Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of UiO-66
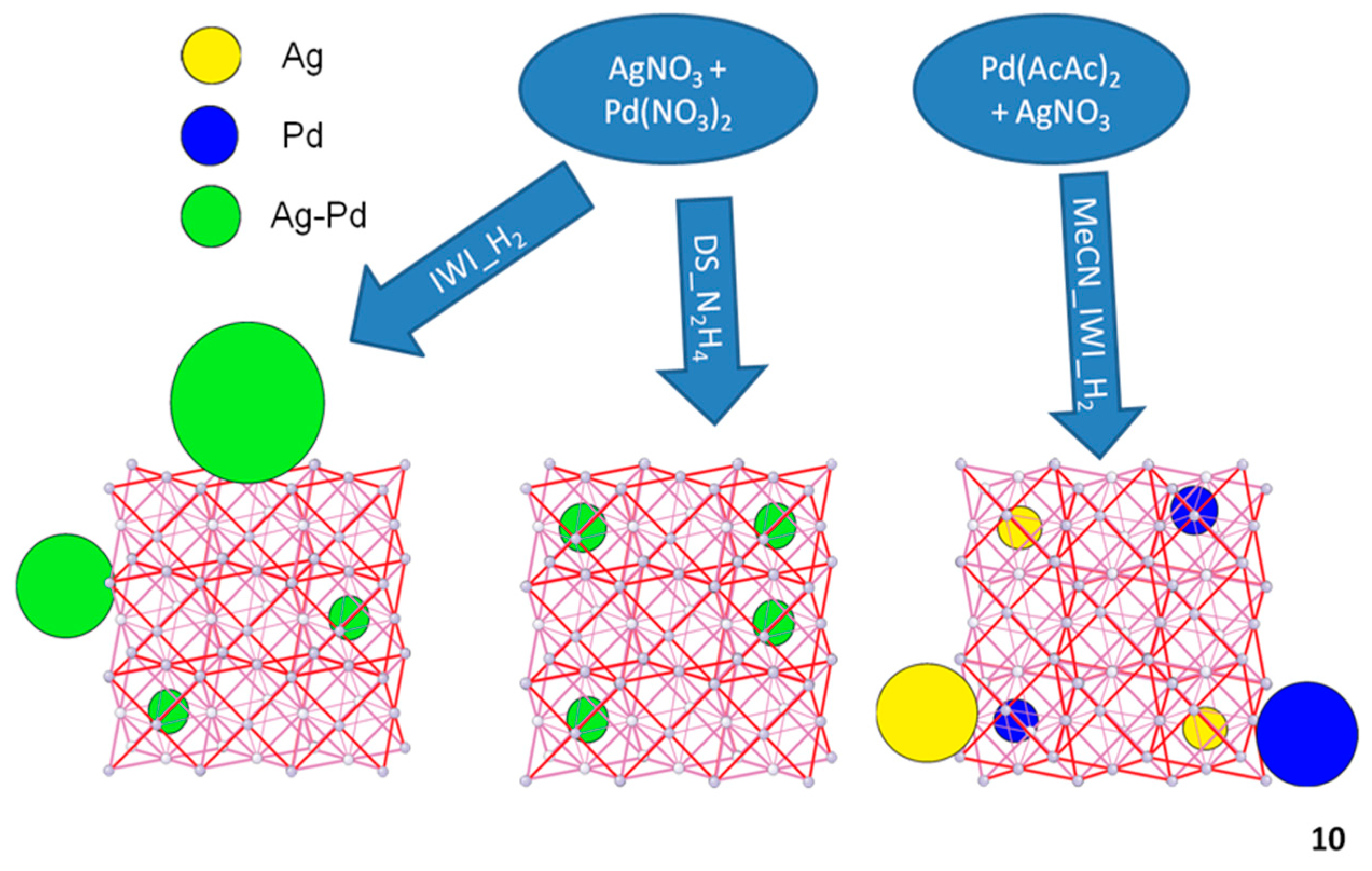
2.2. Synthesis of AgPd@UiO-66
2.2.1. Incipient Wetness Impregnation Method (IWI)
2.2.2. Double Solvent Approach (DS)
2.3. Characterization
2.4. Catalytic Tests
3. Results and Discussion
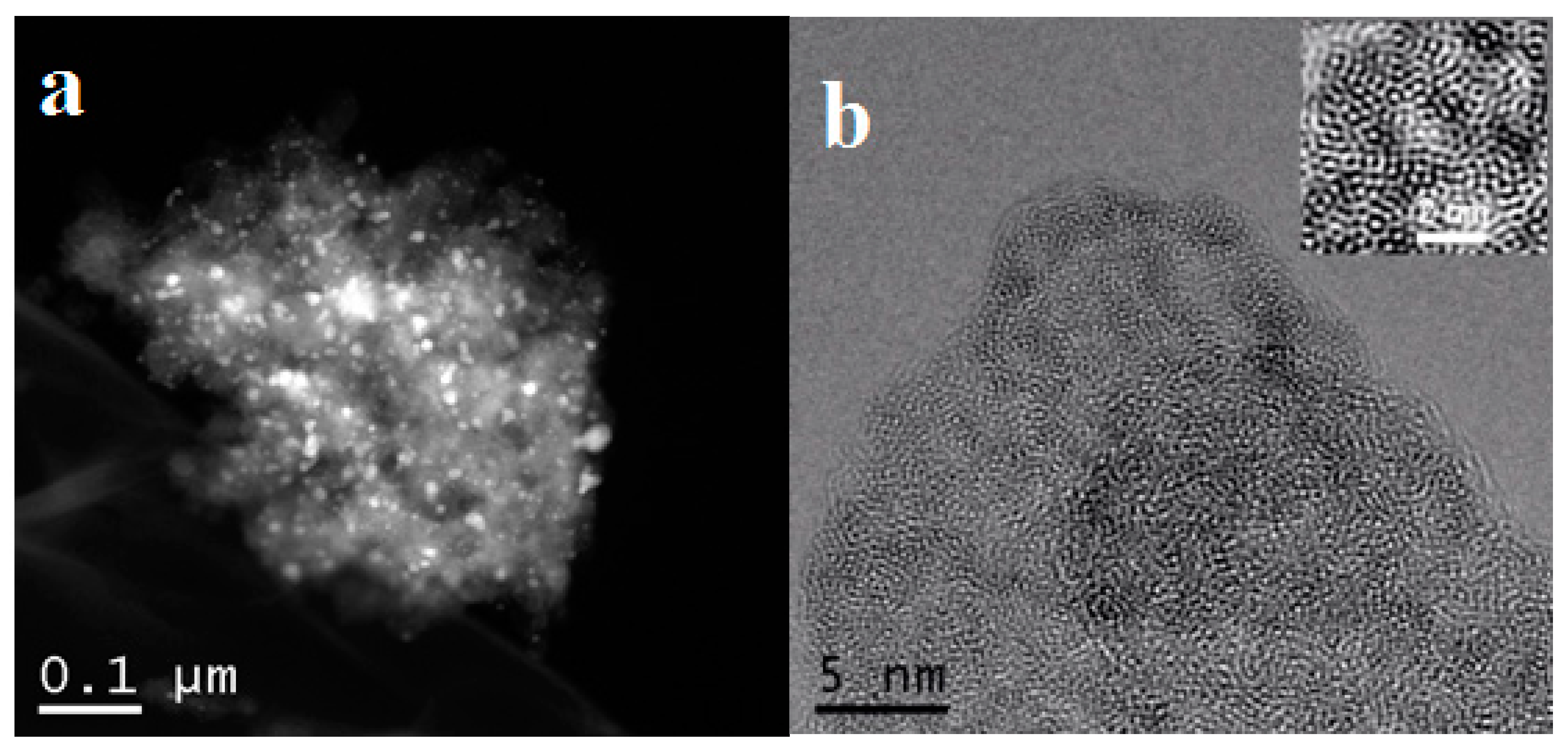
3.1. Comparison of Synthetic Methods
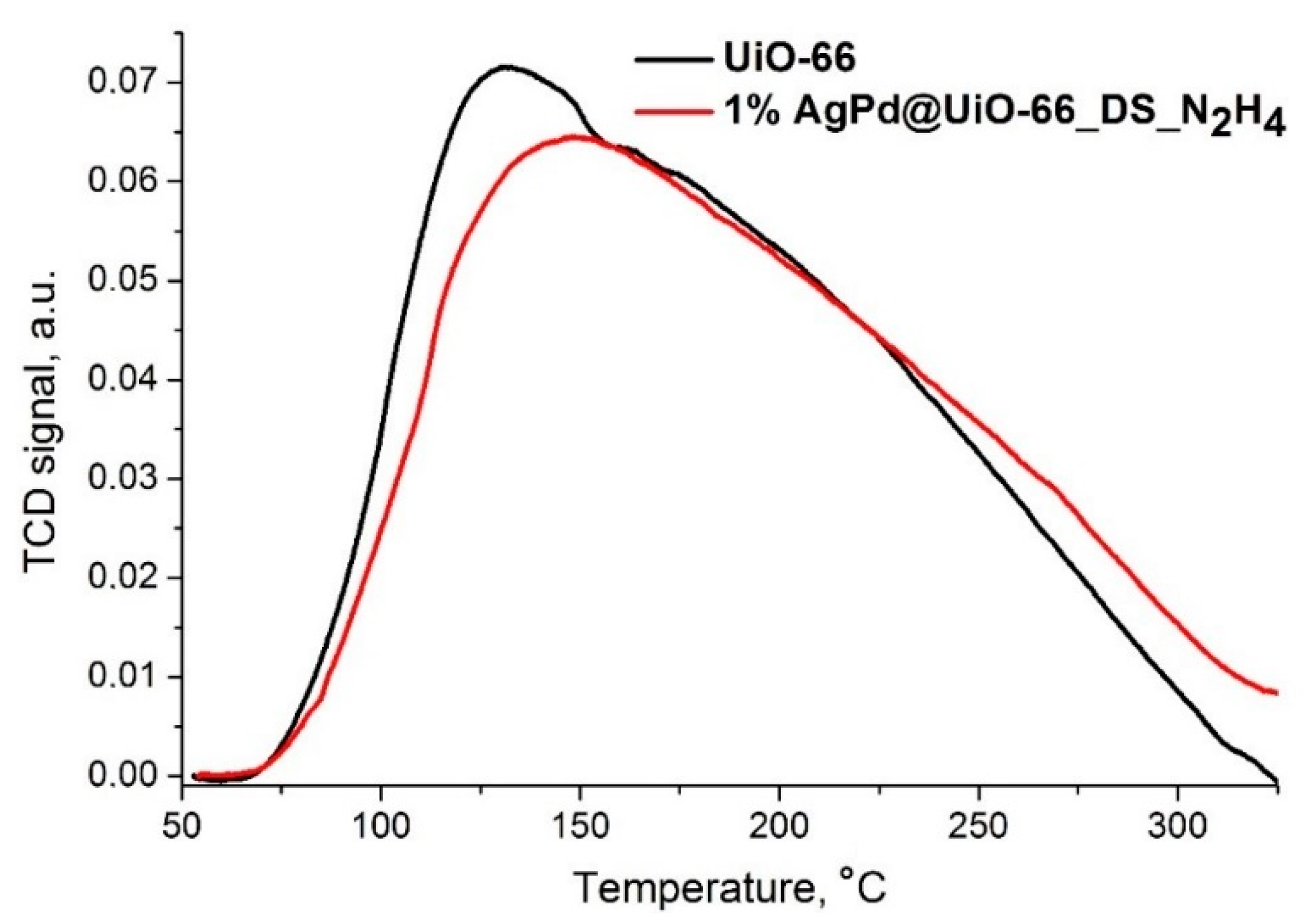
3.2. Acid and Redox Properties
3.3. Effect of Metal Loading
3.4. Catalytic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fang, Z.; Li, H.; Smith, R.L., Jr. Production of Biofuels and Chemicals with Bifunctional Catalysts; Springer Nature: Singapore, 2017. [Google Scholar]
- Timofeev, K.L.; Vodyankina, O.V. Selective oxidation of bio-based platform molecules and its conversion products over metal nanoparticle catalysts: A review. React. Chem. Eng. 2020. [Google Scholar] [CrossRef]
- Feng, Y.; Xue, W.; Yin, H.; Meng, M.; Wang, A.; Liu, S. Selective oxidation of 1,2-propanediol to lactic acid catalyzed by hydroxyapatite-supported Pd and Pd–Ag nanoparticles. RSC Adv. 2015, 5, 106918. [Google Scholar] [CrossRef]
- Albuquerque, E.M.; Borges, L.E.P.; Fraga, M.A.; Sievers, C. Relationship between acid–base properties and the activity of ZrO2-based catalysts for the Cannizzaro reaction of pyruvaldehyde to lactic acid. ChemCatChem 2017, 9, 2675–2683. [Google Scholar] [CrossRef]
- Lin, J.; He, J.; Qi, F.; Zheng, B.; Wang, X.; Yu, B.; Zhou, K.; Zhang, W.; Li, Y.; Chen, Y. In-situ selenization of Co-based metal-organic frameworks as a highly efficient electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2017, 247, 258–264. [Google Scholar] [CrossRef]
- He, J.; Lv, W.; Chen, Y.; Wen, K.; Xu, C.; Zhang, W.; Li, Y.; Qin, W.; He, W. Tellurium-impregnated porous Cobalt-doped carbon polyhedra as superior cathodes for lithium−tellurium batteries. ACS Nano 2017, 11, 8144–8152. [Google Scholar] [CrossRef]
- Rösler, C.; Fisher, R.A. Metal–organic frameworks as hosts for nanoparticles. CrystEngComm 2015, 17, 199–217. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal–organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef]
- Mallat, T.; Baiker, A. Potential of gold nanoparticles for oxidation in fine chemical Synthesis. Annu. Rev. Chem. Biomol. Eng. 2012, 3, 11–28. [Google Scholar] [CrossRef]
- Dimitratos, N.; Lopez-Sanchez, J.A.; Hutchings, G.J. Selective liquid phase oxidation with supported metal nanoparticles. Chem. Sci. 2012, 3, 20–44. [Google Scholar] [CrossRef]
- Torbina, V.V.; Vodyankin, A.A.; Ten, S.; Mamontov, G.V.; Salaev, M.A.; Sobolev, V.I.; Vodyankina, O.V. Ag-Based catalysts in heterogeneous selective oxidation of alcohols: A review. Catalysts 2018, 8, 447. [Google Scholar] [CrossRef]
- Zaid, S.; Skrzynska, E.; Addad, A.; Nandi, S.; Jalowiecki-Duhamel, L.; Girardon, J.-S.; Capron, M. Development of silver based catalysts promoted by noble metal M (M=Au, Pd or Pt) for glycerol oxidation in liquid phase. Top. Catal. 2017, 60, 1072–1081. [Google Scholar] [CrossRef]
- Opieda, I.A.; Kytsya, A.R.; Bazylyak, L.I.; Pobigun, O.I. Silver nanoparticles catalysis of the liquid-phase radical chain oxidation of cumene by molecular oxygen. Theor. Exp. Chem. 2017, 52, 369–374. [Google Scholar] [CrossRef]
- Kaneda, K.; Mitsudome, T. Metal–support cooperative catalysts for environmentally benign molecular transformations. Chem. Rec. 2017, 17, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Fisher, E.; Kenisgberg, L.; Carreira, M.; Fernandez-Gallardo, J.; Baldwin, R.; Contel, M. Water-compatible gold and silver nanoparticles as catalysts for the oxidation of alkenes. Polyhedron 2016, 120, 82–87. [Google Scholar] [CrossRef]
- Vadakkekara, R.; Chakraborty, M.; Parikh, P.A. Room temperature benzaldehyde oxidation using air over gold–silver nanoalloy catalysts. J. Taiwan Inst. Chem. Eng. 2015, 50, 84–92. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Ling, L.; Zhang, R.; Fan, M. Probe into the effects of surface composition and ensemble effect of active sites on the catalytic performance of C2H2 semi-hydrogenation over the Pd-Ag bimetallic catalysts. Chem. Eng. Sci. 2020, 218, 115549–115562. [Google Scholar] [CrossRef]
- Lu, F.; Sun, D.; Jiang, X. Plant-mediated synthesis of AgPd/γ-Al2O3 catalyst for selective hydrogenation of 1,3-butadiene at low temperature. New J. Chem. 2019, 43, 13891–13898. [Google Scholar] [CrossRef]
- Eken Korkut, S.; Küçükkeçeci, H.; Metin, Ö. Mesoporous graphitic carbon nitride/black phosphorus/AgPd alloy nanoparticles ternary nanocomposite: A highly efficient catalyst for the methanolysis of ammonia borane. ACS Appl. Mater. Interfaces 2020, 12, 8130–8139. [Google Scholar] [CrossRef]
- Sun, D.; Li, P.; Yang, B.; Xu, Y.; Huang, J.; Li, Q. Monodisperse AgPd alloy nanoparticles as a highly active catalyst towards the methanolysis of ammonia borane for hydrogen generation. RSC Adv. 2016, 6, 105940–105947. [Google Scholar] [CrossRef]
- Babel, V.; Hiran, B.L. Heterogeneous AgPd alloy nanocatalyst for selective reduction of aromatic nitro compounds using formic acid as hydrogen source. Catal. Lett. 2020, 150, 1865–1869. [Google Scholar] [CrossRef]
- Gao, S.; Feng, T.; Wu, Q.; Feng, C.; Shang, N.; Wang, C. Immobilizing AgPd alloy on Vulcan XC-72 carbon: A novel catalyst for highly efficient hydrogen generation from formaldehyde aqueous solution. RSC Adv. 2016, 6, 105638–105643. [Google Scholar] [CrossRef]
- Shang, N.Z.; Feng, C.; Gao, S.T.; Wang, C. Ag/Pd nanoparticles supported on amine-functionalized metal-organic framework for catalytic hydrolysis of ammonia borane. Int. J. Hydrog. Energy 2016, 41, 944–950. [Google Scholar] [CrossRef]
- Zhang, X.; Shang, N.; Shang, H.; Du, T.; Zhou, X.; Feng, C.; Gao, S.; Wang, C.; Wang, Z. Nitrogen-decorated porous carbon supported AgPd nanoparticles for boosting hydrogen generation from formic acid. Energy Technol. 2019, 7, 140–145. [Google Scholar] [CrossRef]
- Wen, C.; Yin, A.; Dai, W.L. Recent advances in silver-based heterogeneous catalysts for green chemistry processes. Appl. Catal. B 2014, 160, 730–741. [Google Scholar] [CrossRef]
- Besson, S.; Gacoin, T.; Ricolleau, C.; Boilot, J.P. Silver nanoparticle growth in 3D-hexagonal mesoporous silica films. Chem. Commun. 2003, 3, 360–361. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–138051. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, X.-C.; Chang, G.-G.; Ke, S.-C.; Xia, T.; Hu, Z.-Y.; Yang, X.-Y. Synergistic catalysis of Pd nanoparticles with both Lewis and Bronsted acid sites encapsulated within a sulfonated metal–organic frameworks toward one-pot tandem reactions. J. Colloid Interface Sci. 2019, 557, 207–215. [Google Scholar] [CrossRef]
- Vermoortele, F.; Bueken, B.; Van De Voorde, B.; Vandichel, M.; Houthoofd, K.; Vimont, A.; Daturi, M.; Waroquier, M.; Van Speybroeck, V.; Kirschhock, C.; et al. Synthesis modulation as a tool to increase the catalytic activity of metal−organic frameworks: The unique case of UiO-66(Zr). J. Am. Chem. Soc. 2013, 135, 11465–11468. [Google Scholar] [CrossRef]
- Hajek, J.; Vandichel, M.; Van De Voorde, B.; Bueken, B.; De Vos, D.; Waroquier, M.; Van Speybroeck, V. Mechanistic studies of aldol condensations in UiO-66 and UiO-66-NH2 metal organic frameworks. J. Catal. 2015, 331, 1–12. [Google Scholar] [CrossRef]
- De Mello, M.D.; Tsapatsis, M. Selective glucose to fructose isomerization over modified zirconium UiO-66 in alcohol media. ChemCatChem 2018, 10, 2417–2423. [Google Scholar] [CrossRef]
- Liu, X.-H.; Ma, J.-G.; Niu, Z.; Yang, G.-M.; Cheng, P. An efficient nanoscale heterogeneous catalyst for the capture and conversion of carbon dioxide at ambient pressure. Angew. Chem. 2015, 54, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Papurello, R.L.; Fernández, J.L.; Miró, E.E.; Zamaro, J.M. Microreactor with silver-loaded metal-organic framework films for gas-phase reactions. Chem. Eng. J. 2017, 313, 1468–1476. [Google Scholar] [CrossRef]
- Gao, S.-T.; Liu, W.; Feng, C.; Shang, N.-Z.; Wang, C. Ag-Pd alloy supported on amine-functionalized UiO-66 as an efficient synergetic catalyst for dehydrogenation of formic acid at room temperature. RSC Technol. 2016, 6, 869–874. [Google Scholar] [CrossRef]
- Li, Y.-X.; Wei, Z.-Y.; Liu, L.; Gao, M.-L.; Han, Z.-B. Ag nanoparticles supported on UiO-66 for selective oxidation of styrene. Inorg. Chem. Commun. 2018, 88, 47–50. [Google Scholar] [CrossRef]
- Liu, M.; Zhou, L.; Luo, X.; Wan, C.; Xu, L. Recent advances in noble metal catalysts for hydrogen production from ammonia borane. Catalysts 2020, 10, 788. [Google Scholar] [CrossRef]
- Meng, W.; Wen, Y.; Dai, L.; He, Z.; Wang, L. A novel electrochemical sensor for glucose detection based on Ag@ZIF-67 nanocomposite. Sens. Actuators B 2018, 260, 852–860. [Google Scholar] [CrossRef]
- Ali Molla, R.; Ghosh, K.; Banerjee, B.; Iqubal, M.A.; Kundu, S.K.; Islam, S.M.; Bhaumik, A. Silver nanoparticles embedded over porous MOF for CO2 fixation via carboxylation of terminal alkynes at ambient pressure. J. Colloid Interface Sci. 2016, 477, 220–229. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, X.; Yan, Y.; Zhou, J.; Zhang, W.; Tai, X. Bimetallic gold-silver nanoparticles supported on zeolitic imidazolate framework-8 as highly Active heterogenous catalysts for selective oxidation of benzyl alcohol into benzaldehyde. Polymers 2018, 10, 1089. [Google Scholar] [CrossRef]
- Moon, H.R.; Kim, J.H.; Suh, M.P. Redox-Active Porous Metal–Organic Framework Producing Silver Nanoparticles from AgI Ions at Room Temperature. Angew. Chem. Int. Ed. 2005, 44, 1261–1265. [Google Scholar] [CrossRef]
- Houk, R.J.T.; Jacobs, B.W.; El Gabaly, F.; Chang, N.N.; Talin, A.A.; Graham, D.D.; House, S.D.; Robertson, I.M.; Allendorf, M.D. Silver cluster formation, dynamics, and chemistry in metal-organic frameworks. Nano Lett. 2009, 9, 3413–3418. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, P.F.; Yang, L.; Huang, C.Z.; Li, Y.F. Facile in Situ Synthesis of Silver Nanoparticles on the Surface of Metal–Organic Framework for Ultrasensitive Surface-Enhanced Raman Scattering Detection of Dopamine. Anal. Chem. 2015, 87, 12177–12182. [Google Scholar] [CrossRef]
- Han, C.; Yang, J.; Gu, J. Immobilization of silver nanoparticles in Zr-based MOFs: Induction of apoptosis in cancer cells. J. Nanoparticle Res. 2018, 20, 77–88. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, D.; Xu, H.; Mei, J.; Qu, Z.; Liu, P.; Cui, Y.; Yan, N. Combined effects of Ag and UiO-66 for removal of elemental mercury from flue gas. Chemosphere 2018, 197, 65–72. [Google Scholar] [CrossRef]
- Tang, L.; Shi, J.; Wu, H.; Zhang, S.; Liu, H.; Zou, H.; Wu, Y.; Zhao, J.; Jiang, Z. In situ biosynthesis of ultrafine metal nanoparticles within metal-organic framework for efficient heterogeneous catalysis. Nanotechnology 2017, 28, 365604–365631. [Google Scholar] [CrossRef]
- Liu, L.; Tai, X.; Yu, G.; Guo, H.; Meng, Q. Gold and Silver Nanoparticles Supported on Metal-organic Frameworks: A Highly Active Catalyst for Three-component Coupling Reaction. Chem. Res. Chin. Univ. 2016, 32, 443–450. [Google Scholar] [CrossRef]
- Aijaz, A.; Karkamkar, A.; Choi, Y.J.; Tsumori, N.; Rönnebro, E.; Autrye, T.; Shioyama, H.; Xu, Q. Immobilizing Highly Catalytically Active Pt Nanoparticles inside the Pores of Metal−Organic Framework: A Double Solvents Approach. J. Am. Chem. Soc. 2012, 134, 13926–13929. [Google Scholar] [CrossRef]
- Liu, Y.; Jia, S.-Y.; Wu, S.-H.; Li, P.-L.; Liu, C.-J.; Xu, Y.-M.; Qin, F.-X. Synthesis of highly dispersed metallic nanoparticles inside the pores of MIL-101(Cr) via the new double solvent method. Catal. Commun. 2015, 70, 44–48. [Google Scholar] [CrossRef]
- Yadav, M.; Xu, Q. Catalytic chromium reduction using formic acid and metal nanoparticles immobilized in a metal–organic framework. Chem. Commun. 2013, 49, 3327–3329. [Google Scholar] [CrossRef]
- Shang, N.; Gao, S.; Zhou, X.; Feng, C.; Wang, Z.; Wang, C. Palladium nanoparticles encapsulated inside the pores of a metal–organic framework as a highly active catalyst for carbon–carbon cross-coupling. RSC Adv. 2014, 4, 54487–54493. [Google Scholar] [CrossRef]
- Baguc, I.B.; Ertas, I.E.; Yurderi, M.; Bulut, A.; Zahmakiran, M.; Kaya, M. Nanocrystalline Metal Organic Framework (MIL-101) Stabilized Copper Nanoparticles: Highly Efficient Nanocatalyst for the Hydrolytic Dehydrogenation of Methylamine Borane. Inorg. Chim. Acta 2018, 483, 431–439. [Google Scholar] [CrossRef]
- Zhu, Q.-L.; Li, J.; Xu, Q. Immobilizing Metal Nanoparticles to Metal−Organic Frameworks with Size and Location Control for Optimizing Catalytic Performance. J. Am. Chem. Soc. 2013, 135, 10210–10213. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, P.C.; Lu, M. Selective oxidation of benzyl alcohol under solvent-free condition with gold nanoparticles encapsulated in metal-organic framework. Appl. Catal. A 2014, 477, 125–131. [Google Scholar] [CrossRef]
- Nakamura, T.; Magara, H.; Herabani, Y.; Sato, S. Fabrication of silver nanoparticles by highly intense laser irradiation of aqueous solution. Appl. Phys. A 2011, 104, 1021–1024. [Google Scholar] [CrossRef]
- Torbina, V.V.; Nedoseykina, N.S.; Ivanchikova, I.D.; Kholdeeva, O.A.; Vodyankina, O.V. Propylene glycol oxidation with hydrogen peroxide over Zr-containing metal-organic framework UiO-66. Catal. Today 2019, 333, 47–53. [Google Scholar] [CrossRef]
- Patricek, V.; Dusek, M.; Palatinus, L. Crystallographic Computer System JANA2006: General features. Z. Krist. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Henglein, A. Physicochemical Properties of Small Metal Particles in Solution: “Microelectrode” Reactions, Chemisorption, Composite Metal Particles, and the Atom-to-Metal Transition. J. Phys. Chem. 1993, 97, 5457–5471. [Google Scholar] [CrossRef]
- Popov, A.K.; Tanke, R.S.; Brummer, J.; Taft, G.; Loth, M.; Langlois, R.; Wruck, A.; Schmitz, R. Laser-stimulated synthesis of large fractal silver nanoaggregates. Nanotechnology 2006, 17, 1901–1905. [Google Scholar] [CrossRef][Green Version]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and properties of silver nanoparticles: Advances and prospects. Russ. Chem. Rev. 2008, 77, 233–257. [Google Scholar] [CrossRef]
- Mie, G. Annalen der Physik. Ann. Phys. 1908, 25, 377–446. [Google Scholar] [CrossRef]
- Shen, L.; Liang, R.; Luo, M.; Jing, F.; Wu, L. Electronic effects of ligand substitution on metal–organic framework photocatalysts: The case study of UiO-66. Phys. Chem. Chem. Phys. 2015, 17, 117–121. [Google Scholar] [CrossRef]
- Kreibig, U.; Vollmer, M. Optical Properties of Metal Clusters; Springer: Berlin, Germany, 1995. [Google Scholar]
- Gurin, V.S.; Petranovskii, V.P.; Hernandez, M.-A.; Bogdanchikova, N.E.; Alexeenko, A.A. Silver and copper clusters and small particles stabilized within nanoporous silicate-based materials. Mater. Sci. Eng. A 2005, 391, 71–76. [Google Scholar] [CrossRef]
- Bárcia, P.S.; Guimarães, D.; Mendes, P.A.P.; Silva, J.A.C.; Guillerm, V.; Chevreau, H.; Rodrigues, C.; Serre, A.E. Reverse shape selectivity in the adsorption of hexane and xylene isomers in MOF UiO-66. Microporous Mesoporous Mater. 2011, 139, 67–73. [Google Scholar] [CrossRef]
- Yumura, T.; Oda, A.; Torigoe, H.; Itadani, A.; Kuroda, Y.; Wakasugi, T.; Kobayashi, H. Combined Experimental and Computational Approaches To Elucidate the Structures of Silver Clusters inside the ZSM-5 Cavity. J. Phys. Chem. C 2014, 118, 23874–23887. [Google Scholar] [CrossRef]
- Fron, E.; Aghakhani, S.; Baekelant, W.; Grandjean, D.; Coutino-Gonzalez, E.; Van der Auweraer, M.; Roeffaers, M.B.J.; Lievens, P.; Hofkens, J. Structural and Photophysical Characterization of Ag Clusters in LTA Zeolites. J. Phys. Chem. C 2019, 123, 10630–10638. [Google Scholar] [CrossRef]
- Yang, C.-C.; Wan, C.-C.; Wang, Y.-Y. Synthesis of Ag/Pd nanoparticles via reactive micelles as templates and its application to electroless copper deposition. J. Colloid Interface Sci. 2004, 279, 433–439. [Google Scholar] [CrossRef]
- Afanasev, D.S.; Anufrienko, V.F.; Ruzankin, S.F.; Larina, T.V.; Kuznetsova, N.I.; Bukhtiyarov, V.I. Effect of Oxygen Adsorption on the Surface Plasmon Resonance of Oxide-Supported Silver Nanoparticles. Dokl. Phys. Chem. 2011, 436, 23–25. [Google Scholar] [CrossRef]
- Zhou, F.; Lu, N.; Fan, B.; Wang, H.; Li, R. Zirconium-containing UiO-66 as an efficient and reusable catalyst for transesterification of triglyceride with methanol. J. Energy Chem. 2016, 25, 874–879. [Google Scholar] [CrossRef]
- Karski, S.; Witońska, I.; Rogowski, J.; Gołuchowska, J. Interaction between Pd and Ag on the surface of silica. J. Mol. Catal. A Chem. 2005, 240, 155–163. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Liu, X.; Zhu, Q. Synergetic effect of Pd and Ag dispersed on Al2O3 in the selective hydrogenation of acetylene. Appl. Catal. A Gen. 2000, 197, 221–228. [Google Scholar] [CrossRef]
- Gorte, R.J. Design Parameters for Temperature Programmed Desorption from Porous Catalyst. J. Catal. 1982, 75, 164–174. [Google Scholar] [CrossRef]
- Chou, C.-W.; Chu, S.-J.; Chiang, H.-J.; Huang, C.-Y.; Lee, C.-J.; Sheen, S.-R.; Perng, T.P.; Yeh, C.-T. Temperature-Programmed Reduction Study on Calcination of Nano-Palladium. J. Phys. Chem. B 2001, 105, 9113–9117. [Google Scholar] [CrossRef]
- Khan, Z.; Dummer, N.F.; Edwards, J.K. Silver–palladium catalysts for the direct synthesis of hydrogen peroxide. Phil. Trans. R. Soc. 2017, 376, 20170058. [Google Scholar] [CrossRef] [PubMed]
- Hirasawa, S.; Watanabe, H.; Kizuka, T.; Nakagawa, Y.; Tomishige, K. Performance, structure and mechanism of Pd–Ag alloy catalyst for selective oxidation of glycerol to dihydroxyacetone. J. Catal. 2013, 300, 205–216. [Google Scholar] [CrossRef]
- Bondarchuk, I.S.; Mamontov, G.V. Role of PdAg Interface in Pd–Ag/SiO2 Bimetallic Catalysts in Low-Temperature Oxidation of Carbon Monoxide. Kinet. Catal. 2015, 56, 379–385. [Google Scholar] [CrossRef]
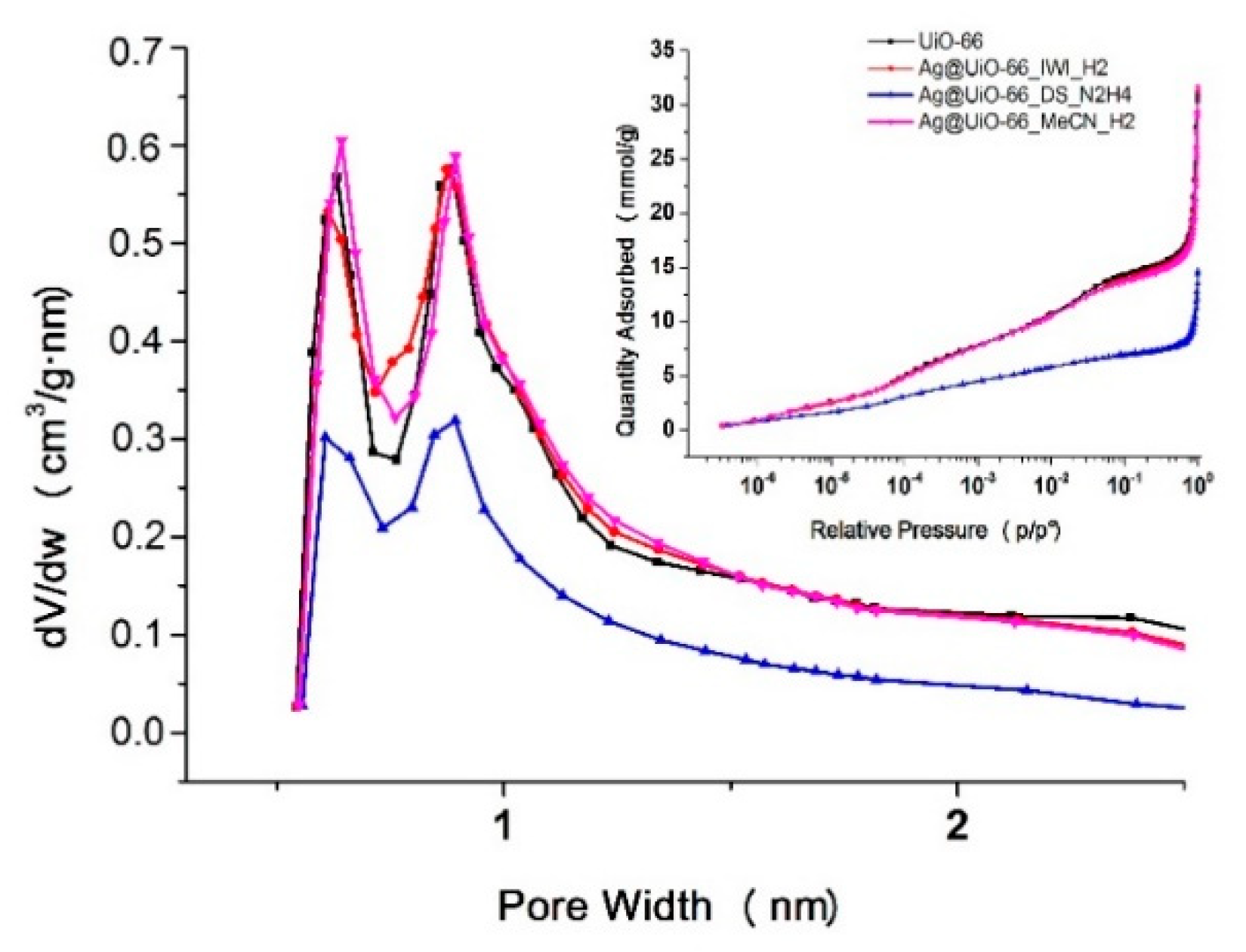
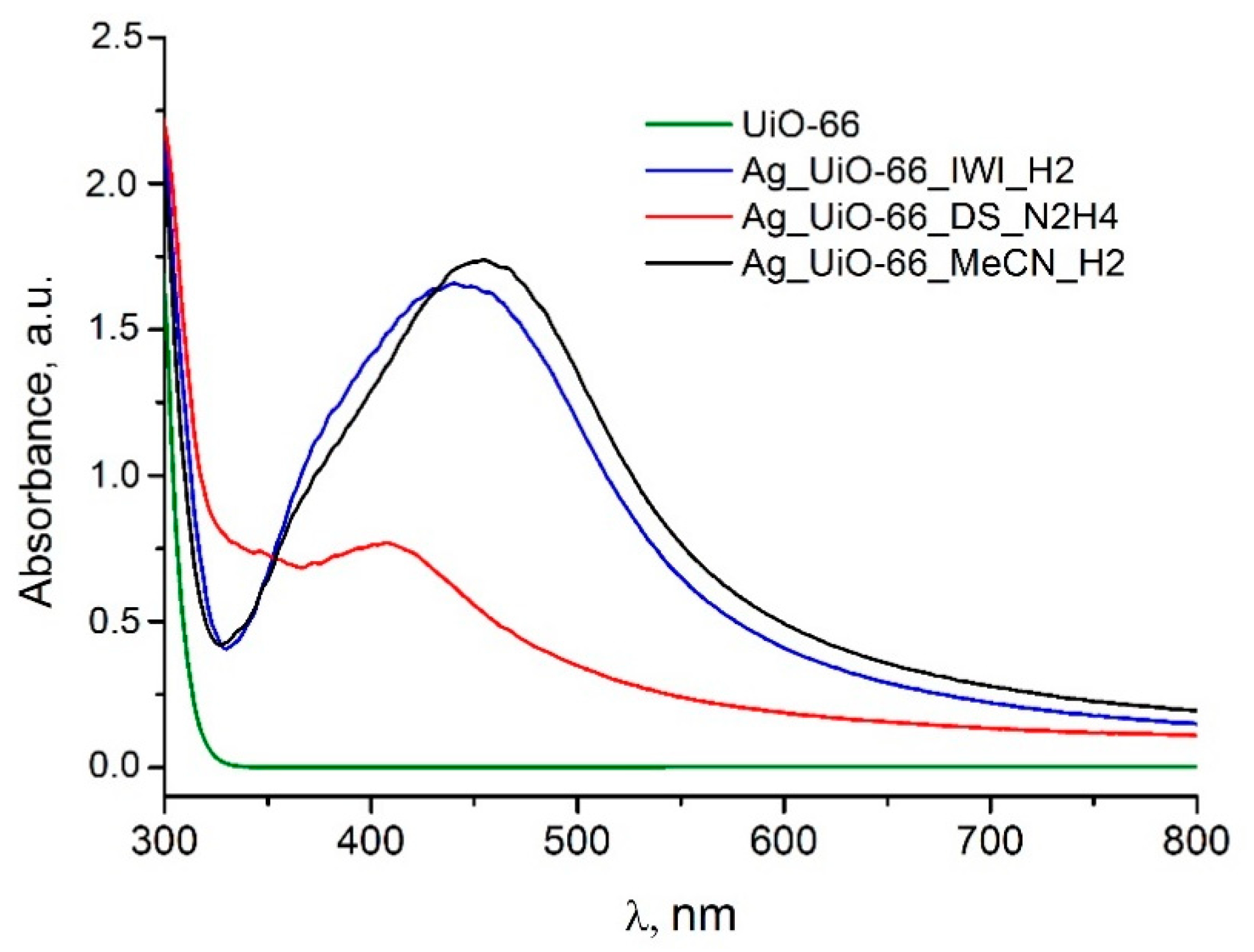
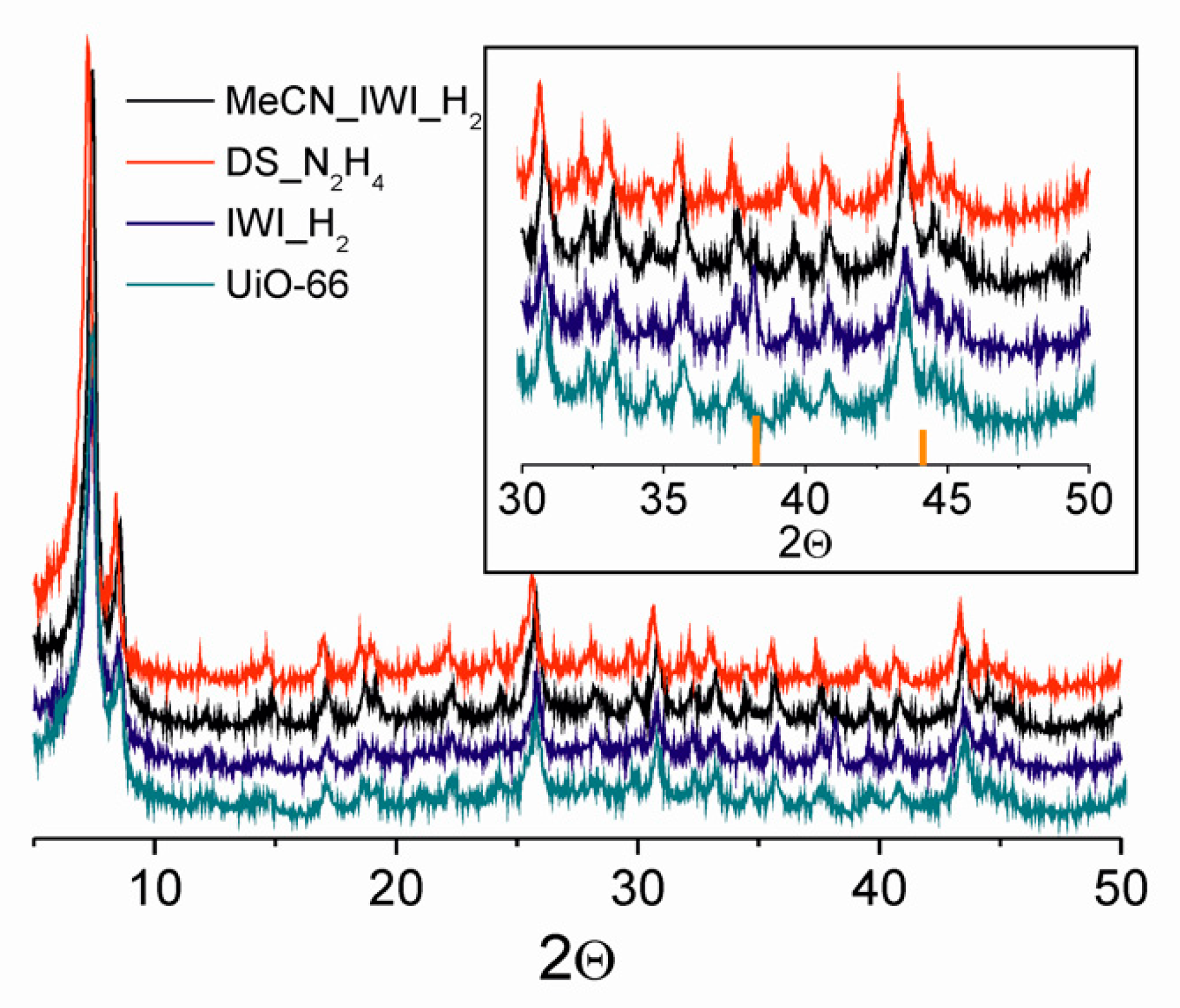
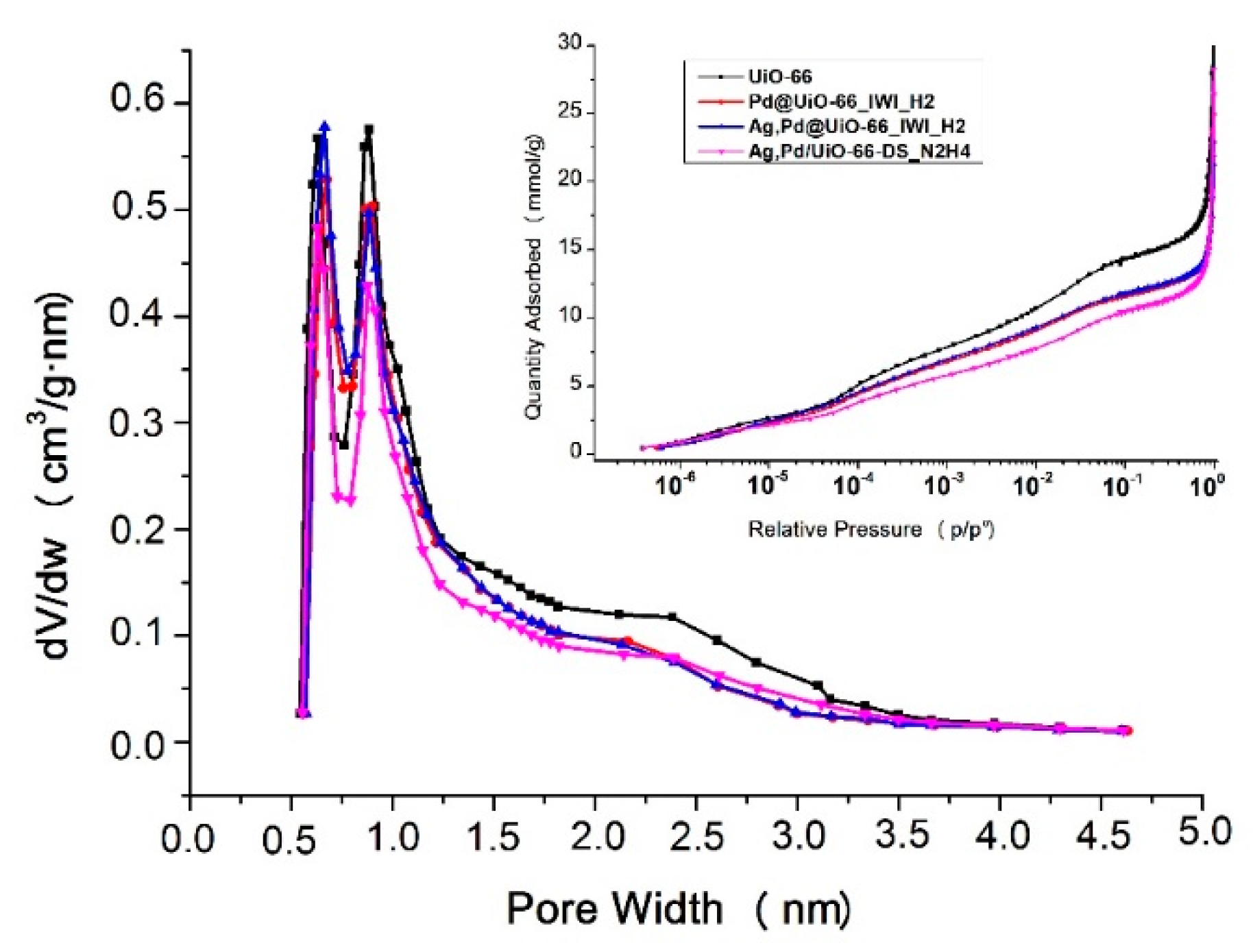



| Abbreviation | Impregnation | Drying | Reduction Conditions |
|---|---|---|---|
| WI_H2 | IWI | 20 h at room temperature | H2/Ar at 200 °C 2 h |
| MeCN_WI_H2 | IWI | 20 h at room temperature | H2/Ar at 200 °C 2 h |
| DS_N2H4 | DS | no drying | N2H4 at room temperature |
| Sample | UiO-66 | WI_H2 | MeCN_WI_H2 | DS_N2H4 |
|---|---|---|---|---|
| SBET, m2/g | 1298 | 1268 | 1246 | 982 |
| Vmicropores a, cm3/g | 0.50 | 0.50 | 0.49 | 0.38 |
| Sample | Area under NH3-TPD Peak, a.u./g | Amount of Lewis Acid Sites, µmol/g |
|---|---|---|
| UiO-66 | 9.22 | 939 |
| AgPd@UiO-66 | 8.32 | 847 |
| № | Preparation Method | Me NPs, wt % | Ag/Pd Ratio | PG/Me | PG Conv. % | LA Sel., % | HA Sel., % | AA Sel., % | FA Sel., % | Other Products* Sel., % |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | WI_H2 | 1 | 1/1 | 9174 | 1.0 | 20.0 | 5 | 0 | 6.7 | 68.7 |
| 2 | MeCN_WI_H2 | 1 | 1/1 | 9174 | 5.0 | 0 | 0 | 75 | 8.3 | 16.7 |
| 3 | DS_N2H4 | 1 | 1/1 | 9174 | 7 | 35.7 | 10 | 0 | 17.6 | 36.7 |
| 4 | DS_N2H4 | 1 | 85/15 | 9174 | 6 | 80 | 15 | 0 | 2.8 | 2.2 |
| 5 | DS_N2H4 | 1 | 15/85 | 9174 | 2 | 9 | 15 | 25 | 13.3 | 37.7 |
| 6 | DS_N2H4 | 1 | 0/1 | 9174 | 1 | 0 | 0 | 0 | 0 | 100 |
| 7 | DS_N2H4 | 0.5 | 85/15 | 18,348 | 2.5 | 64 | 20 | 0 | 4.5 | 11.5 |
| 8 | DS_N2H4 | 3 | 85/15 | 3058 | 10 | 5 | 30 | 4 | 4.7 | 56.3 |
| 9 | DS_N2H4 | 5 | 85/15 | 1834 | 12 | 2.5 | 41.7 | 8.7 | 1.4 | 54.3 |
| 10 | UiO-66 | 0 | 0 | 300 ** | 48 | 77 | 0 | 10 | 5 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ten, S.; Torbina, V.V.; Zaikovskii, V.I.; Kulinich, S.A.; Vodyankina, O.V. Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid. Materials 2020, 13, 5471. https://doi.org/10.3390/ma13235471
Ten S, Torbina VV, Zaikovskii VI, Kulinich SA, Vodyankina OV. Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid. Materials. 2020; 13(23):5471. https://doi.org/10.3390/ma13235471
Chicago/Turabian StyleTen, Sergey, Viktoriia V. Torbina, Vladimir I. Zaikovskii, Sergei A. Kulinich, and Olga V. Vodyankina. 2020. "Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid" Materials 13, no. 23: 5471. https://doi.org/10.3390/ma13235471
APA StyleTen, S., Torbina, V. V., Zaikovskii, V. I., Kulinich, S. A., & Vodyankina, O. V. (2020). Bimetallic AgPd/UiO-66 Hybrid Catalysts for Propylene Glycol Oxidation into Lactic Acid. Materials, 13(23), 5471. https://doi.org/10.3390/ma13235471







