1. Introduction
Smart windows that use the thermochromic property of vanadium dioxide (VO
2) have been highlighted as a promising energy saving technology due to their selective control over the transmission of heat rays that enhances energy saving efficiency [
1]. At 68 °C or below, VO
2 has a monoclinic structure (M) with a semiconducting property, and at temperatures above 68 °C, its crystal structure is transformed to a tetragonal rutile structure, accompanying a change in its electrical property from semiconducting to metallic [
2]. This metal-insulator transition (MIT) enables VO
2 to selectively reflect near-infrared light (NIR) due to its metallic property above its MIT temperature while transmitting visible light [
3]. To practically apply the thermochromic property of VO
2 to smart windows that can control sunlight transmission at room temperature, decreasing the MIT temperature of VO
2 down to around room temperature is very much required. The doping of VO
2 (M) with tungsten (W) atoms has been a promising route for reducing the MIT temperature of VO
2 (M) [
4,
5,
6,
7]. When tungsten atoms are doped to the VO
2, it is expected that the original V
4+-V
4+ pairs in VO
2 (M) are converted to V
3+-V
4+ and V
3+-W
6+ pairs by electron donation from W to the neighboring V ions. As a result, the concentration of free electrons is increased via electron donation, and the monoclinic structure is distorted due to the insertion of larger doped W
6+ ions with an ionic radius of 64 pm than V
4+ ions with an ionic radius of 49.5 pm. Thus, the MIT temperature of pristine VO
2 is decreased, making W
xVO
2 (M) an ideal candidate for thermochromic window materials as it can reflect NIR at room temperature while transmitting visible light [
8,
9].
A variety of methods, including hydrothermal synthesis with an autoclave at a high pressure [
10], chemical vapor deposition (CVD) [
11], sputtering [
12,
13], and ion implantation [
14], have been utilized to synthesize W
xVO
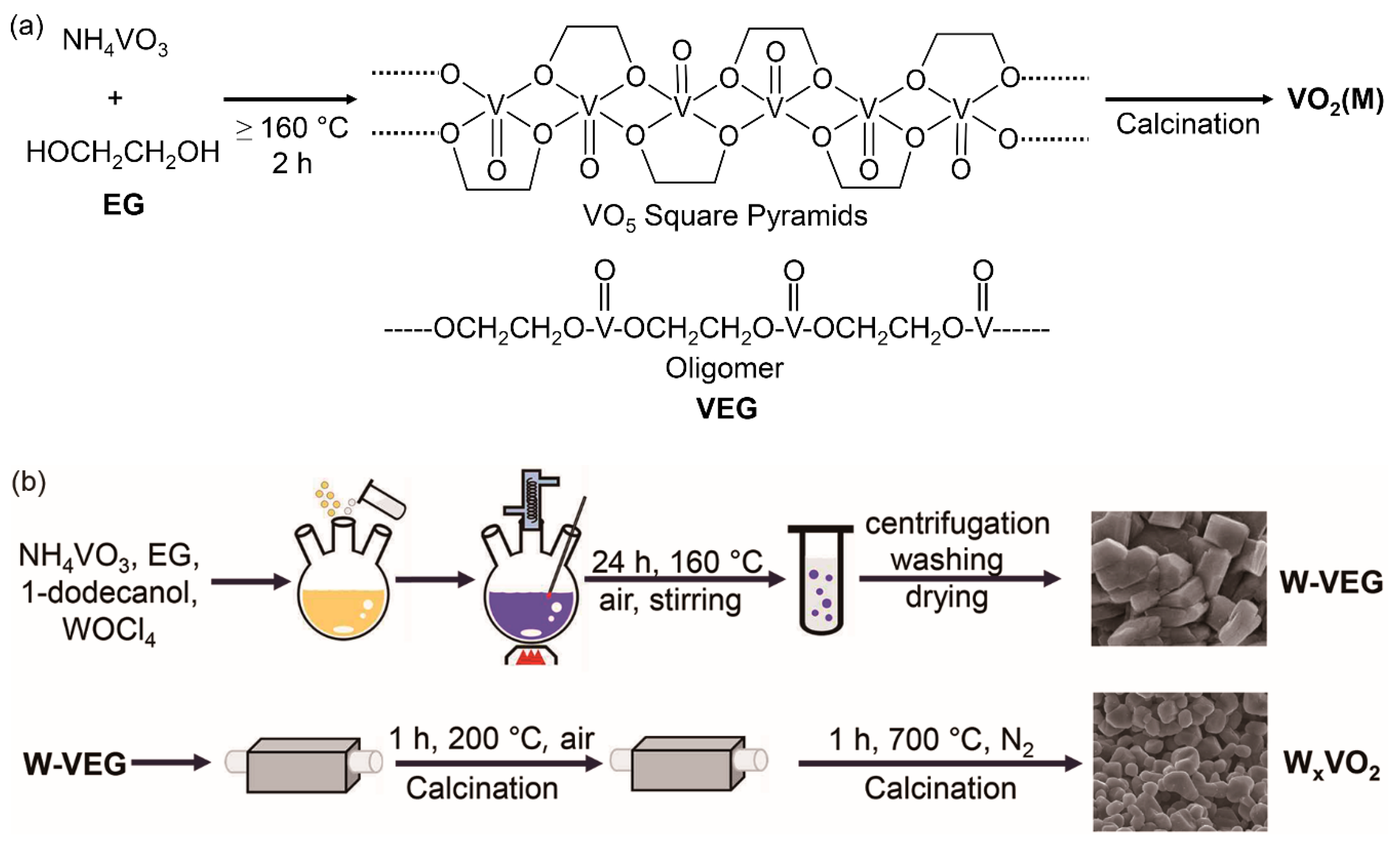
2 (M). However, these methods require complicated experimental parameter controls or special devices, thereby restricting the extension of these methods to large-scale production at a low cost. Thus, it is highly necessary to develop a convenient process that utilizes atmospheric pressure in air at a low processing temperature. A candidate method for resolving these issues is the thermolysis of vanadyl glycolate to synthesize VO
2 (M) [
15,
16]. In this method, an alcohol with two or more hydroxyl groups and high boiling points, such as ethylene glycol (EG) with a boiling point of 197 °C, is mixed with a cheap vanadium precursor, such as ammonium metavanadate (NH
4VO
3), and the resulting solution mixture is heated over 160 °C in atmospheric air followed by thermolysis of precipitates at a low pyrolytic temperature of 200 °C to produce VO
2 (M) (
Scheme 1). In this polyol method, EG works as a stabilizer that limits particle growth and prevents aggregation [
17], as a coupling agent that can further lead vanadyl ethyleneglycol glycolate (VEG) to oligomer with a repeat unit of VO(OCH
2CH
2O) [
18], and as a reducing agent when the VEG is calcinated [
19]. The crystal structure of VEG can also be depicted by the one-dimensional chain of the VO
5 square pyramids in the VO(OCH
2CH
2O) structure by edge sharing (
Scheme 1) [
19]. When using this method to make VEG, special devices are not required, and subsequent calcination can be carried out via a conventional sintering process, thereby suggesting its possibility for mass production at a low cost. Although the polyol method shows great potential for the facile synthesis of W
xVO
2, it is difficult to find reports about the polyol method being applied to synthesize W
xVO
2 nanoparticles. This lack of research seems to be because a special synthesis for tungsten precursors that can be coupled within VEG is necessary or because the insertion of the large W
6+ ions into the monoclinic VO
2 (M) at an atmospheric pressure is difficult. Thus, the synthesis of W
xVO
2 using the polyol method via thermolysis in atmospheric air remains challenging.
In this study, we present a modified polyol method using 1-dodecanol as an additive to synthesize W
xVO
2 (M). 1-Dodecanol has a boiling point of 259 °C, which is high enough for the polyol method, and can act as both the solvent and capping agent, as shown in inorganic nanoparticle synthesis [
20]. Unlike the original polyol method, we found that tungsten could be doped into VO
2 (M) with the addition of 1-dodecanol, thereby reducing the MIT temperature of the resulting W
xVO
2 (M). We investigated the influence of the mixing ratios of EG and 1-dodecanol and the amounts of tungsten precursor on the synthesis of W
xVO
2. Our results suggest a procedure to dope tungsten into VO
2 (M) via the polyol method.
3. Results and Discussion
We used WOCl
4 as a tungsten precursor to synthesize W
xVO
2 (M) via the polyol method based on ammonium metavanadate and EG because WOCl
4 could be readily combined with EG via an exothermic reaction between WOCl
4 and hydroxyl groups that make byproducts of HCl. Thus, one might expect that WOCl
4 combined with EG participates in the formation of VEG, thereby resulting in the insertion of tungsten atoms into VO
2 (M) during calcination. However, when 2.77 mol% of WOCl
4 to NH
4VO
3 was added into the reaction mixture of ammonium metavanadate and EG at the 1:0 mixing ratio of EG to 1-dodecanol, the final product gained after calcination did not show a highly crystalline structure in its XRD pattern, as shown in
Figure 1a. Additionally, the XRD pattern was not matched with that of VO
2 (M). This result can be ascribed to the incomplete synthesis of linear VEG via a condensation reaction between NH
4VO
3 and EG because WOCl
4 with four reactive W-Cl functionalities to EGs can interrupt the formation of the linear VEG.
We presumed that the simple addition of 1-dodecanol as a co-solvent could resolve this issue because 1-dodecanol might act as a partial capping agent for the highly reactive W-Cl functionality in WOCl
4. To investigate the capping effect of 1-dodecanol on WOCl
4, we carried out the synthesis of W
xVO
2 by varying the molar mixing ratio of EG to 1-dodecanol at 1:0.5, 1:1, and 1:2 at fixed amounts of NH
4VO
3 to WOCl
4 at 10 and 0.277 mmol, respectively.
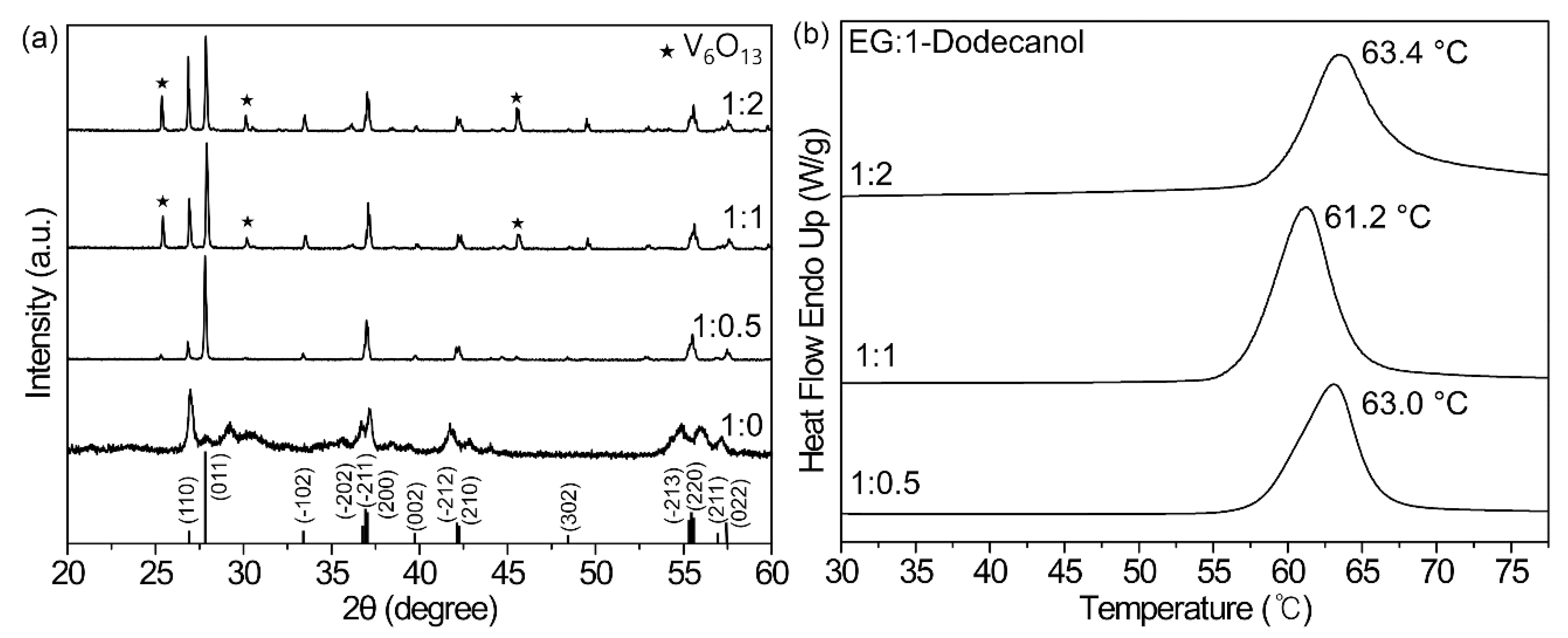
Figure 1a shows the XRD patterns of the tungsten-doped vanadium oxides produced by controlling the molar ratio of EG:1-dodecanol. The observed diffraction peaks at all three ratios of 1:0.5, 1:1 and 1:2 indicate the monoclinic vanadium dioxide VO
2 (M) crystal structure reported in JCPDS No. 43-1051 [
21]. Particularly, well-developed crystallinity was seen in the main VO
2(M) peaks at 2θ angles of 26.8°, 27.8° 33.4°, 37.0°, 39.8°, 42.1°, 42.2°, 55.5°, and 57.4° for (110), (011), (−102), (−202; −211; 200), (002), (−212; 210), (−213; 220; 211), and (022) planes, respectively. However, diffraction peaks of V
6O
13 at 2θ = 25.3°, 30.1°, and 45.6° for (101), (400), and (005), respectively, were also clearly observed in the XRD patterns of the compounds synthesized at ratios of 1:1 and 1:2, and were in agreement with the reference data (JCPDS No. 25-1251) [
22]. The appearance of the oxidized V
6O
13 peaks originates from the increase in oxygen contents in W-VEG as the molar ratio of 1-dodecanol increases. It appears that excess oxygen atoms incorporated into the VO
2 structure chemically interact with vanadium atoms to form a new oxidation state of V
6O
13, as stated in literature [
23].
The decrease in MIT temperatures with the addition of tungsten precursors in varying molar ratios of EG to 1-dodecanol at the fixed amount of WOCl
4 to NH
4VO
3 was confirmed by measuring the DSC thermogram. In
Figure 1b, the DSC curves show that the MIT temperatures were 63.03, 61.23, and 63.44 °C at mixing ratios of 1:0.5, 1:1, and 1:2 for EG to 1-dodecanol. These results show an obvious drop from 68 °C, the original MIT temperature of VO
2 (M), and indicate successful tungsten doping via the polyol method. Furthermore, it should be noted that W
xVO
2 prepared from the 1:1 mixture of EG to 1-dodecanol has the lowest MIT temperature, although the fixed amount of WOCl
4 was used for all calcinated samples.
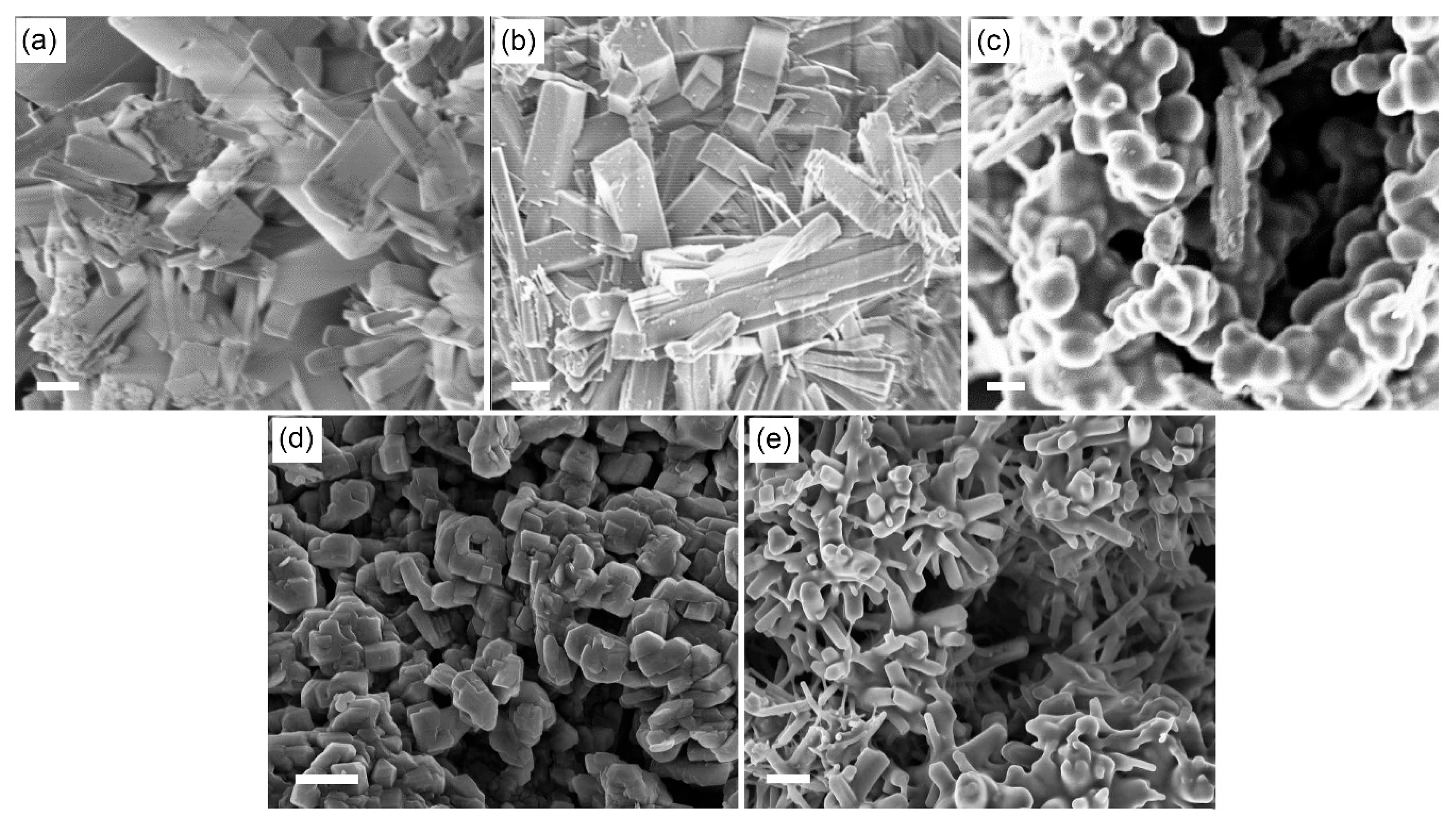
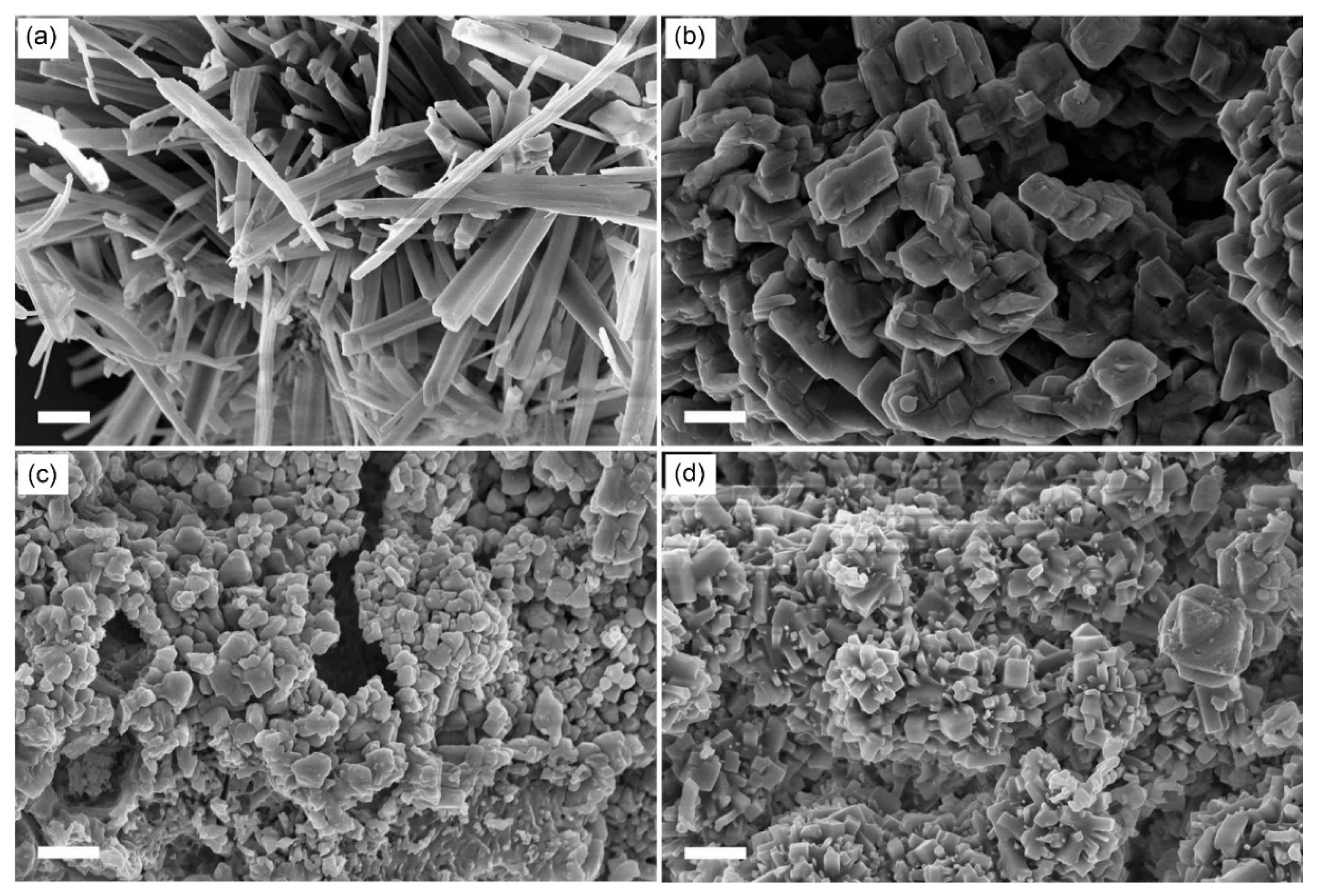
To investigate the origination of these variations in the MIT temperatures, we measured SEM images of VEG synthesized without using 1-dodecanol and WOCl
4 as a reference and W-VEGs prepared with varying mixing ratios of EG to 1-dodecanol at the fixed WOCl
4 concentration of 2.77 mol% relative to NH
4VO
3 (
Figure 2). The SEM images of VEG in
Figure 2a looks like a mixture of square rods at the nano‒ and micrometer scales, which is similar to nanorods found in the literature [
24]. This morphology reflects VEG crystals based on one dimensional chain of square pyramids sharing edges with the formula VO(OCH
2CH
2O) [
19], as shown in
Scheme 1. When 1-dodecanol was not used, the SEM image of W-VEG, as can be seen in
Figure 2b, showed microcrystals with rod-like morphologies and a square cross section. The side length of the square cross section was around 1 μm. This morphology did not significantly deviate from that of VEG without using the tungsten precursor, as can be seen in
Figure 2a. With the addition of 1-dodecanol at 1:0.5 and 1:2, as shown in
Figure 2c,e, the edges of the crystals were smeared, and the resulting morphology was a mixture of rods and smeared aggregates. The SEM image at 1:1 shown in
Figure 2d shows morphologies with sharp edges, but the sizes and lengths of the microcrystals were significantly decreased in comparison to those at a 1:0 ratio of 1-dodecanol.
One hypothesis to explain the variation in the morphologies of W-VEG microcrystals is that WOCl
4 reacted with 1-dodecanol, which significantly influenced the crystal morphologies. The reaction between NH
4VO
3 and EG is like the oligomerization of two monomers, resulting in VEG with a one-dimensional chain of VO
5 square pyramids, as shown in
Scheme 1 and the literature [
18]. Our results suggest that the WOCl
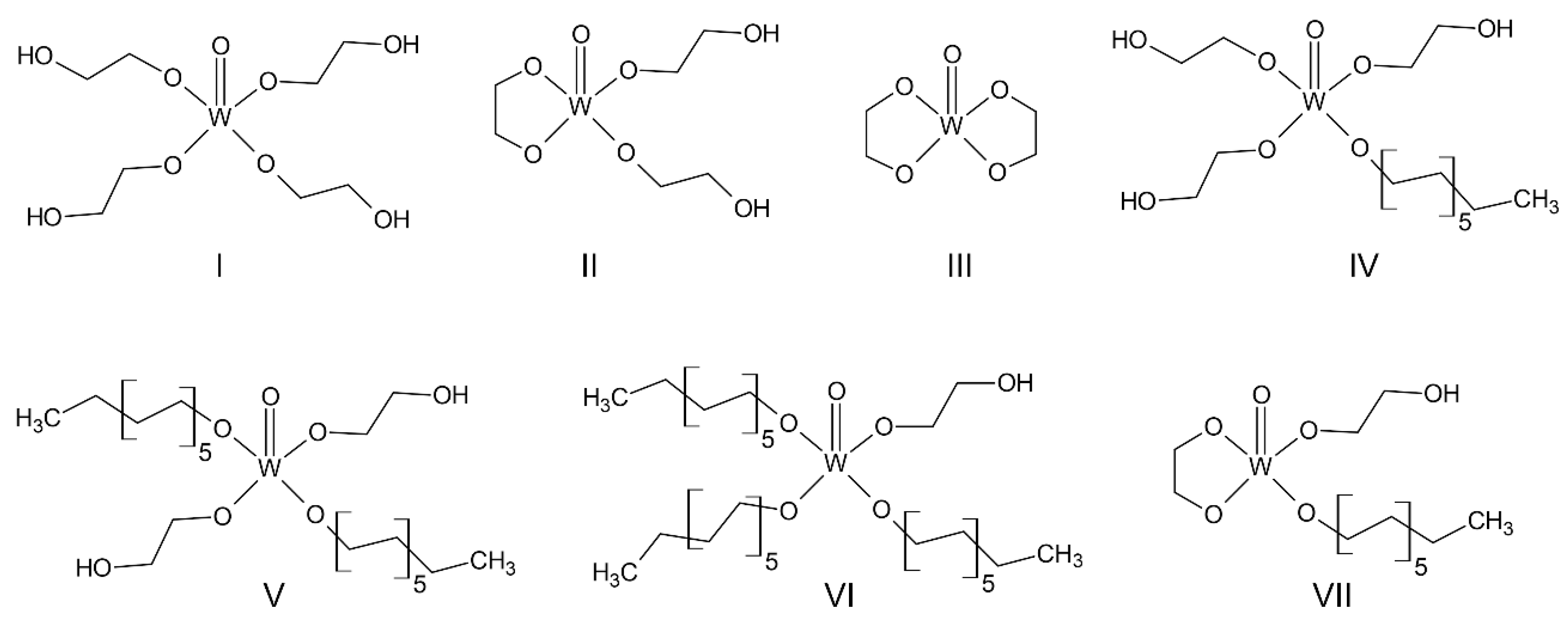
4 combined with 1-dodecanol might interrupt the growth of VEG in one dimension, resulting in the decrease in tungsten doping after calcination of the W-VEG, and when at the 1:1 molar mixing ratio, such an interruption might be alleviated. In the mixture of EG and 1-dodecanol, WOCl
4 can form many different chemical structures shown in
Scheme 2. Without using 1-dodecanol, species I, II, and III are primarily formed, and species I might hinder the formation of the one-dimensional chain of the VO
5 square pyramids due to its multiple functionalities. Species IV, V, VI, and VII, which can be produced by the reaction of WOCl
4 with both of EG and 1-dodecanol, should also significantly influence the final morphologies of W-VEGs. In particular, species V might be the main product of the reaction between WOCl
4 and the mixture of EG and 1-dodecanol at a 1:1 ratio due to the statistically equal opportunity for the reaction, which occurs only if the reactivities of WOCl
4 to EG and 1-dodecanol are identical; this in turn can be responsible for the improved tungsten doping.
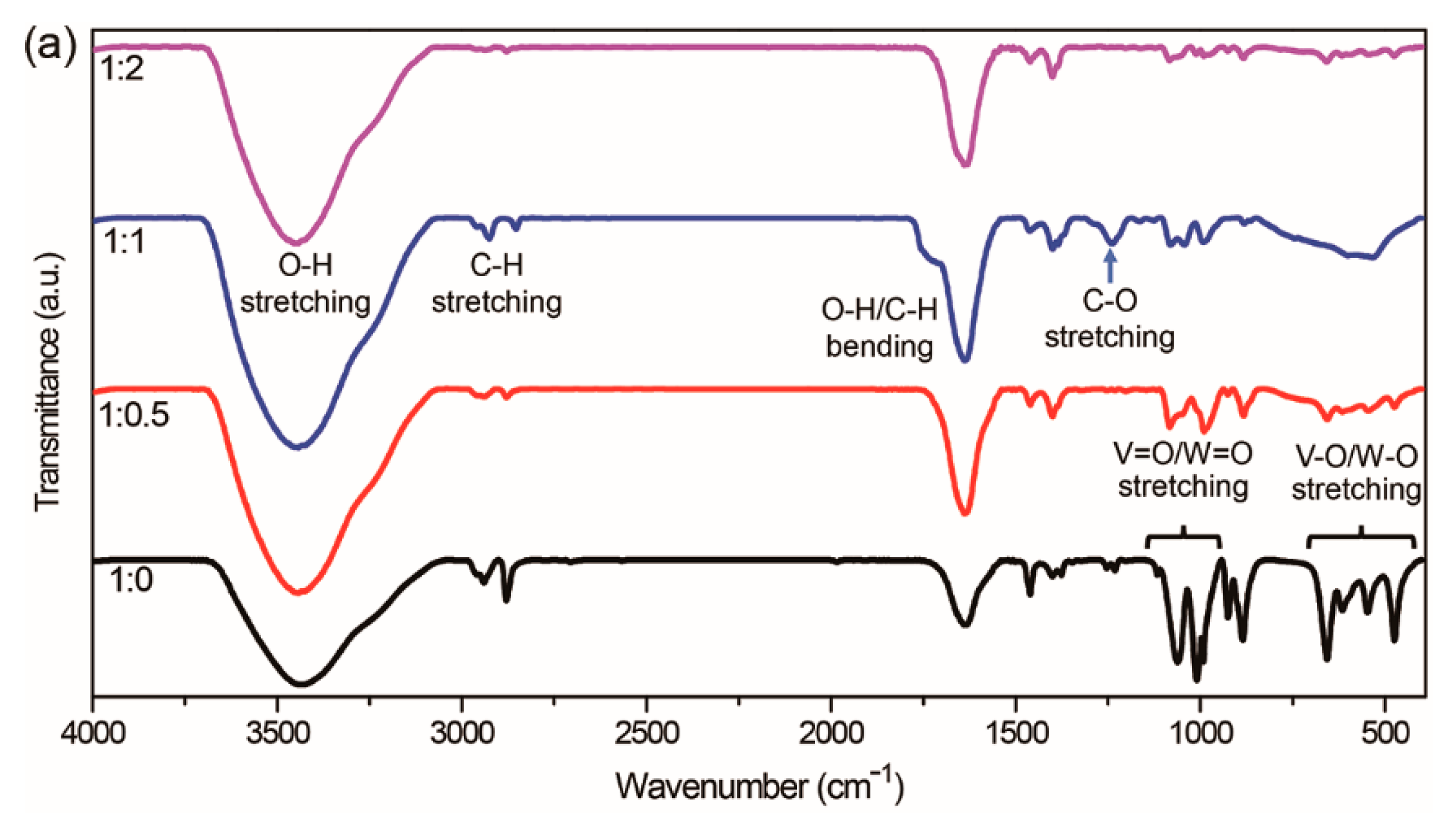
To further investigate the credibility of our hypothesis and speculations and the effects of the addition of 1-dodecanol, we examined the FT-IR spectra of W-VEGs prepared at different mixing ratios of EG to 1-dodecanol. The FT-IR spectra of W-VEGs shown in
Figure 3a in a range of 4000 to 400 cm
−1 clearly present IR bands of O-H stretching (~ 3450 cm
−1), C-H stretching (3000 to 2800 cm
−1), O-H/C-H bending (1800 to 1500 cm
−1), C-O stretching (1239.2 cm
−1), V = O/W = O stretching (1100 to 950 cm
−1), and V-O/W-O stretching (700 to 400 cm
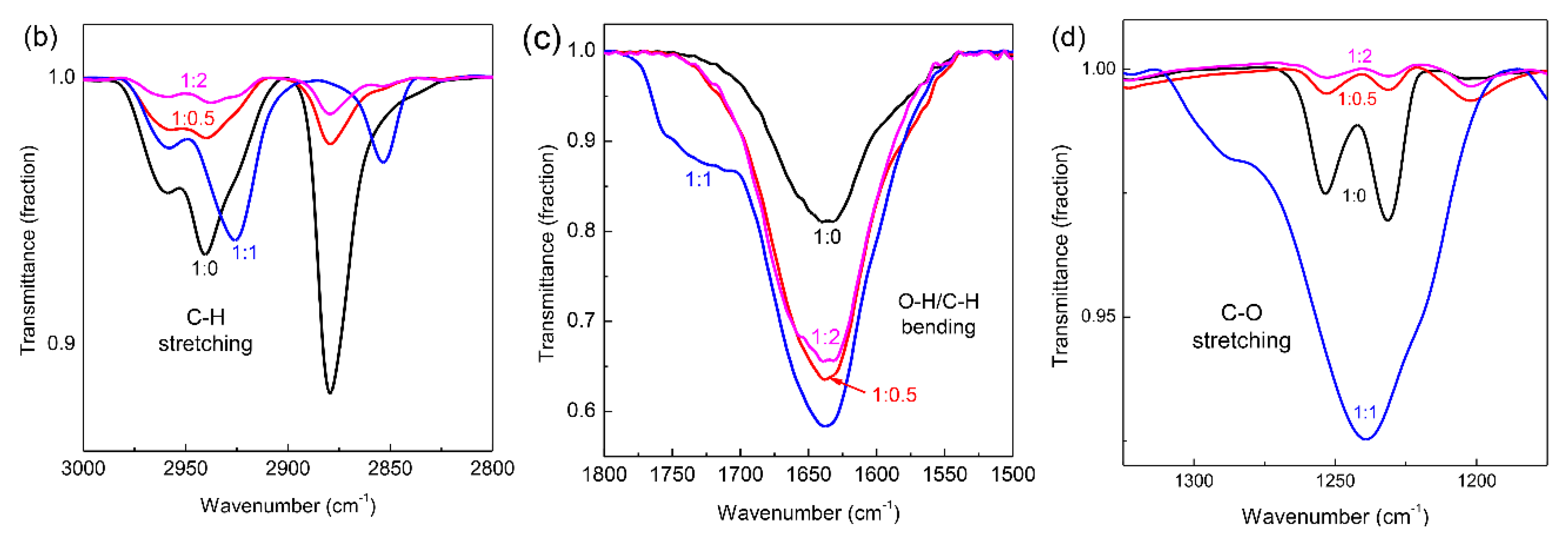
−1). We found that the FT-IR spectrum of W-VEG prepared at the 1:1 mixing ratio of EG to 1-dodecanol was distinctly different from those of other W-VEGs. When we observed the C-H stretching bands in the range of 3000 to 2800 cm
−1, the peak positions of W-VEGs prepared at 1:1 were around 2958.6, 2925.8, and 2853.5 cm
−1 while those at 1:0, 1:0.5, and 1:2 appeared at around 2958.6, 2940.3, and 2879.5 cm
−1, as shown in
Figure 3b. The IR bands that appeared in the range of 1800 to 1500 cm
−1 are ascribed to the overlapping spectra of O-H bending (~1670–1620 cm
−1) and C-H bending in the EG units (~ 1598 cm
−1) [
25,
26,
27]. In
Figure 3c, a shoulder peak in the range of 1790 to 1700 cm
−1 appeared for W-VEG at the 1:1 mixing ratio, while only a strong band centered at around 1638 cm
−1 was shown for all the other W-VEGs at the 1:0, 1:0.5, and 1:2 ratios. In addition, a strong IR band characteristic for C-O stretching appeared at 1239.2 cm
−1 and a shoulder appeared at around 1286 cm
−1 in the FT-IR spectrum of W-VEG at the 1:1 ratio. In contrast, two independent peaks at 1253.6 and 1231.5 cm
−1 appeared in the FT-IR spectra of W-VEGs at the 1:0, 1:0.5, and 1:2 ratios. All these variations in the FT-IR spectrum of W-VEG at the 1:1 ratio strongly suggest that the dodecyloxy moiety combined with WOCl
4 was incorporated into the W-VEG structure, thereby distinctly changing the peak positions of C-H stretching, O-H/C-H bending, and C-O stretching. One of the most plausible mechanisms to explain these results can be the reaction of NH
4VO
3 and EG with WOCl
4 combined with both EG and 1-dodecanol to form W-VEG.
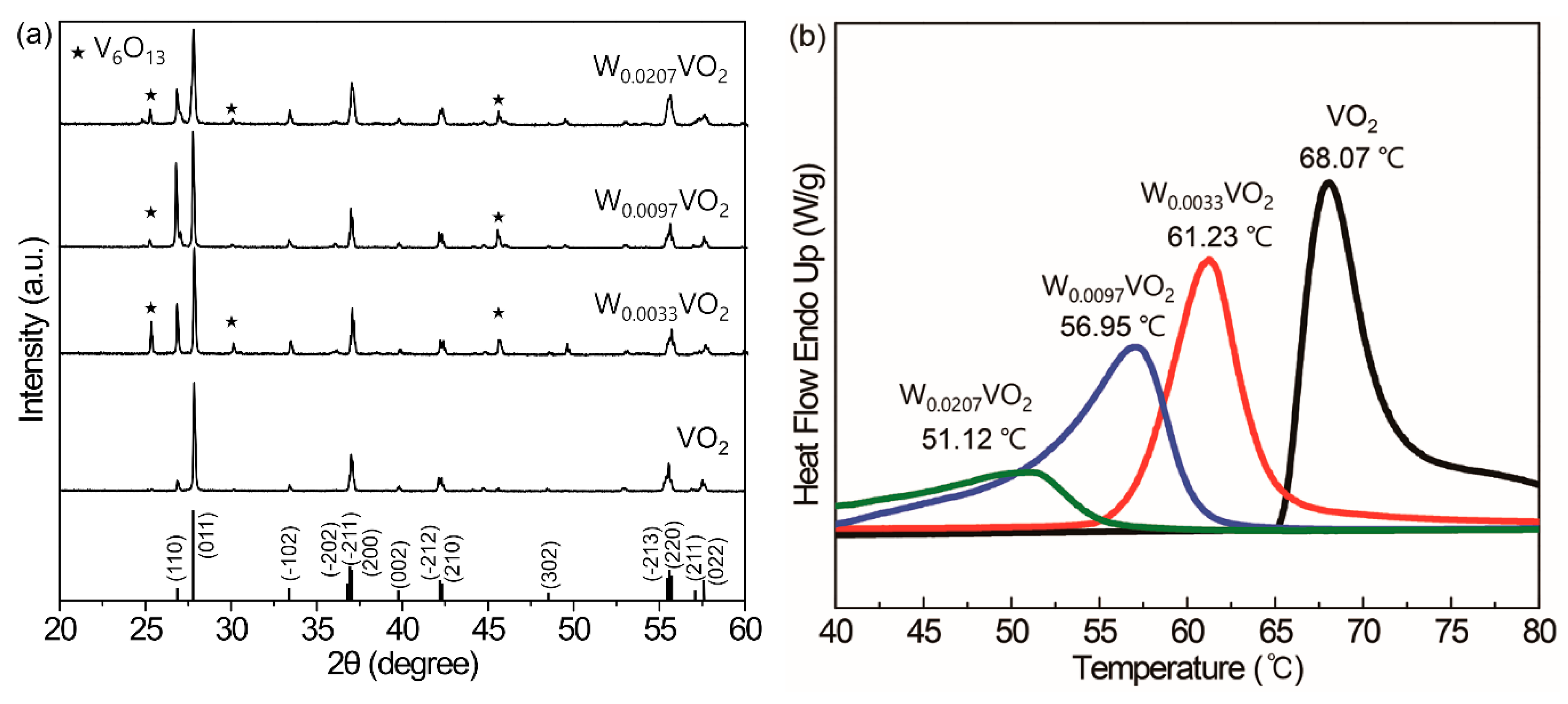
Then, we investigated the effects of the amount of tungsten precursor on the changes in the MIT temperature of W
xVO
2. We fixed the molar mixing ratio of EG to 1-dodecanol at 1:1 for further investigation. At the fixed conditions of NH
4VO
3, EG, and 1-dodecanol, the increasing concentrations of WOCl
4 were used for the synthesis of W
xVO
2. All XRD patterns of W
xVO
2 synthesized at 0, 2.77, 5.53, and 11.08 mol% of WOCl
4 to NH
4VO
3 in
Figure 4a show that the VO
2 (M) phase (JCPDS No. 43-1051) was successfully synthesized, although there exists a portion of the V
6O
13 phase with the addition of WOCl
4. With the further addition of WOCl
4 beyond 11.08 mol%, only V
6O
13 was synthesized (data not shown), indicating the oxidation of VO
2 (M) due to the excessive addition of WOCl
4. The MIT temperatures decreased from 68.07, 61.23, and 56.95 down to 51.12 °C at WOCl
4 concentrations of 0, 2.77, 5.53, and 11.08 mol%, respectively, clearly showing the effects of tungsten doping (
Figure 4b). This decrease in the MIT temperature is ascribed to the formation of the rutile phase VO
2 (R) with the W doping, as indicated in literature [
28]. The enhancement of (110) peak at 2θ = 26.8° is characteristic for the VO
2 (R) phase [
28], and clearly shown in the XRD patterns of W
xVO
2, supporting this mechanism. It should also be noted that the full width at half maximum (FWHM) of the melting transitions in the DSC thermograms (
Figure 4b) drastically increased with the addition of WOCl
4 from 3.9, 4.4, 6.2, to 11.4 °C. These results show that the crystallinity of W
xVO
2 was significantly decreased with increasing concentrations of WOCl
4. Furthermore, the intensity of the melting peak drastically decreased with the increasing W doping, indicating that the activation energy for the MIT was reduced. In the monoclinic VO
2 structure, V-V intervals are alternative (2.65 and 3.12 Å) while those in the rutile VO
2 above the MIT are symmetric (2.87 Å). When V atoms are partially substituted with W atoms, the V-V intervals are shrunk, decreasing the structural differences between the monoclinic and rutile structure, and thereby reducing the activation energy [
29]. The amounts of tungsten doped to vanadium in W
xVO
2 (M) were 0.33, 0.97, and 2.07 mol%, as determined by ICP-AES measurements (
Table 1). These figures represent significantly decreased amounts in comparison to the tungsten precursor WOCl
4. Hereafter, the calcinated W
xVO
2 from W-VEGs prepared at WOCl
4 concentrations of 0, 2.77, 5.53, and 11.08 mol% are denoted by W
0.0033VO
2, W
0.0097VO
2, and W
0.0207VO
2, respectively. Our results showed that only 10~20% of tungsten added into the reaction mixtures was incorporated into the final W
xVO
2 compounds and indicate that a significant portion of WOCl
4 that reacted with EG and 1-dodecanol did not participate in the formation of W-VEGs. We presumed that many of the species shown in
Scheme 2 might not be appropriate for the formation of W-VEGs.
To examine the origination of these broadening melting peaks resulting from an increase in the WOCl
4, we analyzed W-VEGs by measuring SEM images.
Figure 5a shows that the VEG synthesized at the 1:1 mixing ratio of EG to 1-dodecanol without adding the WOCl
4. The VEG has a nanowire morphology with a square or rectangular cross section. The nanowire morphology with a high aspect ratio over 10 is clearly different from that of VEG with a nanorod or microrod morphology prepared without using 1-dodecanol, which can be observed in
Figure 2a. These results demonstrate that 1-dodecanol acts as a capping agent to block a specific crystal plane, thereby enhancing one-dimensional growth when VEG is synthesized. With the addition of WOCl
4, the nanowire morphology disappears, presenting irregular crystal morphologies, as shown in
Figure 5b–d. The crystal sizes of W-VEGs decreased from the micrometer-scale at 2.77 mol% to the nanometer-scale at 5.53 and 11.08 mol% of WOCl
4, as shown in
Figure 5b–d, respectively. We ascribe this disappearance of the nanowire morphology and the decrease in the crystal sizes to the increasing portion of dodecyloxy moieties in W-VEGs. When the bulky alkyl side chains are incorporated into the crystal structure of W-VEGs, they might act as defects to the formation of the one-dimensional chain-like structures shown in
Scheme 1 and the crystallization of the chains.
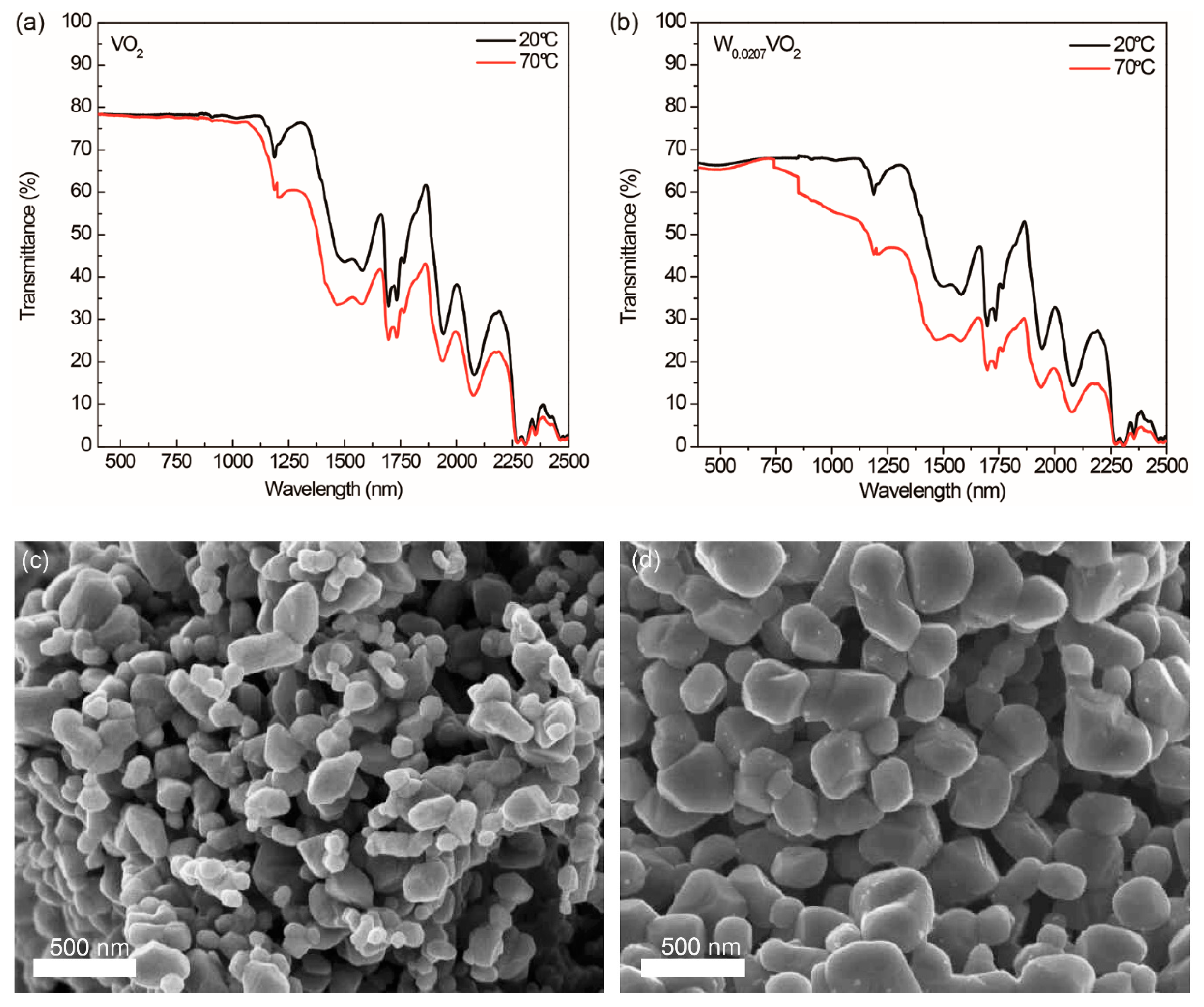
W
xVO
2 compounds prepared via our modified polyol method utilizing 1-dodecanol present distinct thermochromic properties. We measured the transmittance spectra of the VO
2 and W
0.0207VO
2 crystals dispersed in ethanol at 20 and 70 °C as two representatives. When the UV-Vis-NIR curves of 20 and 70°C were compared, there were no significant changes in the range of visible light shorter than 780 nm. However, in the NIR region, we observed that the transmittances of ethanol dispersions of VO
2 and W
0.0207VO
2 at 70 °C were significantly decreased from those at 20 °C. The solar modulation
Tsol at each temperature was calculated by Equation (1), where
φ(
λ) and
Tr(
λ) are the solar irradiation spectrum for an air mass of 1.5 (corresponding to the sun standing 37° above the horizontal) [
30] and transmittance at specific wavelengths
λ, respectively, and Δ
Tsol was estimated by Equation (2) [
9]. The luminous transmittance was also calculated by Equation (3) where
φlum(
λ) is the spectral sensitivity of the light-adapted eye [
18]. The estimated Δ
Tsol of VO
2 without tungsten doping was 2.5% and that of W
0.0207VO
2 was 21.8%. The usefulness of our modified polyol method utilizing WOCl
4 and 1-dodecanol to prepare tungsten-doped VO
2 is evidenced by the significantly enhanced solar modulation of the ethanol dispersion of W
0.0207VO
2 with the manifestation of the tungsten doping. The luminous transmittance of W
0.0207VO
2 at 20 °C estimated by Equation (3) was 66.8% while that of VO
2 was 78.2%. This decrease in the luminous transmittance with the tungsten doping can be ascribed to serious light scattering of W
0.0207VO
2 in comparison to VO
2. Sizes of VO
2 and W
0.0207VO
2 nanoparticles estimated by analyzing their SEM images shown in
Figure 6c,d are 156 ± 81 and 287 ± 84 nm, respectively. These results indicate that the W
0.0207VO
2 nanoparticles with bigger sizes than the VO
2 nanoparticles significantly scatter light, resulting in the decrease in transmittance. It should be noted that the average crystallite sizes of VO
2 and V
6O
13 estimated by using XRD patterns in
Figure 4a and the Scherrer equation [
31] are 63.8 and 36.2 nm, respectively. The bigger size of the W
0.0207VO
2 nanoparticles than their crystallite sizes indicate that the nanoparticles have polycrystalline textures. On the other hand, the thermochromic properties gained in our study are compared with those of W
xVO
2 with the MIT temperature (
Tc) in a range of 40–45 °C in
Table 2. The
Tlum at 20 °C and Δ
Tsol of W
0.0207VO
2 in this study are higher than those of reported materials except the
Tlum at 20 °C of the W-doped VO
2/polyvinylpyrrolidone coating as presented in the
Table 2 [
32,
33,
34,
35]. This comparison suggests that W
xVO
2 materials in our study have a great potential for thermochromic applications.