Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Characterization
3. Results and Discussion
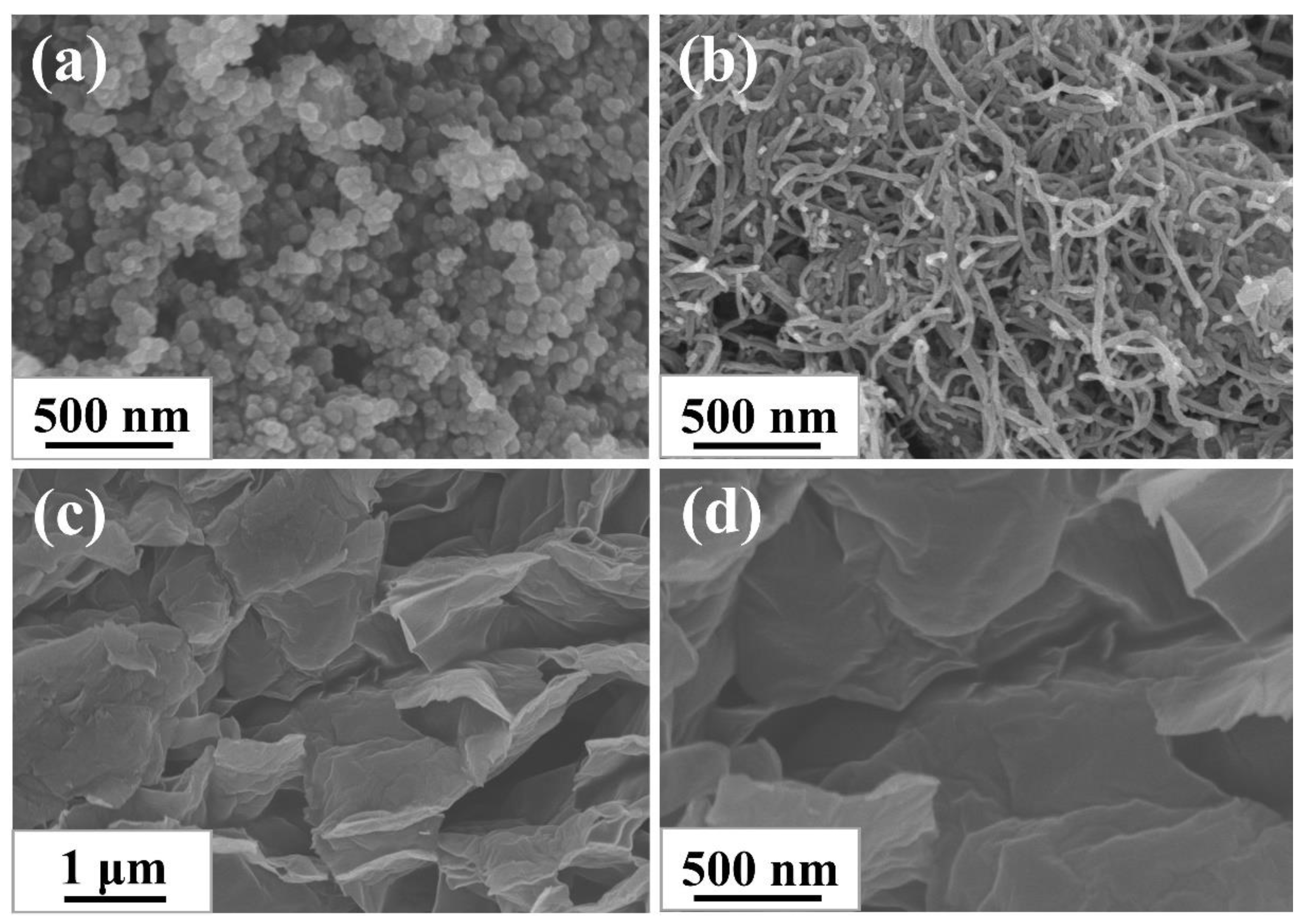
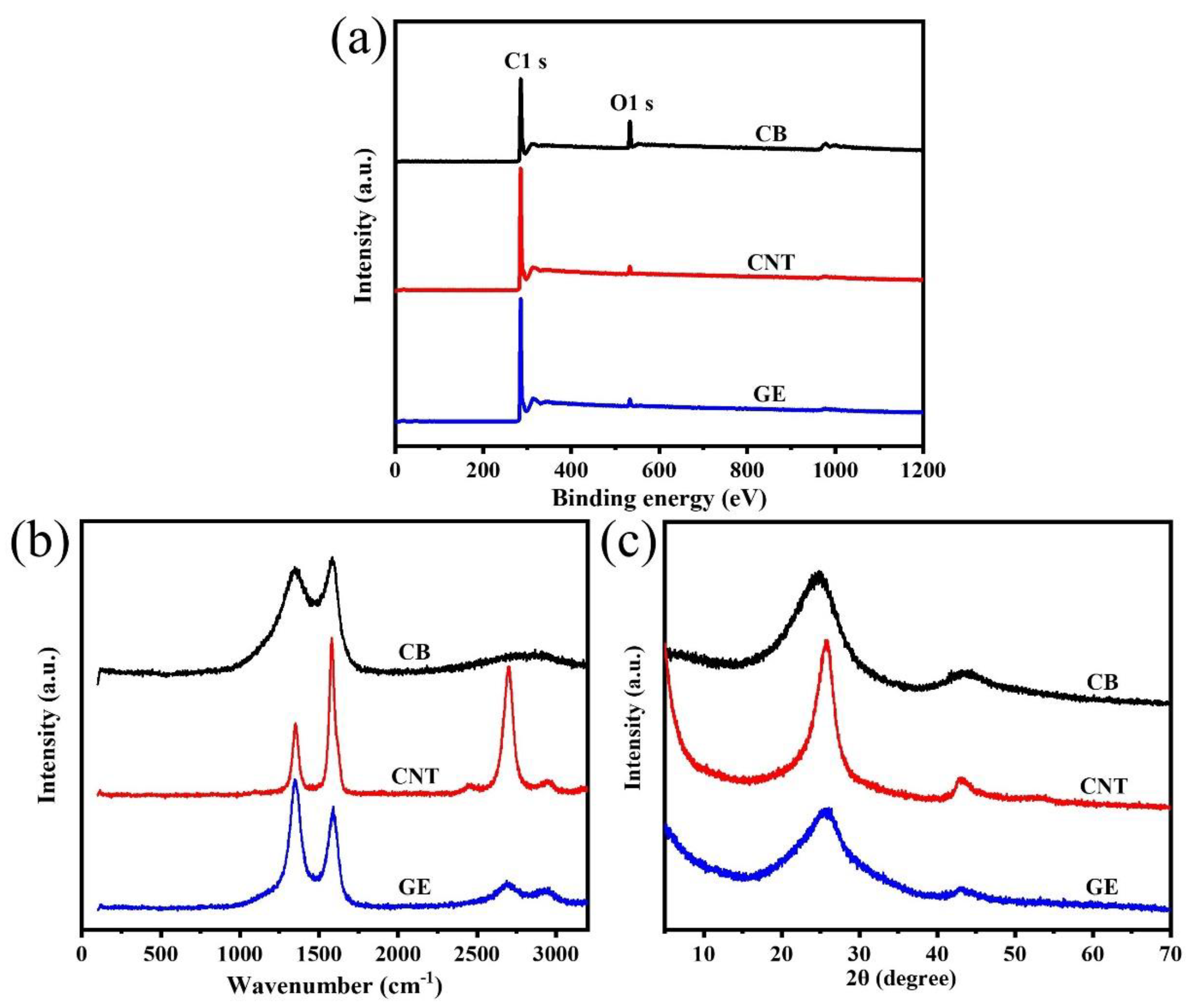
3.1. The Morphology and Structural Characteristics of CB, CNTs and GE
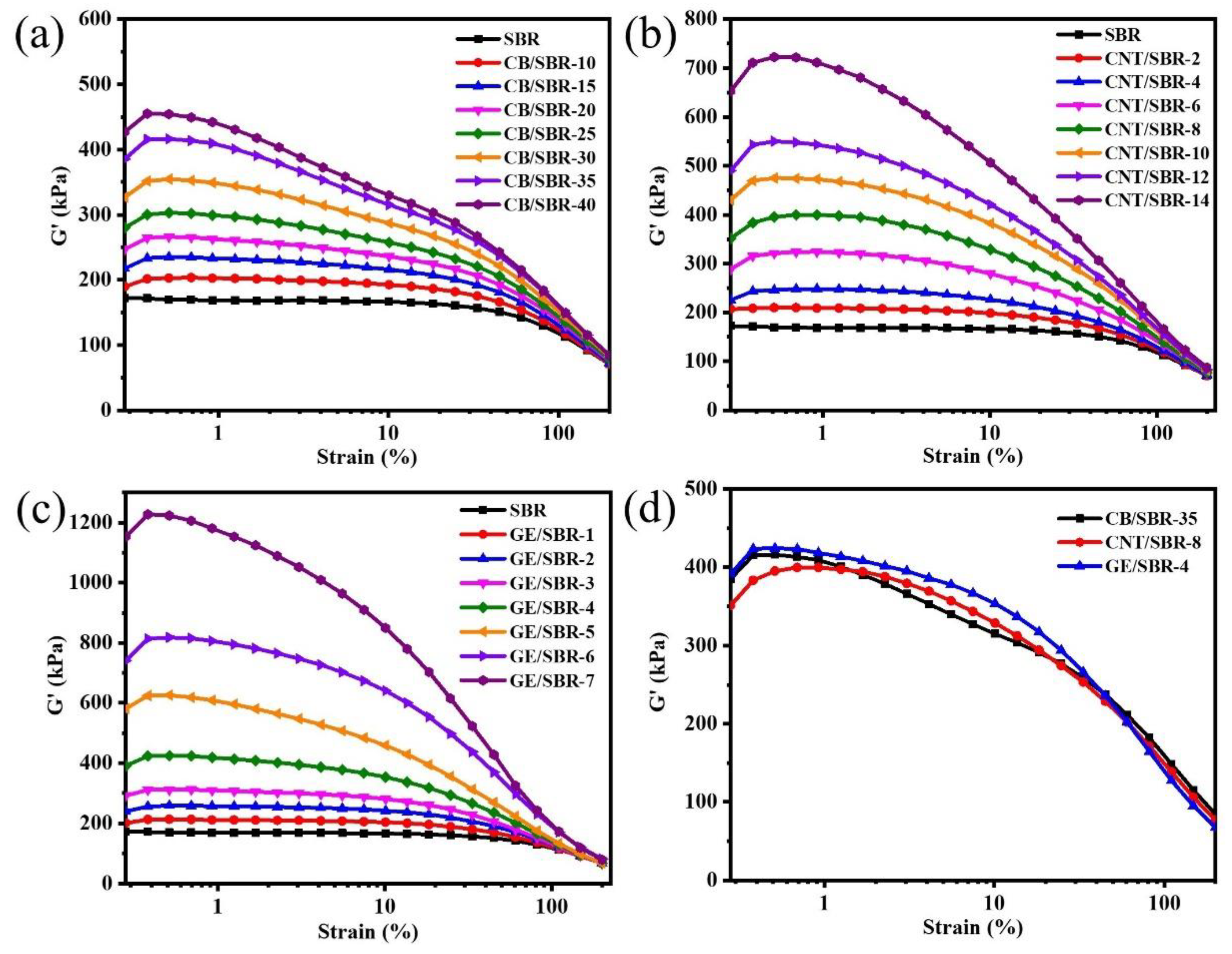
3.2. Filler Networks in SBR Composites
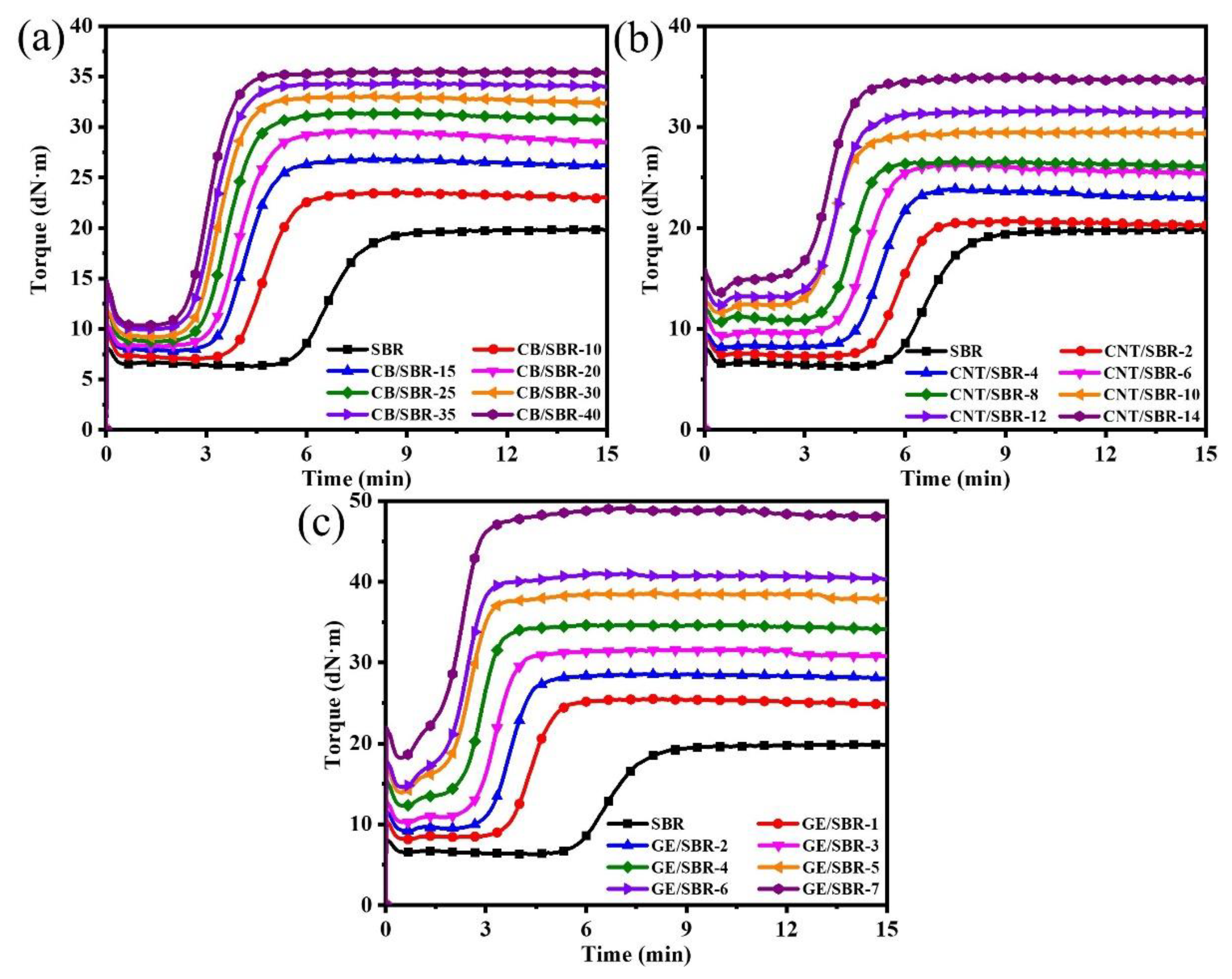
3.3. Curing Characteristics and Crosslinking Density of SBR Composites
3.4. The Microstructure of SBR Composites
3.5. Mechanical and Thermal Properties of SBR Composites
3.6. Gas Barrier Properties of SBR Composites
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Papageorgiou, D.G.; Li, Z.L.; Liu, M.F.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.N.; Yan, X. A review on the damping properties of fiber reinforced polymer composites. J. Ind. Text. 2020, 49, 693–721. [Google Scholar] [CrossRef]
- Wei, H.G.G.; Wang, H.; Li, A.; Cui, D.P.P.; Zhao, Z.N.N.; Chu, L.Q.Q.; Wei, X.; Wang, L.; Pan, D.; Fan, J.C.; et al. Multifunctions of Polymer Nanocomposites: Environmental Remediation, Electromagnetic Interference Shielding, And Sensing Applications. Chemnanomat 2020, 6, 174–184. [Google Scholar] [CrossRef]
- Chlanda, A.; Oberbek, P.; Heljak, M.; Kijenska-Gawronska, E.; Bolek, T.; Gloc, M.; John, L.; Janeta, M.; Wozniak, M.J. Fabrication, multi-scale characterization and in-vitro evaluation of porous hybrid bioactive glass polymer-coated scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 94, 516–523. [Google Scholar] [CrossRef]
- John, Ł.; Janeta, M.; Rajczakowska, M.; Ejfler, J.; Łydżba, D.; Szafert, S. Synthesis and microstructural properties of the scaffold based on a 3-(trimethoxysilyl)propyl methacrylate–POSS hybrid towards potential tissue engineering applications. RSC Adv. 2016, 6, 66037–66047. [Google Scholar] [CrossRef]
- Arpornwichanop, T.; Polpanich, D.; Thiramanas, R.; Suteewong, T.; Tangboriboonrat, P. PMMA-N,N,N-trimethyl chitosan nanoparticles for fabrication of antibacterial natural rubber latex gloves. Carbohydr. Polym. 2014, 109, 1–6. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, J.; Hou, G.; Ma, J.; Wang, W.; Wei, F.; Zhang, L. From nano to giant? Designing carbon nanotubes for rubber reinforcement and their applications for high performance tires. Compos. Sci. Technol. 2016, 137, 94–101. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, B.; Li, X.; Wang, W.; Tian, M.; Fan, Z.; Zhang, L. Enhanced adhesion property of aramid fibers by polyphenol-metal iron complexation and silane grafting. J. Adhes. 2019, 1–15. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y. Enhanced mechanical and thermal properties of SBR composites by introducing graphene oxide nanosheets decorated with silica particles. Compos. Part A Appl. Sci. Manuf. 2017, 102, 236–242. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Wu, S.; Wang, W.; Zhang, L. Novel percolation phenomena and mechanism of strengthening elastomers by nanofillers. Phys. Chem. Chem. Phys. 2010, 12, 3014–3030. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Zhang, Z.; Xian, Y.; Lin, Y.; Ji, X.; Lu, Y.; Zhang, L. Construction of interconnected Al2O3 doped rGO network in natural rubber nanocomposites to achieve significant thermal conductivity and mechanical strength enhancement. Compos. Sci. Technol. 2020, 186, 107930. [Google Scholar] [CrossRef]
- Lin, J.; Luo, Y.; Zhong, B.; Hu, D.; Jia, Z.; Jia, D. Enhanced interfacial interaction and antioxidative behavior of novel halloysite nanotubes/silica hybrid supported antioxidant in styrene-butadiene rubber. Appl. Surf. Sci. 2018, 441, 798–806. [Google Scholar] [CrossRef]
- Dobashi, R.; Matsunaga, K.; Tajima, M. Effects of fullerene derivatives on the gas permeability of thermoplastic polyurethane elastomers. J. Appl. Polym. Sci. 2014, 131, 39986. [Google Scholar] [CrossRef]
- Smith, A.P.; Crossley, S.D.; Gruber, T.C. Computational Investigation of the Effects of Spherical Filler Morphology and Loading on Diffusion Tortuosity and Rubber Permeability. Rubber Chem. Technol. 2013, 86, 175–189. [Google Scholar] [CrossRef]
- Padhi, S.; Raju Achary, P.G.; Nayak, N.C. Molecular transport behaviour of organic solvents through halloysite nanotubes filled ethylene–vinyl acetate copolymer. Bull. Mater. Sci. 2015, 38, 925–933. [Google Scholar] [CrossRef][Green Version]
- Zhang, Y.; Liu, Q.; Zhang, S.; Zhang, Y.; Cheng, H. Gas barrier properties and mechanism of kaolin/styrene–butadiene rubber nanocomposites. Appl. Clay Sci. 2015, 111, 37–43. [Google Scholar] [CrossRef]
- Kumar, S.K.; Castro, M.; Saiter, A.; Delbreilh, L.; Feller, J.F.; Thomas, S.; Grohens, Y. Development of poly(isobutylene-co-isoprene)/reduced graphene oxide nanocomposites for barrier, dielectric and sensing applications. Mater. Lett. 2013, 96, 109–112. [Google Scholar] [CrossRef]
- Maria, H.J.; Thomas, M.G.; Morreale, M.; La Mantia, F.P.; Nzihou, A.; Joseph, K.; Rouxel, D.; Fernandes, S.C.M.; Kalarikkal, N.; Thomas, S. Gas Barrier, Rheological and Mechanical Properties of Immiscible Natural Rubber/Acrylonitrile Butadiene Rubber/Organoclay (NR/NBR/Organoclay) Blend Nanocomposites. Materials 2020, 13, 2654. [Google Scholar] [CrossRef]
- Fan, Y.R.; Fowler, G.D.; Zhao, M. The past, present and future of carbon black as a rubber reinforcing filler—A review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.Y.; Zhao, L.F.; He, H.F.; Shao, X.Y.; Fang, G.B.; Wan, Z.G.; Zeng, R.C. Research progress of graphene-based rubber nanocomposites. Polym. Compos. 2018, 39, 1006–1022. [Google Scholar] [CrossRef]
- Bokobza, L. Multiwall carbon nanotube elastomeric composites: A review. Polymer 2007, 48, 4907–4920. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Zaborski, M. Effect of different carbon fillers on the properties of nitrile rubber composites. Compos. Interfaces 2018, 26, 729–750. [Google Scholar] [CrossRef]
- Kumar, V.; Lee, G.; Singh, K.; Choi, J.; Lee, D.-J. Structure-property relationship in silicone rubber nanocomposites reinforced with carbon nanomaterials for sensors and actuators. Sens. Actuators A Phys. 2020, 303, 111712. [Google Scholar] [CrossRef]
- Hu, H.Q.; Gao, Q.Q.; Tian, G.N.; Hong, S.; Zhao, J.; Zhao, Y.X. The influence of topology and morphology of fillers on the conductivity and mechanical properties of rubber composites. J. Polym. Res. 2018, 25, 87. [Google Scholar] [CrossRef]
- Dong, B.; Zhang, L.; Wu, Y. Influences of different dimensional carbon-based nanofillers on fracture and fatigue resistance of natural rubber composites. Polym. Test. 2017, 63, 281–288. [Google Scholar] [CrossRef]
- Sujith, A.; Unnikrishnan, G. Barrier properties of natural rubber/ethylene vinyl acetate/carbon black composites. J. Mater. Sci. 2005, 40, 4625–4640. [Google Scholar] [CrossRef]
- Wang, Z.F.; Wang, B.; Qi, N.; Zhang, H.F.; Zhang, L.Q. Influence of fillers on free volume and gas barrier properties in styrene-butadiene rubber studied by positrons. Polymer 2005, 46, 719–724. [Google Scholar] [CrossRef]
- Guadagno, L.; Vertuccio, L.; Sorrentino, A.; Raimondo, M.; Naddeo, C.; Vittoria, V.; Iannuzzo, G.; Calvi, E.; Russo, S. Mechanical and barrier properties of epoxy resin filled with multi-walled carbon nanotubes. Carbon 2009, 47, 2419–2430. [Google Scholar] [CrossRef]
- Varghese, J. Tailoring the mechanical and gas barrier properties of nanocomposites by incorporating a MWCNT/CuS hybrid nanofiller. New J. Chem. 2019, 43, 14051–14056. [Google Scholar] [CrossRef]
- Lin, Y.; Liu, S.; Liu, L. A new approach to construct three dimensional segregated graphene structures in rubber composites for enhanced conductive, mechanical and barrier properties. J. Mater. Chem. C 2016, 4, 2353–2358. [Google Scholar] [CrossRef]
- Van Amerongen, G.J. The effect of fillers on the permeability of rubber to gases. Rubber Chem. Technol. 1955, 28, 821–832. [Google Scholar] [CrossRef]
- Cadambi, R.M.; Ghassemieh, E. Optimized process for the inclusion of carbon nanotubes in elastomers with improved thermal and mechanical properties. J. Appl. Polym. Sci. 2011, 124, 4993–5001. [Google Scholar] [CrossRef]
- Varghese, T.V.; Ajith Kumar, H.; Anitha, S.; Ratheesh, S.; Rajeev, R.S.; Lakshmana Rao, V. Reinforcement of acrylonitrile butadiene rubber using pristine few layer graphene and its hybrid fillers. Carbon 2013, 61, 476–486. [Google Scholar] [CrossRef]
- Qu, L.L.; Yu, G.Z.; Wang, L.L.; Li, C.Q.; Zhao, Q.S.; Li, J. Effect of filler-elastomer interactions on the mechanical and nonlinear viscoelastic behaviors of chemically modified silica-reinforced solution-polymerized styrene butadiene rubber. J. Appl. Polym. Sci. 2012, 126, 116–126. [Google Scholar] [CrossRef]
- Heise, H.M.; Kuckuk, R.; Ojha, A.K.; Srivastava, A.; Srivastava, V.; Asthana, B.P. Characterisation of carbonaceous materials using Raman spectroscopy: A comparison of carbon nanotube filters, single- and multi-walled nanotubes, graphitised porous carbon and graphite. J. Raman Spectrosc. 2009, 40, 344–353. [Google Scholar] [CrossRef]
- Fröhlich, J.; Niedermeier, W.; Luginsland, H.D. The effect of filler–filler and filler–elastomer interaction on rubber reinforcement. Compos. Part A Appl. Sci. Manuf. 2005, 36, 449–460. [Google Scholar] [CrossRef]
- Liu, X.; Kuang, W.; Guo, B. Preparation of rubber/graphene oxide composites with in-situ interfacial design. Polymer 2015, 56, 553–562. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Saphiannikova, M.; Fritzsche, J.; Lorenz, H.; Klüppel, M.; Heinrich, G. Modified and unmodified multiwalled carbon nanotubes in high performance solution-styrene–butadiene and butadiene rubber blends. Polymer 2008, 49, 5276–5283. [Google Scholar] [CrossRef]
- Araby, S.; Zhang, L.; Kuan, H.-C.; Dai, J.-B.; Majewski, P.; Ma, J. A novel approach to electrically and thermally conductive elastomers using graphene. Polymer 2013, 54, 3663–3670. [Google Scholar] [CrossRef]
- Berki, P.; Gobl, R.; Karger-Kocsis, J. Structure and properties of styrene-butadiene rubber (SBR) with pyrolytic and industrial carbon black. Polym. Test. 2017, 61, 404–415. [Google Scholar] [CrossRef]
- Xu, Z.; Jerrams, S.; Guo, H.; Zhou, Y.; Jiang, L.; Gao, Y.; Zhang, L.; Liu, L.; Wen, S. Influence of graphene oxide and carbon nanotubes on the fatigue properties of silica/styrene-butadiene rubber composites under uniaxial and multiaxial cyclic loading. Int. J. Fatigue 2020, 131, 105388. [Google Scholar] [CrossRef]


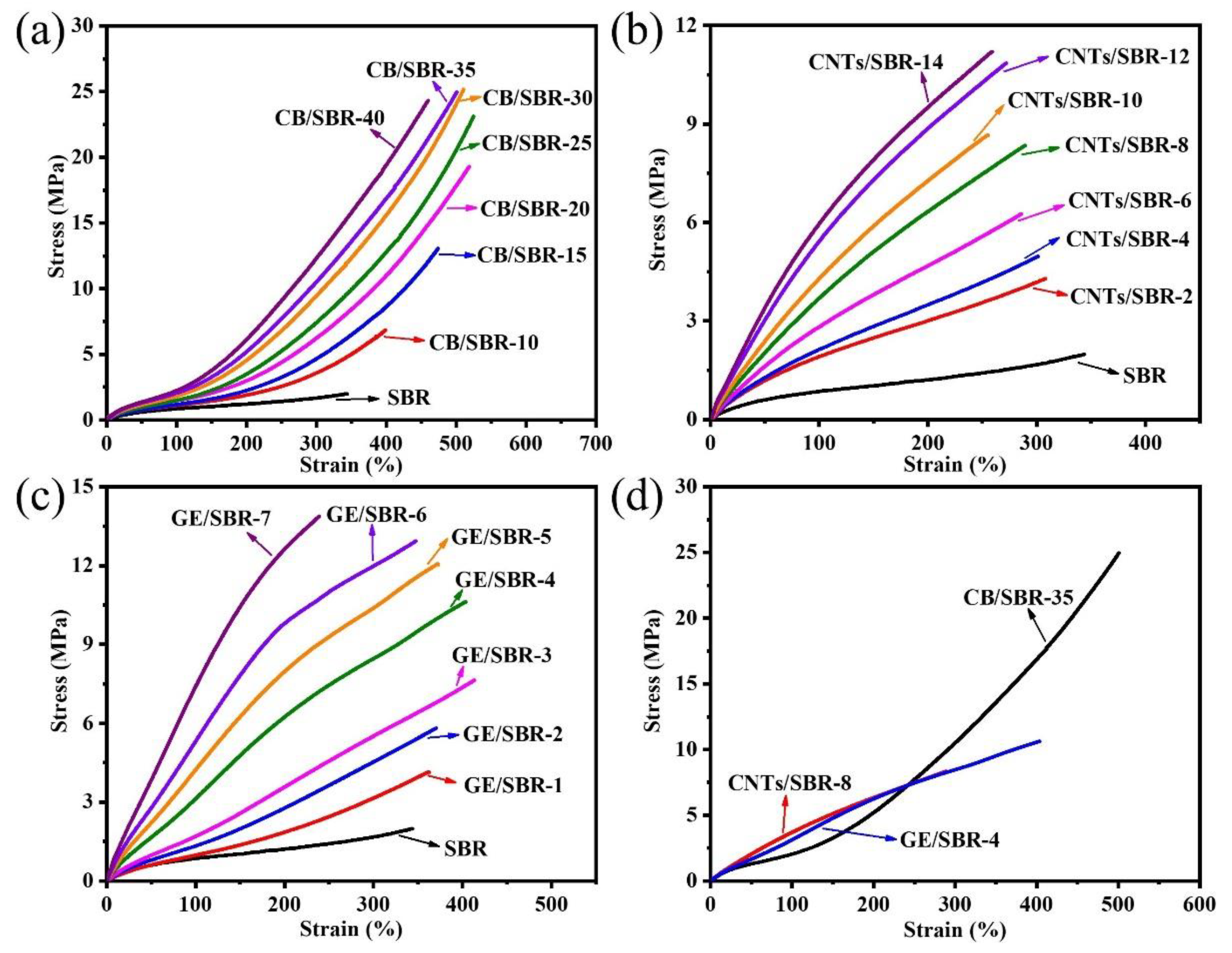
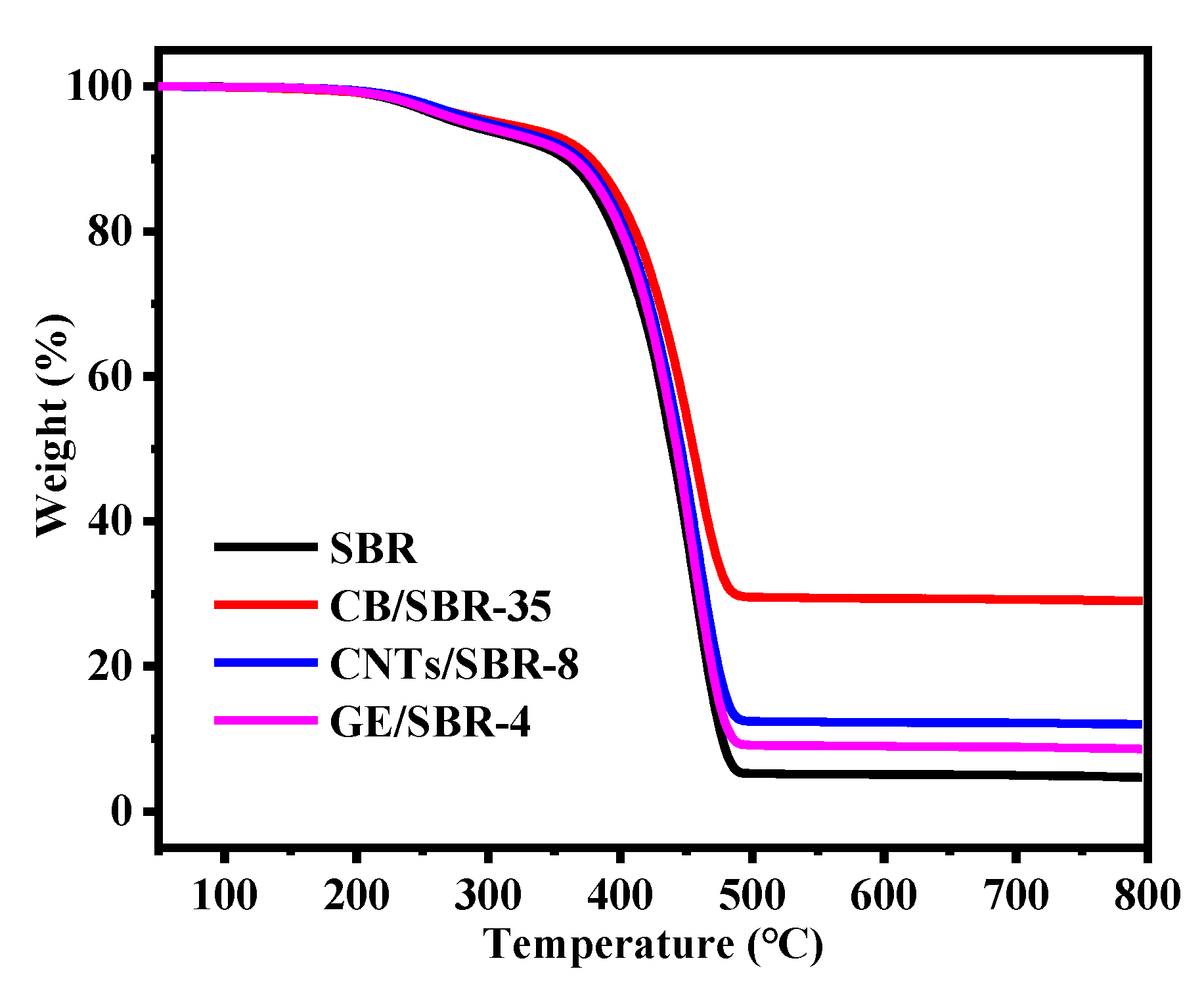
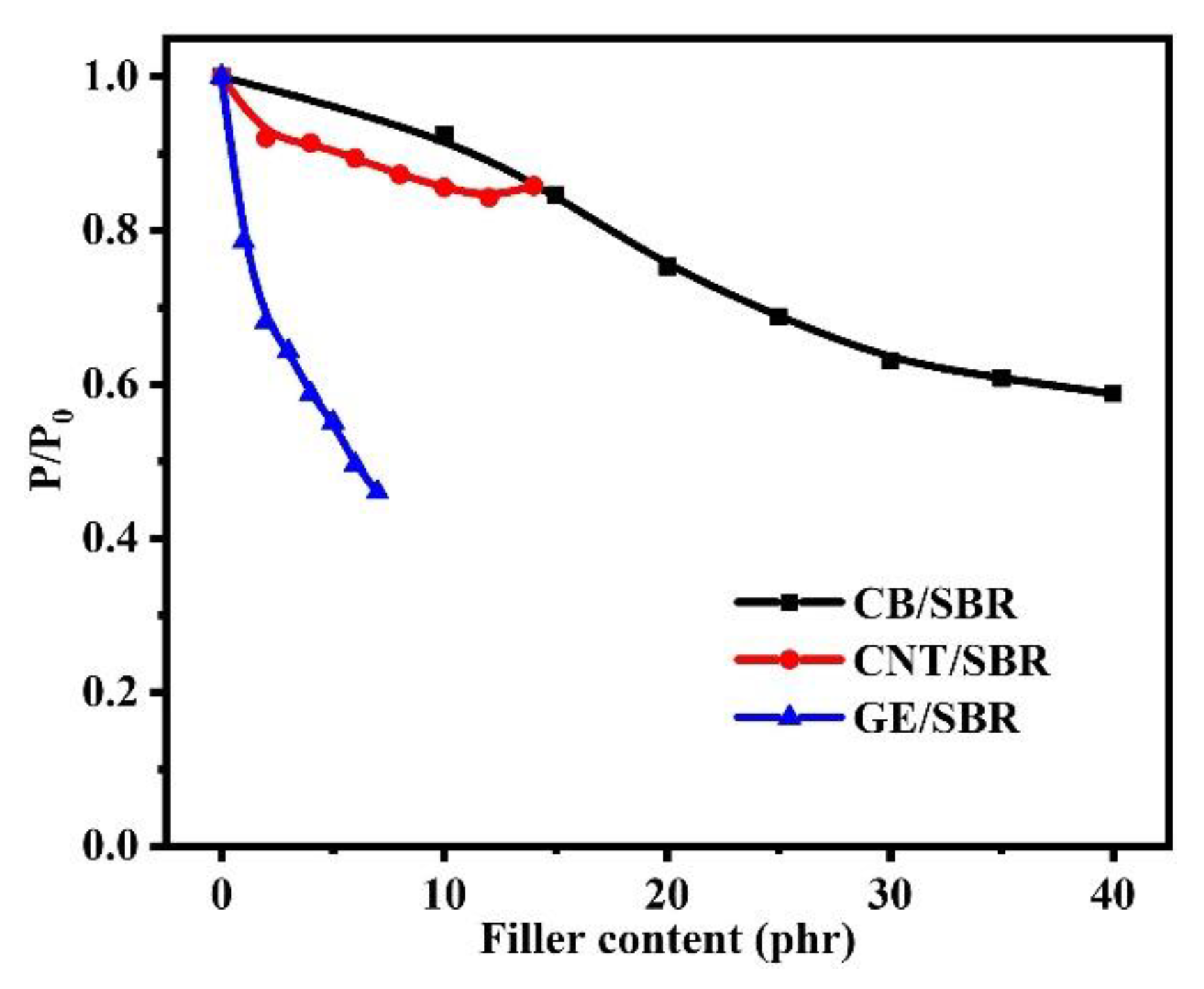
| Samples | SBR | CB/SBR-35 | CNTs/SBR-8 | GE/SBR-4 |
|---|---|---|---|---|
| Tensile strength (MPa) | 2.0 ± 0.5 | 25.0 ± 1.8 | 8.3 ± 1.2 | 10.6 ± 1.4 |
| Tear strength (kN/m) | 7.4 ± 1.6 | 39.4 ± 3.1 | 30.7 ± 2.4 | 32.5 ± 2.9 |
| Elongation at break (%) | 344 ± 9 | 501 ± 18 | 289 ± 11 | 403 ± 14 |
| Modulus at 100% strain (MPa) | 0.8 ± 0.1 | 2.0 ± 0.1 | 3.7 ± 0.2 | 3.1 ± 0.2 |
| Modulus at 300% strain (MPa) | 1.7 ± 0.1 | 10.5 ± 0.6 | -- | 8.5 ± 0.3 |
| Shore A hardness | 48 ± 1 | 61 ± 1 | 62 ± 1 | 66 ± 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wen, S.; Zhang, R.; Xu, Z.; Zheng, L.; Liu, L. Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites. Materials 2020, 13, 5416. https://doi.org/10.3390/ma13235416
Wen S, Zhang R, Xu Z, Zheng L, Liu L. Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites. Materials. 2020; 13(23):5416. https://doi.org/10.3390/ma13235416
Chicago/Turabian StyleWen, Shipeng, Rui Zhang, Zongchao Xu, Long Zheng, and Li Liu. 2020. "Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites" Materials 13, no. 23: 5416. https://doi.org/10.3390/ma13235416
APA StyleWen, S., Zhang, R., Xu, Z., Zheng, L., & Liu, L. (2020). Effect of the Topology of Carbon-Based Nanofillers on the Filler Networks and Gas Barrier Properties of Rubber Composites. Materials, 13(23), 5416. https://doi.org/10.3390/ma13235416




