Striped Non-Uniform Corrosion Behavior of Non-Equiatomic FeMnCoCr High-Entropy Alloy Prepared by Laser Melting Deposition in 0.1 M H2SO4 Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.2. Electrochemical Measurements
2.3. Sample Characterisation
3. Results
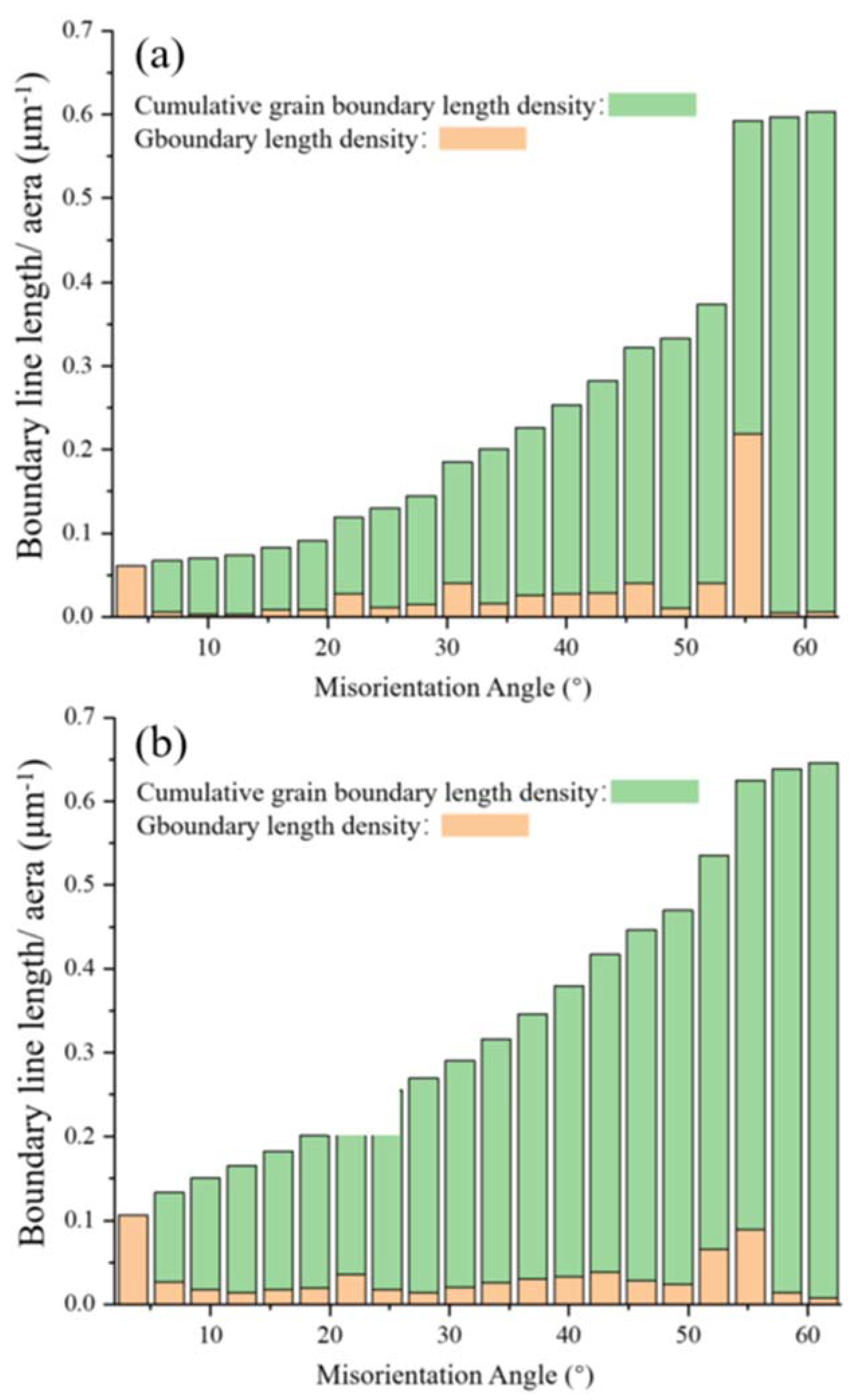
3.1. Characterisation of the Initial Microstructure
3.2. Electrochemical Analyses
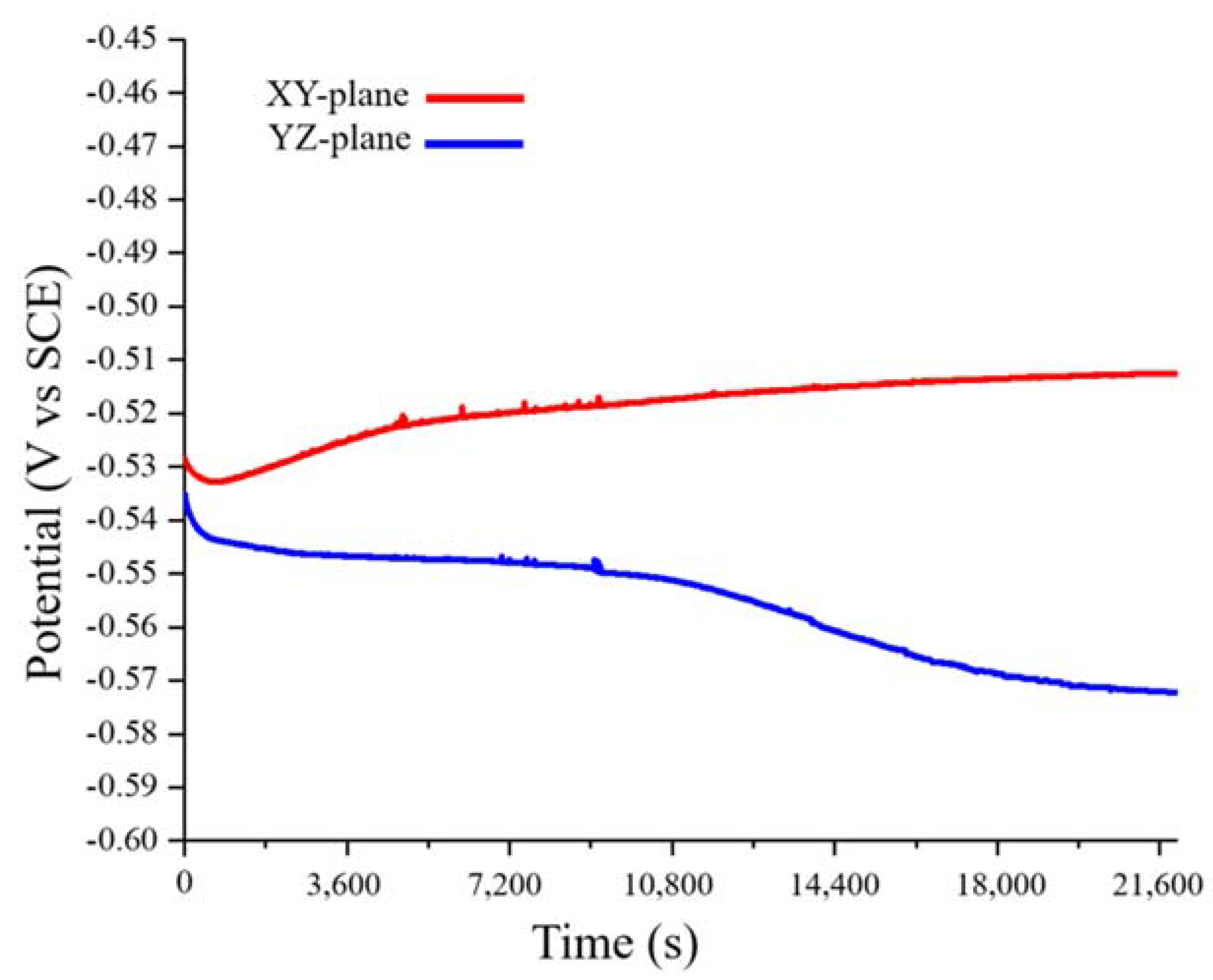
3.2.1. Open Circuit Potential Measurement
3.2.2. Potentiodynamic Polarization
3.2.3. Electrochemical Impedance Spectroscopy
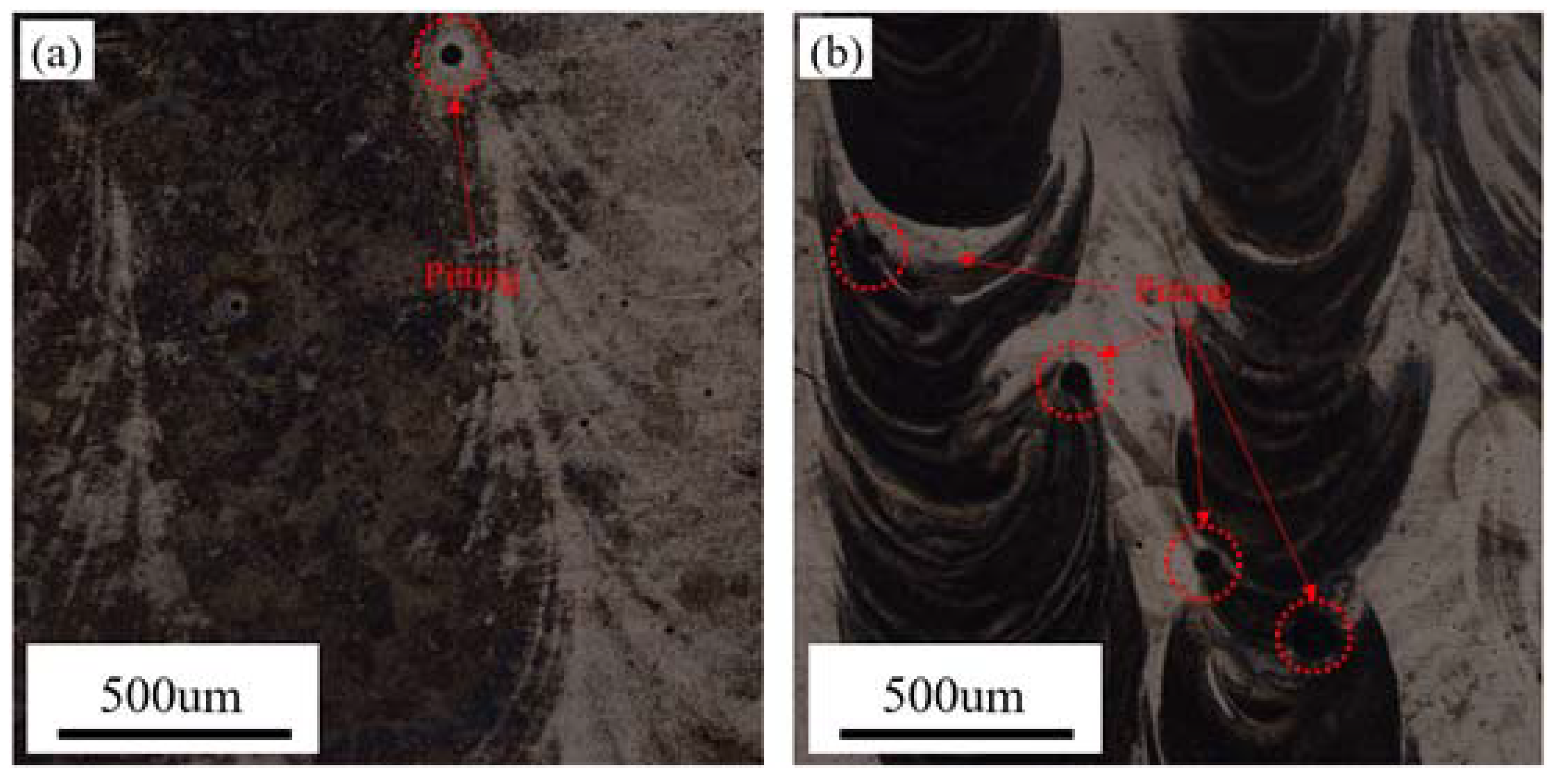
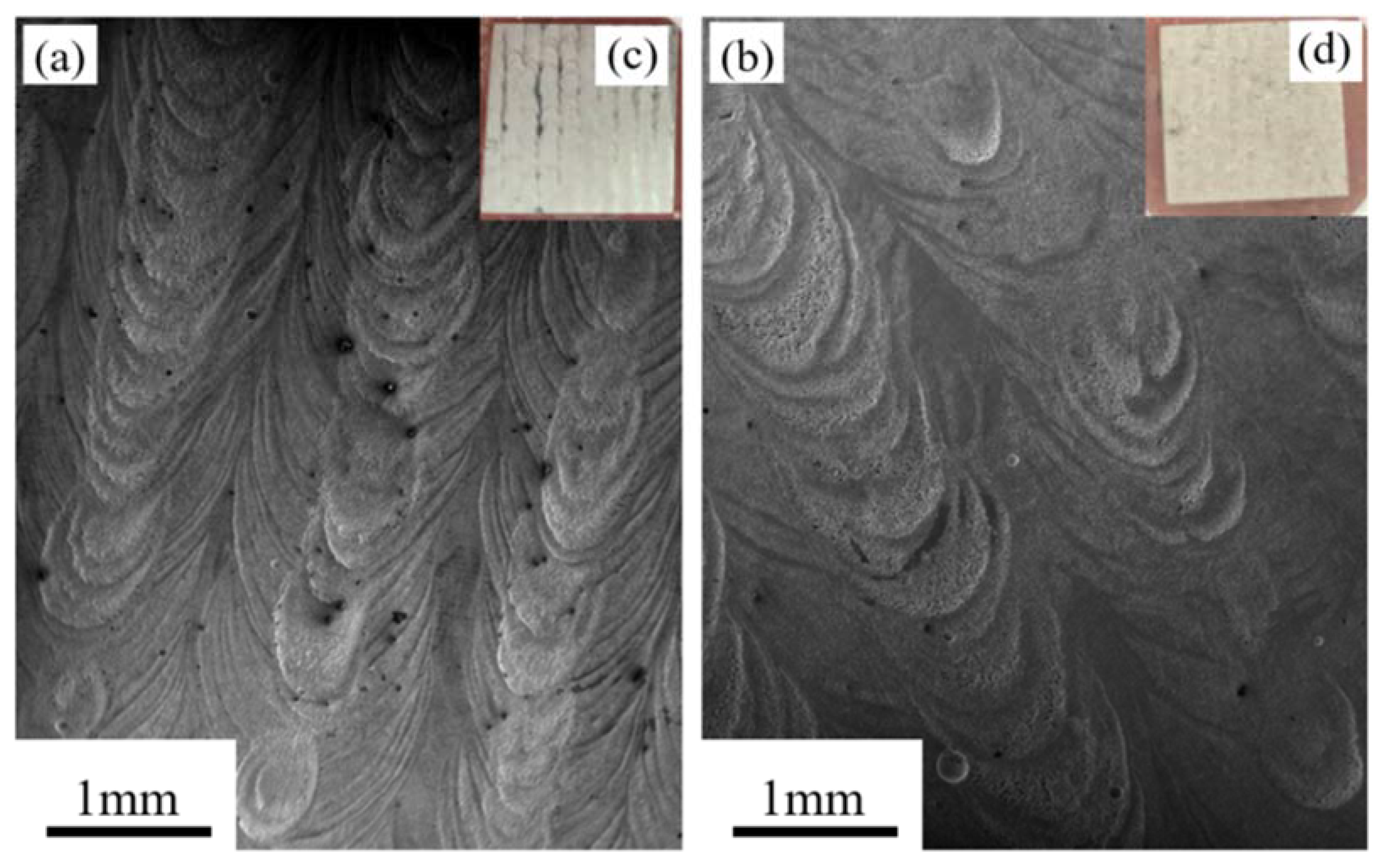
3.3. Morphology of the Corroded Surfaces
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Data Availability
References
- Quiambao, K.F.; McDonnell, S.J.; Schreiber, D.K.; Gerard, A.Y.; Freedy, K.M.; Lu, P.; Saal, J.E.; Frankel, G.S.; Scully, J.R. Passivation of a corrosion resistant high entropy alloy in non-oxidizing sulfate solutions. Acta Mater. 2019, 164, 362–376. [Google Scholar] [CrossRef]
- Park, N.; Lee, B.-J.; Tsuji, N. The phase stability of equiatomic CoCrFeMnNi high-entropy alloy: Comparison between experiment and calculation results. J. Alloys Compd. 2017, 719, 189–193. [Google Scholar] [CrossRef]
- Jodi, D.E.; Park, J.; Park, N. Precipitate behavior in nitrogen-containing CoCrNi medium-entropy alloys. Mater. Charact. 2019, 157, 109888. [Google Scholar] [CrossRef]
- Santodonato, L.J.; Zhang, Y.; Feygenson, M.; Parish, C.M.; Gao, M.C.; Weber, R.J.K.; Neuefeind, J.C.; Tang, Z.; Liaw, P.K. Deviation from high-entropy configurations in the atomic distributions of a multi-principal-element alloy. Nat. Commun. 2015, 6, 5964. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Mao, M.M.; Wang, J.; Gludovatz, B.; Zhang, Z.; Mao, S.X.; George, E.P.; Yu, Q.; Ritchie, R.O. Nanoscale origins of the damage tolerance of the high-entropy alloy CrMnFeCoNi. Nat. Commun. 2015, 6, 10143. [Google Scholar] [CrossRef]
- Gludovatz, B.; Hohenwarter, A.; Catoor, D.; Chang, E.H.; George, E.P.; Ritchie, R.O. A fracture-resistant high-entropy alloy for cryogenic applications. Science 2014, 345, 1153–1158. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yuan, T.; Tsai, C.W.; Yeh, J.W.; Lundin, C.D.; Liaw, P.K. Fatigue behavior of a wrought Al0.5CoCrCuFeNi two-phase high-entropy alloy. Acta Mater. 2015, 99, 247–258. [Google Scholar] [CrossRef]
- Li, Z.; Pradeep, K.G.; Deng, Y.; Raabe, D.; Tasan, C.C. Metastable high-entropy dual-phase alloys overcome the strength–ductility trade-off. Nature 2016, 534, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.N.; Senkova, S.V.; Woodward, C. Effect of aluminum on the microstructure and properties of two refractory high-entropy alloys. Acta Mater. 2014, 68, 214–228. [Google Scholar] [CrossRef]
- Hemphill, M.A.; Yuan, T.; Wang, G.Y.; Yeh, J.W.; Tsai, C.W.; Chuang, A.; Liaw, P.K. Fatigue behavior of Al0.5CoCrCuFeNi high entropy alloys. Acta Mater. 2012, 60, 5723–5734. [Google Scholar] [CrossRef]
- Su, J.; Raabe, D.; Li, Z. Hierarchical microstructure design to tune the mechanical behavior of an interstitial TRIP-TWIP high-entropy alloy. Acta Mater. 2019, 163, 40–54. [Google Scholar] [CrossRef]
- Li, Z.; Tasan, C.C.; Pradeep, K.G.; Raabe, D. A TRIP-assisted dual-phase high-entropy alloy: Grain size and phase fraction effects on deformation behavior. Acta Mater. 2017, 131, 323–335. [Google Scholar] [CrossRef]
- Bu, Y.; Li, Z.; Liu, J.; Wang, H.; Raabe, D.; Yang, W. Nonbasal Slip Systems Enable a Strong and Ductile Hexagonal-Close-Packed High-Entropy Phase. Phys. Rev. Lett. 2019, 122, 075502. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, Y. Thermodynamics-kinetics of twinning/martensitic transformation in Fe50Mn30Co10Cr10 high-entropy alloy during adiabatic shearing. Scr. Mater. 2020, 181, 115–120. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Z.; He, J.; Luan, J.; Wang, Z.; Liu, B.; Liu, Y.; Chen, M.; Liu, C. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Santo, L. Laser cladding of metals: A review. Int. J. Surf. Sci. Eng. 2008, 2, 327. [Google Scholar] [CrossRef]
- Guo, P.; Zou, B.; Huang, C.; Gao, H. Study on microstructure, mechanical properties and machinability of efficiently additive manufactured AISI 316L stainless steel by high-power direct laser deposition. J. Mater. Process. Technol. 2017, 240, 12–22. [Google Scholar] [CrossRef]
- Olakanmi, E.O.; Cochrane, R.F.; Dalgarno, K.W. A review on selective laser sintering/melting (SLS/SLM) of aluminium alloy powders: Processing, microstructure, and properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Garibaldi, M.; Ashcroft, I.; Simonelli, M.; Hague, R. Metallurgy of high-silicon steel parts produced using Selective Laser Melting. Acta Mater. 2016, 110, 207–216. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Elwany, A.; Yadollahi, A.; Thompson, S.M.; Bian, L.; Shamsaei, N. Mechanical properties and microstructural characterization of selective laser melted 17-4 PH stainless steel. Rapid Prototyp. J. 2017, 23, 280–294. [Google Scholar] [CrossRef]
- Li, S.; Hassanin, H.; Attallah, M.M.; Adkins, N.J.E.; Essa, K. The development of TiNi-based negative Poisson’s ratio structure using selective laser melting. Acta Mater. 2016, 105, 75–83. [Google Scholar] [CrossRef]
- El-Desouky, A.; Carter, M.; Mahmoudi, M.; Elwany, A.; Leblanc, S. Influences of energy density on microstructure and consolidation of selective laser melted bismuth telluride thermoelectric powder. J. Manuf. Process. 2017, 25, 411–417. [Google Scholar] [CrossRef]
- Jia, Q.; Gu, D. Selective laser melting additive manufacturing of Inconel 718 superalloy parts: Densification, microstructure and properties. J. Alloys Compd. 2014, 585, 713–721. [Google Scholar] [CrossRef]
- Franco, B.E.; Ma, J.; Loveall, B.; Tapia, G.A.; Karayagiz, K.; Liu, J.; Elwany, A.; Arroyave, R.; Karaman, I. A Sensory Material Approach for Reducing Variability in Additively Manufactured Metal Parts. Sci. Rep. 2017, 7, 3604. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Qin, Z.; Dong, P.; Mi, Y.; Gong, D.; Li, W. Microstructure and corrosion properties of FeCoNiCrMn high entropy alloy coatings prepared by high speed laser cladding and ultrasonic surface mechanical rolling treatment. Mater. Lett. 2020, 259, 126769. [Google Scholar] [CrossRef]
- Kuwabara, K.; Shiratori, H.; Fujieda, T.; Yamanaka, K.; Koizumi, Y.; Chiba, A. Mechanical and corrosion properties of AlCoCrFeNi high-entropy alloy fabricated with selective electron beam melting. Addit. Manuf. 2018, 23, 264–271. [Google Scholar] [CrossRef]
- Fujieda, T.; Chen, M.; Shiratori, H.; Kuwabara, K.; Yamanaka, K.; Koizumi, Y.; Chiba, A.; Watanabe, S. Mechanical and corrosion properties of CoCrFeNiTi-based high-entropy alloy additive manufactured using selective laser melting. Addit. Manuf. 2019, 25, 412–420. [Google Scholar] [CrossRef]
- Kumar, N.; Fusco, M.; Komarasamy, M.; Mishra, R.S.; Bourham, M.; Murty, K.L. Understanding effect of 3.5 wt.% NaCl on the corrosion of Al0.1CoCrFeNi high-entropy alloy. J. Nucl. Mater. 2017, 495, 154–163. [Google Scholar] [CrossRef]
- Li, T.; Swanson, O.J.; Frankel, G.S.; Gerard, A.Y.; Lu, P.; Saal, J.E.; Scully, J.R. Localized corrosion behavior of a single-phase non-equimolar high entropy alloy. Electrochim. Acta 2019, 306, 71–84. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Xu, H.; Zang, J.; Yuan, Y.; Zhou, Y.; Tian, P.; Wang, Y. In-situ assembly from graphene encapsulated CoCrFeMnNi high-entropy alloy nanoparticles for improvement corrosion resistance and mechanical properties in metal matrix composites. J. Alloys Compd. 2019, 811, 152082. [Google Scholar] [CrossRef]
- Shi, Y.; Collins, L.; Feng, R.; Zhang, C.; Balke, N.; Liaw, P.K.; Yang, B. Homogenization of AlxCoCrFeNi high-entropy alloys with improved corrosion resistance. Corros. Sci. 2018, 133, 120–131. [Google Scholar] [CrossRef]
- Agustianingrum, M.P.; Latief, F.H.; Park, N.; Lee, U. Thermal oxidation characteristics of Fex(CoCrMnNi)100-x medium and high-entropy alloys. Intermetallics 2020, 120, 106757. [Google Scholar] [CrossRef]
- Hiromoto, S.; Onodera, E.; Chiba, A.; Asami, K.; Hanawa, T. Microstructure and corrosion behaviour in biological environments of the new forged low-Ni Co-Cr-Mo alloys. Biomaterials 2005, 26, 4912–4923. [Google Scholar] [CrossRef]
- Wang, N.; Wang, R.; Feng, Y.; Xiong, W.; Zhang, J.; Deng, M. Discharge and corrosion behaviour of Mg-Li-Al-Ce-Y-Zn alloy as the anode for Mg-air battery. Corros. Sci. 2016, 112, 13–24. [Google Scholar] [CrossRef]
- Brito, C.; Vida, T.; Freitas, E.S.; Cheung, N.; Spinelli, J.E.; Garcia, A. Cellular/dendritic arrays and intermetallic phases affecting corrosion and mechanical resistances of an Al-Mg-Si alloy. J. Alloys Compd. 2016, 673, 220–230. [Google Scholar] [CrossRef]
- Rehim, S.S.A.; Hassan, H.H.; Amin, M.A. Corrosion and corrosion inhibition of Al and some alloys in sulphate solutions containing halide ions investigated by an impedance technique. Appl. Surf. Sci. 2002, 187, 279–290. [Google Scholar] [CrossRef]
- Barcia, O.E. Mass-Transport Study for the Electrodissolution of Copper in 1M Hydrochloric Acid Solution by Impedance. J. Electrochem. Soc. 1993, 140, 2825–2832. [Google Scholar] [CrossRef]
- Deslouis, C.; Tribollet, B.; Mengoli, G.; Musiani, M.M. Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation. J. Appl. Electrochem. 1988, 18, 374–383. [Google Scholar] [CrossRef]
- Veloz, M.; González, I. Electrochemical study of carbon steel corrosion in buffered acetic acid solutions with chlorides and H2S. Electrochim. Acta 2002, 48, 135–144. [Google Scholar] [CrossRef]
- Amin, M.A.; Abd El-Rehim, S.S.; El-Sherbini, E.E.F.; Bayoumi, R.S. The inhibition of low carbon steel corrosion in hydrochloric acid solutions by succinic acid. Part I. Weight loss, polarization, EIS, PZC, EDX and SEM studies. Electrochim. Acta 2007, 52, 3588–3600. [Google Scholar] [CrossRef]
- Ye, Q.; Feng, K.; Li, Z.; Lu, F.; Li, R.; Huang, J.; Wu, Y. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Lins, V.D.F.C.; de Andrade Reis, G.F.; de Araujo, C.R.; Matencio, T. Electrochemical impedance spectroscopy and linear polarization applied to evaluation of porosity of phosphate conversion coatings on electrogalvanized steels. Appl. Surf. Sci. 2006, 253, 2875–2884. [Google Scholar] [CrossRef]
- Jägle, E.A.; Choi, P.-P.; Van Humbeeck, J.; Raabe, D. Precipitation and austenite reversion behavior of a maraging steel produced by selective laser melting. J. Mater. Res. 2014, 29, 2072–2079. [Google Scholar] [CrossRef]
- Kelly, S.M.; Kampe, S.L. Microstructural evolution in laser-deposited multilayer Ti-6Al-4V builds: Part II. Thermal Modeling. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2004, 35, 1869–1879. [Google Scholar] [CrossRef]
- Inturi, R.B.; Szklarska-Smialowska, Z. Dependence of the pitting potential of Al alloys on solubility of alloying element oxides. Corros. Sci. 1993, 34, 705–710. [Google Scholar] [CrossRef]
- Afshari, V.; Dehghanian, C. Effects of grain size on the electrochemical corrosion behaviour of electrodeposited nanocrystalline Fe coatings in alkaline solution. Corros. Sci. 2009, 51, 1844–1849. [Google Scholar] [CrossRef]
- Wang, Z.; Palmer, T.A.; Beese, A.M. Effect of processing parameters on microstructure and tensile properties of austenitic stainless steel 304L made by directed energy deposition additive manufacturing. Acta Mater. 2016, 110, 226–235. [Google Scholar] [CrossRef]
- Pokharel, R.; Balogh, L.; Brown, D.W.; Clausen, B.; Gray, G.T.; Livescu, V.; Vogel, S.C.; Takajo, S. Signatures of the unique microstructure of additively manufactured steel observed via diffraction. Scr. Mater. 2018, 155, 16–20. [Google Scholar] [CrossRef]
- Pham, M.S.; Dovgyy, B.; Hooper, P.A. Twinning induced plasticity in austenitic stainless steel 316 L made by additive manufacturing. Mater. Sci. Eng. A 2017, 704, 102–111. [Google Scholar] [CrossRef]
- Wang, S.; Shen, C.B.; Long, K.; Yang, H.Y.; Wang, F.H.; Zhang, Z.D. Preparation and electrochemical corrosion behavior of bulk nanocrystalline ingot iron in HCl acid solution. J. Phys. Chem. B 2005, 109, 2499–2503. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.G.; Shen, C.B.; Long, K.; Zhang, T.; Wang, F.H.; Zhang, Z.D. The electrochemical corrosion of bulk nanocrystalline ingot iron in acidic sulfate solution. J. Phys. Chem. B 2006, 110, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Zeiger, W.; Schneider, M.; Worch, H. Passivity and pitting corrosion of a nanocrystalline FeAl8-alloy. Mater. Sci. Forum 1998, 269, 833–836. [Google Scholar] [CrossRef]
- Hadzima, B.; Janeček, M.; Estrin, Y.; Kim, H.S. Microstructure and corrosion properties of ultrafine-grained interstitial free steel. Mater. Sci. Eng. A 2007, 462, 243–247. [Google Scholar] [CrossRef]
- El Kedim, O.; Cao, H.S.; Fluzin, P. Hardness and corrosion performance of nanocrystalline iron powder prepared by ball milling: Comparison with different iron obtained by conventional methods. Mater. Sci. Forum 1999, 312, 635–640. [Google Scholar] [CrossRef]
- Alvarez, M.G.; Vazquez, S.M.; Moya, J.; Sirkin, H. Anodic behaviour of Fe73.5Si13.5−xAlxB9Nb3Cu1 (x = 0–2) amorphous, nanostructured and crystalline alloys. Scr. Mater. 2001, 44, 507–512. [Google Scholar] [CrossRef]
- Pisarek, M.; Kędzierzawski, P.; Janik-Czachor, M.; Kurzydłowski, K.J. Effect of hydrostatic extrusion on passivity breakdown on 303 austenitic stainless steel in chloride solution. J. Solid State Electrochem. 2008, 13, 283–291. [Google Scholar] [CrossRef]
- Li, Y.; Wang, F.; Liu, G. Grain size effect on the electrochemical corrosion behavior of surface nanocrystallized low-carbon steel. Corrosion 2004, 60, 891–896. [Google Scholar] [CrossRef]
- Hao, Y.; Deng, B.; Zhong, C.; Jiang, Y.M.; Li, J. Effect of surface mechanical attrition treatment on corrosion behavior of 316 stainless steel. J. Iron Steel Res. Int. 2009, 16, 68–72. [Google Scholar] [CrossRef]
- Zeiger, W.; Schneider, M.; Scharnweber, D.; Worch, H. Corrosion behaviour of a nanocrystalline FeA18 alloy. Nanostruct. Mater. 1995, 6, 1013–1016. [Google Scholar] [CrossRef]
- Zhou, C.; Hu, S.; Shi, Q.; Tao, H.; Song, Y.; Zheng, J.; Xu, P.; Zhang, L. Improvement of corrosion resistance of SS316L manufactured by selective laser melting through subcritical annealing. Corros. Sci. 2020, 164, 108353. [Google Scholar] [CrossRef]
- Foroulis, Z.A.; Uhlig, H.H. Effect of Cold-Work on Corrosion of Iron and Steel in Hydrochloric Acid. J. Electrochem. Soc. 1964, 111, 522–528. [Google Scholar] [CrossRef]
- Goldstein, E.M. The Corrosion and Oxidation of Metals: Scientific Principles and Practical Applications (Evans, Ulick R.). J. Chem. Educ. 1960, 37, 662. [Google Scholar] [CrossRef]
- Greene, N.D.; Saltzman, G.A. Effect of Plastic Deformation on the Corrosion of Iron and Steel. Corrosion 1964, 20, 293t–298t. [Google Scholar] [CrossRef]
- Salvati, E.; Lunt, A.J.G.; Ying, S.; Sui, T.; Zhang, H.J.; Heason, C.; Baxter, G.; Korsunsky, A.M. Eigenstrain reconstruction of residual strains in an additively manufactured and shot peened nickel superalloy compressor blade. Comput. Methods Appl. Mech. Eng. 2017, 320, 335–351. [Google Scholar] [CrossRef]
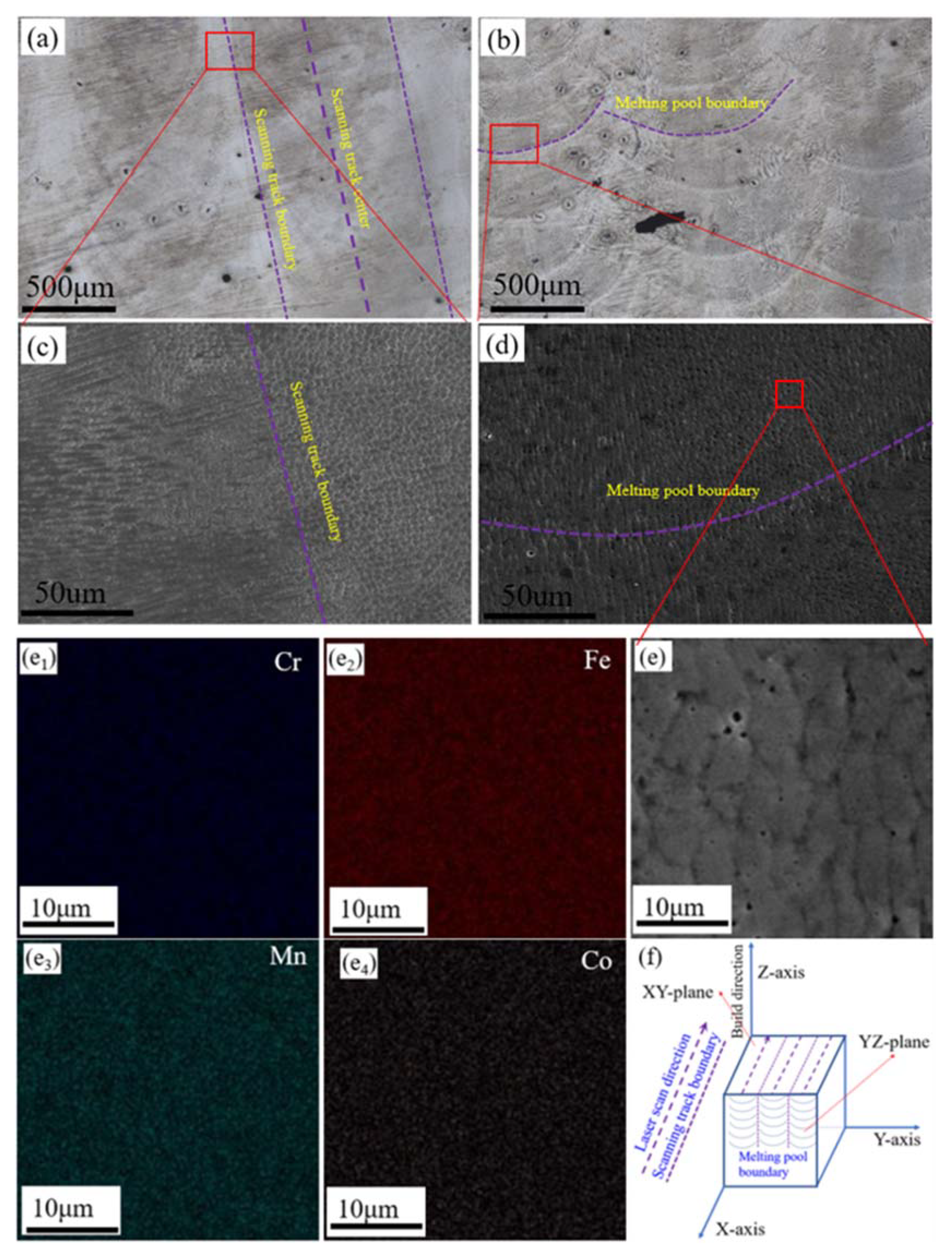
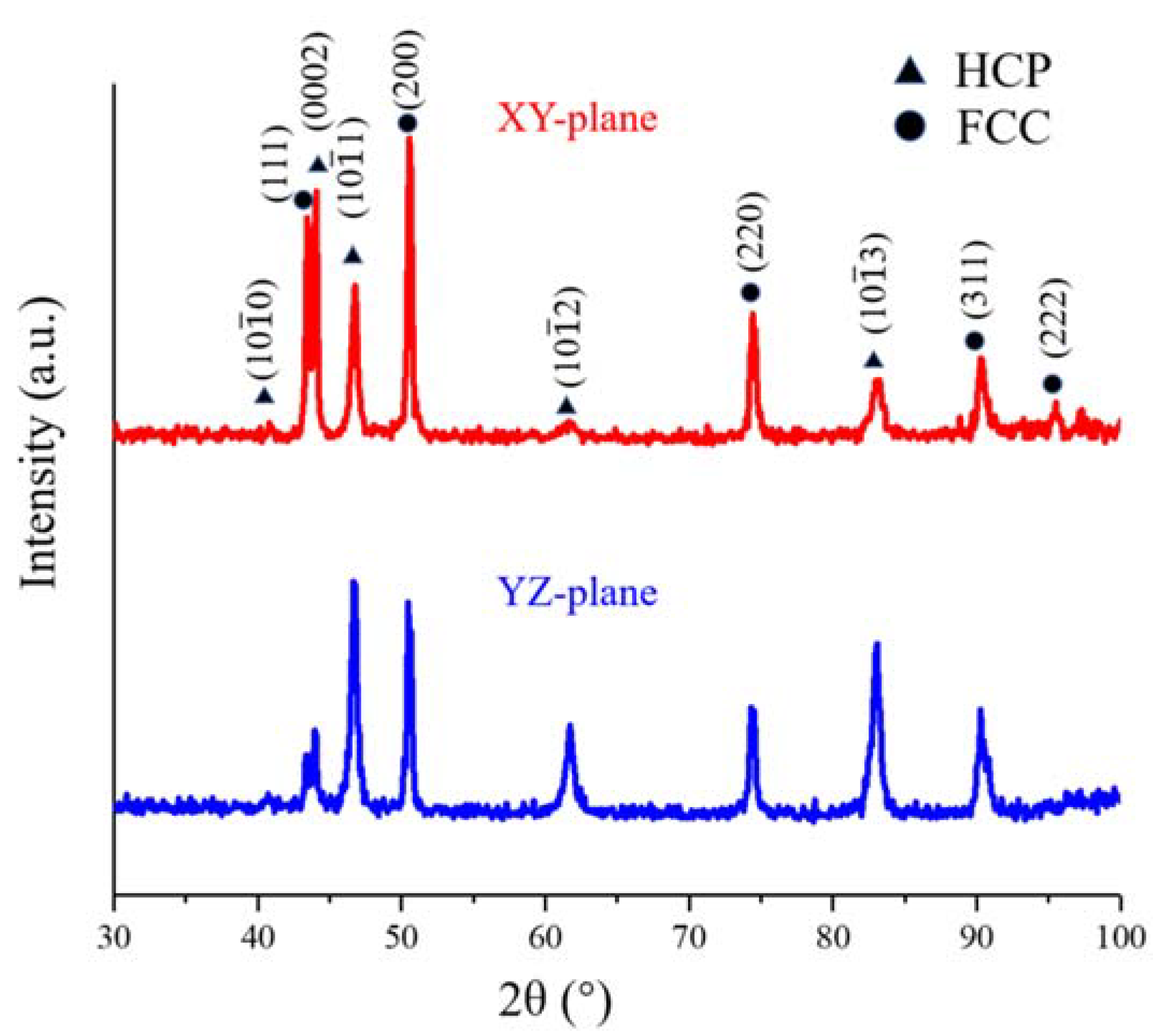
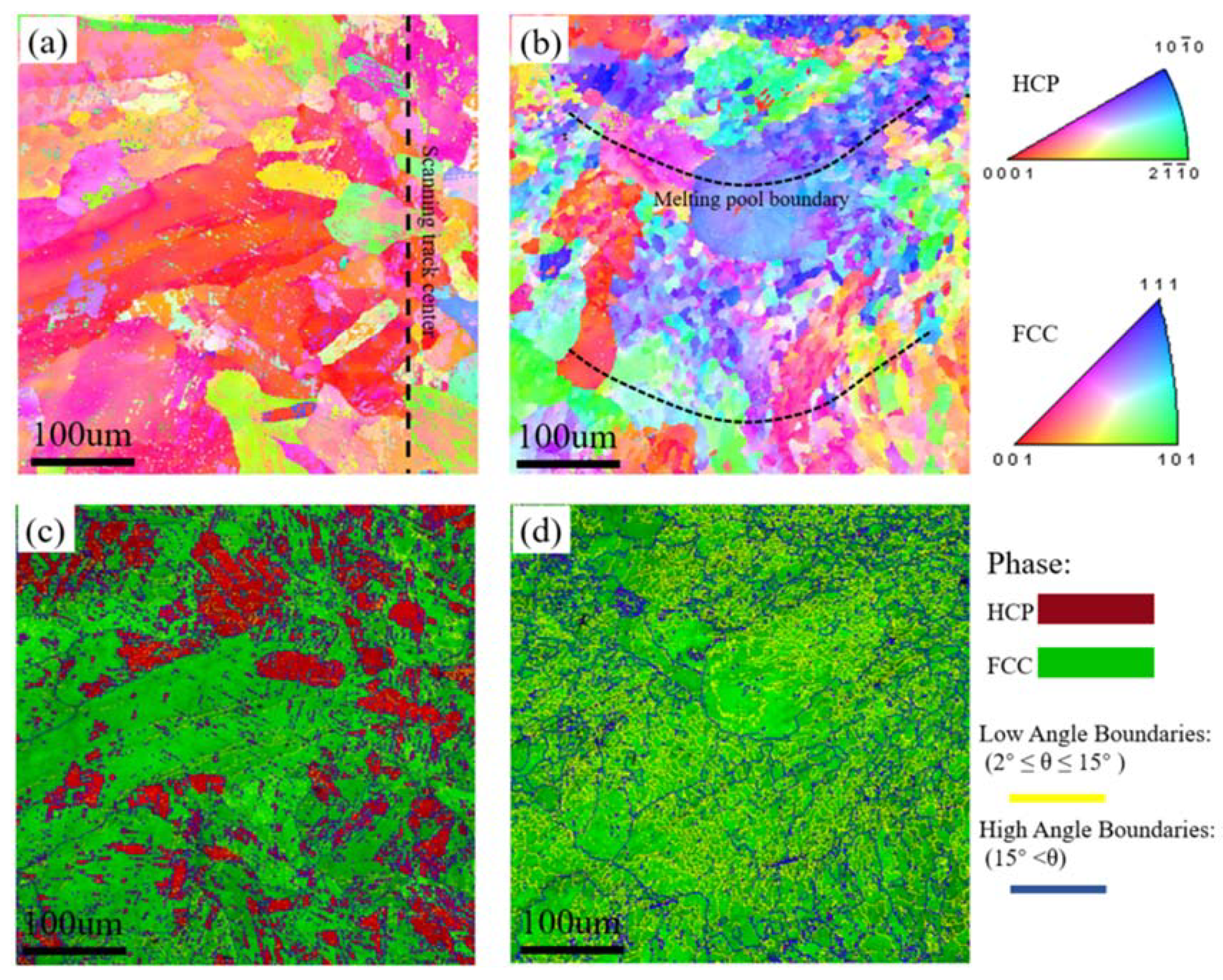
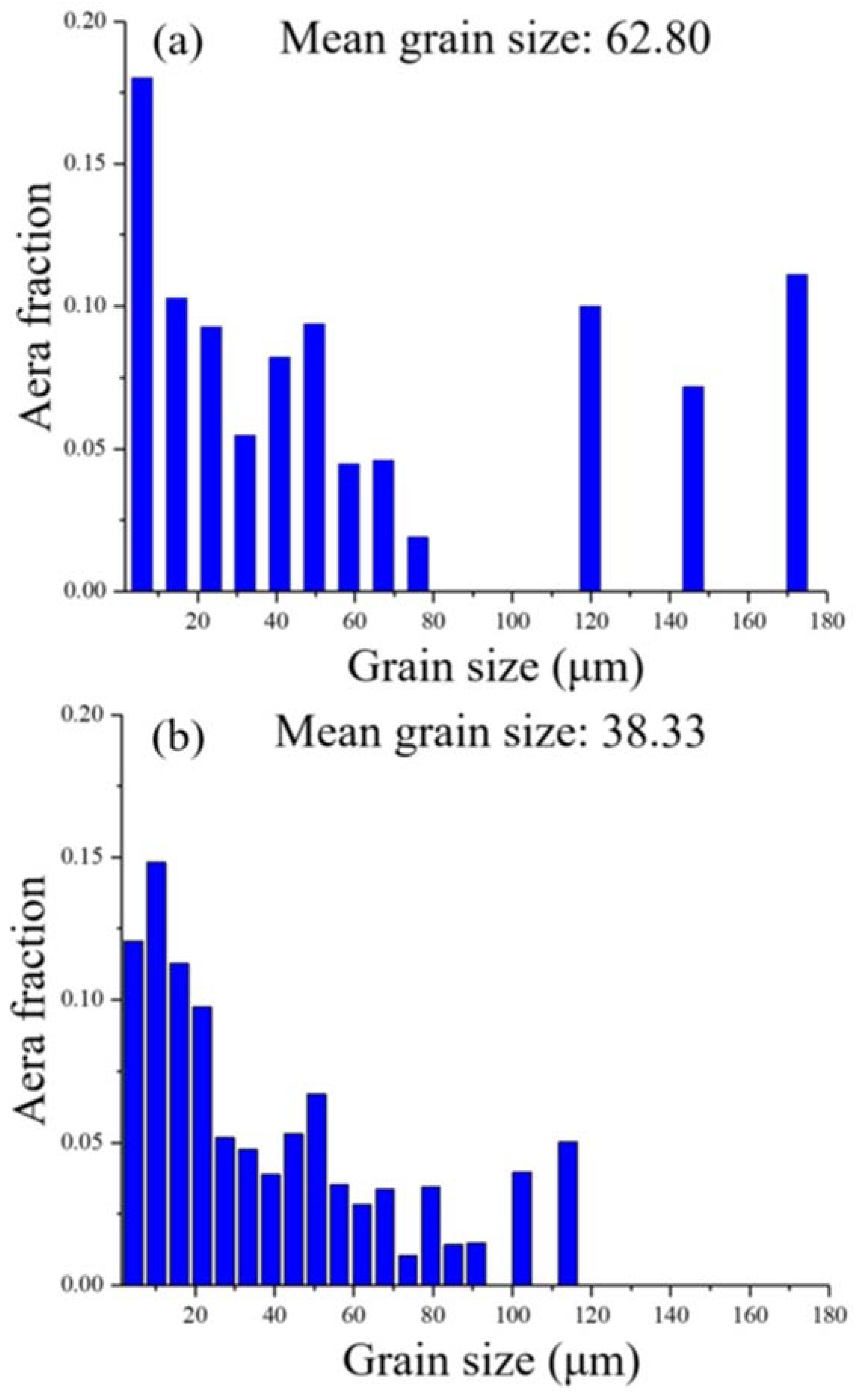



| Alloys | Fe | Mn | Co | Cr | Ni | Si | Zr | C | O | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe50Mn30Co10Cr10 | 52.3 | 28.2 | 9.44 | 9.89 | 0.0008 | <0.001 | <0.00001 | <0.0002 | 0.003 | 0.00019 | 0.00016 |
| Sample | Ecorr (mV) | Icorr (mA cm−2) | βa (mV dec−1) | −βc (mV dec−1) | Rp (Ω cm−2) | Epp (mV) | Icrit (mA cm−2) | Erp (mV) | Eb (mV) |
|---|---|---|---|---|---|---|---|---|---|
| XY-plane | −509 | 0.6216 | 66.25 | 174.64 | 16.6 | 20 | 65.14 | 1060 | 1500 |
| YZ-plane | −570 | 2.513 | 177.59 | 207.60 | 33.6 | 13 | 55.68 | 1060 | 1500 |
| Sample | Rs (Ω cm−2) | Qdl (l0−4 Ω−1 cm−2 sn) | ndl | Rct (Ω cm−2) | RL (Ω cm−2) | L (H cm−2) | χ2 × 10−3 |
|---|---|---|---|---|---|---|---|
| XY-plane | 9.352 | 2.41 | 0.90778 | 14.63 | 3.952 | 7.23 | 3.035 |
| YZ-plane | 9.486 | 1.61 | 0.90407 | 11 | 2.947 | 0.2 | 2.787 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Yuan, T.; Li, R. Striped Non-Uniform Corrosion Behavior of Non-Equiatomic FeMnCoCr High-Entropy Alloy Prepared by Laser Melting Deposition in 0.1 M H2SO4 Solution. Materials 2020, 13, 5554. https://doi.org/10.3390/ma13235554
Zhang Z, Yuan T, Li R. Striped Non-Uniform Corrosion Behavior of Non-Equiatomic FeMnCoCr High-Entropy Alloy Prepared by Laser Melting Deposition in 0.1 M H2SO4 Solution. Materials. 2020; 13(23):5554. https://doi.org/10.3390/ma13235554
Chicago/Turabian StyleZhang, Zhijian, Tiechui Yuan, and Ruidi Li. 2020. "Striped Non-Uniform Corrosion Behavior of Non-Equiatomic FeMnCoCr High-Entropy Alloy Prepared by Laser Melting Deposition in 0.1 M H2SO4 Solution" Materials 13, no. 23: 5554. https://doi.org/10.3390/ma13235554
APA StyleZhang, Z., Yuan, T., & Li, R. (2020). Striped Non-Uniform Corrosion Behavior of Non-Equiatomic FeMnCoCr High-Entropy Alloy Prepared by Laser Melting Deposition in 0.1 M H2SO4 Solution. Materials, 13(23), 5554. https://doi.org/10.3390/ma13235554





