Abstract
In situ high-temperature Raman spectra of polycrystalline KBi(MoO4)2 were recorded from room temperature to 1073 K. Thermal stability of the monoclinic KBi(MoO4)2 was examined by temperature-dependent XRD. The monoclinic phase transformed into the scheelite tetragonal structure at 833 K, and then to the monoclinic phase at 773 K. Quantum chemistry ab initio calculation was performed to simulate the Raman spectra of the structure of KBi(MoO4)2 high-temperature melt. The experimental Raman band at 1023 K was deconvoluted into seven Gaussian peaks, and the calculated results were in good agreement with the experimental data. Therefore, the vibrational modes of Raman peaks of molten KBi(MoO4)2 were assigned. It was confirmed that the isolated structure of [Bi(MoO4)2]− monomer, consisting of Mo6+ centers and Bi3+ sub-centers connected by edge-sharing, mainly exists in the melt of KBi(MoO4)2.
1. Introduction
Double molybdates have attracted extensive interest due to their potential applications in various fields of science and technology [1]. Compounds with the general formula ARE(MO4)2 (A = Li, Na, K, RE = rare earth or Bi, and M = W or Mo) have wide application as hosts for lasers, fluorescence, and scintillating materials [2,3,4,5], due to their excellent chemical durability in air atmosphere, high rare earth ion admittance, large absorption, and emission cross-sections of rare earth ions in their lattice [6,7,8,9,10,11,12,13,14,15,16,17]. Double molybdates of monovalent and trivalent cations have the following three characteristics reported by Isupov [18]: (i) there are many combinations of monovalent (Li, Na, K, Rb, Cs, Tl, Ag) and trivalent (Al, Sc, Fe, Ga, Y, In, La-Lu, Bi) elements in compounds, (ii) the number of crystal types of these compounds is large, and (iii) there are numerous reconstructive phase transitions which differ from the displacement transitions. Regarding the discovery, synthesis, and physicochemical examination of double molybdates with all possible compositions and different cation valences, relevant research began intensively decades ago and has been continuously developing with undiminished interest [19,20,21,22,23].
Much attention has been paid to the rare earth doped bismuth molybdates and tungstates. In particular, double potassium bismuth molybdate KBi(MoO4)2 are generally used as promising hosts for a variety of luminescent RE3+ ions, such as the doped Cr3+:KCrxBi1-x(MoO4)2 system [24,25,26]. It was reported that the KBi(MoO4)2 ceramic showed a distorted scheelite structure and a very low sintering temperature around 903 K [27]. Infrared and Raman spectroscopy were used by Hanuza et al. [28] to obtain the distribution of vibrational levels, the symmetry, and assignment to the respective normal modes of KBi(MoO4)2 crystal. The transformation of α-KBi(MoO4)2 into the disordered CaWO4 structure took place continuously and was accompanied by a very weak endothermic effect [29]. High-temperature melt is the mother liquor of crystal growth; however, the structure of double molybdate melt still remains unknown.
This study attempts to derive the structure information of the melt of KBi(MoO4)2 from high-temperature Raman spectroscopy, mainly including the structural units in atomic scale and the existing form of multi-molecular clusters structure. In the present paper, we report the temperature-dependent Raman spectroscopy studies of polycrystalline KBi(MoO4)2 from room temperature to 1073 K in order to obtain information on the structural changes that occur in this material. High-temperature X-ray diffraction (XRD) was also carried out to characterize the thermal stability of the room temperature phase. Quantum chemistry ab initio calculation was carried out to explore the structure of KBi(MoO4)2 in the molten state.
2. Materials and Methods
The starting materials of K2CO3, Bi2O3, and MoO3 (analytically pure from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were used in the experiment without any purification or treatment. The synthesis of KBi(MoO4)2 compound was achieved via the melting method, in which the stoichiometric ratio of the original reagents was mixed, uniformly ground, and then heated in a platinum crucible. The temperature control settings are shown in Table 1.

Table 1.
The temperature control settings used for the synthesis of KBi(MoO4)2 crystal.
The phase identification was performed using a D8 Advance diffractometer (Bruker AXS, Karlsruhe, Germany) with Cu Kα radiation in the Bragg–Brentano geometry mode. Temperature-dependent XRD data were collected from room temperature (RT) up to 933 K under air atmosphere in the 2θ range of 5–70° with a step size of 0.016° and an acquisition time of 0.5 s/step. The powder sample was placed in a platinum-lined corundum sample holder and then heated in a high-temperature chamber (HTK 1200N, Anton Paar, Austria). The collected diffraction data were analyzed using the JADE 6.0 software with the JCPDS-ICDD Powder Diffraction File database.
In situ high-temperature Raman spectra of KBi(MoO4)2 crystal were recorded with a laser confocal Raman spectrometer (LabRAM HR800, Horiba Jobin Y’von, France), which was equipped with an intensity charge coupled device (ICCD) detector for signal enhancement at high temperature. The microscopic heating furnace (Linkam, TS1500, Tadworth, UK) was used to achieve the heating and cooling (the rate controlled at 5 K/min) of the sample in the temperature range from RT to 1073 K with a precision of ±1 K. Each Raman spectrum was collected after keeping the sample for 10 min at the given temperature, which was helpful for elimination of the hysteresis of structural changes induced by temperature. The 532 nm line of a Q-switch pulsed SHG-Nd:YAG laser (Coherent, Santa Clara, CA, USA) was used as an excitation source. In this study, the slits were set for a resolution of about 1 cm−1.
In order to explore the melt structure of KBi(MoO4)2, a series of typical structural units and their multi-molecular clusters structure were designed. Ab initio calculation, a powerful quantum chemistry program for studying finite scale systems [30,31], was performed to study the short-range ordered clusters structure and their properties dependent on this scale of order. The geometry configuration of the designed structural models in the melt of KBi(MoO4)2 was first optimized before simulating their vibrational Raman spectra using the Gaussian 09 software package. A pseudopotential basis set of LanL2DZ [32,33] and the method of Restricted Hartree–Fock (RHF) [34] were adopted. The single point energy of the cluster structure models with the singlet spin was selected to obtain the ground state.
3. Results and Discussion
3.1. Temperature-Dependent XRD Spectra
At room temperature and in atmospheric pressure, the crystallization of KBi(MoO4)2 is monoclinic belonging to the space group P21/c (C2h5), with twelve molecules in the unit cell. It is isostructural with α-KSm(MoO4)2 for which a = 16.69, b = 23.85, c = 5.30, and β = 91.3° [28,35]. Some studies have indicated that at temperatures close to the melting or decomposition point, most double molybdates show the tetragonal scheelite structure with a centrosymmetric space group (SG) I41/a (No. 88) [17]. The structure of KBi(MoO4)2 is reported to be similar to the distorted scheelite-type (CaWO4) structure [36].
High-temperature XRD of the KBi(MoO4)2 was performed in order to examine the thermal stability of the monoclinic phase, as is shown in Figure 1. Upon heating, nearly all the diffraction peaks shifted to lower Bragg angles. The XRD pattern changed significantly from 783 K to 833 K, especially the disappearance of the peaks in the 2θ range of 27.2–27.5°, 32–34°, 44–48°, and 54–57°, and the new appearance of the peaks at 27.3°, 32.8°, and 44.5°, which is recognized as the characteristics of the scheelite tetragonal structure. In the low Bragg angle range (<25°), the diffraction peaks disappeared completely at 833 K. That is, as the temperature increased, the split peaks that represent the monoclinic symmetry gradually merged into one, and the ratio of the peak intensities changed with the transformation into a tetragonal structure. The splitting gradually disappeared toward 833 K, and the structure became fully tetragonal with the I41/a space group. The order–disorder transformation of Bi3+ and K+ cations gradually occurred in the crystal structure. Some earlier studies indicated that the low-temperature monoclinic phase of KBi(MoO4)2 continuously transforms to the high-temperature disordered tetragonal scheelite structure in the temperature range ΔT ≥ 150 K and is completed at 933 K, accompanied by a very weak endothermic effect [29,37]. The observed diffraction change from monoclinic to tetragonal is consistent with those reported data. After the temperature decreased to 773 K, a diffraction pattern of monoclinic appeared, but there was still a certain difference from the diffraction spectrum of 783 K. This is probably because the phase structure could not be completely restored due to the faster cooling rate (10 K/min). Therefore, it can be inferred that the phase transition from monoclinic to tetragonal may be reversible at about 773 K when the cooling rate is slow enough. After cooling down to room temperature, the diffraction pattern showed monoclinic reflections. However, the positions of the diffraction peaks after cooling to room temperature had a shift, the intensities of the peaks were lower, and the half-widths of the peaks became larger than those at room temperature before heating. This demonstrates that the monoclinic structure was reobtained, but not completely.
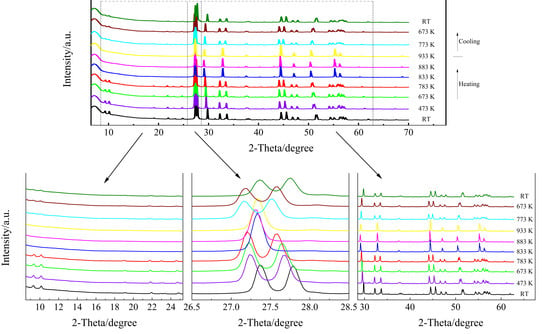
Figure 1.
High-temperature XRD patterns of KBi(MoO4)2, where RT stands for room temperature.
3.2. Temperature-Dependent Raman Spectra
For the monoclinic KBi(MoO4)2, according to the factor group analysis (FGA), the 432 vibrational modes were predicted: Γ = 108Ag + 108Bg + 108Au + 108Bu where Au + 2Bu are acoustic modes. There are 429 optical zonecenter modes, 216 of which are Raman-active only and 213 of which are IR-active only. It is reported that the predicted vibrational modes were distributed among 54Ag + 54Bg + 54Au + 54Bu internal modes, 18Ag + 18Bg + 18Au + 18Bu librational modes, and 36Ag + 36Bg + 35Au + 34Bu translational modes [28]. According to selection rules, only Ag and Bg are Raman-active.
Raman spectra of KBi(MoO4)2 crystal at room temperature are present in Figure 2. It is obvious that the number of vibrational modes observed is much smaller than that predicted for the KBi(MoO4)2, which is caused by the polycrystalline samples in the measurement. When it comes to solid-state Raman band distribution, a more appropriate distinction is made between internal and external lattice vibration modes. The most intense lines at 952 and 866 cm−1 are attributed to the symmetric and asymmetric stretching vibrations of MoO4, respectively. The Raman band in the middle frequency region of 580–750 cm−1 is characteristic of the vibrations of double MoO2Mo and single bridge MoOMo, which formed due to the intermolecular interactions that exist in the unit cell. The assignment of the major Raman vibrational modes of monoclinic KBi(MoO4)2 is displayed in Table 2.
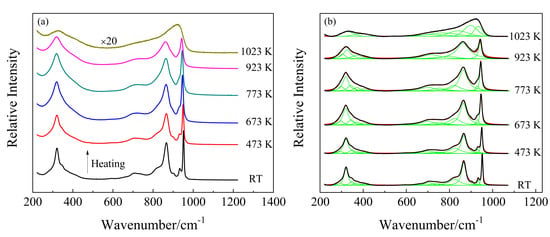
Figure 2.
(a) Temperature-dependent Raman spectra of polycrystalline KBi(MoO4)2 from room temperature to 1023 K; (b) deconvolution of Raman spectra by Gaussian (1023 K) and Lorentz (from RT to 923 K) function as a function of temperature.

Table 2.
The assignment of major vibrational modes of crystalline KBi(MoO4)2.
In situ high-temperature Raman spectra of KBi(MoO4)2 from room temperature to 1023 K in the wavenumber range of 200–1200 cm−1 are shown in Figure 2a. As the temperature increased from room temperature to 923 K, the overall line shapes of the Raman spectra had no obvious change, whereas the full width at half maximum (FWHM) of nearly all the Raman peaks increased and their intensities decreased, which is shown more clearly in Figure 3b. With temperature increasing from RT to 773 K, the adjacent weak and shoulder Raman peaks merged with the major one. It can be seen from Figure 3a that the modes located at 347, 373, 404, 704, 823, 866, 933, and 952 cm−1 exhibited a decrease in wavenumber, whereas the modes of 294, 593, and 739 cm−1 showed a linear increase with increasing temperature. The structure gradually relaxed with broadening distribution of atomic bond distances and bond angles due to thermal expansion and thermal disorder. With a further temperature increase to 923 K, the vibrational modes of 342, 401, 822, and 930 cm−1 (monoclinic phase at 773 K) disappeared, while the modes of 298, 319, 700, and 751 cm−1 increased obviously in wavenumber. The Raman response was still very well-defined at 923 K, but it was suddenly strongly diffused at 1023 K. At first sight, such spectral evolution should be related to structural phase transitions. Combined with the high-temperature XRD results, it can be determined that at 833 K the monoclinic phase of KBi(MoO4)2 to the tetragonal transformation occurred. A similar pseudo-tetragonal lattice distortion appearing in KBi(MoO4)2, which was attributed to the ordering of the K+ and Bi3+ ions [37], may explain the slow and continuous change of Raman spectra before this transition. Figure 2b demonstrates the deconvolution of Raman spectra corresponding to the experimental temperatures by Gaussian and Lorentz function for the molten spectrum and the other spectra, respectively. The spectrum of the KBi(MoO4)2 melt was deconvoluted into seven Gaussian Raman peaks at 1023 K. The dramatic changes in the Raman spectra from 923 K to 1023 K were due to the solid–liquid phase transformation, and the tetragonal crystal structure was destroyed and in a completely molten state.
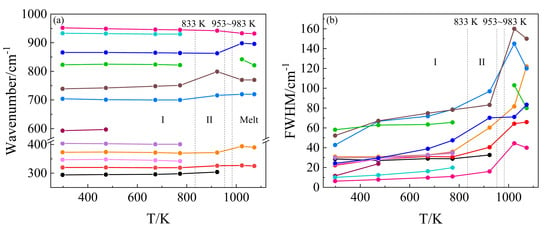
Figure 3.
(a) Wavenumber versus temperature and (b) full width at half maximum (FWHM) versus temperature plot for the major stretching and bending vibrational modes of KBi(MoO4)2. The vertical dashed lines indicate the reported temperature [27,37] at which the phase transition (phase I: monoclinic, phase II: tetragonal, and the melting point of 953–983 K) takes place.
3.3. Structure of Molten KBi(MoO4)2
The Raman spectrum of the molten KBi(MoO4)2 at 1023 K is shown in Figure 4. The whole envelope was deconvoluted by Gaussian function after subtracting the baseline of the spectrum. The anion motifs of isolated [MoO4]2− tetrahedra have been reported to exist in the melt of alkali metal monomolybdates [38,39]. KBi(MoO4)2 has the same chemical ratio as K2MoO4; however, their band shapes and positions are quite different. This indicates that the melt structure of KBi(MoO4)2 cannot be the simple monomer of [MoO4]2−. Nevertheless, on the basis of the distribution of different structural units previously studied [40], the six coordinated [MoO6]6− cannot be the primary structure in the melt of KBi(MoO4)2. From the positions of the deconvoluted Raman peaks, the four coordinated tetrahedral [MoO4]2− can be confirmed to be present in the melt.
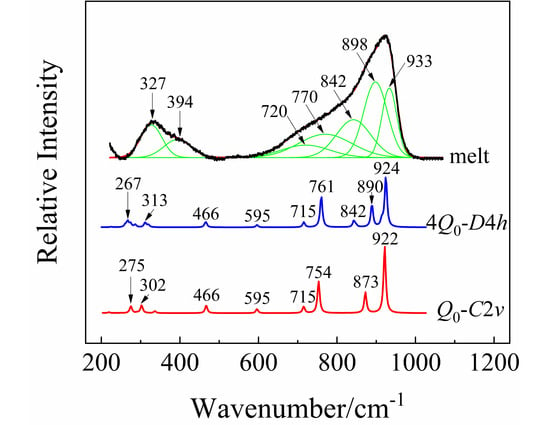
Figure 4.
The deconvolution of Raman spectrum for the melt of KBi(MoO4)2 at 1023 K by Gaussian function, and the calculated Raman spectra by quantum chemistry ab initio calculation, where Q0 and 4Q0 represent the structural unit and the corresponding multi-molecular clusters structure containing four structural units.
In order to further examine the structural unit and its multi-molecular clusters structure in the melt of KBi(MoO4)2, quantum chemistry ab initio calculation was performed for the optimization and simulation of the vibrational properties of the designed structure. Considering the large electronegativity of the heavier Bi atom, the [MoO4]2− group is more likely to be coordinated to Bi3+ than K+. Therefore, a model of “double-center” structure with K+ acting as the charge compensation was proposed and illustrated in Figure 5a. Due to the strong attraction of Bi to electrons, it will compete for the non-bridging oxygens around Mo atoms, thereby increasing the number of bridging oxygens in the system. Note that Bi is a sub-center, while Mo is still the center of the entire structural system. The oxygen atoms coordinated to Bi are also shared by the [MoO4]2− tetrahedra. Taking into account the effect of multi-molecular cluster structure, the corresponding multi-molecular cluster containing four structural units was also built and is exhibited in Figure 5b,c. The calculated Raman spectra of the cluster structure models by ab initio calculation are shown in Figure 4 after being corrected by a wavenumber scaling factor of 0.8555 and normalizing the intensity. Through comparative analysis, the calculated Raman frequencies and intensities are consistent with the data measured experimentally.
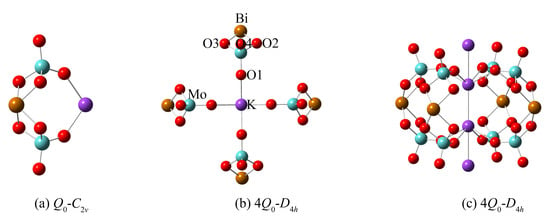
Figure 5.
(a) The designed structural units and (b,c) the corresponding multi-molecular clusters structure for the ab initio calculation of molten Raman spectra of KBi(MoO4)2. Here, (b,c) represent the same cluster structure containing four molecules viewed from the in-plane and out-of-plane directions. The elements are labeled (Mo, light blue; K, violet; Bi, light brown; O, red) in the diagram of (b).
As can be seen from Figure 5, the structure models of anion motifs (a) represent a monomer (Q0) molecule of KBi(MoO4)2 with C2v symmetry, and (b,c) represent the multi-molecular cluster structure with higher D4h symmetry which consists of four monomers (4Q0). In the anionic multi-molecular cluster structure, the environment of each Q0 is equivalent. Figure 4 shows the calculated Raman spectra of a different number of model clusters of KBi(MoO4)2. The calculation result demonstrates that as the number of model clusters increases, the frequency of the characteristic peaks shifts toward a high wavenumber, which has been observed earlier caused by the multi-molecular cluster effect. Therefore, considering the environmental effects of cations, the more reliable multi-molecular cluster structure is closer to the real structure of the melt of KBi(MoO4)2. Through the combination of the experimental and calculation data, it is proved that the structure of the [Bi(MoO4)2]− monomer with Mo6+ centers and Bi3+ sub-centers primarily presents in the melt of KBi(MoO4)2 molybdate and is distributed in a short-range ordered and long-range disordered state.
In Figure 4, the fitted Raman peak at 933 cm−1 originates from the symmetric stretching vibrations of non-bridging oxygens of Mo-O4. The peaks located at 898 and 842 cm−1 are attributed to the asymmetric stretching vibrations of non-bridging oxygens of Mo-O1. The peak position at 761 cm−1 from calculation clearly shows the vibrations of the Bi-O bond with an oxygen coordination number of 4. The wavenumber region of 450–770 cm−1 mainly involves the vibrations of Bi-O in the structure. Table 3 lists the experimental and calculated wavenumbers of the major vibrational modes and their assignment in the melt of KBi(MoO4)2. For instance, the peak at 770 cm−1 was assigned to the symmetric scissor vibrations of Bi-O2 and Bi-O3, whereas the asymmetric scissor vibrations of Bi-O are around 720 cm−1. The peaks at about 394 and 327 cm−1 are caused by the asymmetric stretching and wagging vibrations of Mo-O in the clusters structure.

Table 3.
The attribution of major vibrational modes in the melt of KBi(MoO4)2.
4. Conclusions
In situ high-temperature Raman spectroscopy and quantum chemistry ab initio calculation were applied to investigate the structure present in the melt of KBi(MoO4)2 molybdates. The temperature-induced phase transition from monoclinic to the scheelite tetragonal structure was observed to occur at 833 K by temperature-dependent XRD. The calculated results via the ab initio method are in good agreement with the experimental Raman data, demonstrating the reliability of the assignment of the observed Raman bands for the molten KBi(MoO4)2 at 1023 K. The isolated structure of [Bi(MoO4)2]− monomer anion, in which the oxygen coordination numbers of Mo and Bi atoms are both four and connected to each other by edge-sharing, is confirmed to exist primarily in the melt of KBi(MoO4)2. The study on the melt structure of KBi(MoO4)2 makes it possible to examine clusters structure composed of different complexes and Mz+ cations and opens up a new way for the exploration of the development of novel laser host materials.
Author Contributions
Conceptualization, M.W.; methodology and formal analysis, M.W., C.W., and J.Y.; software, M.W. and J.W.; data curation, M.W. and X.G.; writing—original draft preparation, M.W.; writing—review and editing, M.W., C.W., J.W., L.L., X.G., X.T., F.Z., and J.Y.; visualization, M.W., X.T., and F.Z.; supervision, J.Y. and L.L.; funding acquisition, M.W. and J.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 21773152) and Shanghai Committee of Science and Technology, China (Grant No. 12520709200). Wang thanks the China Postdoctoral Science Foundation (Grant No. 2019M661460) for support.
Conflicts of Interest
The authors declare no competing financial interest.
References
- Hizhnyi, Y.; Nedilko, S.; Chornii, V.; Nikolaenko, T.; Zatovsky, I.; Terebilenko, K.; Boiko, R. Electronic structure and luminescence mechanisms in MIMIII(MoO4)2 molybdates. In Proceedings of the IEEE International Conference on Oxide Materials for Electronic Engineering (OMEE), Lviv, Ukraine, 3–7 September 2012; pp. 135–136. [Google Scholar] [CrossRef]
- Yang, J.; Fu, P.; Lin, Z. Preparation of KBi(MoO4)2 nanocrystallite by solvothermal process and its gas-sensing properties. Mater. Res. Express 2018, 5, 065033. [Google Scholar] [CrossRef]
- Vasylkiv, Y.; Kvasnyuk, O.; Shopa, Y.; Vlokh, R. Optical activity caused by torsion stresses: The case of NaBi(MoO4)2 crystals. J. Opt. Soc. Am. A 2013, 30, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Ryadun, A.A.; Nadolinny, V.A.; Tsydypova, B.N.; Pavlyuk, A.A. Electron paramagnetic resonance and photoluminescence of NaBi(MoO4)2 crystals doped with gadolinium ions. Phys. Solid State 2015, 57, 1188–1191. [Google Scholar] [CrossRef]
- Adhikari, R.; Joshi, B.; Narro-García, R.; De la Rosa, E.; Lee, S.W. Microwave hydrothermal synthesis and infrared to visible upconversion luminescence of Er3+/Yb3+ co-doped bismuth molybdate nanopowder. J. Lumin. 2014, 145, 866–871. [Google Scholar] [CrossRef]
- Mateos, X.; Solé, R.; Gavaldà, J.; Aguiló, M.; Massons, J.; Díaz, F. Crystal growth, optical and spectroscopic characterisation of monoclinic KY(WO4)2 co-doped with Er3+ and Yb3+. Opt. Mater. 2006, 28, 423–431. [Google Scholar] [CrossRef]
- Kasprowicz, D.; Drozdowski, M.; Majchrowski, A.; Michalski, E. Spectroscopic properties of KGd(WO4)2: (Er, Yb) single crystals studied by Brillouin scattering method. Opt. Mater. 2007, 30, 152–154. [Google Scholar] [CrossRef]
- Mateos, X.; Pujol, M.C.; Güell, F.; Solé, R.; Gavaldà, J.; Aguiló, M.; Díaz, F.; Massons, J. Sensitization of Er3+ emission at 1.5μm by Yb3+ in KYb(WO4)2 single crystals. Phys. Rev. B 2002, 66, 214104. [Google Scholar] [CrossRef]
- Cheng, Z.X.; Zhang, S.J.; Song, F.; Guo, H.C.; Han, J.R.; Chen, H.C. Optical spectroscopy of Yb/Er codoped NaY(WO4)2 crystal. J. Phys. Chem. Solids 2002, 63, 2011–2017. [Google Scholar] [CrossRef]
- Song, F.; Tan, H.; Shang, M.R.; Zhang, G.Y.; Cheng, Z.X.; Chen, H.C. Spectra characteristics of Er3+ doped NaY(WO4)2 crystal. Acta Phys. Sin. 2002, 51, 2375–2379. [Google Scholar]
- Huang, J.H.; Gong, X.H.; Chen, Y.J.; Lin, Y.F.; Liao, J.S.; Chen, X.Y.; Luo, Z.D.; Huang, Y.D. Polarized spectral properties of Er3+ ions in NaGd(WO4)2 crystal. Appl. Phys. B 2007, 89, 73–80. [Google Scholar] [CrossRef]
- Lu, X.; You, Z.; Li, J.; Zhu, Z.; Jia, G.; Wu, B.; Tu, C. The optical properties of Er3+ doped NaY(MoO4)2 crystal for laser applications around 1.5 μm. J. Alloys Compd. 2006, 426, 352–356. [Google Scholar] [CrossRef]
- Kuz’micheva, G.M.; Lis, D.A.; Subbotin, K.A.; Rybakov, V.B.; Zharikov, E.V. Growth and structural X-ray investigations of scheelite-like single crystals Er, Ce:NaLa(MoO4)2 and Yb:NaGd(WO4)2. J. Cryst. Growth 2005, 275, e1835–e1842. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Zhang, L.; Wang, G. Growth, thermal and spectroscopic characterization of Er3+:NaY(MoO4)2 crystal. J. Cryst. Growth 2006, 293, 157–161. [Google Scholar] [CrossRef]
- Li, X.; Lin, Z.; Zhang, L.; Wang, G. Growth, thermal and spectral properties of Nd3+-doped NaGd(MoO4)2 crystal. J. Cryst. Growth 2006, 290, 670–673. [Google Scholar] [CrossRef]
- Sardar, D.K.; Russell, C.C., III; Yow, R.M.; Gruber, J.B.; Zandi, B.; Kokanyan, E.P. Spectroscopic analysis of the Er3+ (4f11) absorption intensities in NaBi(WO4)2. J. Appl. Phys. 2004, 95, 1180–1184. [Google Scholar] [CrossRef]
- Rico, M.; Méndez-Blas, A.; Volkov, V.; Monge, M.Á.; Cascales, C.; Zaldo, C.; Kling, A.; Fernández-Díaz, M.T. Polarization and local disorder effects on the properties of Er3+-doped XBi(YO4)2, X = Li or Na and Y = W or Mo, crystalline tunable laser hosts. J. Opt. Soc. Am. B 2006, 23, 2066–2078. [Google Scholar] [CrossRef]
- Isupov, V.A. Binary molybdates and tungstates of monoand trivalent elements as possible ferroelastics and ferroelectrics. Ferroelectrics 2005, 321, 63–90. [Google Scholar] [CrossRef]
- Wang, M.; You, J.; Sobol, A.A.; Lu, L.; Wang, J.; Xie, Y. In-situ studies of structure transformation and Al coordination of KAl(MoO4)2 during heating by high temperature Raman and 27Al NMR spectroscopies. Materials 2017, 10, 310. [Google Scholar] [CrossRef]
- Li, K.; Deun, R.V. Low-temperature solid-state synthesis and upconversion luminescence properties in (Na/Li)Bi(MoO4)2:Yb3+,Er3+ and color tuning in (Na/Li)Bi(MoO4)2:Yb3+,Ho3+,Ce3+ phosphors. Inorg. Chem. 2019, 58, 6821–6831. [Google Scholar] [CrossRef]
- Amarasinghe, D.K.; Perera, S.S.; Rabuffetti, F.A. Rotational disorder in scheelite-type NaRE(MO4)2 (RE = Rare-Earth, Y; M = Mo, W). Cryst. Growth Des. 2020, 20, 3442–3448. [Google Scholar] [CrossRef]
- Atuchin, V.V.; Aleksandrovsky, A.S.; Bazarov, B.G.; Bazarova, J.G.; Chimitova, O.D.; Denisenko, Y.G.; Gavrilova, T.A.; Krylov, A.S.; Maximovskiy, E.A.; Molokeev, M.S.; et al. Exploration of structural, vibrational and spectroscopic properties of self-activated orthorhombic double molybdate RbEu(MoO4)2 with isolated MoO4 units. J. Alloys Compd. 2019, 785, 692–697. [Google Scholar] [CrossRef]
- Dudnikova, V.B.; Zharikov, E.V.; Eremin, N.N. Local structure of molybdates solid solutions containing europium by results of atomistic simulation. Mater. Today Commun. 2020, 23, 101180. [Google Scholar] [CrossRef]
- Voda, M.; Balda, R.; Sáez de Ocáriz, I.; Lacha, L.M.; Illarramendi, M.A.; Fernández, J. Spectroscopic properties of rare earths in K5Bi1-x(RE)x(MoO4)4 crystals. J. Alloys Compd. 1998, 275–277, 214–218. [Google Scholar] [CrossRef]
- Huang, X.; Wang, G. Growth and optical characteristics of Er3+:LiLa(MoO4)2 crystal. J. Alloys Compd. 2009, 475, 693–697. [Google Scholar] [CrossRef]
- Yan, B.; Wu, J.H. NaY(MoO4)2:Eu3+ and NaY0.9Bi0.1(MoO4)2:Eu3+ submicrometer phosphors: Hydrothermal synthesis assisted by room temperature-solid state reaction. microstructure and photoluminescence. Mater. Chem. Phys. 2009, 116, 67–71. [Google Scholar] [CrossRef]
- Zhou, D.; Pang, L.X.; Guo, J.; Wang, H.; Yao, X.; Randall, C. Phase evolution, phase transition, Raman spectra, infrared spectra, and microwave dielectric properties of low temperature firing (K0.5xBi1−0.5x)(MoxV1−x)O4 ceramics with scheelite related structure. Inorg. Chem. 2011, 50, 12733–12738. [Google Scholar] [CrossRef]
- Hanuza, J.; Maczka, M.; van der Maas, J.H. Vibrational characteristics of the single-bridge MoOMo and double-bridge MoO2Mo intermolecular interactions-polarized infrared and Raman spectra of monoclinic KBi(MoO4)2 single crystal. Vib. Spectrosc. 1995, 8, 417–423. [Google Scholar] [CrossRef]
- Klevtsov, P.V.; Klevtsova, R.F. Polymorphism of the double molybdates and tungstates of mono-and trivalent metals with the composition M+R3+(EO4)2. J. Struct. Chem. 1977, 18, 339–355. [Google Scholar] [CrossRef]
- You, J.L.; Jiang, G.C.; Hou, H.Y.; Chen, H.; Wu, Y.Q.; Xu, K.D. Quantum chemistry study on superstructure and Raman spectra of binary sodium silicates. J. Raman Spectrosc. 2005, 36, 237–249. [Google Scholar] [CrossRef]
- Labet, V.; Colomban, P. Vibrational properties of silicates: A cluster model able to reproduce the effect of “SiO4” polymerization on Raman intensities. J. Non Cryst. Solids 2013, 370, 10–17. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Lee, T.J.; Jayatilaka, D. An open-shell restricted Hartree—Fock perturbation theory based on symmetric spin orbitals. Chem. Phys. Lett. 1993, 201, 1–10. [Google Scholar] [CrossRef]
- Hamann, D.R.; Schlüter, M.; Chiang, C. Norm-conserving pseudopotentials. Phys. Rev. Lett. 1979, 43, 1494. [Google Scholar] [CrossRef]
- Kisel, N.G.; Mokhosoev, M.V. Potassium and bismuth double molybdates. Ukr. Khim. Zh. 1972, 38, 743–745. [Google Scholar]
- Klevtsov, P.V.; Vinokurov, V.A.; Klevtsova, R.F. Double molybdates and tungstates of alkali metals with bismuth, M+Bi(TO4)2. Kristallografiya 1973, 18, 1192–1197. [Google Scholar]
- Klevtsov, P.V.; Vinokurov, V.A. Phase transformation in KBi(MoO4)2 crystals. Kristallografiya 1974, 19, 763–767. [Google Scholar]
- Voronko, Y.K.; Sobol, A.A.; Shukshin, V.E. Raman scattering study of molten alkali-metal molybdates and tungstates rich in basic oxides. Inorg. Mater. 2014, 50, 844–849. [Google Scholar] [CrossRef]
- Wang, M.; You, J.; Sobol, A.A.; Wang, J.; Wu, J.; Lv, X. Temperature-dependent Raman spectroscopic studies of microstructure present in dipotassium molybdate crystals and their melts. J. Raman Spectrosc. 2016, 47, 1259–1265. [Google Scholar] [CrossRef]
- Wang, M.; Simon, P.; Lu, L.; Sobol, A.A.; Wang, J.; Wan, S.; You, J. Quantitative studies on the structure of molten binary potassium molybdates by in situ Raman spectroscopy and quantum chemistry ab initio calculations. Anal. Chem. 2018, 90, 9085–9092. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).