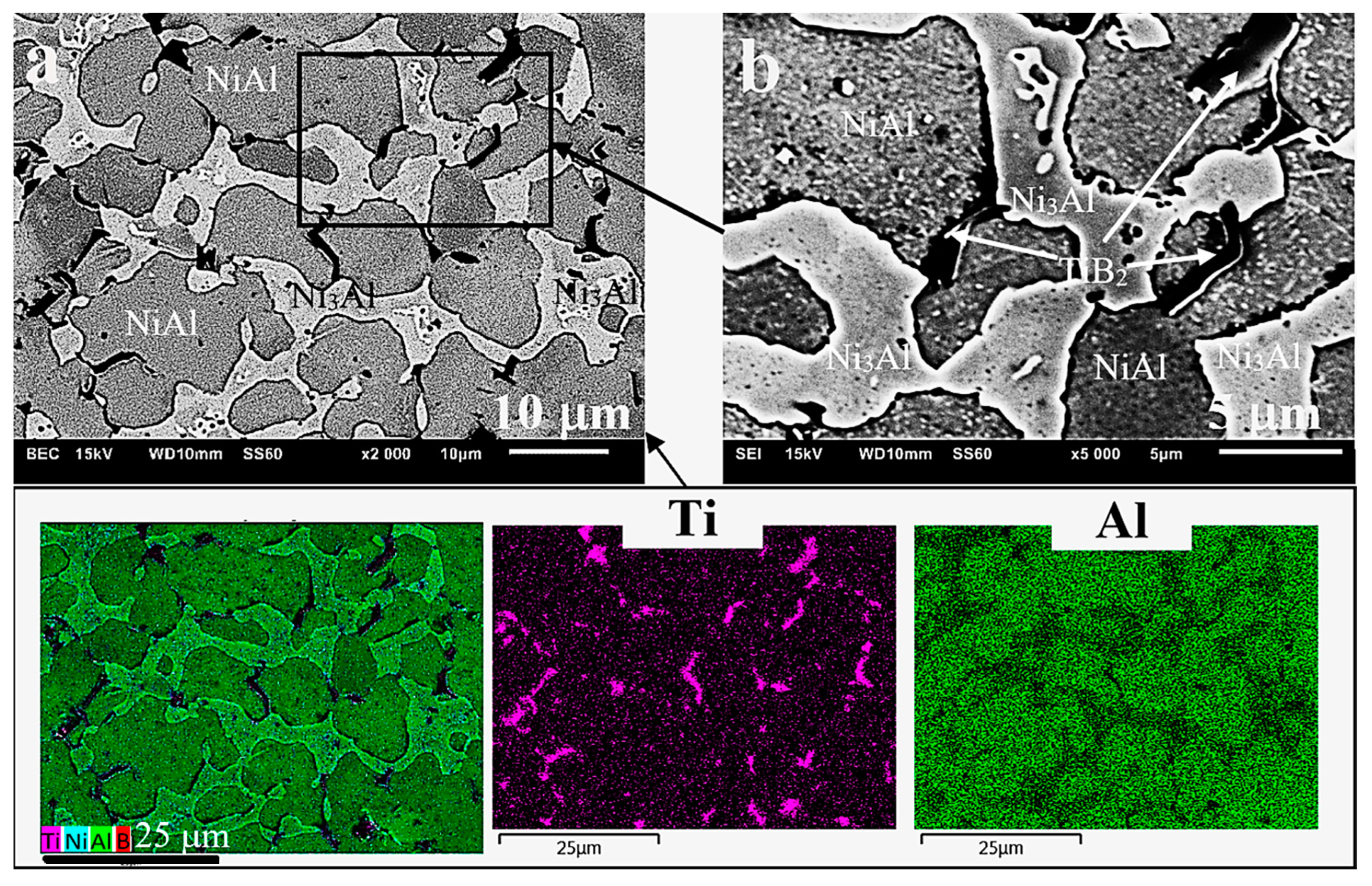
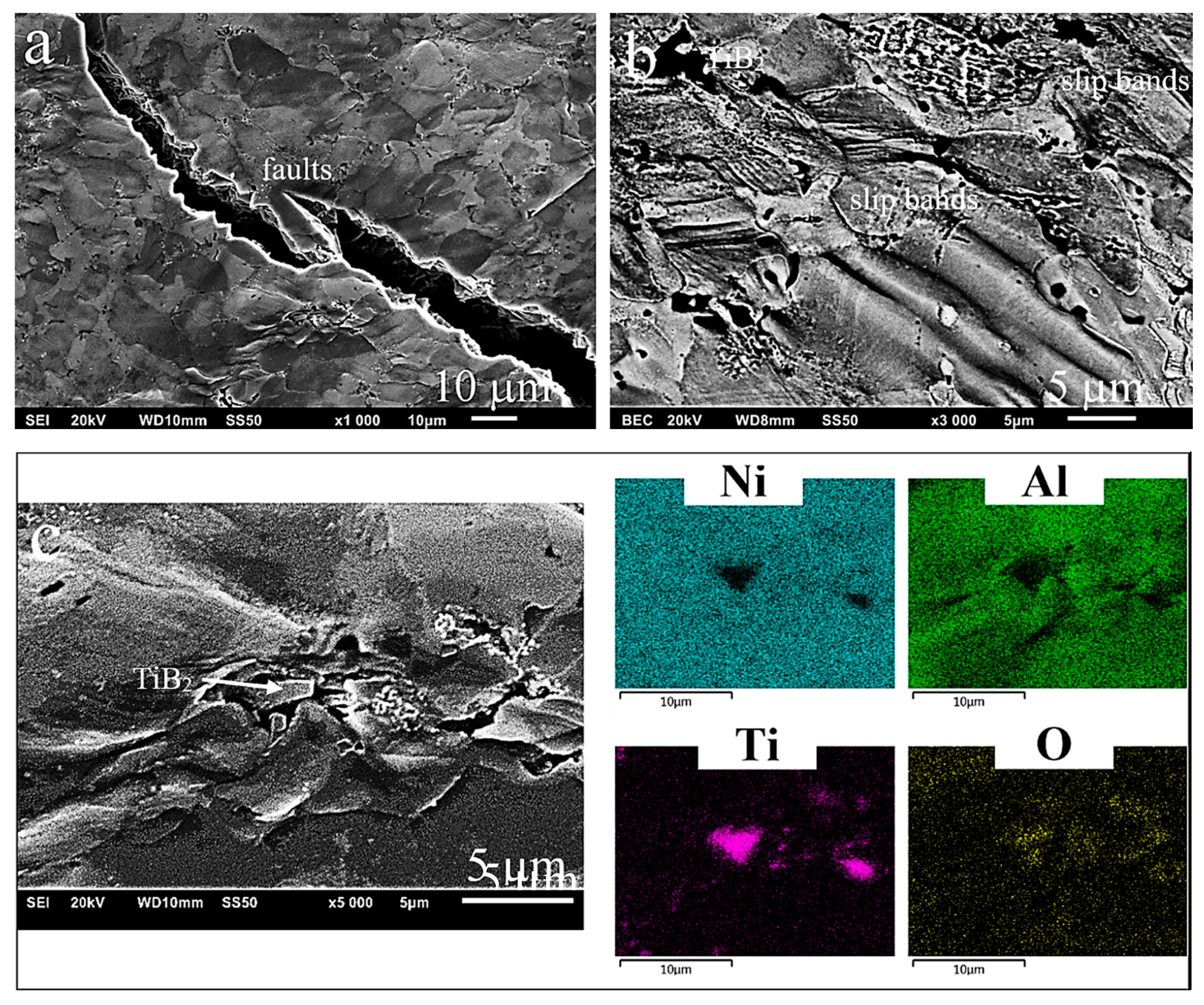
3.2. Microstructure
3.3. Evaluation of the Properties of the Produced Sinters
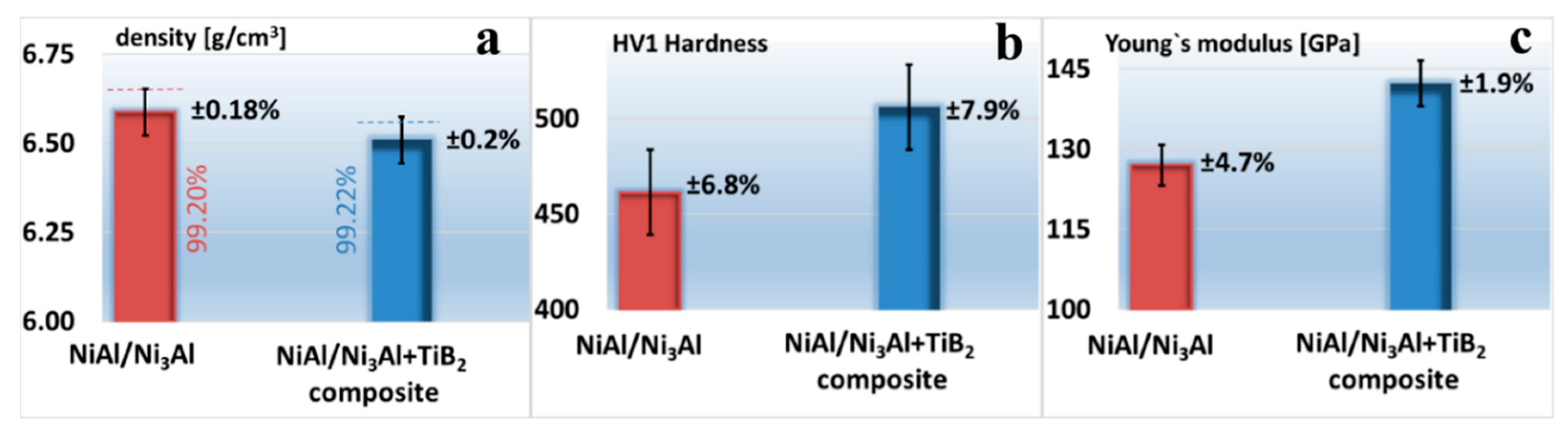
3.3.1. Density, Hardness and Young’s Modulus
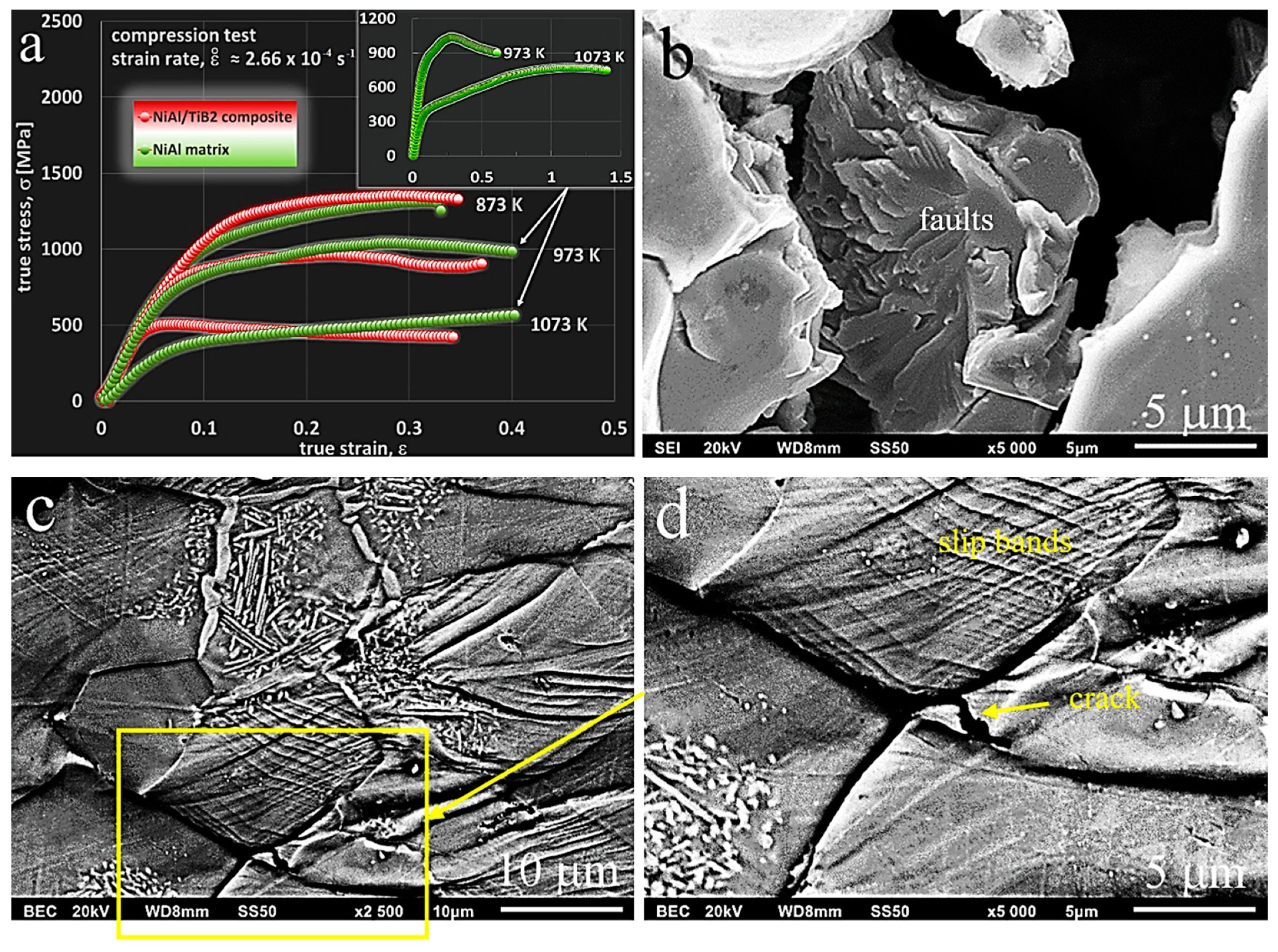
3.3.2. Room-Temperature Compression Tests
3.3.3. High-Temperature Compression Tests
3.3.4. Tribological Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miracle, D. Overview No. 104 The physical and mechanical properties of NiAl. Acta Metall. Mater. 1993, 41, 649–684. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Nathal, M.V. Physical and mechanical properties of the B2 compound NiAl. Int. Mater. Rev. 1993, 38, 193–232. [Google Scholar] [CrossRef]
- Bochenek, K.; Basista, M. Advances in processing of NiAl intermetallic alloys and composites for high temperature aerospace applications. Prog. Aerosp. Sci. 2015, 79, 136–146. [Google Scholar] [CrossRef]
- Czeppe, T.; Wierzbinski, S. Structure and mechanical properties of NiAl and Ni3Al-based alloys. Int. J. Mech. Sci. 2000, 42, 1499–1518. [Google Scholar] [CrossRef]
- Lasalmonie, A. Intermetallics: Why is it so difficult to introduce them in gas turbine engines? Intermetallics 2006, 14, 1123–1129. [Google Scholar] [CrossRef]
- Hyjek, P.; Sulima, I. Microstructure and mechanical properties of Ni-Al-Ti-B alloy. Ores Non-Ferr. Met. 2015, 60, 211–217. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Hoyle, T. Brittle-to-ductile transition in polycrystalline NiAl. Acta Mater. 1997, 45, 4193–4204. [Google Scholar] [CrossRef]
- Dongmei, J.; Dongliang, L. Superplasticity of single-phase Ni-40Al intermetallics with large grains. J. Mater. Sci. Lett. 2002, 21, 505–508. [Google Scholar] [CrossRef]
- Hu, J.; Lin, D.-L. Plastic deformation behavior in dual-phase Ni–31Al intermetallics at elevated temperature. Mater. Sci. Eng. A 2008, 490, 157–161. [Google Scholar] [CrossRef]
- Kainuma, R.; Imano, S.; Ohtani, H.; Ishida, K. Microstructural evolution in ductile (B2) + y’(L12) Ni-Al-Fe alloys. Intermetallics 1996, 4, 37–45. [Google Scholar] [CrossRef]
- Aruna, S.T.; Mukasyan, A.S. Combustion synthesis and nanomaterials. Curr. Opin. Solid State Mater. Sci. 2008, 12, 44–50. [Google Scholar] [CrossRef]
- Michalcová, A.; Vojtech, D.; Kubatík, T.F.; Novák, P.; Dvorák, P.; Svobodová, P.; Marek, I. NiAl intermetallic prepared with reactive sintering and subsequent powder-metallurgical plasma-sintering compaction. Mater. Technol. 2016, 50, 447–450. [Google Scholar]
- Saheb, N.; Iqbal, Z.; Khalil, A.; Hakeem, A.S.; Al Aqeeli, N.; Laoui, T.; Al-Qutub, A.; Kirchner, R. Spark Plasma Sintering of Metals and Metal Matrix Nanocomposites: A Review. J. Nanomater. 2012, 2012. [Google Scholar] [CrossRef]
- Collet, R.; Le Gallet, S.; Charlot, F.; Lay, S.; Chaix, J.; Bernard, F. Oxide reduction effects in SPS processing of Cu atomized powder containing oxide inclusions. Mater. Chem. Phys. 2016, 173, 498–507. [Google Scholar] [CrossRef]
- A Munir, Z.; Quach, D.V.; Ohyanagi, M. Electric Current Activation of Sintering: A Review of the Pulsed Electric Current Sintering Process. J. Am. Ceram. Soc. 2010, 94. [Google Scholar] [CrossRef]
- Suarez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Hennicke, J.; Kirchner, R.; Kessel, H.U. Challenges and Opportunities for Spark Plasma Sintering: A Key Technology for a New Generation of Materials. Sinter. Appl. 2013. [Google Scholar] [CrossRef]
- Kelly, J.P.; Graeve, O.A. Spark Plasma Sintering as an Approach to Manufacture Bulk Materials: Feasibility and Cost Savings. JOM 2014, 67, 29–33. [Google Scholar] [CrossRef]
- Morsi, K. Combustion synthesis and the electric field: A review. Int. J. Self-Propagating High-Temp. Synth. 2017, 26, 199–209. [Google Scholar] [CrossRef]
- Varma, A.; Rogachev, A.S.; Mukasyan, A.S.; Hwang, S. Combustion Synthesis of Advanced Materials: Principles and Applications. Adv. Chem. Eng. 1998, 24, 79–226. [Google Scholar] [CrossRef]
- Naplocha, K. Self-Propagating High-Temperature Synthesis (SHS) of Intermetallic Matrix Composites. In Intermetallic Matrix Composites; Elsevier: Amsterdam, The Netherlands, 2018; pp. 203–220. [Google Scholar]
- Levashov, E.A.; Mukasyan, A.S.; Rogachev, A.S.; Shtansky, D.V. Self-propagating high-temperature synthesis of advanced materials and coatings. Int. Mater. Rev. 2016, 62, 203–239. [Google Scholar] [CrossRef]
- Mukasyan, A.S.; Manukyan, K.V. Chapter 4—One- and Two-Dimensional Nanostructures Prepared by Combustion Synthesis. In Micro and Nano Technologies. Nanomaterials Synthesis; Elsevier: Amsterdam, The Netherlands, 2019; pp. 85–120. [Google Scholar] [CrossRef]
- Strutt, E.R.; Olevsky, E.A.; Meyers, M.A. Combustion synthesis/quasi-isostatic pressing of TiC-NiTi cermets: Processing and mechanical response. J. Mater. Sci. 2008, 43, 6513–6526. [Google Scholar] [CrossRef]
- Misra, A.; Gibala, R.; Noebe, R.D. Deformation and fracture behavior of a directionally solidified β/γ′ Ni-30 At. pct Al alloy. Metall. Mater. Trans. A 1999, 30, 1003–1015. [Google Scholar] [CrossRef]
- Kim, S.; Oh, M.; Hirano, T.; Wee, D. Phase transformation and microstructure of NiAl/Ni3Al alloys containing Ti. Scr. Mater. 2003, 48, 443–448. [Google Scholar] [CrossRef]
- Chen, T.-H.; Wu, J.-H. Mechanical Behavior and Fracture Properties of NiAl Intermetallic Alloy with Different Copper Contents. Appl. Sci. 2016, 6, 70. [Google Scholar] [CrossRef]
- Song, S.H.; Kim, S.H.; Hong, Y.H.; Oh, M.H.; Wee, D.M. Phase transformation and microstructural features of NiAl/Ni3Al two-phase alloys containing ternary elements. Met. Mater. Int. 2004, 10, 307–312. [Google Scholar] [CrossRef]
- Sulima, I.; Putyra, P.; Hyjek, P.; Tokarski, T. Effect of SPS parameters on densification and properties of steel matrix composites. Adv. Powder Technol. 2015, 26, 1152–1161. [Google Scholar] [CrossRef]
- Hyjek, P.; Sulima, I.; Jaworska, L. Characteristics of the NiAl/Ni3Al Matrix Composite with TiB2 Particles Fabricated By High Pressure—High Temperature Sintering. Arch. Met. Mater. 2017, 62, 1511–1520. [Google Scholar] [CrossRef]
- Philpot, K.A.; Munir, Z.A.; Holt, J.B. An investigation of the synthesis of nickel aluminides through gasless combustion. J. Mater. Sci. 1987, 22, 159–169. [Google Scholar] [CrossRef]
- Brunetti, C.; Belotti, L.; Miyoshi, M.; Pintaude, G.; D’Oliveira, A.S.C. Influence of Fe on the room and high-temperature sliding wear of NiAl coatings. Surf. Coat. Technol. 2014, 258, 160–167. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, M.C.; Lee, J.H.; Oh, M.H.; Wee, D.M. Microstructure control in two-phase (B2 + L12) Ni–Al–Fe alloys by addition of carbon. Mater. Sci. Eng. A 2002, 329, 668–674. [Google Scholar] [CrossRef]
- Cui, H.; Wei, N.; Zeng, L.-L.; Wang, X.-B.; Tang, H.-J. Microstructure and formation mechanism of Ni-Al intermetallic compounds fabricated by reaction synthesis. Trans. Nonferrous Met. Soc. China 2013, 23, 1639–1645. [Google Scholar] [CrossRef]
- Colín, J.; Serna-Barquera, S.A.; Campillo, B.; Rodríguez, R.; Juárez-Islas, J. Effect of Cu additions over the lattice parameter and hardness of the NiAl intermetallic compound. J. Alloys Compd. 2010, 489, 26–29. [Google Scholar] [CrossRef]
- Fan, Q.; Chai, H.; Jin, Z. Dissolution-precipitation mechanism of self-propagating high-temperature synthesis of mononickel aluminide. Intermetallics 2001, 9, 609–619. [Google Scholar] [CrossRef]
- Subramanan, P.R.; Mendiratta, M.G.; Miracle, D.B. Microstructures and Mechanical Behavior of NiAl-Mo and NiAl-Mo-Ti Two-Phase Alloys. Metall. Mater. Trans. A 1994, 25, 2769–2781. [Google Scholar] [CrossRef]
- Song, S.; Kim, S.; Oh, M.; Lee, J.; Wee, D.-M. Effects of ternary elements on phase transformation and microstructure of NiAl/Ni3Al two-phase alloys. Intermetallics 2005, 13, 203–210. [Google Scholar] [CrossRef]
- Wen, J.; Cui, H.; Wei, N.; Song, X.; Zhang, G.; Wang, C.; Song, Q. Effect of phase composition and microstructure on the corrosion resistance of Ni-Al intermetallic compounds. J. Alloys Compd. 2017, 695, 2424–2433. [Google Scholar] [CrossRef]
- Özdemir, Ö.; Zeytin, S.; Bindal, C. Characterization of two-phase nickel aluminides produced by pressure-assisted combustion synthesis. Vacuum 2008, 82, 311–315. [Google Scholar] [CrossRef]
- Torres, R.; Lepienski, C.M.; Moore, J.J.; Reimanis, I.E. Influence of the Processing Route in the Microstructure and Mechanical Properties of NiAl/TiB2 Composites Produced by Combustion Synthesis. Met. Mater. Trans. A 2009, 40, 187–195. [Google Scholar] [CrossRef]
- Hyjek, P.; Sulima, I.; Pałka, P.; Jaworska, L. Production of titanium diboride-reinforced NiAl/Ni3Al materials by SPS process. In Proceedings of the 25th International Conference on Metallurgy and Materials, METAL 2016, Brno, Czech Republic, 25–27 May 2016. [Google Scholar]
- Chmielewski, M.; Nosewicz, S.; Pietrzak, K.; Rojek, J.; Strojny-Nędza, A.; Mackiewicz, S.; Dutkiewicz, J. Sintering Behavior and Mechanical Properties of NiAl, Al2O3, and NiAl-Al2O3 Composites. J. Mater. Eng. Perform. 2014, 23, 3875–3886. [Google Scholar] [CrossRef]
- Liu, E.; Jia, J.; Bai, Y.; Wang, W.; Gao, Y. Study on preparation and mechanical property of nanocrystalline NiAl intermetallic. Mater. Des. 2014, 53, 596–601. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, K.; Huang, Z. The synthesis and characterization of ultrafine grain NiAl intermetallic. Adv. Powder Technol. 2012, 23, 366–371. [Google Scholar] [CrossRef]
- Hayun, S.; Ionash, E.; Kalabukhov, S.; Frage, N.; Zaretsky, E.B. Strength of ceramic–metal joints measured in planar impact experiments. J. Mater. Sci. 2018, 53, 8211–8220. [Google Scholar] [CrossRef]
- Miriyev, A.; Grützner, S.; Kruger, L.; Kalabukhov, S.; Frage, N. Bonding of TRIP-Steel/Al2O3-(3Y)-TZP Composites and (3Y)-TZP Ceramic by a Spark Plasma Sintering (SPS) Apparatus. Materials 2016, 9, 558. [Google Scholar] [CrossRef] [PubMed]
- A Olevsky, E.; Froyen, L. Impact of Thermal Diffusion on Densification during SPS. J. Am. Ceram. Soc. 2009, 92, S122–S132. [Google Scholar] [CrossRef]
- Sharma, N.; Alam, S.N.; Ray, B.C. Fundamentals of Spark Plasma Sintering (SPS): An Ideal Processing Technique for Fabrication of Metal Matrix Nanocomposites. In Spark Plasma Sintering of Materials; Cavaliere, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-Assisted Sintering Technology/Spark Plasma Sintering: Mechanisms, Materials, and Technology Developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Dudina, D.V.; Mukherjee, A. Reactive Spark Plasma Sintering: Successes and Challenges of Nanomaterial Synthesis. J. Nanomater. 2013, 2013, 625218. [Google Scholar] [CrossRef]
- Franceschin, G.; Flores-Martínez, N.; Vázquez-Victorio, G.; Ammar, S.; Valenzuela, R. Sintering and Reactive Sintering by Spark Plasma Sintering (SPS). Sinter. Funct. Mater. 2017. [Google Scholar] [CrossRef]
- Salamon, D.; Eriksson, M.; Nygren, M.; Shen, Z. Homogeneous TiB2 Ceramics Achieved by Electric Current-Assisted Self-Propagating Reaction Sintering. J. Am. Ceram. Soc. 2007, 90, 3303–3306. [Google Scholar] [CrossRef]
- Noebe, R.D.; Bowman, R.R.; Kim, J.T.; Larsen, M.; Gibala, R. The potential for ductility enhancement from surface and interface dislocation sources in NiAl. In High Temperature Aluminides and Intermetallics; Wang, S.H., Ed.; TMS: Warrendale, PA, USA, 1990; pp. 271–300. [Google Scholar]
- Zhao, H.; Qiu, F.; Jin, S.; Jiang, Q. High work-hardening effect of the pure NiAl intermetallic compound fabricated by the combustion synthesis and hot pressing technique. Mater. Lett. 2011, 65, 2604–2606. [Google Scholar] [CrossRef]
- Du, X.H.; Huang, J.C.; Wu, B.L. Compressive Mechanical Properties of NiAl/Cr(Nb) Alloy Prepared by Rapid Solidification Processing. Adv. Eng. Mater. 2007, 9, 684–688. [Google Scholar] [CrossRef]
- Bochenek, K.; Węglewski, W.; Morgiel, J.; Maj, M.; Basista, M. Enhancement of fracture toughness of hot-pressed NiAl-Re material by aluminum oxide addition. Mater. Sci. Eng. A 2020, 790, 139670. [Google Scholar] [CrossRef]
- Hyjek, P.; Sulima, I.; Jaworska, L. Application of SHS in the Manufacture of (NiAl/Ni3Al)/TiB2 Composite. Metall. Mater. Trans. A 2019, 50, 3724–3735. [Google Scholar] [CrossRef]
- Ma, J.; Fan, C.; Sun, Q.; Li, Q.; Zhu, S.; Liu, W.; Yang, J. Tribological behavior of NiAl-based intermetallic compounds in artificial seawater. Tribol. Int. 2020, 153, 106612. [Google Scholar] [CrossRef]
- Zeumer, B.; Sauthoff, G. Deformation behaviour of intermetallic NiAl-Ta alloys with strengthening Laves phase for high-temperature applications III. Effects of alloying with Cr. Intermetallics 1998, 6, 451–460. [Google Scholar] [CrossRef]
- Chen, R.; Guo, J.; Zhou, W.; Zhou, J. Brittle-to-ductile transition of a multiphase intermetallic alloy based on NiAl. Intermetallics 2000, 8, 663–667. [Google Scholar] [CrossRef]
- Sakata, T.; Yasuda, H.; Umakoshi, Y. Formation of deformation bands and precipitation of Ni 3 Al(γ′) phase in NiAl(β)-based single crystals by thermomechanical processing. Intermetallics 2002, 10, 139–147. [Google Scholar] [CrossRef]
- Tang, L.-Z.; Zhang, Z.-G.; Li, S.-S.; Gong, S.-K. Mechanical behaviors of NiAl-Cr(Mo)-based near eutectic alloy with Ti, Hf, Nb and W additions. Trans. Nonferrous Met. Soc. China 2010, 20, 212–216. [Google Scholar] [CrossRef]
- Whittenberger, J.D.; Viswanadham, R.K.; Mannan, S.K.; Sprissler, B. Elevated temperature slow plastic deformation of NiAl-TiB2 particulate composites at 1200 and 1300K. J. Mater. Sci. 1990, 25, 35–44. [Google Scholar] [CrossRef]
- Sulima, I.; Hyjek, P.; Jaworska, L.; Perek-Nowak, M. Influence of ZrB2 on Microstructure and Properties of Steel Matrix Composites Prepared by Spark Plasma Sintering. Materials 2020, 13, 2459. [Google Scholar] [CrossRef]
- Szala, M.; Dudek, A.; Maruszczyk, A.; Walczak, M.; Chmiel, J.; Kowal, M. Effect of Atmospheric Plasma Sprayed TiO2–10% NiAl Cermet Coating Thickness on Cavitation Erosion, Sliding and Abrasive Wear Resistance. Acta Phys. Pol. A 2019, 136. [Google Scholar] [CrossRef]
- Xiao, Y.; Shi, X.; Zhai, W.; Yao, J.; Xu, Z.; Chen, L.; Zhu, Q. Tribological Performance of NiAl Self-lubricating Matrix Composite with Addition of Graphene at Different Loads. J. Mater. Eng. Perform. 2015, 24, 2866–2874. [Google Scholar] [CrossRef]
- Yu, Y.; Zhou, J.; Ren, S.; Wang, L.; Xin, B.; Cao, S. Tribological properties of laser cladding NiAl intermetallic compound coatings at elevated temperatures. Tribol. Int. 2016, 104, 321–327. [Google Scholar] [CrossRef]
- Stott, F.; Wood, G. The influence of oxides on the friction and wear of alloys. Tribol. Int. 1978, 11, 211–218. [Google Scholar] [CrossRef]
- Zhu, S.; Bi, Q.; Niu, M.; Yang, J.; Liu, W. Tribological behavior of NiAl matrix composites with addition of oxides at high temperatures. Wear 2012, 274–275, 423–434. [Google Scholar] [CrossRef]





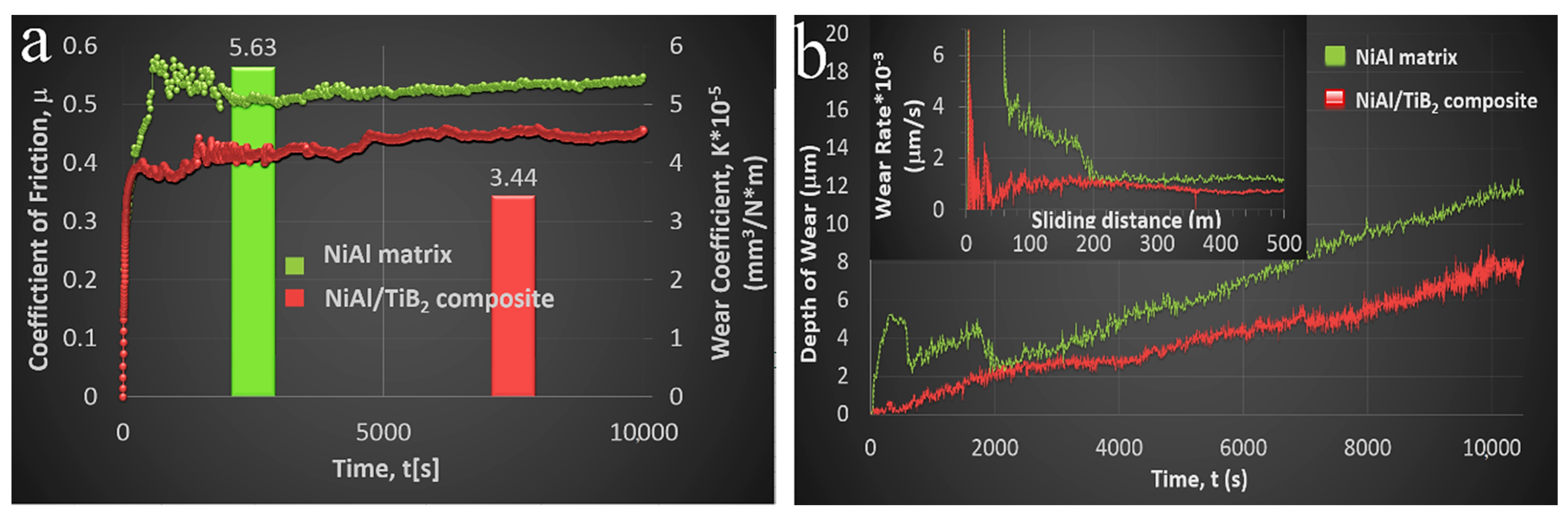



| Load Applied [N] | Radius of the Wear Track [mm] | RPM | Ball Parameters | Chamber Parameters | Test Duration [s] | ||
|---|---|---|---|---|---|---|---|
| Temp. [K] | Humidity [%] | Medium | |||||
| 5.0 | 5.0 | 192.0 | Material—Al2O3 Diameter-1/8” (in) | 298 | 38.2 | Air | 10,000 |
| Material | Test Temperature [K] | Compressive Offset Yield Strength σc0.2 [MPa] | Compressive Strength for Max. True Stress/def. Level σcmax [MPa]/ɛc | Compressive Strength σc [MPa] | Plastic Deformation Ac [%] |
|---|---|---|---|---|---|
| NiAl/Ni3Al matrix | 296 | 1150 | 3105/0.43 | 3207 | 34.2 |
| 873 | 1130 | 1830/0.32 | 1842 | 25 | |
| 973 | 800 | 1395/0.28 | not destroyed | not destroyed | |
| 1073 | 390 | 2377/1.12 | not destroyed | not destroyed | |
| NiAl/Ni3Al + TiB2 composite | 296 | 1340 | 3024/0.33 | 3079 | 27.8 |
| 873 | 1270 | 1816/0.29 | 1891 | 23.9 | |
| 973 | 890 | 1210/0.22 | not destroyed | not destroyed | |
| 1073 | 480 | 534/0.07 | not destroyed | not destroyed |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyjek, P.; Sulima, I.; Malczewski, P.; Bryła, K.; Jaworska, L. Effect of Reactive SPS on the Microstructure and Properties of a Dual-Phase Ni-Al Intermetallic Compound and Ni-Al-TiB2 Composite. Materials 2020, 13, 5668. https://doi.org/10.3390/ma13245668
Hyjek P, Sulima I, Malczewski P, Bryła K, Jaworska L. Effect of Reactive SPS on the Microstructure and Properties of a Dual-Phase Ni-Al Intermetallic Compound and Ni-Al-TiB2 Composite. Materials. 2020; 13(24):5668. https://doi.org/10.3390/ma13245668
Chicago/Turabian StyleHyjek, Paweł, Iwona Sulima, Piotr Malczewski, Krzysztof Bryła, and Lucyna Jaworska. 2020. "Effect of Reactive SPS on the Microstructure and Properties of a Dual-Phase Ni-Al Intermetallic Compound and Ni-Al-TiB2 Composite" Materials 13, no. 24: 5668. https://doi.org/10.3390/ma13245668
APA StyleHyjek, P., Sulima, I., Malczewski, P., Bryła, K., & Jaworska, L. (2020). Effect of Reactive SPS on the Microstructure and Properties of a Dual-Phase Ni-Al Intermetallic Compound and Ni-Al-TiB2 Composite. Materials, 13(24), 5668. https://doi.org/10.3390/ma13245668







