Simple and Eco-Friendly Route from Agro-Food Waste to Water Pollutants Removal
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
Preparation of Carbon Material
2.2. Methods
2.2.1. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.2.2. Raman Spectroscopy
2.2.3. Elemental Analysis (EA)
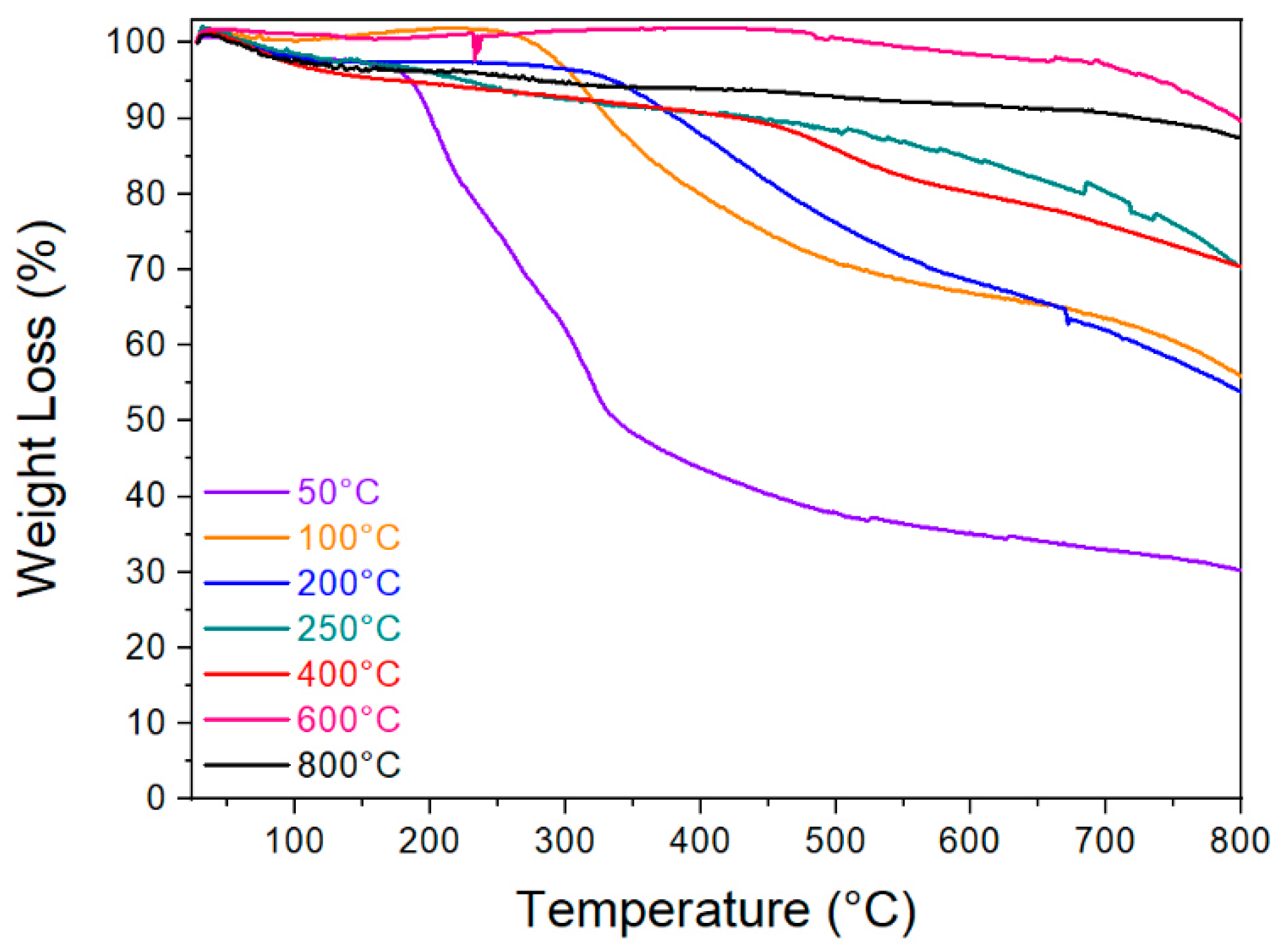
2.2.4. Thermogravimetric Analysis (TGA)
2.2.5. Scanning Electron Microscopy (SEM)
2.2.6. X-Ray Diffraction (XRD)
2.2.7. Positron Annihilation Lifetime Spectroscopy (PALS)
2.2.8. Evaluation of Adsorption Properties of the Carbon Material
3. Results
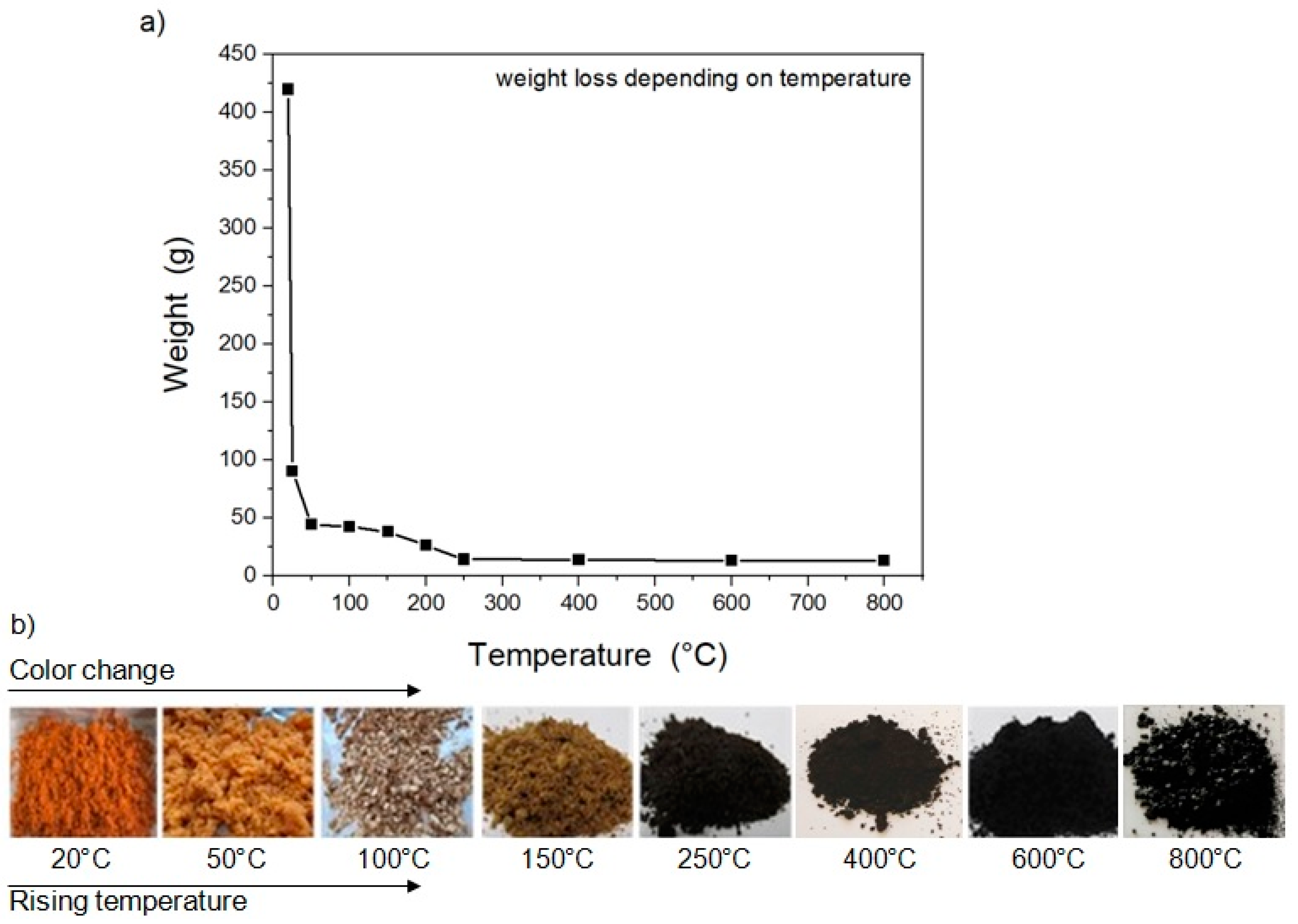
3.1. Preparation of the Carbon-Based Material from the Carrot Pulp
3.2. Characterization of Investigated Material
3.2.1. Thermal Analysis
3.2.2. Raman Spectroscopy Analysis
3.2.3. Elemental Analysis
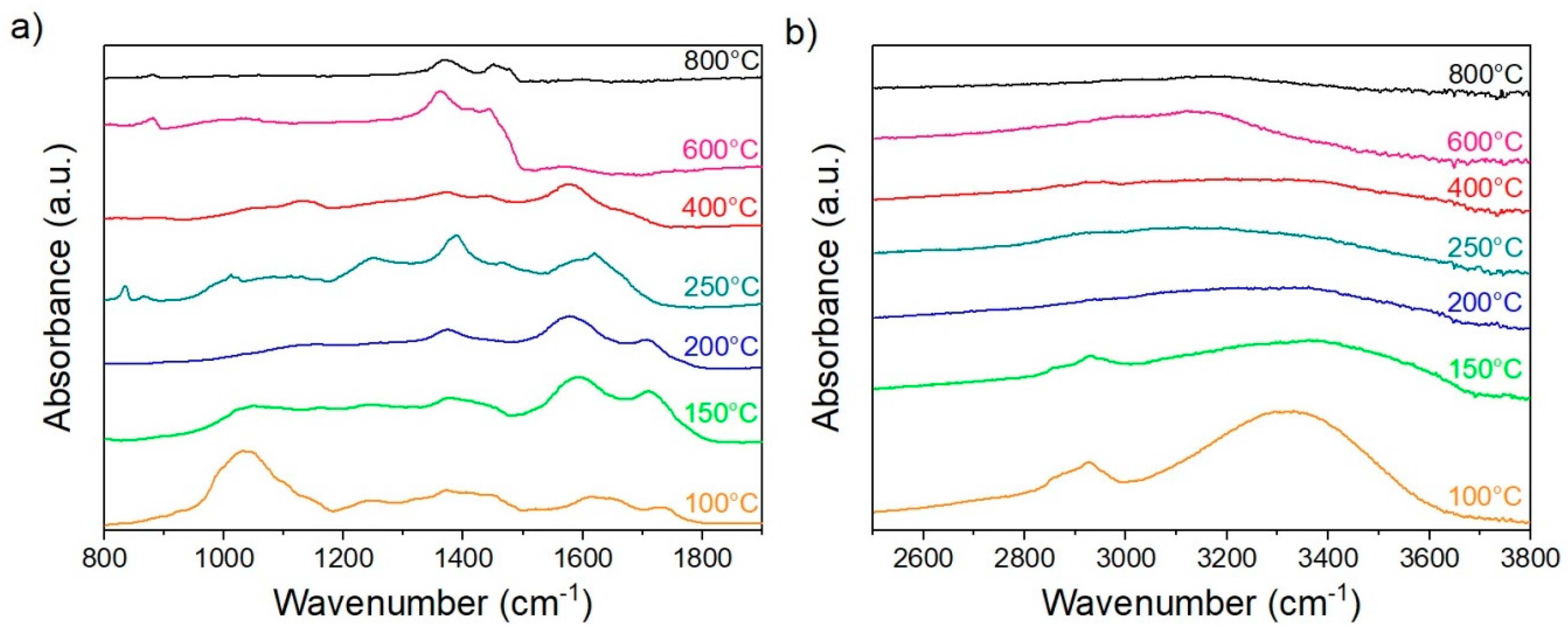
3.2.4. FTIR Analysis
3.2.5. Morphological Analysis
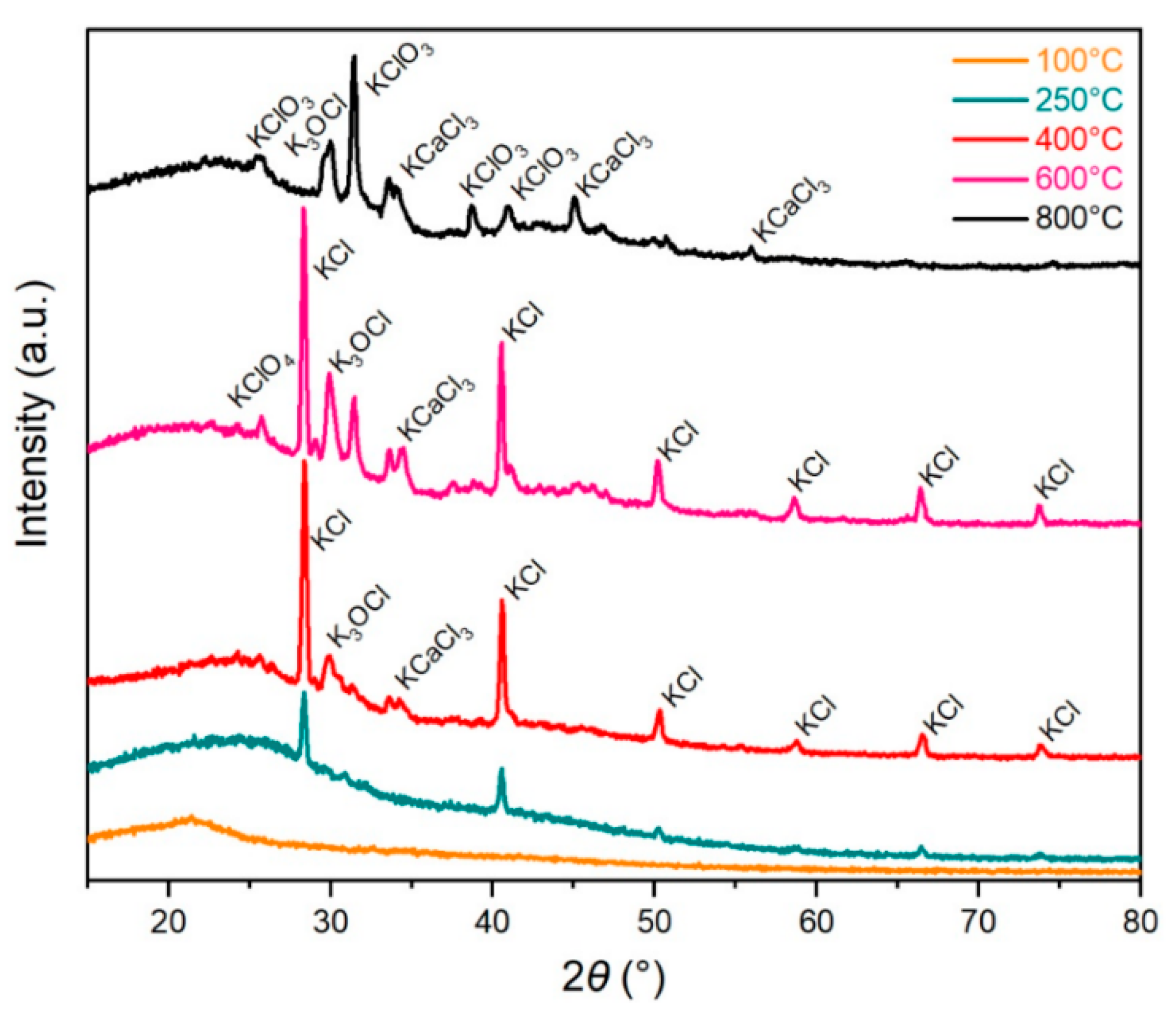
3.2.6. X-Ray Diffraction Analysis
3.3. Organic Pollutants Removal from the Water
Free Volume Analysis
4. Discussion
5. Conclusions
- The eco-friendly and straightforward process can be used to fabricate carbon sorbent to purify wastewater with significant efficiency.
- Thermal treatment leads to the production the carbon-based material with a maximum carbon content of 75.5% from an alternative natural resource without using additional chemicals.
- There is a significant change of the cell wall morphology with treatment temperature in the phase’s development phase. Furthermore, SEM and XRD analysis reveal different inorganic salts on the surface of carbonized carrot pulp, which could be products of molten salt of eutectic KCl–CaCl2 products of surface organic-potassium salt complexes.
- The CP treated at a temperature of 800 °C (CCP) could adsorb the organic dye (RhB and PhB). The removal efficiency of the CCP sample increased by increasing time independently of the dye type. The removal efficiency of the cationic type of dye is more than 99%, and the anionic kind of dye is more than 94% into the CP-based carbon material.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arya, M.; Lee, N.; Pellegrino, S. Ultralight Structures for Space Solar Power Satellites. 2020. Available online: http://www.mananarya.com/docs/2016_sdm_arya.pdf (accessed on 3 September 2020).
- Kromoser, D.D.B.; Preinstorfer, D.D.T.P.; Kollegger, J. Building lightweight structures with carbon-fiber-reinforced polymer--reinforced ultra-high-performance concrete: Research approach, construction materials, and conceptual design of three building components. Struct. Concr. 2019, 20, 730–744. [Google Scholar] [CrossRef]
- Elanchezhian, C.; Ramnath, B.V.; Ramakrishnan, G.; Raghavendra, K.S.; Muralidharan, M.; Kishore, V. Review on metal matrix composites for marine applications. Mater. Today Proc. 2018, 5, 1211–1218. [Google Scholar] [CrossRef]
- Deo, R.; Starnes, J.; Holzwarth, R. Low-cost composite materials and structures for aircraft applications. In Proceedings of the Low Cost Composite Structures and Cost Effective Application of Titanium Alloys in Military Platforms, Loen, Norway, 7–11 May 2001; pp. 1–11. [Google Scholar]
- Maria, M. Advanced composite materials of the future in aerospace industry. INCAS Bull. 2013, 5, 139–150. [Google Scholar] [CrossRef]
- Kochar, G.K.; Sharma, K.K. Fiber content and its composition in commonly consumed Indian vegetables and fruits. J. Food Sci. Technol. 1992, 29, 187–190. [Google Scholar]
- Olenic, L.; Mihailescu, G.; Pruneanu, S.; Lupu, D.; Biris, A.R.; Mărgineanu, P.; Garabagiu, S.; Mihǎilescu, G. Investigation of carbon nanofibers as support for bioactive substances. J. Mater. Sci. Mater. Electron. 2008, 20, 177–183. [Google Scholar] [CrossRef]
- Saito, N.; Aoki, K.; Usui, Y.; Shimizu, M.; Hara, K.; Narita, N.; Ogihara, N.; Nakamura, K.; Ishigaki, N.; Kato, H.; et al. Application of carbon fibers to biomaterials: A new era of nano-level control of carbon fibers after 30-years of development. Chem. Soc. Rev. 2011, 40, 3824–3834. [Google Scholar] [CrossRef]
- Aoki, K.; Usui, Y.; Narita, N.; Ogiwara, N.; Iashigaki, N.; Nakamura, K.; Kato, H.; Sano, K.; Ogiwara, N.; Kametani, K.; et al. A Thin Carbon--Fiber Web as a Scaffold for Bone-Tissue Regeneration. Small 2009, 5, 1540–1546. [Google Scholar] [CrossRef]
- Tran, P.A.; Zhang, L.; Webster, T.J. Carbon nanofibers and carbon nanotubes in regenerative medicine. Adv. Drug Deliv. Rev. 2009, 61, 1097–1114. [Google Scholar] [CrossRef]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Bin Ruslan, M.S.; Ping, C.S. Investigation on Carbon Fiber Composites as Architectural EMI Shielding Material. Adv. Mater. Res. 2013, 748, 309–313. [Google Scholar] [CrossRef]
- Gamage, S.J.P.; Yang, K.; Braveenth, R.; Raagulan, K.; Kim, H.S.; Kim, J.S.; Yang, C.-M.; Jung, M.J.; Chai, K.Y. MWCNT Coated Free-Standing Carbon Fiber Fabric for Enhanced Performance in EMI Shielding with a Higher Absolute EMI SE. Materials 2017, 10, 1350. [Google Scholar] [CrossRef]
- Vendittozzi, C.; Sindoni, G.; Paris, C.; Del Marmo, P.P. Application of an FBG sensors system for structural health monitoring and high-performance trimming on racing yacht. In Proceedings of the 2011 Fifth International Conference on Sensing Technology, Palmerston North, New Zealand, 28 November–1 December 2011; pp. 617–622. [Google Scholar]
- Coupe, D. Woven reinforcements for composites. In Composite Reinforcements for Optimum Performance; Boisse, P., Ed.; Woodhead Publishing: Cambridge, UK, 2011; Chapter 4; pp. 89–115. [Google Scholar]
- Sullivan, R.A. Automotive carbon fiber: Opportunities and challenges. JOM 2006, 58, 77–79. [Google Scholar] [CrossRef]
- Crow, R. Carbon Fibre in Mass Automotive Applications. In Proceedings of the Challenges and Drivers for composites. Franco-British Symposium on Composite Materials, London, UK, 28 April 2015; pp. 1–12. [Google Scholar]
- Ishikawa, T.; Amaoka, K.; Masubuchi, Y.; Yamamoto, T.; Yamanaka, A.; Arai, M.; Takahashi, J. Overview of automotive structural composites technology developments in Japan. Compos. Sci. Technol. 2018, 155, 221–246. [Google Scholar] [CrossRef]
- Cakici, M.; Kakarla, R.R.; Alonso-Marroquin, F. Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem. Eng. J. 2017, 309, 151–158. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Zi, Y.; Wang, S.; Li, Z.; Zheng, L.; Yi, F.; Li, S.; Wang, Z.L. A Flexible Fiber-Based Supercapacitor-Triboelectric-Nanogenerator Power System for Wearable Electronics. Adv. Mater. 2015, 27, 4830–4836. [Google Scholar] [CrossRef]
- Samimi, F.; Babapoor, A.; Azizi, M.; Karimi, G. Thermal management analysis of a Li-ion battery cell using phase change material loaded with carbon fibers. Energy 2016, 96, 355–371. [Google Scholar] [CrossRef]
- Lee, T.; Ooi, C.H.; Othman, R.; Yeoh, F.Y. Activated carbon fiber - the hybrid of carbon fiber and activated carbon. Rev. Adv. Mater. Sci. 2014, 36, 118–136. [Google Scholar]
- Liu, F.; Dai, Y.; Zhang, S.; Li, J.; Zhao, C.; Wang, Y.; Liu, C.; Sun, J. Modification and application of mesoporous carbon adsorbent for removal of endocrine disruptor bisphenol A in aqueous solutions. J. Mater. Sci. 2017, 53, 2337–2350. [Google Scholar] [CrossRef]
- Hu, S.-C.; Shiue, A.; Chang, S.-M.; Chang, Y.-T.; Tseng, C.-H.; Mao, C.-C.; Hsieh, A.; Chan, A. Removal of carbon dioxide in the indoor environment with sorption-type air filters. Int. J. Low-Carbon Technol. 2016, 12, 330–334. [Google Scholar] [CrossRef]
- Fujishige, M.; Yoshida, I.; Toya, Y.; Banba, Y.; Oshida, K.-I.; Tanaka, Y.-S.; Dulyaseree, P.; Wongwiriyapan, W.; Takeuchi, K. Preparation of activated carbon from bamboo-cellulose fiber and its use for EDLC electrode material. J. Environ. Chem. Eng. 2017, 5, 1801–1808. [Google Scholar] [CrossRef]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Wu, Q.; Liang, D.; Ma, X.; Luc, S.; Xiang, Y. Chitosan-based activated carbon as economic and efficient sustainable material for capacitive deionization of low salinity water. RSC Adv. 2019, 9, 26676–26684. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Hu, H.; Liu, S.; Cai, Y.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. Ultrahigh-surface-area hierarchical porous carbon from chitosan: Acetic acid mediated efficient synthesis and its application in superior supercapacitors. J. Mater. Chem. A 2017, 5, 24775–24781. [Google Scholar] [CrossRef]
- Taer, E. Production of an Activated Carbon from a Banana Stem and its application as electrode materials for Supercapacitors. Int. J. Electrochem. Sci. 2018, 13, 8428–8439. [Google Scholar] [CrossRef]
- Xie, Z.; Guan, W.; Ji, F.; Song, Z.; Zhao, Y. Production of Biologically Activated Carbon from Orange Peel and Landfill Leachate Subsequent Treatment Technology. J. Chem. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Tovar, A.K.; Godínez, L.A. Sustainable Sorbent Materials Obtained from Orange Peel as an Alternative for Water Treatment. Wastewater. Water Qual. 2018, 11, 201–217. [Google Scholar]
- Ouhammou, M.; Lahnine, L.; Mghazli, S.; Hidar, N.; Bouchdoug, M.; Jaouad, A.; Mandi, L.; Mahrouz, M. Valorisation of cellulosic waste basic cactus to prepare activated carbon. J. Saudi Soc. Agric. Sci. 2019, 18, 133–140. [Google Scholar] [CrossRef]
- Saleem, J.; Bin Shahid, U.; Hijab, M.; Mackey, H.; McKay, G. Production and applications of activated carbons as adsorbents from olive stones. Biomass-Convers. Biorefin. 2019, 9, 775–802. [Google Scholar] [CrossRef]
- Sesuk, T.; Tammawat, P.; Jivaganont, P.; Somton, K.; Limthongkul, P.; Kobsiriphat, W. Activated carbon derived from coconut coir pith as high performance supercapacitor electrode material. J. Energy Storage 2019, 25, 100910. [Google Scholar] [CrossRef]
- Taer, E. Activated Carbon Electrode Made From Coconut Husk Waste for Supercapacitor Application. Int. J. Electrochem. Sci. 2018, 13, 12072–12084. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, H.; Cheng, C.; Li, K.; Zhang, J.; Zhang, C.; Li, Y. Preparation and characterization of activated carbon from wool waste and the comparison of muffle furnace and microwave heating methods. Powder Technol. 2013, 249, 234–240. [Google Scholar] [CrossRef]
- Ahmed, M.; Islam, A.; Asif, M.; Hameed, B. Human hair-derived high surface area porous carbon material for the adsorption isotherm and kinetics of tetracycline antibiotics. Bioresour. Technol. 2017, 243, 778–784. [Google Scholar] [CrossRef]
- Djilani, C.; Zaghdoudi, R.; Djazi, F.; Bouchekima, B.; Lallam, A.; Magri, P. Preparation and characterisation of activated carbon from animal bones and its application for removal of organic micropollutants from aqueous solution. Desalinisation Water Treat. 2016, 57, 25070–25079. [Google Scholar] [CrossRef]
- Nwankwo, I.H.; Nwaiwu, N.E.; Nwabanne, J.T. Production and characterization of activated carbon from animal bone. Am. J. Eng. Res. 2018, 7, 335–341. [Google Scholar]
- Khan, M.R.; Gotoh, Y.; Morikawa, H.; Miura, M.; Fujimori, Y.; Nagura, M. Carbon fiber from natural biopolymer Bombyx mori silk fibroin with iodine treatment. Carbon 2007, 45, 1035–1042. [Google Scholar] [CrossRef]
- Khamkeaw, A.; Jongsomjit, B.; Robison, J.; Phisalaphong, M. Activated carbon from bacterial cellulose as an effective adsorbent for removing dye from aqueous solution. Sep. Sci. Technol. 2019, 54, 2180–2193. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, J.; Zhao, L.; Zhou, Z.; Qiu, C.; Li, Q. Adsorption of Rhodamine B from Aqueous Solution by Goat Manure Biochar: Kinetics, Isotherms, and Thermodynamic Studies. Pol. J. Environ. Stud. 2020, 4, 2721–2730. [Google Scholar] [CrossRef]
- Lu, H.; Zhao, X.S. Biomass-derived carbon electrode materials for supercapacitors. Sustain. Energy Fuels 2017, 1, 1265–1281. [Google Scholar] [CrossRef]
- Javaid, A. Activated carbon fiber for energy storage. In Activated Carbon Fiber and Textiles; Woodhead Publishing: Cambridge, UK, 2017; pp. 281–303. [Google Scholar]
- Semitekolos, D.; Trompeta, A.-F.A.; Santos, R.M.; Martins, M.S.S.; Dos Santos, C.M.; Iglesias, V.; Böhm, R.; Gong, G.; Chiminelli, A.; Verpoest, I.; et al. Research and Development in Carbon Fibers and Advanced High-Performance Composites Supply Chain in Europe: A Roadmap for Challenges and the Industrial Uptake. J. Compos. Sci. 2019, 3, 86. [Google Scholar] [CrossRef]
- Jamee, R.; Siddique, R. Biodegradation of synthetic dyes of textile effluent by microorganisms: An environmentally and economically sustainable approach. Eur. J. Microbiol. Immunol. 2019, 9, 114–118. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Hoang, V.C.; Gomes, V.G.; Dinh, K.N. Ni- and P-doped carbon from waste biomass: A sustainable multifunctional electrode for oxygen reduction, oxygen evolution and hydrogen evolution reactions. Electrochim. Acta 2019, 314, 49–60. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Chemin. 2012, 4, 17. [Google Scholar] [CrossRef]
- Kansy, J. Microcomputer program for analysis of positron annihilation lifetime spectra. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 1996, 374, 235–244. [Google Scholar] [CrossRef]
- Du, H.; Fuh, R.-C.A.; Li, J.; Corkan, L.A.; Lindsey, J.S. PhotochemCAD: A Computer-Aided Design and Research Tool in Photochemistry. Photochem. Photobiol. 1998, 68, 141–142. [Google Scholar] [CrossRef]
- Litman, Y.; Rodríguez, H.B.; Román, E.S. Tuning the concentration of dye loaded polymer films for maximum photosensitization efficiency: Phloxine B in poly(2-hydroxyethyl methacrylate). Photochem. Photobiol. Sci. 2016, 15, 80–85. [Google Scholar] [CrossRef]
- Li, L.; Zhong, Q.; Kim, N.D.; Ruan, G.; Yang, Y.; Gao, C.; Fei, H.; Li, Y.; Ji, Y.; Tour, J.M. Nitrogen-doped carbonized cotton for highly flexible supercapacitors. Carbon 2016, 105, 260–267. [Google Scholar] [CrossRef]
- Taylor, R.; Siva, S.B.V.; Sreekanth, P.S.R. Carbon Matrix Composites. In Reference Module in Materials Science and Materials Engineering, 1st ed.; Hashmi, S., Ed.; Elsevier: Oxford, UK, 2017; pp. 1–40. [Google Scholar]
- Shibahara, Y.; Sodaye, H.S.; Akiyama, Y.; Nishijima, S.; Honda, Y.; Isoyama, G.; Tagawa, S. Effect of humidity and temperature on polymer electrolyte membrane (Nafion 117) studied by positron annihilation spectroscopy. J. Power Sources 2010, 195, 5934–5937. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Santana, R.M.C. Structural differences between wood species: Evidence from chemical composition, FTIR spectroscopy, and thermogravimetric analysis. J. Appl. Polym. Sci. 2012, 126, E337–E344. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of hemicellulose, cellulose and lignin pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot—A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Stevulova, N.; Hospodarova, V.; Estokova, A. Study of Thermal Analysis of Selected Cellulose Fibres. Geosci. Eng. 2016, 62, 18–21. [Google Scholar] [CrossRef]
- Hintsho, N.; Shaikjee, A.; Masenda, H.; Naidoo, D.; Billing, D.G.; Franklyn, P.; Durbach, S.H. Direct synthesis of carbon nanofibers from South African coal fly ash. Nanoscale Res. Lett. 2014, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Jagdale, P.; Koumoulos, E.P.; Cannavaro, I.; Khan, A.; Castellino, M.; Dragatogiannis, D.A.; Tagliaferro, A.; Charitidis, C. Towards green carbon fibre manufacturing from waste cotton: A microstructural and physical property investigation. Manuf. Rev. 2017, 4, 10. [Google Scholar] [CrossRef]
- Vollmannova, A.; Musilova, J.; Urminska, D. Chémia Potravín, 1st ed.; Slovenská Poľnohospodárska Univerzita v Nitre: Nitra, Slovakia, 2018; pp. 84–231. [Google Scholar]
- Bachrun, S.; AyuRizka, N.; Annisa, S.; Arif, H. Preparation and characterization of activated carbon from sugarcane bagasse by physical activation with CO2gas. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2016; Volume 105, p. 012027. [Google Scholar] [CrossRef]
- Varanasi, S.; Henzel, L.; Sharman, S.; Batchelor, W.; Garnier, G. Producing nanofibres from carrots with a chemical-free process. Carbohydr. Polym. 2018, 184, 307–314. [Google Scholar] [CrossRef]
- Müller, G.; Bartholme, M.; Kharazipour, A.; Polle, A. FTIR-ATR Spectroscopic analysis of changes in fiber properties during insulating fiberboard manufacture of beech wood. Wood Fiber Sci. 2008, 40, 532–543. [Google Scholar]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- van Dalen, G.; Voda, A.; Duijster, A.; van Vliet, L.; Vergeldt, F.; van der Sman, R.; van As, H.; van Duynhoven, J. Multi-length scale structural imaging of freeze-dried carrots and their rehydration behaviour. In Proceedings of the Inside Food Symposium, Leuven, Belgium, 9–12 April 2013; pp. 1–7. [Google Scholar]
- Cheng, Y.; Li, B.; Huang, Y.; Wang, Y.; Chen, J.; Wei, D.; Feng, Y.; Jia, D.; Zhou, Y. Molten salt synthesis of nitrogen and oxygen enriched hierarchically porous carbons derived from biomass via rapid microwave carbonization for high voltage supercapacitors. Appl. Surf. Sci. 2018, 439, 712–723. [Google Scholar] [CrossRef]
- Chen, Z.-G.; Guo, X.-Y.; Wu, T. A novel dehydration technique for carrot slices implementing ultrasound and vacuum drying methods. Ultrason. Sonochem. 2016, 30, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Baker, J.O.; Himmel, M.E.; Parilla, P.A.; Johnson, D.K. Cellulose crystallinity index: Measurement techniques and their impact on interpreting cellulase performance. Biotechnol. Biofuels 2010, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Socha, A.M.; Plummer, S.P.; Stavila, V.; Simmons, B.; Singh, S. Comparison of sugar content for ionic liquid pretreated Douglas-fir woodchips and forestry residues. Biotechnol. Biofuels 2013, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.P.; Reinhardt, F.W. Phase diagrams for the binary systems CaCl2-KCl and CaCl2-CaCrO4. Thermochim. Acta 1975, 12, 309–314. [Google Scholar] [CrossRef]
- Mims, C.A.; Pabst, J.K. Role of surface salt complexes in alkali-catalysed carbon gasification. Fuel 1983, 62, 176–179. [Google Scholar] [CrossRef]
- Wood, B.J.; Sancier, K.M. The Mechanism of the Catalytic Gasification of Coal Char: A Critical Review. Catal. Rev. 1984, 26, 233–279. [Google Scholar] [CrossRef]
- Hussain, G.; Rees, G.J. Effect of additives on the decomposition of KClO4. Fuel 1991, 70, 1399–1401. [Google Scholar] [CrossRef]
- Martins, S.; Fernandes, J.B.; Mojumdar, S.C. Catalysed thermal decomposition of KClO3 and carbon gasification. J. Therm. Anal. Calorim. 2014, 119, 831–835. [Google Scholar] [CrossRef]
- Chakrabarti, K.; Nambissan, P.; Mukherjee, C.; Bardhan, K.; Kim, C.; Yang, K. Positron annihilation spectroscopic studies of the influence of heat treatment on defect evolution in hybrid MWCNT-polyacrylonitrile-based carbon fibers. Carbon 2007, 45, 2777–2782. [Google Scholar] [CrossRef]
- Jean, Y.C.; Lu, X.; Lou, Y.; Bharathi, A.; Sundar, C.S.; Lyu, Y.; Hor, P.H.; Chu, C.W. Positron annihilation inC60. Phys. Rev. B 1992, 45, 12126–12129. [Google Scholar] [CrossRef]
- Dryzek, J.; Pamuła, E.; Błażewicz, S. Positron Annihilation in Carbon Fibers. Phys. Status Solidi (a) 1995, 151, 39–46. [Google Scholar] [CrossRef]
- Kilic, B.; Simsek, E.B.; Turkdogan, S.; Demircivi, P.; Tuna, Ö.; Mucur, S.P.; Berek, D. Carbon nanofiber based CuO nanorod counter electrode for enhanced solar cell performance and adsorptive photocatalytic activity. J. Nanopart. Res. 2020, 22. [Google Scholar] [CrossRef]
- Rice-Evans, P.C.; Haynes, C.E.; Al-Qaradawi, I.; El Khangi, F.A.R.; Evans, H.E.; Smith, D.L. Positronium production at a carbon-oxygen interface. Phys. Rev. B 1992, 46, 14178–14181. [Google Scholar] [CrossRef] [PubMed]
- Eldrup, M.; Lightbody, D.; Sherwood, J. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Tao, S.J. Positronium Annihilation in Molecular Substances. J. Chem. Phys. 1972, 56, 5499–5510. [Google Scholar] [CrossRef]
- Liao, K.-S.; Chen, H.; Awad, S.; Yuan, J.-P.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y.; Hu, C.-C.; Jean, Y.C. Determination of Free-Volume Properties in Polymers Without Orthopositronium Components in Positron Annihilation Lifetime Spectroscopy. Macromolecules 2011, 44, 6818–6826. [Google Scholar] [CrossRef]
- Zgardzińska, B.; Chołubek, G.; Jarosz, B.; Wysogląd, K.; Gorgol, M.; Goździuk, M.; Jasińska, B. Studies on healthy and neoplastic tissues using positron annihilation lifetime spectroscopy and focused histopathological imaging. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef]
- Yuan, Q.; Su, C.; Cao, Y.; Wu, K.; Xu, J.; Yang, S. Rhodamine loading and releasing behavior of hydrogen-bonded poly(vinylpyrrolidone)/poly(acrylic acid) film. Colloids Surf. A Physicochem. Eng. Asp. 2014, 456, 153–159. [Google Scholar] [CrossRef]
- Dukali, R.; Radovic, I.; Stojanovic, D.; Sevic, D.; Radojevic, V.; Jocic, D.; Aleksic, R. Electrospinning of laser dye Rhodamine B-doped poly(methyl methacrylate) nanofibers. J. Serbian Chem. Soc. 2014, 79, 867–880. [Google Scholar] [CrossRef]
- Pereira, L.; Alves, M. Dyes—Environmental impact and remediation. In Environmental Strategies for Sustainable Development, Strategies for Sustainability; Malik, A., Grohmann, E., Eds.; Springer Science+Busines Media, B.V.: Heidelberg, Germany, 2012; pp. 111–162. [Google Scholar] [CrossRef]
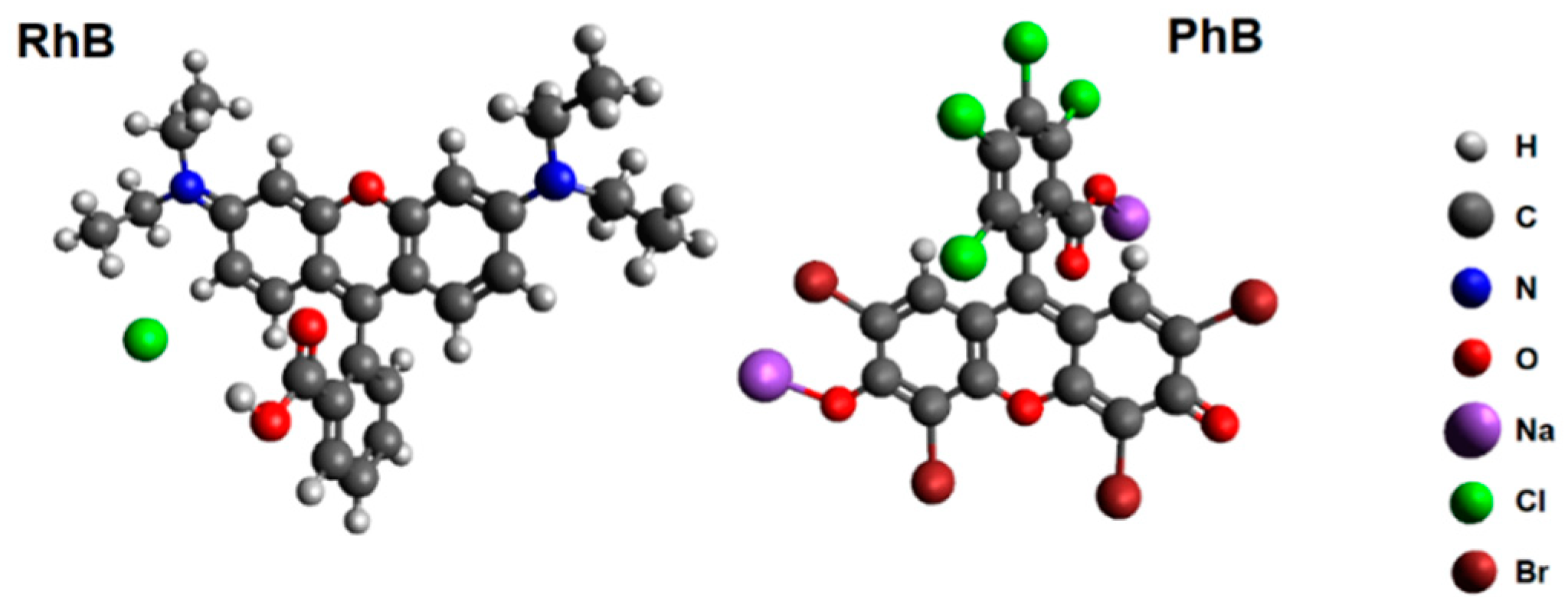





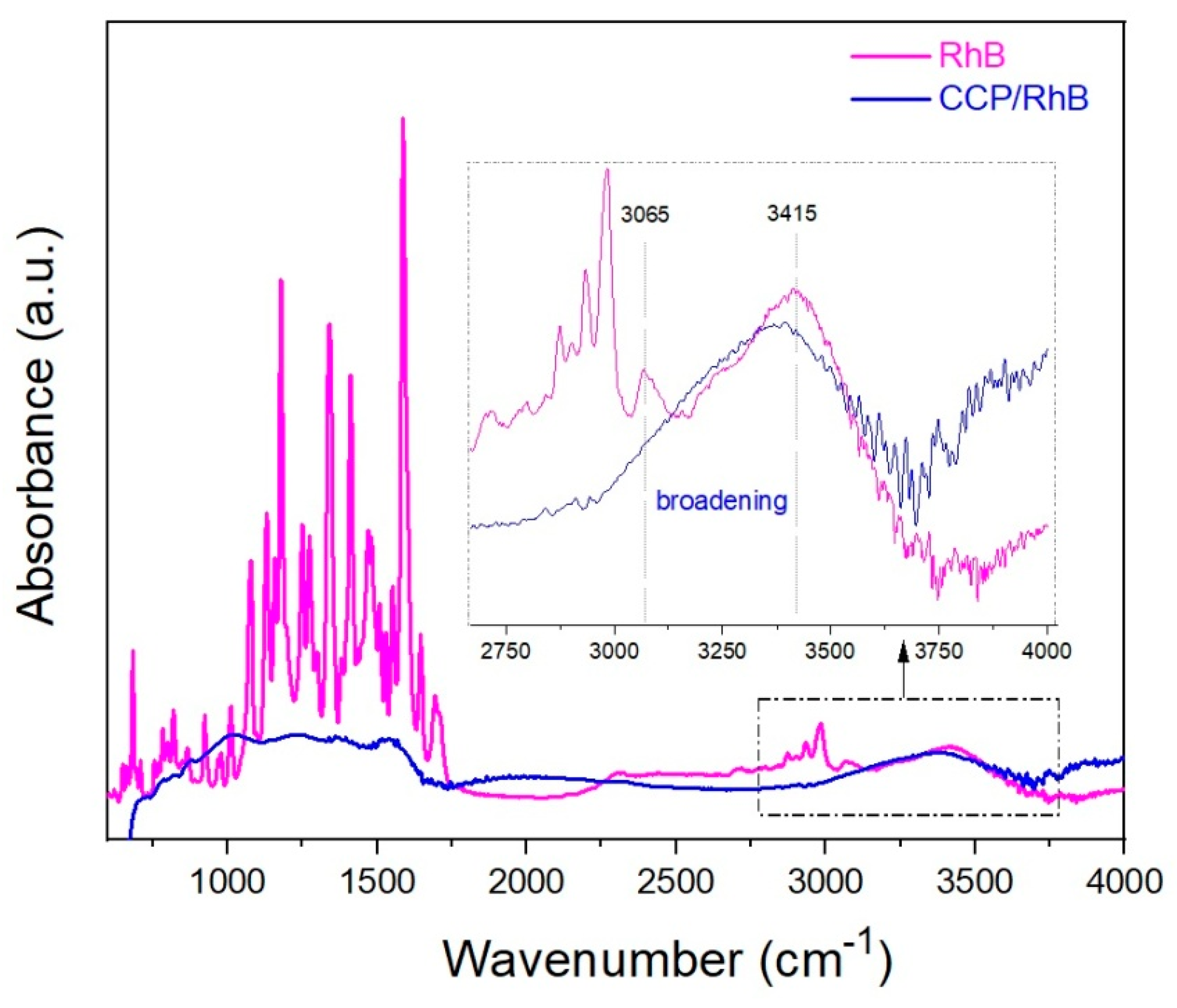
| Mixture | c (dye) (mol·L−1) | cm (CCP) (g·L−1) | n (dye)/m (CCP) (µmol·g−1) |
|---|---|---|---|
| I. | 10−5 | 6.25 | 1.60 |
| II. | 10−5 | 25 | 0.40 |
| Treatment Temp. (°C) | 50 | 100 | 200 | 250 | 400 | 600 | 800 |
|---|---|---|---|---|---|---|---|
| Mass Yield (%) | 22.37 | 43.67 | 44.14 | 53.67 | 63.15 | 76.20 | 80.37 |
| Temperature (°C) | ID | λD (cm−1) | IG | λG (cm−1) | ID/IG |
|---|---|---|---|---|---|
| 100 | – | – | – | – | – |
| 150 | 547.8 | 1355 | 735.5 | 1576 | 0.74 |
| 200 | 666.6 | 1351 | 818.2 | 1575 | 0.81 |
| 250 | 580.5 | 1353 | 643.5 | 1576 | 0.90 |
| 400 | 241.5 | 1337 | 253.2 | 1571 | 0.95 |
| 600 | 628.6 | 1326 | 561.9 | 1557 | 1.12 |
| 800 | 672.5 | 1333 | 622.9 | 1573 | 1.09 |
| Temperature (°C) | C | N | O | H |
|---|---|---|---|---|
| 100 | 42.0 | 0.8 | 51.5 | 5.7 |
| 150 | 51.5 | 1.5 | 43.1 | 3.9 |
| 200 | 54.2 | 2.0 | 40.6 | 3.2 |
| 250 | 55.0 | 2.1 | 41.0 | 1.9 |
| 400 | 66.2 | 2.4 | 28.5 | 2.9 |
| 600 | 68.6 | 2.3 | 27.6 | 1.5 |
| 800 | 75.5 | 1.9 | 21.8 | 0.8 |
| Wavenumber (cm−1) | Bands |
|---|---|
| 3000–3700 | –OH Stretching |
| 2700–2900 | –CH3, –CH2 Stretching |
| 1732 | C=O in Hemicellulose |
| 1649 | C–O–H Absorbed and C–O Conjugated |
| 1543 | Aromatic Skeletal Vibration C=O Stretch |
| 1460 | C–H Deformation, Asymmetric at –CH3 and –CH2 |
| 1424 | Aromatic Skeletal Vibration Combined with C–H in-Plane Deformation |
| 1372 | C–H Deformation |
| 1235 | Syringyl Rings and C= in Lignin |
| 1109 | –OH Activation in Cellulose and Hemicellulose |
| 1076 | C–H, C–O Deformation |
| 1056 | C–O Stretching in Cellulose and Lignin |
| 1031 | Aromatic C–H in-Plane Deformation, C–O Deformation, and Primary Alcohol |
| 998 | C–O–H Stretching in Cellulose and Hemicellulose |
| Researched Material | t2 (ns) | I2 (%) | R (Å) |
|---|---|---|---|
| CCP | 0.410 ± 0.004 | 54 ± 2 | 2.9 |
| RhB | 0.359 ± 0.003 | 71 ± 2 | 2.4 |
| CCP/RhB | 0.365 ± 0.002 | 79 ± 1 | 2.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opálková Šišková, A.; Dvorák, T.; Šimonová Baranyaiová, T.; Šimon, E.; Eckstein Andicsová, A.; Švajdlenková, H.; Opálek, A.; Krížik, P.; Nosko, M. Simple and Eco-Friendly Route from Agro-Food Waste to Water Pollutants Removal. Materials 2020, 13, 5424. https://doi.org/10.3390/ma13235424
Opálková Šišková A, Dvorák T, Šimonová Baranyaiová T, Šimon E, Eckstein Andicsová A, Švajdlenková H, Opálek A, Krížik P, Nosko M. Simple and Eco-Friendly Route from Agro-Food Waste to Water Pollutants Removal. Materials. 2020; 13(23):5424. https://doi.org/10.3390/ma13235424
Chicago/Turabian StyleOpálková Šišková, Alena, Tomáš Dvorák, Tímea Šimonová Baranyaiová, Erik Šimon, Anita Eckstein Andicsová, Helena Švajdlenková, Andrej Opálek, Peter Krížik, and Martin Nosko. 2020. "Simple and Eco-Friendly Route from Agro-Food Waste to Water Pollutants Removal" Materials 13, no. 23: 5424. https://doi.org/10.3390/ma13235424
APA StyleOpálková Šišková, A., Dvorák, T., Šimonová Baranyaiová, T., Šimon, E., Eckstein Andicsová, A., Švajdlenková, H., Opálek, A., Krížik, P., & Nosko, M. (2020). Simple and Eco-Friendly Route from Agro-Food Waste to Water Pollutants Removal. Materials, 13(23), 5424. https://doi.org/10.3390/ma13235424







