Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
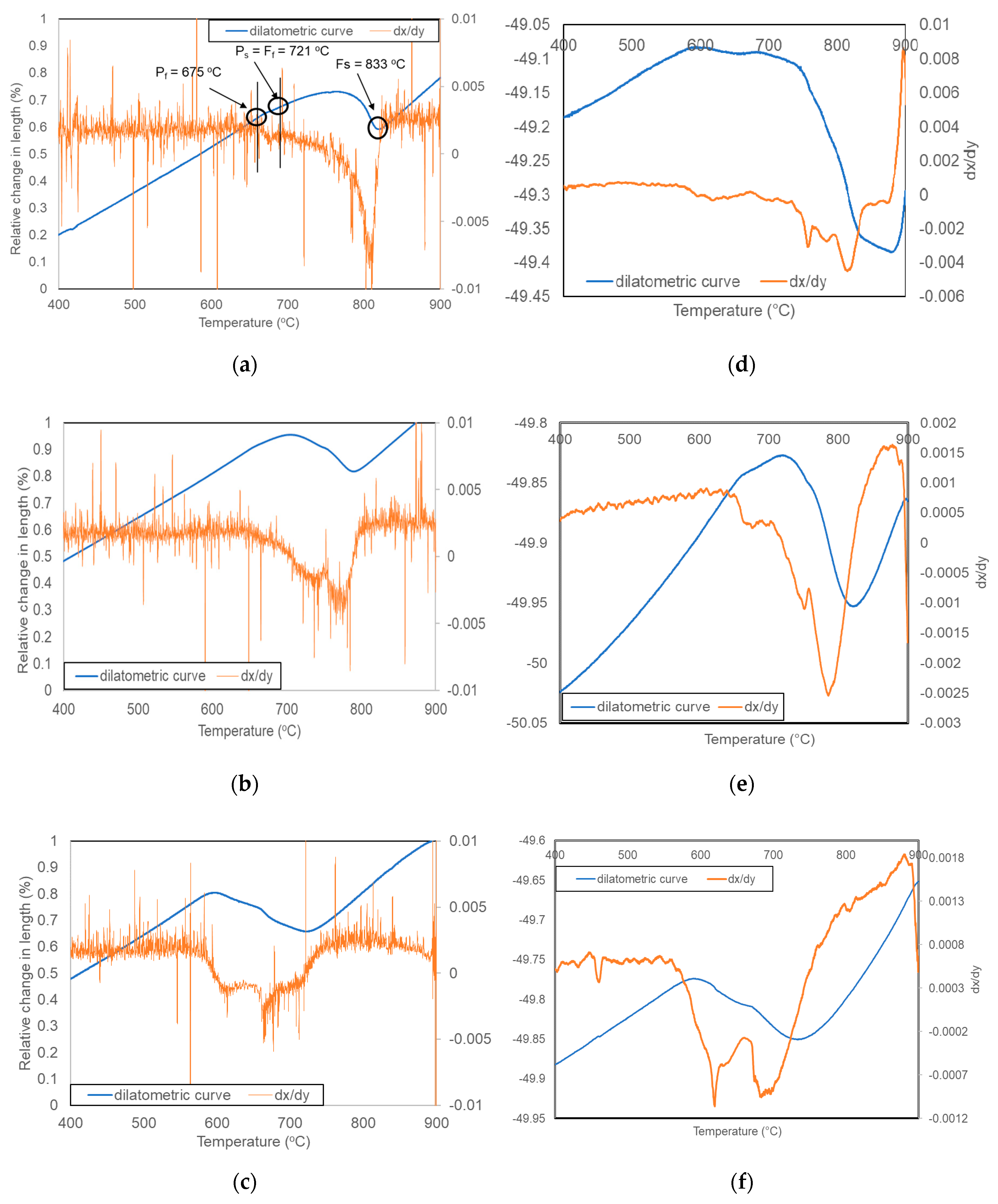
3.1. Dilatometric Analysis
3.2. Microstructure Evolution
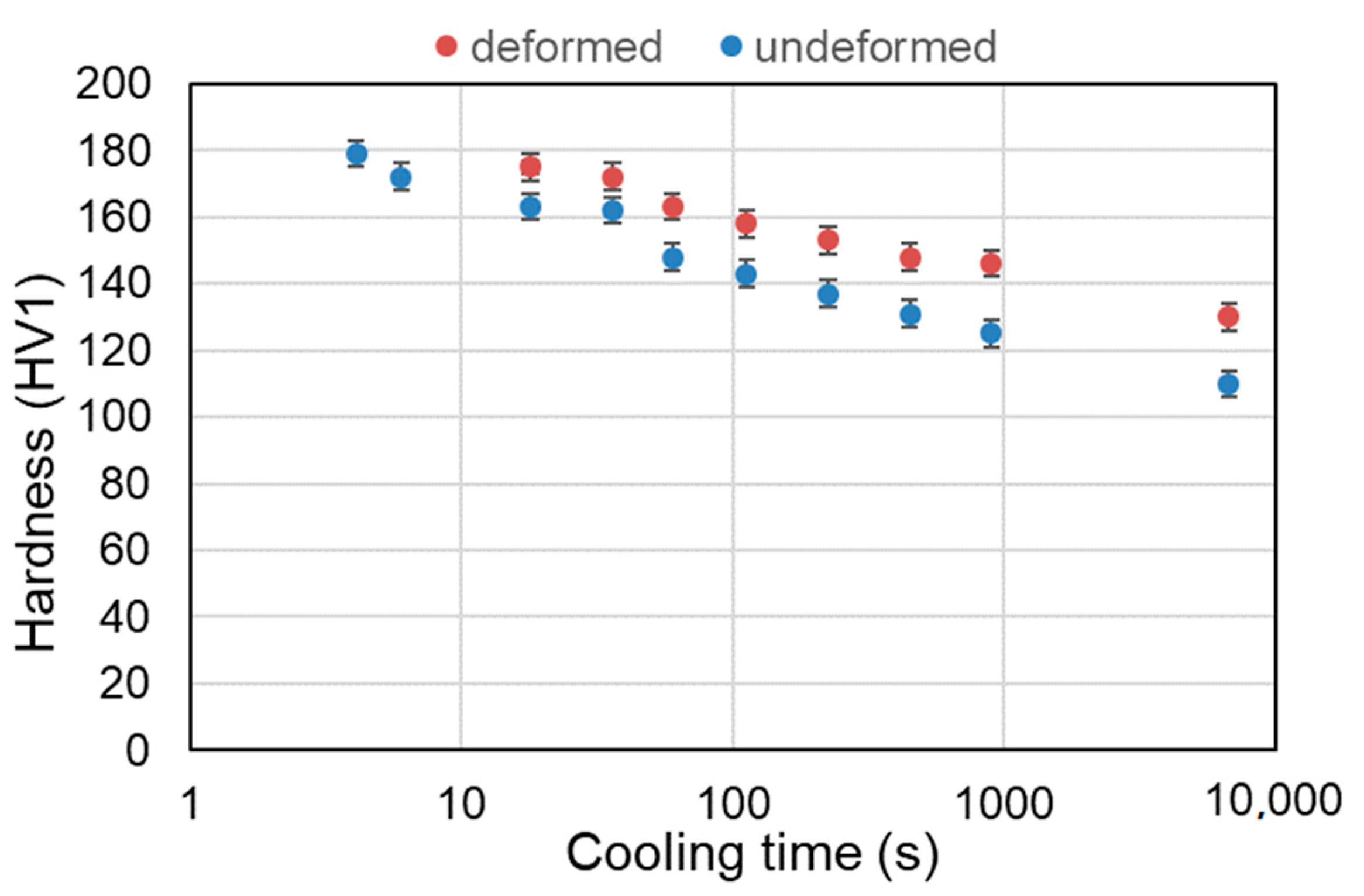
3.3. Hardness Analysis
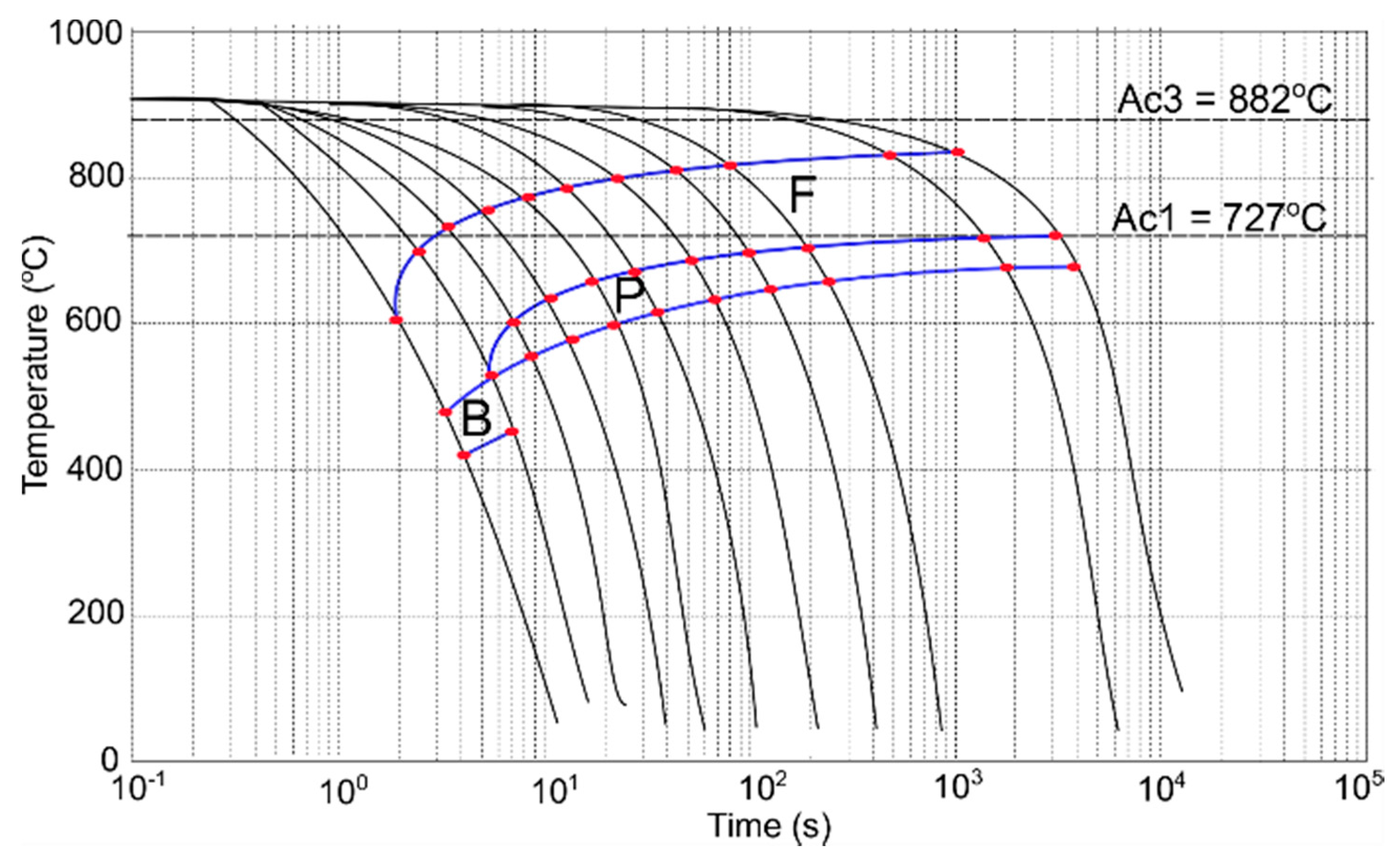
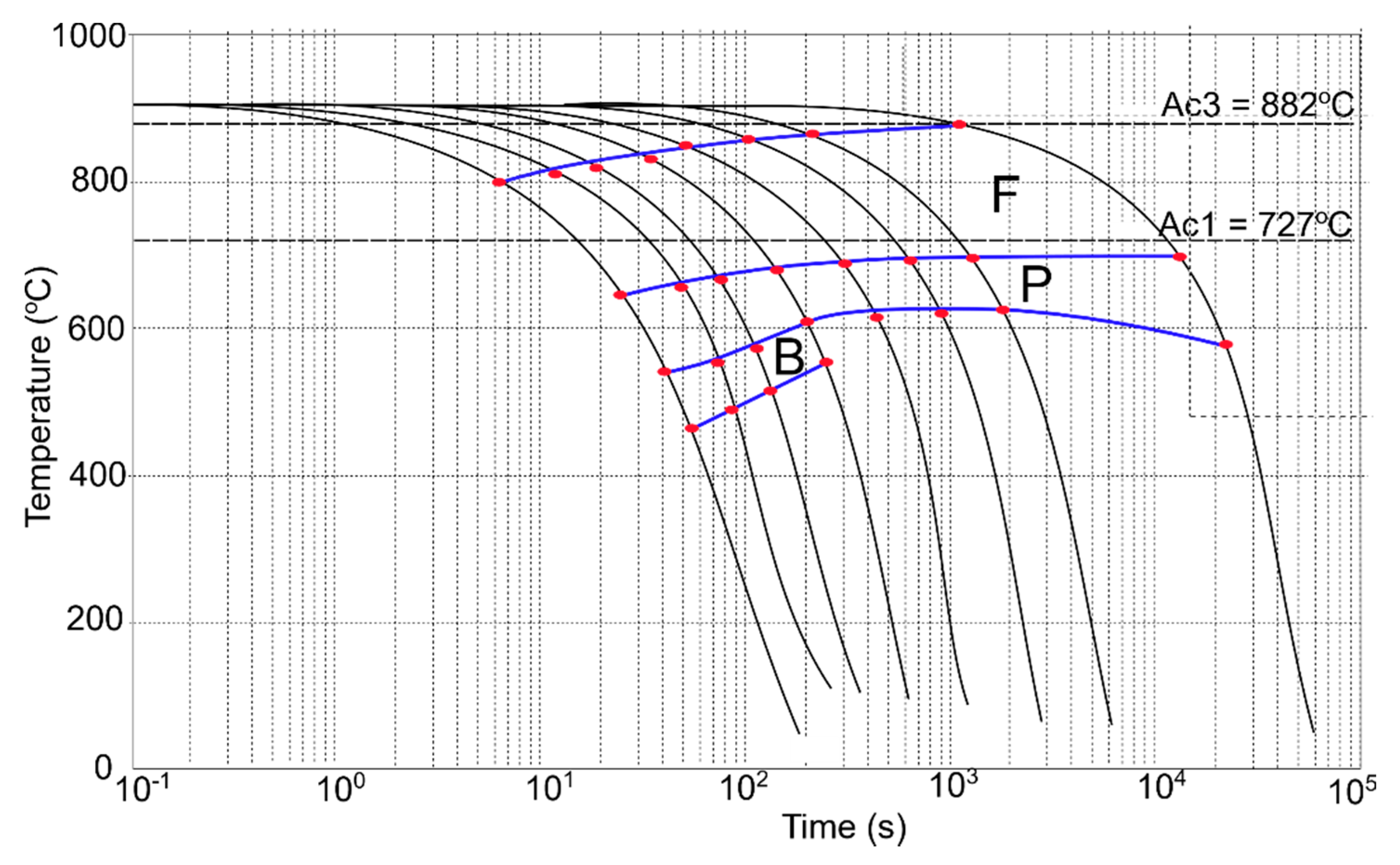
3.4. CCT and DCCT Diagrams
4. Conclusions
- The analyzed low-C steel is suitable for the production of ferrite-based microstructures because of its low carbon content and resulting in low hardenability.
- The hot deformation performed before cooling increases the diffusion rate of elements and highly influences the phase transformation kinetics.
- The deformation shifts the phase transformation product regions to higher temperatures and slower cooling rates.
- The deformation causes an increase of strain-induced preferable nucleation places for ferrite, pearlite, and bainite formation, which are diffusional or semi-diffusional (bainite) phase transformations.
- The necessary minimal cooling rate for bainite formation strongly decreases after deformation from 150 to 8 °C/s.
- The plastic deformation substantially enhanced grain refinement in the whole range of applied cooling rates.
- The hardness of the steel increases along with the increasing cooling rate, due to progressive grain refinement and lower transformation start temperatures, also inducing smaller grain sizes.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Inoue, T.; Ueji, R. Improvement of strength, toughness and ductility in ultrafine-grained low-carbon steel processed by warm bi-axial rolling. Mater. Sci. Eng. A 2020, 786, 139415. [Google Scholar] [CrossRef]
- Ahmed, M.; Bepari, M.M.A.; Galib, R.H. Effects of Chromium and Nickel Additions on the Austenite Grain Coarsening of Low Carbon Structural Steels Containing 0.13% C. Appl. Mech. Mater. 2016, 860, 152–157. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Paul, G.; Somani, M.; Porter, D.; Bracke, L.; Latz, A.; Garcia De Andres, C.; Caballero, F. Transferring nanoscale bainite concept to lower C contents: A perspective. Metals 2017, 7, 159. [Google Scholar] [CrossRef]
- Gorka, J.; Opiela, M. Structure and properties of high-strength low-alloy steel melted by the laser beam. Mater. Perform. Charact. 2019, 8, 1–10. [Google Scholar] [CrossRef]
- Dobrzański, L.A.; Czaja, M.; Borek, W.; Labisz, K.; Tański, T. Influence of Hot-Working Conditions on a Structure of X11MnSiAl17-1-3 Steel. Adv. Mater. Res. 2014, 1036, 122–127. [Google Scholar] [CrossRef]
- Caballero, F.G.; Allain, S.; Cornide, J.; Puerta Velásquez, J.D.; Garcia-Mateo, C.; Miller, M.K. Design of cold rolled and continuous annealed carbide-free bainitic steels for automotive application. Mater. Des. 2013, 49, 667–680. [Google Scholar] [CrossRef]
- Kawulok, R.; Opěla, P.; Schindler, I.; Kawulok, P.; Mizera, J.; Rusz, S.; Olszar, M.; Čmiel, K.M. The Influence of a Cooling Rate on the Evolution of Microstructure and Hardness of the Steel 27MnCrB5. Arch. Metall. Mater. 2018, 63, 907–914. [Google Scholar] [CrossRef]
- Grajcar, A.; Kuziak, R.; Zalecki, W. Designing of cooling conditions for Si-Al microalloyed TRIP steel on the basis of DCCT diagrams. J. Achiev. Mater. Manuf. Eng. 2011, 45, 115–124. [Google Scholar]
- Kučerová, L.; Opatová, K.; Káňa, J.; Jirková, H. High versatility of niobium alloyed AHSS. Rch. Metall. Mater. 2017, 62, 1485–1491. [Google Scholar] [CrossRef]
- Yin, S.; Sun, X.; Liu, Q.; Zhang, Z. Influence of deformation on transformation of low-carbon and high Nb-containing steel during continuous cooling. J. Iron Steel Res. Int. 2010, 17, 43–47. [Google Scholar] [CrossRef]
- Olasolo, M.; Uranga, P.; Rodriguez-Ibabe, J.M.; López, B. Effect of austenite microstructure and cooling rate on transformation characteristics in a low carbon Nb–V microalloyed steel. Mater. Sci. Eng. A 2011, 528, 2559–2569. [Google Scholar] [CrossRef]
- Eghbali, B.; Abdollah-Zadeh, A. Deformation-induced ferrite transformation in a low carbon Nb–Ti microalloyed steel. Mater. Des. 2007, 28, 1021–1026. [Google Scholar] [CrossRef]
- Nürnberger, F.; Grydin, O.; Schaper, M.; Bach, F.-W.; Koczurkiewicz, B.; Milenin, A. Microstructure Transformations in Tempering Steels during Continuous Cooling from Hot Forging Temperatures. Steel Res. Int. 2010, 81, 224–233. [Google Scholar] [CrossRef]
- Grajcar, A.; Lesz, S. Influence of Nb microaddition on a microstructure of low-alloyed steels with increased manganese content. Mater. Sci. Forum 2012, 706, 2124–2129. [Google Scholar] [CrossRef]
- Bai, D.Q.; Yue, S.; Maccagno, T.M.; Jonas, J.J. Effect of Deformation and Cooling Rate on the Microstructures of Low Carbon Nb-B Steels. ISIJ Int. 1998, 38, 371–379. [Google Scholar] [CrossRef]
- Junhua, K.; Lin, Z.; Bin, G.; Pinghe, L.; Aihua, W.; Changsheng, X. Influence of Mo content on microstructure and mechanical properties of high strength pipeline steel. Mater. Des. 2004, 25, 723–728. [Google Scholar] [CrossRef]
- Pawłowski, B.; Bała, P.; Dziurka, R. Improper interpretation of dilatometric data for cooling transformation in steels. Arch. Metall. Mater. 2014, 59, 1159–1161. [Google Scholar] [CrossRef]
- Sourmail, T.; Garcia-Mateo, C.; Caballero, F.G.; Cazottes, S.; Epicier, T.; Danoix, F.; Milbourn, D. The Influence of Vanadium on Ferrite and Bainite Formation in a Medium Carbon Steel. Metall. Mat. Trans. A 2017, 48, 3985–3996. [Google Scholar] [CrossRef]
- Trzaska, J.; Jagiełło, A.; Dobrzański, L.A. The calculation of CCT diagrams for engineering steels. Arch. Mater. Sci. Eng. 2009, 39, 13–20. [Google Scholar]
- ASTM A1033-04. Standard Practice for Quantitative Measurement and Reporting of Hypoeutectoid Carbon and Low-Alloy Steel Phase Transformations; ASTM International: West Conshohocken, PA, USA, 2004; Available online: https://www.astm.org/ (accessed on 25 March 2020).
- ASTM E3-11. Standard Guide for Preparation of Metallographic Specimens; ASTM International: West Conshohocken, PA, USA, 2011; Available online: http://www.astm.org/ (accessed on 20 October 2020).
- Calvo, J.; Jung, I.-H.; Elwazri, A.M.; Bai, D.; Yue, S. Influence of the chemical composition on transformation behaviour of low carbon microalloyed steels. Mater. Sci. Eng. A 2009, 520, 90–96. [Google Scholar] [CrossRef]
- Denis, S.; Gautier, E.; Simon, A.; Beck, G. Stress–phase-transformation interactions–basic principles, modelling, and calculation of internal stresses. Mater. Sci. Technol. 1985, 1, 805–814. [Google Scholar] [CrossRef]
- Barcellona, A.; Palmeri, D. Effect of Plastic Hot Deformation on the Hardness and Continuous Cooling Transformations of 22MnB5 Microalloyed Boron Steel. Metall. Mater. Trans. A 2009, 40, 1160–1174. [Google Scholar] [CrossRef]
- Opiela, M.; Grajcar, A. Elaboration of forging conditions on the basis of the precipitation analysis of MX type phase in microalloyed steels. Arch. Civ. Mech. Eng. 2012, 12, 427–435. [Google Scholar] [CrossRef]
- Li, H.-P.; Jiang, R.; He, L.-F.; Yang, H.; Wang, C.; Zhang, C.-Z. Influence of Deformation Degree and Cooling Rate on Microstructure and Phase Transformation Temperature of B1500HS Steel. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 33–47. [Google Scholar] [CrossRef]
- Nakajima, K.; Apel, M.; Steinbach, I. The role of carbon diffusion in ferrite on the kinetics of cooperative growth of pearlite: A multi-phase field study. Acta Materialia 2006, 54, 3665–3672. [Google Scholar] [CrossRef]
- Zhang, M.; Li, L.; Fu, R.Y.; Krizan, D.; De Cooman, B.C. Continuous cooling transformation diagrams and properties of micro-alloyed TRIP steels. Mater. Sci. Eng. A 2006, 438–440, 296–299. [Google Scholar] [CrossRef]
- Liu, T.; Long, M.; Fan, H.; Cheng, D.; Chen, H.; Duan, H.; Jiang, W.; He, W. Dilatometric determination of four criticial temperatures and phase transition fraction for austenite decomposition in hypo-eutectoid steels using peak separation method. J. Mater. Rese. 2018, 33, 1–11. [Google Scholar] [CrossRef]
- Fan, L.; Zhou, D.; Wang, T.; Li, S.; Wang, Q. Tensile properties of an acicular ferrite and martensite/austenite constituent steel with varying cooling rates. Mater. Sci. Eng. A 2014, 590, 224–231. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Qiao, Z.; Guo, Q.; Liu, C.; Yu, L.; Li, H. Effects of cooling rates on δ-ferrite/γ-austenite formation and martensitic transformation in modified ferritic heat resistant steel. Fusion Eng. Des. 2017, 125, 354–360. [Google Scholar] [CrossRef]
- Inoue, T.; Torizuka, S.; Nagai, K. Formation of uniformly fine grained ferrite structure through multidirectional deformation. Mater. Sci. Technol. 2001, 17, 1329–1338. [Google Scholar] [CrossRef]
- ASTM E112-13. Standard Test Methods for Determining Average Grain Size; ASTM International: West Conshohocken, PA, USA, 2013; Available online: http://www.astm.org/ (accessed on 20 October 2020).
- Qi, Y.; Li, J.; Shi, C.; Zhu, Q. Continuous Cooling Transformation of Undeformed and Deformed High Strength Crack-Arrest Steel Plates for Large Container Ships. High Temp. Mater. Process. 2019, 38, 183–191. [Google Scholar] [CrossRef]
- Zhao, M.-C.; Yang, K.; Xiao, F.-R.; Shan, Y.-Y. Continuous cooling transformation of undeformed and deformed low carbon pipeline steels. Mater. Sci. Eng. A 2003, 355, 126–136. [Google Scholar] [CrossRef]
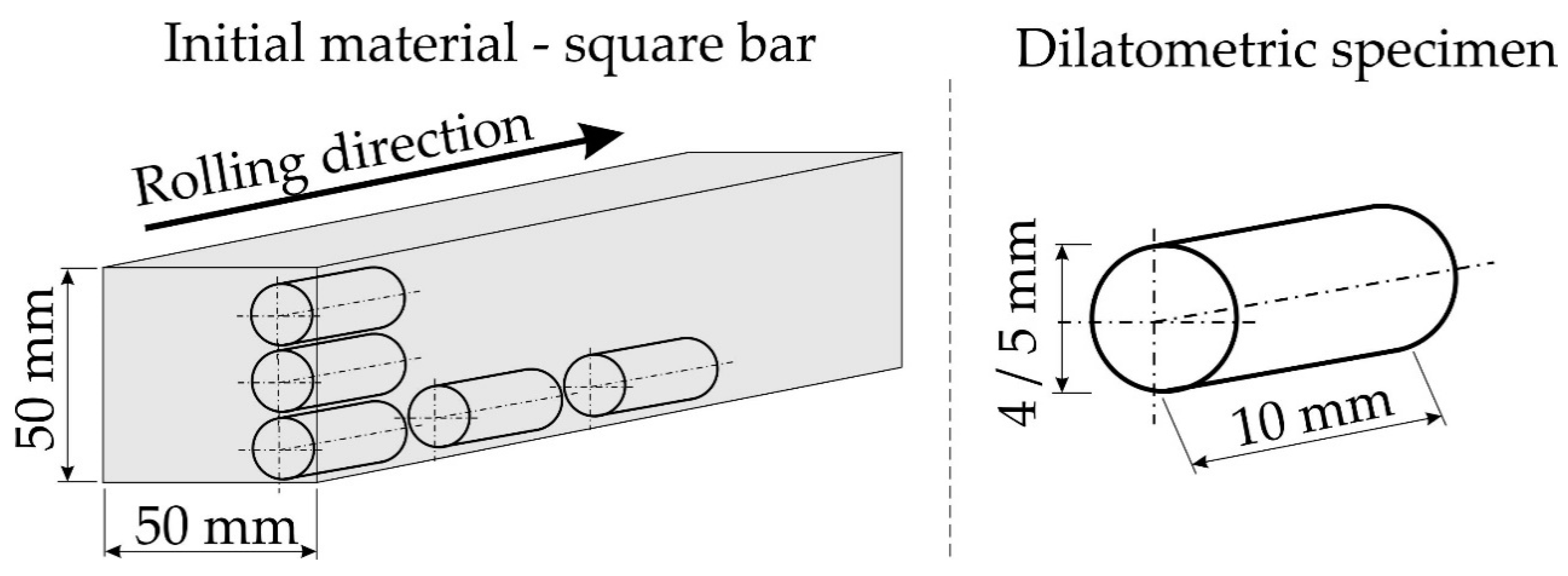
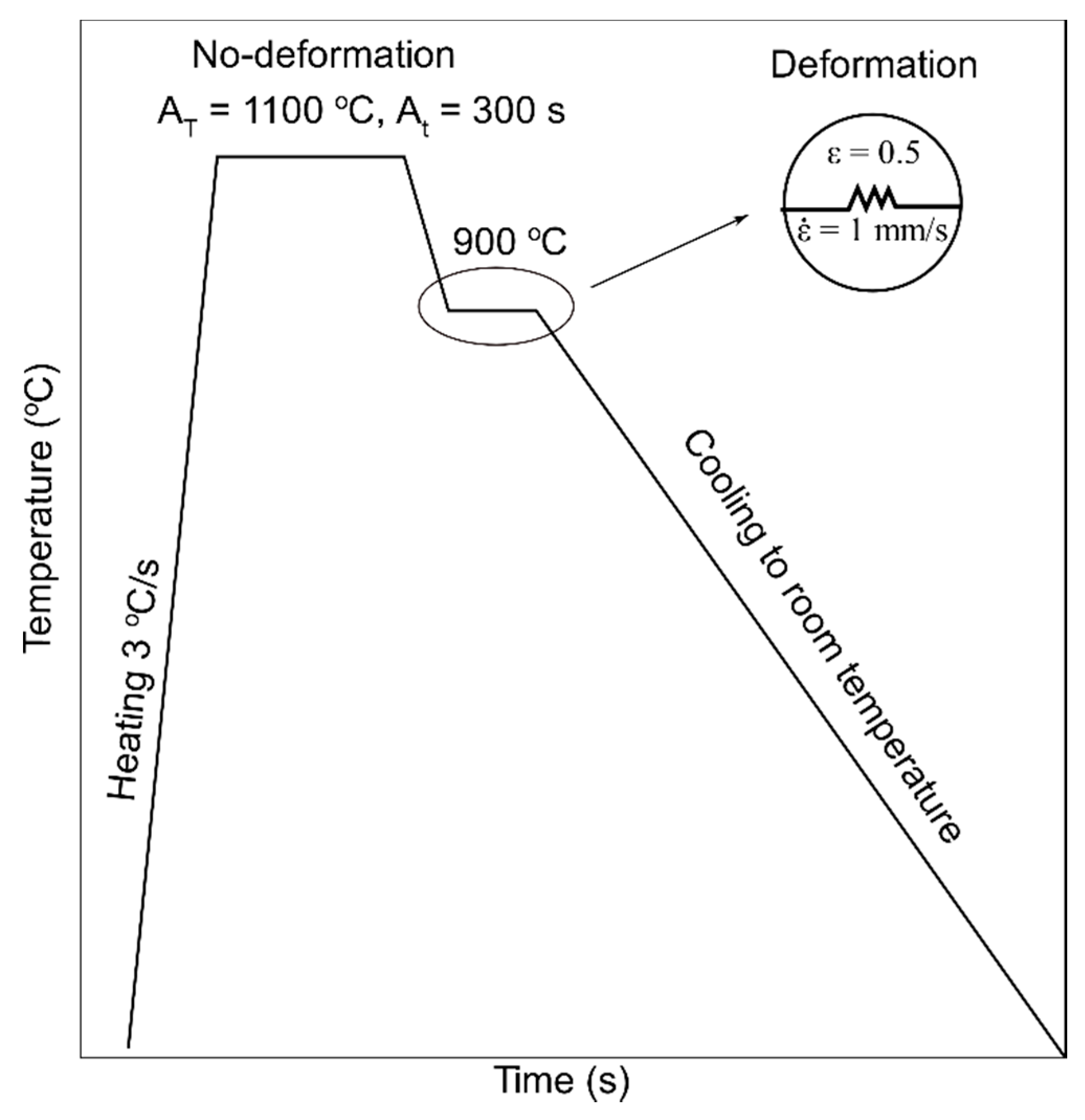
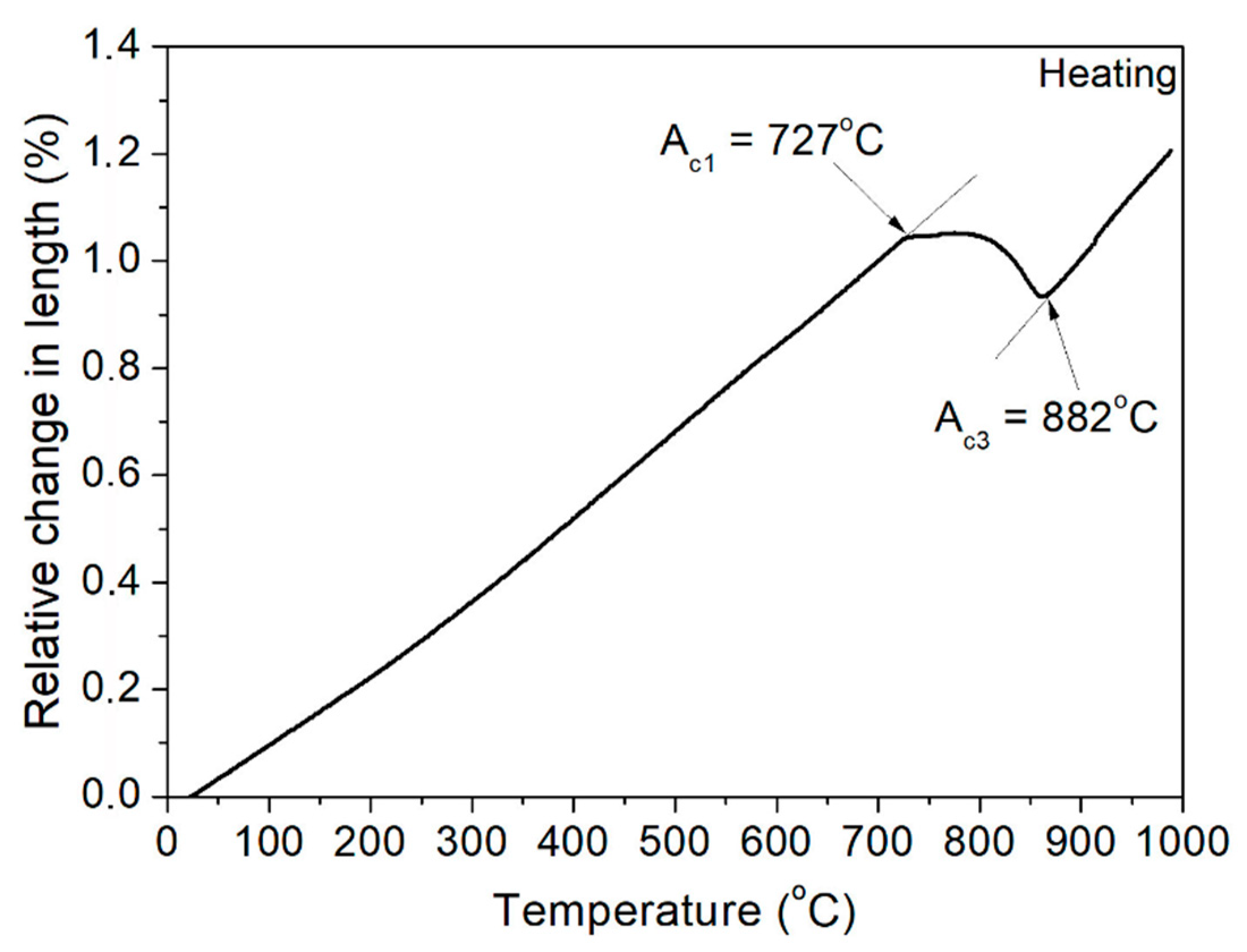
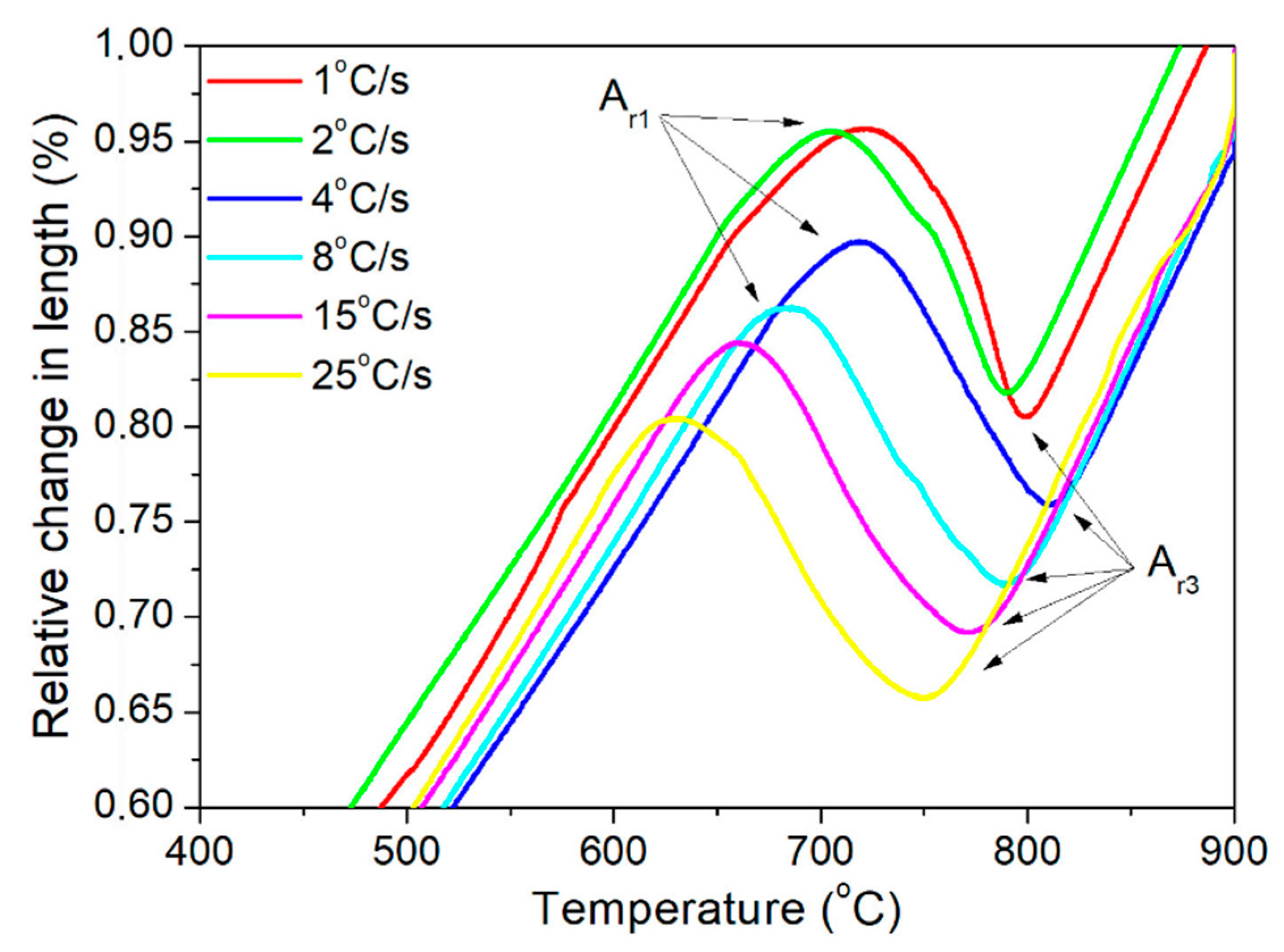
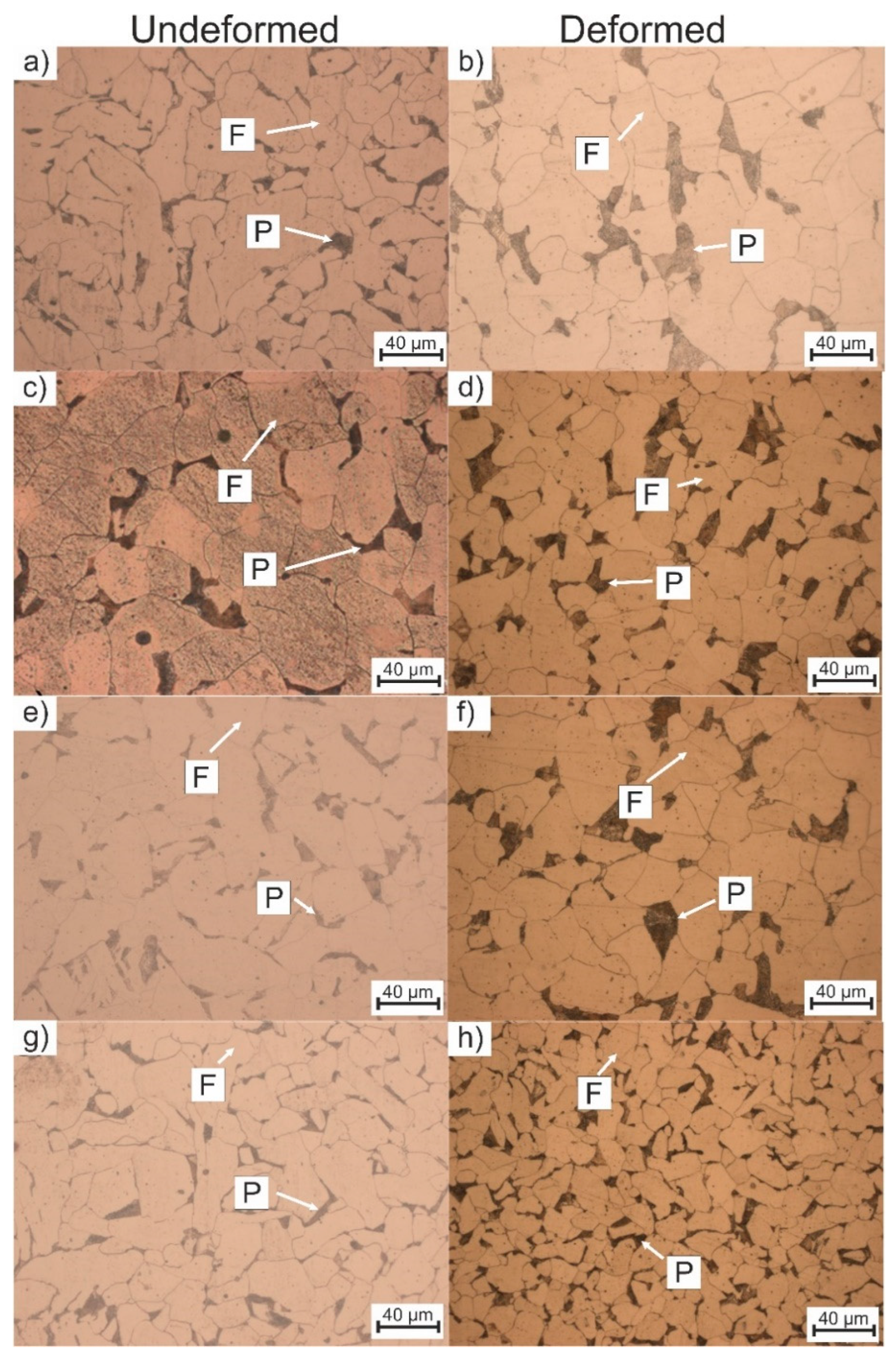
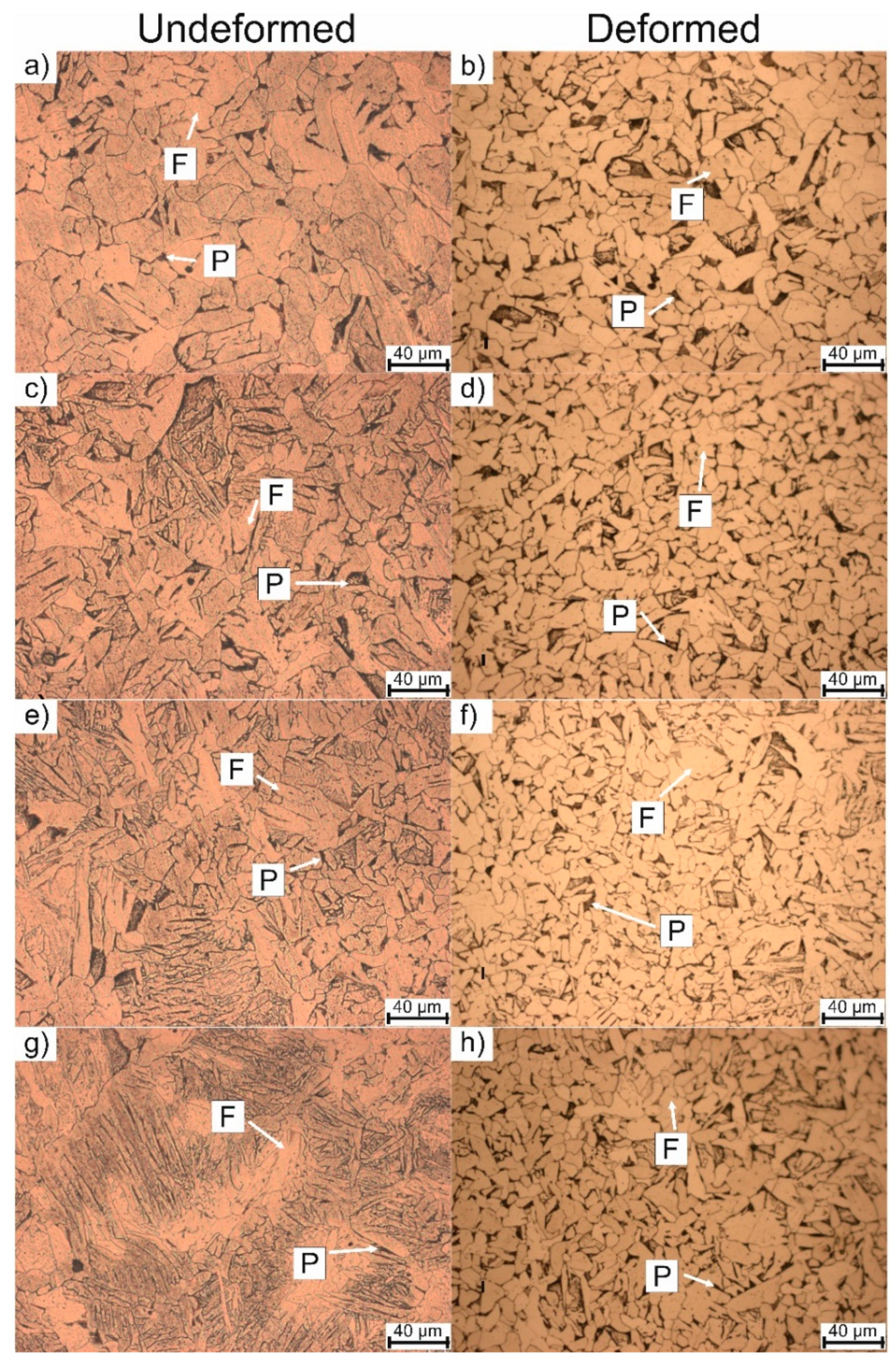
| Sample | Undeformed | Deformed |
|---|---|---|
| 1 | 0.125 °C/s | 0.125 °C/s |
| 2 | 1 °C/s | 1 °C/s |
| 3 | 2 °C/s | 2 °C/s |
| 4 | 4 °C/s | 4 °C/s |
| 5 | 8 °C/s | 8 °C/s |
| 6 | 15 °C/s | 15 °C/s |
| 7 | 25 °C/s | 25 °C/s |
| 8 | 50 °C/s | 50 °C/s |
| 9 | 150 °C/s | - |
| 10 | 220 °C/s | - |
| Cooling Rate, °C/s | 0.125 | 1 | 2 | 4 | 8 | 15 | 25 | 50 |
|---|---|---|---|---|---|---|---|---|
| ND, µm | 53 | 50 | 34 | 34 | 34 | 19 | 15 | LM |
| D, µm | 53 | 49 | 30 | 30 | 30 | 15 | 14 | 13 |
| Cooling Rate | Hardness, HV1 | |
|---|---|---|
| Non-Deformed | Deformed | |
| 8 °C/min | 110 ± 3 | 130 ± 4 |
| 1 °C/s | 125 ± 3 | 146 ± 3 |
| 2 °C/s | 131 ± 3 | 148 ± 3 |
| 4 °C/s | 137 ± 3 | 153 ± 4 |
| 8 °C/s | 143 ± 4 | 158 ± 3 |
| 15 °C/s | 148 ± 3 | 163 ± 3 |
| 25 °C/s | 162 ± 3 | 172 ± 3 |
| 50 °C/s | 163 ± 4 | 175 ± 3 |
| 150 °C/s | 172 ± 3 | - |
| 220 °C/s | 179 ± 3 | - |
| Vcooling °C/s | Hardness HV1 | Transformation Start and Finish Temperatures | |||||
|---|---|---|---|---|---|---|---|
| Bs | Bf | Ps | Pf | Fs | Ff | ||
| 220 | 179 | 482 | 415 | - | - | 605 | 482 |
| 150 | 172 | 550 | 516 | - | - | 673 | 550 |
| 50 | 163 | - | - | 602 | 550 | 698 | 602 |
| 25 | 162 | - | - | 637 | 582 | 748 | 637 |
| 15 | 148 | - | - | 650 | 609 | 764 | 650 |
| 8 | 143 | - | - | 683 | 618 | 789 | 680 |
| 4 | 137 | - | - | 682 | 640 | 797 | 682 |
| 2 | 131 | - | - | 692 | 648 | 806 | 692 |
| 1 | 125 | - | - | 707 | 653 | 811 | 707 |
| 0.125 | 110 | - | - | 714 | 672 | 826 | 714 |
| Vcooling °C/s | Hardness HV1 | Transformation Start and Finish Temperatures | |||||
|---|---|---|---|---|---|---|---|
| Bs | Bf | Ps | Pf | Fs | Ff | ||
| 50 | 172 | 541 | 470 | 660 | 541 | 800 | 660 |
| 25 | 165 | 560 | 485 | 660 | 560 | 810 | 660 |
| 15 | 163 | 569 | 492 | 668 | 569 | 820 | 668 |
| 8 | 158 | 626 | 542 | 673 | 626 | 820 | 673 |
| 4 | 153 | - | - | 680 | 641 | 857 | 680 |
| 2 | 148 | - | - | 682 | 639 | 860 | 682 |
| 1 | 146 | - | - | 683 | 642 | 862 | 683 |
| 0.125 | 130 | - | - | 685 | 576 | 882 | 685 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morawiec, M.; Skowronek, A.; Król, M.; Grajcar, A. Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel. Materials 2020, 13, 5443. https://doi.org/10.3390/ma13235443
Morawiec M, Skowronek A, Król M, Grajcar A. Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel. Materials. 2020; 13(23):5443. https://doi.org/10.3390/ma13235443
Chicago/Turabian StyleMorawiec, Mateusz, Adam Skowronek, Mariusz Król, and Adam Grajcar. 2020. "Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel" Materials 13, no. 23: 5443. https://doi.org/10.3390/ma13235443
APA StyleMorawiec, M., Skowronek, A., Król, M., & Grajcar, A. (2020). Dilatometric Analysis of the Austenite Decomposition in Undeformed and Deformed Low-Carbon Structural Steel. Materials, 13(23), 5443. https://doi.org/10.3390/ma13235443






