Assessment of Effects of Laser Light Combining Three Wavelengths (450, 520 and 640 nm) on Temperature Increase and Depth of Tissue Lesions in an Ex Vivo Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Laser Irradiation
2.2. Tissue
2.3. Temperature Measurement
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Patents
Appendix A
| Wavelength/ DutyCycle | B 10% | B 50% | B 80% | B 100% | G 10% | G 50% | G 80% | G 100% | R 10% | R 50% | R 80% | R 100% | RGB 10% | RGB 50% | RGB 80% | BG 10% | BG 50% | BG 80% | BG 100% | BR 10% | BR 50% | BR 80% | BR 100% | GR 10% | GR 50% | GR 80% | GR 100% | RGB 100% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B10% | 0.634 | 0.000 | 0.000 | 1.000 | 0.000 | 0.012 | 0.000 | 1.000 | 0.689 | 0.189 | 0.007 | 1.000 | 0.000 | 0.000 | 1.000 | 0.030 | 0.000 | 0.000 | 1.000 | 0.005 | 0.000 | 0.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| B50% | 0.634 | 0.000 | 0.000 | 0.634 | 0.914 | 1.000 | 0.000 | 0.634 | 1.000 | 1.000 | 1.000 | 0.634 | 0.000 | 0.000 | 0.634 | 1.000 | 0.061 | 0.000 | 0.634 | 0.999 | 0.038 | 0.000 | 0.634 | 1.000 | 0.438 | 0.310 | 0.000 | |
| B80% | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.016 | 0.244 | 0.000 | 0.000 | 0.009 | 0.947 | 0.000 | 0.000 | 0.016 | 0.411 | 0.000 | 0.000 | 0.000 | 0.001 | 0.023 | |
| B100% | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 | 0.076 | 0.000 | 0.000 | 0.043 | 0.740 | 0.000 | 0.000 | 0.068 | 0.740 | 0.000 | 0.000 | 0.002 | 0.005 | 0.005 | |
| G10% | 1.000 | 0.634 | 0.000 | 0.000 | 0.000 | 0.012 | 0.000 | 1.000 | 0.689 | 0.189 | 0.007 | 1.000 | 0.000 | 0.000 | 1.000 | 0.030 | 0.000 | 0.000 | 1.000 | 0.005 | 0.000 | 0.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| G50% | 0.000 | 0.914 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.885 | 0.999 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 | 0.689 | 0.000 | 1.000 | 1.000 | 1.000 | 0.000 | |
| G80% | 0.012 | 1.000 | 0.000 | 0.000 | 0.012 | 1.000 | 0.000 | 0.012 | 1.000 | 1.000 | 1.000 | 0.012 | 0.000 | 0.000 | 0.012 | 1.000 | 0.914 | 0.000 | 0.012 | 1.000 | 0.850 | 0.117 | 0.012 | 1.000 | 1.000 | 0.998 | 0.000 | |
| G100% | 0.000 | 0.000 | 1.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | 0.225 | 0.000 | 0.000 | 0.011 | 0.937 | 0.000 | 0.000 | 0.018 | 0.438 | 0.000 | 0.000 | 0.000 | 0.001 | 0.020 | |
| R10% | 1.000 | 0.634 | 0.000 | 0.000 | 1.000 | 0.000 | 0.012 | 0.000 | 0.689 | 0.189 | 0.007 | 1.000 | 0.000 | 0.000 | 1.000 | 0.030 | 0.000 | 0.000 | 1.000 | 0.005 | 0.000 | 0.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| R50% | 0.689 | 1.000 | 0.000 | 0.000 | 0.689 | 0.885 | 1.000 | 0.000 | 0.689 | 1.000 | 0.999 | 0.689 | 0.000 | 0.000 | 0.689 | 1.000 | 0.049 | 0.000 | 0.689 | 0.998 | 0.030 | 0.000 | 0.689 | 1.000 | 0.384 | 0.265 | 0.000 | |
| R80% | 0.189 | 1.000 | 0.000 | 0.000 | 0.189 | 0.999 | 1.000 | 0.000 | 0.189 | 1.000 | 1.000 | 0.189 | 0.000 | 0.000 | 0.189 | 1.000 | 0.334 | 0.000 | 0.189 | 1.000 | 0.244 | 0.006 | 0.189 | 1.000 | 0.885 | 0.788 | 0.000 | |
| R100% | 0.007 | 1.000 | 0.000 | 0.000 | 0.007 | 1.000 | 1.000 | 0.000 | 0.007 | 0.999 | 1.000 | 0.007 | 0.000 | 0.000 | 0.007 | 1.000 | 0.956 | 0.000 | 0.007 | 1.000 | 0.914 | 0.172 | 0.007 | 1.000 | 1.000 | 1.000 | 0.000 | |
| RGB10% | 1.000 | 0.634 | 0.000 | 0.000 | 1.000 | 0.000 | 0.012 | 0.000 | 1.000 | 0.689 | 0.189 | 0.007 | 0.000 | 0.000 | 1.000 | 0.030 | 0.000 | 0.000 | 1.000 | 0.005 | 0.000 | 0.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| RGB50% | 0.000 | 0.000 | 0.016 | 0.003 | 0.000 | 0.000 | 0.000 | 0.014 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.990 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | |
| RGB80% | 0.000 | 0.000 | 0.244 | 0.076 | 0.000 | 0.000 | 0.000 | 0.225 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | |
| BG10% | 1.000 | 0.634 | 0.000 | 0.000 | 1.000 | 0.000 | 0.012 | 0.000 | 1.000 | 0.689 | 0.189 | 0.007 | 1.000 | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 | 1.000 | 0.005 | 0.000 | 0.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| BG50% | 0.030 | 1.000 | 0.000 | 0.000 | 0.030 | 1.000 | 1.000 | 0.000 | 0.030 | 1.000 | 1.000 | 1.000 | 0.030 | 0.000 | 0.000 | 0.030 | 0.788 | 0.000 | 0.030 | 1.000 | 0.689 | 0.055 | 0.030 | 1.000 | 0.997 | 0.988 | 0.000 | |
| BG80% | 0.000 | 0.061 | 0.009 | 0.043 | 0.000 | 1.000 | 0.914 | 0.011 | 0.000 | 0.049 | 0.334 | 0.956 | 0.000 | 0.000 | 0.000 | 0.000 | 0.788 | 0.000 | 0.000 | 0.970 | 1.000 | 1.000 | 0.000 | 0.634 | 1.000 | 1.000 | 0.000 | |
| BG100% | 0.000 | 0.000 | 0.947 | 0.740 | 0.000 | 0.000 | 0.000 | 0.937 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.990 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.996 | |
| BR10% | 1.000 | 0.634 | 0.000 | 0.000 | 1.000 | 0.000 | 0.012 | 0.000 | 1.000 | 0.689 | 0.189 | 0.007 | 1.000 | 0.000 | 0.000 | 1.000 | 0.030 | 0.000 | 0.000 | 0.005 | 0.000 | 0.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| BR50% | 0.005 | 0.999 | 0.000 | 0.000 | 0.005 | 1.000 | 1.000 | 0.000 | 0.005 | 0.998 | 1.000 | 1.000 | 0.005 | 0.000 | 0.000 | 0.005 | 1.000 | 0.970 | 0.000 | 0.005 | 0.937 | 0.206 | 0.005 | 1.000 | 1.000 | 1.000 | 0.000 | |
| BR80% | 0.000 | 0.038 | 0.016 | 0.068 | 0.000 | 1.000 | 0.850 | 0.018 | 0.000 | 0.030 | 0.244 | 0.914 | 0.000 | 0.000 | 0.000 | 0.000 | 0.689 | 1.000 | 0.000 | 0.000 | 0.937 | 1.000 | 0.000 | 0.521 | 1.000 | 1.000 | 0.000 | |
| BR100% | 0.000 | 0.000 | 0.411 | 0.740 | 0.000 | 0.689 | 0.117 | 0.438 | 0.000 | 0.000 | 0.006 | 0.172 | 0.000 | 0.000 | 0.000 | 0.000 | 0.055 | 1.000 | 0.000 | 0.000 | 0.206 | 1.000 | 0.000 | 0.026 | 0.984 | 0.996 | 0.000 | |
| GR10% | 1.000 | 0.634 | 0.000 | 0.000 | 1.000 | 0.000 | 0.012 | 0.000 | 1.000 | 0.689 | 0.189 | 0.007 | 1.000 | 0.000 | 0.000 | 1.000 | 0.030 | 0.000 | 0.000 | 1.000 | 0.005 | 0.000 | 0.000 | 0.061 | 0.000 | 0.000 | 0.000 | |
| GR50% | 0.061 | 1.000 | 0.000 | 0.000 | 0.061 | 1.000 | 1.000 | 0.000 | 0.061 | 1.000 | 1.000 | 1.000 | 0.061 | 0.000 | 0.000 | 0.061 | 1.000 | 0.634 | 0.000 | 0.061 | 1.000 | 0.521 | 0.026 | 0.061 | 0.984 | 0.956 | 0.000 | |
| GR80% | 0.000 | 0.438 | 0.000 | 0.002 | 0.000 | 1.000 | 1.000 | 0.000 | 0.000 | 0.384 | 0.885 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.997 | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 | 0.984 | 0.000 | 0.984 | 1.000 | 0.000 | |
| GR100% | 0.000 | 0.310 | 0.001 | 0.005 | 0.000 | 1.000 | 0.998 | 0.001 | 0.000 | 0.265 | 0.788 | 1.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.988 | 1.000 | 0.000 | 0.000 | 1.000 | 1.000 | 0.996 | 0.000 | 0.956 | 1.000 | 0.000 | |
| RGB100% | 0.000 | 0.000 | 0.023 | 0.005 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 1.000 | 1.000 | 0.000 | 0.000 | 0.000 | 0.996 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |

References
- Tunç, S.K.; Yayli, N.Z.; Talmac, A.C.; Feslihan, E.; Akbal, D. Clinical comparison of the use of ER,CR:YSGG and diode lasers in second stage implants surgery. Saudi Med J. 2019, 40, 490–498. [Google Scholar] [CrossRef]
- Monteiro, L.; Delgado, M.-L.; Garcês, F.; Machado, M.; Ferreira, F.; Martins, M.; Salazar, F.; Pacheco, J.-J. A histological evaluation of the surgical margins from human oral fibrous-epithelial lesions excised with CO2 laser, Diode laser, Er:YAG laser, Nd:YAG laser, electrosurgical scalpel and cold scalpel. Med. Oral Patol. Oral y Cir. Bucal 2019, 24, e271–e280. [Google Scholar] [CrossRef]
- Al-Majmaie, R.; Al-Rubeai, M.; Rice, J.H.; Kennedy, E.; Zerulla, D. AFM-based bivariate morphological discrimination of apoptosis induced by photodynamic therapy using photosensitizer-functionalized gold nanoparticles. RSC Adv. 2015, 5, 82983–82991. [Google Scholar] [CrossRef]
- Lim, H.-S. Development and optimization of a diode laser for photodynamic therapy. Laser Ther. 2011, 20, 195–203. [Google Scholar] [CrossRef]
- Khalkhal, E.; Razzaghi, M.; Rostami-Nejad, M.; Rezaei-Tavirani, M.; Beigvandi, H.H.; Tavirani, M.R. Evaluation of Laser Effects on the Human Body After Laser Therapy. J. Lasers Med. Sci. 2020, 11, 91–97. [Google Scholar] [CrossRef]
- Alster, T.S.; Husain, Z. The role of lasers and intense pulsed light technology in dermatology. Clin. Cosmet. Investig. Dermatol. 2016, 9, 29–40. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.S.; Ajlal, S. Applications of Light Amplification by Stimulated Emission of Radiation (Lasers) for Restorative Dentistry. Med. Princ. Pr. 2016, 25, 201–211. [Google Scholar] [CrossRef]
- Goel, A.; A, G. Clinical applications of Q-switched NdYAG laser. Indian J. Dermatol. Venereol. Leprol. 2008, 74, 682. [Google Scholar] [CrossRef]
- Yates, B.; Que, S.K.T.; D’Souza, L.; Suchecki, J.; Finch, J. Laser treatment of periocular skin conditions. Clin. Dermatol. 2015, 33, 197–206. [Google Scholar] [CrossRef]
- Cobb, C.M. Lasers in Periodontics: A Review of the Literature. J. Periodontol. 2006, 77, 545–564. [Google Scholar] [CrossRef]
- Lee, H.C.; Childs, J.; Chung, K.Y.; Park, J.; Hong, J.; Bin Cho, S. Pattern analysis of 532- and 1,064-nm picosecond-domain laser-induced immediate tissue reactions in ex vivo pigmented micropig skin. Sci. Rep. 2019, 9, 4186. [Google Scholar] [CrossRef]
- Namour, M.; El Mobadder, M.; Magnin, D.; Peremans, A.; Verspecht, T.; Teughels, W.; Lamard, L.; Nammour, S.; Rompen, E.; Mobadder, E. Q-Switch Nd:YAG Laser-Assisted Decontamination of Implant Surface. Dent. J. 2019, 7, 99. [Google Scholar] [CrossRef]
- Namour, M.; Verspecht, T.; El Mobadder, M.; Teughels, W.; Peremans, A.; Nammour, S.; Rompen, E. Q-Switch Nd:YAG Laser-Assisted Elimination of Multi-Species Biofilm on Titanium Surfaces. Materials 2020, 13, 1573. [Google Scholar] [CrossRef]
- Barua, S. Laser-tissue interaction in tattoo removal by Q-switched lasers. J. Cutan. Aesthetic Surg. 2015, 8, 5–8. [Google Scholar] [CrossRef]
- Cencic, B.; Lukac, M.; Marincek, M.; Vizintin, Z. High Fluence, High Beam Quality Q-Switched Nd:YAG Laser with Optoflex Delivery System for Treating Benign Pigmented Lesions and Tattoos. J. Laser Health Acad. 2010, 1, 9–18. [Google Scholar]
- Ivonyak, Y.; Piechal, B.; Mrozowicz, M.; Bercha, A.; Trzeciakowski, W. Note: Coupling of multiple laser diodes into a multi-mode fiber. Rev. Sci. Instrum. 2014, 85, 36106. [Google Scholar] [CrossRef]
- Fekrazad, R.; Asefi, S.; Eslaminejad, M.B.; Taghiyar, L.; Bordbar, S.; Hamblin, M.R. Photobiomodulation with single and combination laser wavelengths on bone marrow mesenchymal stem cells: proliferation and differentiation to bone or cartilage. Lasers Med. Sci. 2019, 34, 115–126. [Google Scholar] [CrossRef]
- Fornaini, C.; Merigo, E.; Vescovi, P.; Bonanini, M.; Antonietti, W.; Leoci, L.; Lagori, G.; Meleti, M. Different laser wavelengths comparison in the second-stage implant surgery: an ex vivo study. Lasers Med. Sci. 2014, 30, 1631–1639. [Google Scholar] [CrossRef]
- Romeo, U.; Del Vecchio, A.; Russo, C.; Palaia, G.; Gaimari, G.; Arnabat-Dominguez, J.; España, A.-J.; Palaia, G. Laser treatment of 13 benign oral vascular lesions by three different surgical techniques. Med. Oral Patol. Oral y Cir. Bucal 2013, 18, e279–e284. [Google Scholar] [CrossRef]
- Kamalski, D.M.; Wegner, I.; Tange, R.A.; Vincent, R.; Stegeman, I.; Van Der Heijden, G.J.M.; Grolman, W. Outcomes of Different Laser Types in Laser-assisted Stapedotomy. Otol. Neurotol. 2014, 35, 1046–1051. [Google Scholar] [CrossRef]
- Anderson, R.R.; Parrish, J.A. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science 1983, 220, 524–527. [Google Scholar] [CrossRef]
- Li, D.; Wang, G.; He, Y.; Kelly, K.; Wu, W.; Wang, Y.; Ying, Z. A two-temperature model for selective photothermolysis laser treatment of port wine stains. Appl. Therm. Eng. 2013, 59, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Bodendorf, M.O.; Grunewald, S.; Wetzig, T.; Simon, J.C.; Paasch, U. Fractional laser skin therapy. J. Dtsch. Dermatol. Ges. 2009, 7, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kasai, K. Picosecond Laser Treatment for Tattoos and Benign Cutaneous Pigmented Lesions (Secondary publication). Laser Ther. 2017, 26, 274–281. [Google Scholar] [CrossRef]
- Belikov, A.; Skrypnik, A.V. Soft tissue cutting efficiency by 980 nm laser with carbon-, erbium-, and titanium-doped optothermal fiber converters. Lasers Surg. Med. 2019, 51, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Martelli, F.S.; De Leo, A.; Zinno, S. Laser in Odontostomatologia – Applicazioni Cliniche; Elsevier Masson: Paris, France, 2000; pp. 59–72. (In Italian) [Google Scholar]
- Matys, J.; Grzech-Leśniak, K.; Flieger, R.; Dominiak, M. Assessment of an Impact of a Diode Laser Mode with Wavelength of 980 nm on a Temperature Rise Measured by Means of k-02 Thermocouple: Preliminary Results. Dent. Med Probl. 2016, 53, 345–351. [Google Scholar] [CrossRef]
- Fornaini, C.; Merigo, E.; Sozzi, M.; Rocca, J.-P.; Poli, F.; Selleri, S.; Cucinotta, A. Four different diode lasers comparison on soft tissues surgery: a preliminary ex vivo study. Laser Ther. 2016, 25, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Meesters, A.A.; Pitassi, L.H.U.; Campos, V.; Wolkerstorfer, A.; Dierickx, C.C. Transcutaneous laser treatment of leg veins. Lasers Med. Sci. 2013, 29, 481–492. [Google Scholar] [CrossRef]
- Romeo, U.; Del Vecchio, A.; Ripari, F.; Palaia, G.; Chiappafreddo, C.; Tenore, G.; Visca, P. Effects of different laser devices on oral soft tissues: in vitro experience. J. Oral Laser Appl. 2007, 7, 155–159. [Google Scholar]
- Azevedo, A.-S.; Monteiro, L.S.; Ferreira, F.; Delgado, M.L.; Garcês, F.; Carreira, S.; Martins, M.; Suarez-Quintanilla, J. In vitro histological evaluation of the surgical margins made by different laser wavelengths in tongue tissues. J. Clin. Exp. Dent. 2016, 8, e388–e396. [Google Scholar] [CrossRef]
- Omi, T.; Numano, K. The Role of the CO2 Laser and Fractional CO2 Laser in Dermatology. Laser Ther. 2014, 23, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Palaia, G.; Pergolini, D.; D’Alessandro, L.; Carletti, R.; Del Vecchio, A.; Tenore, G.; Di Gioia, C.R.T.; Romeo, U. Histological Effects of an Innovative 445 Nm Blue Laser During Oral Soft Tissue Biopsy. Int. J. Environ. Res. Public Heal. 2020, 17, 2651. [Google Scholar] [CrossRef] [PubMed]
- Cadavid, A.M.H.; De Campos, W.G.; Aranha, A.C.C.; Lemos-Junior, C.A. Efficacy of Photocoagulation of Vascular Malformations in the Oral Mucosa Using Nd. J. Craniofacial Surg. 2018, 29, e614–e617. [Google Scholar] [CrossRef] [PubMed]
- Azma, E.; Razaghi, M. Laser Treatment of Oral and Maxillofacial Hemangioma. J. Lasers Med. Sci. 2018, 9, 228–232. [Google Scholar] [CrossRef]
- Bacci, C.; Sacchetto, L.; Zanette, G.; Sivolella, S. Diode laser to treat small oral vascular malformations: A prospective case series study. Lasers Surg. Med. 2018, 50, 111–116. [Google Scholar] [CrossRef]
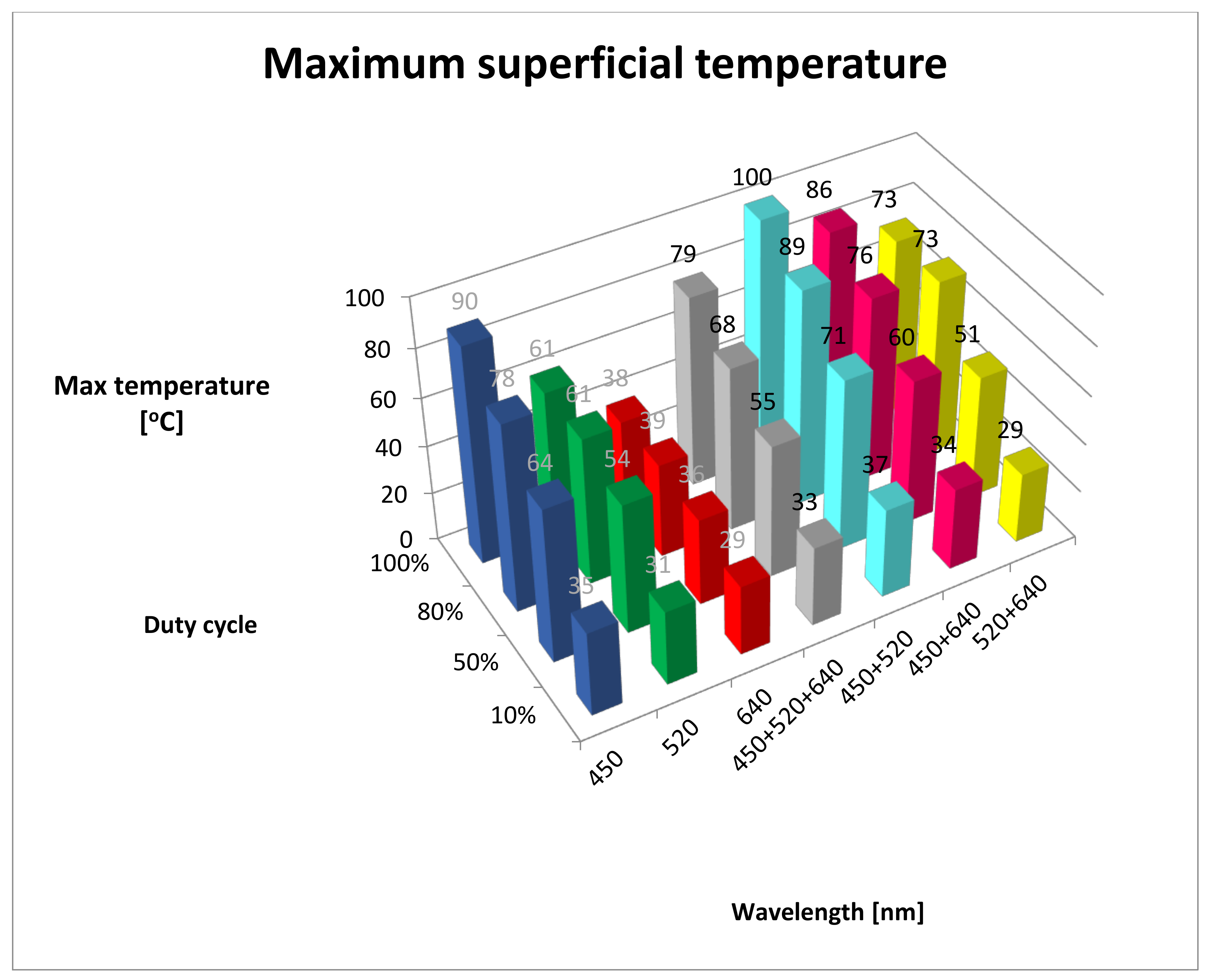
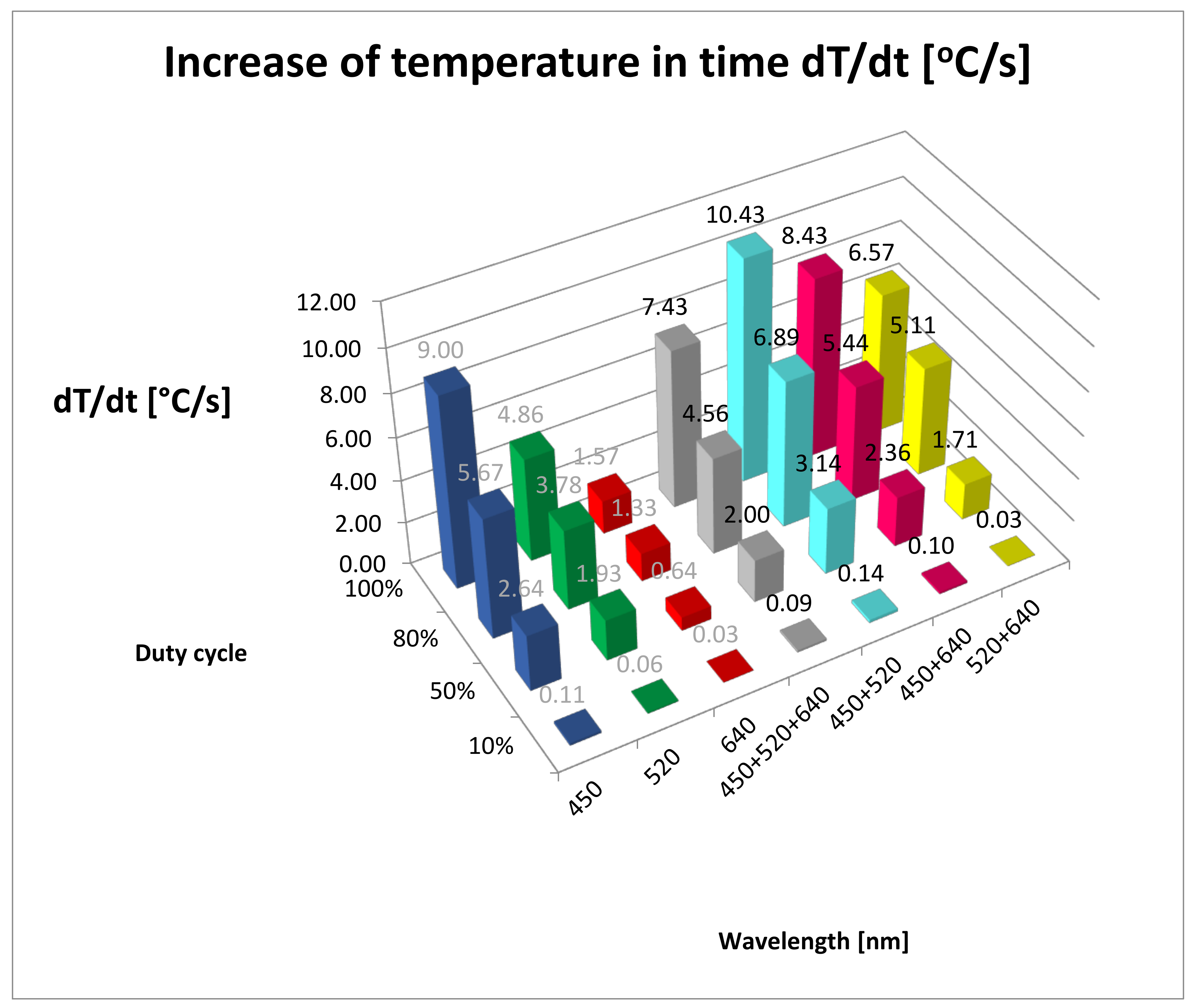
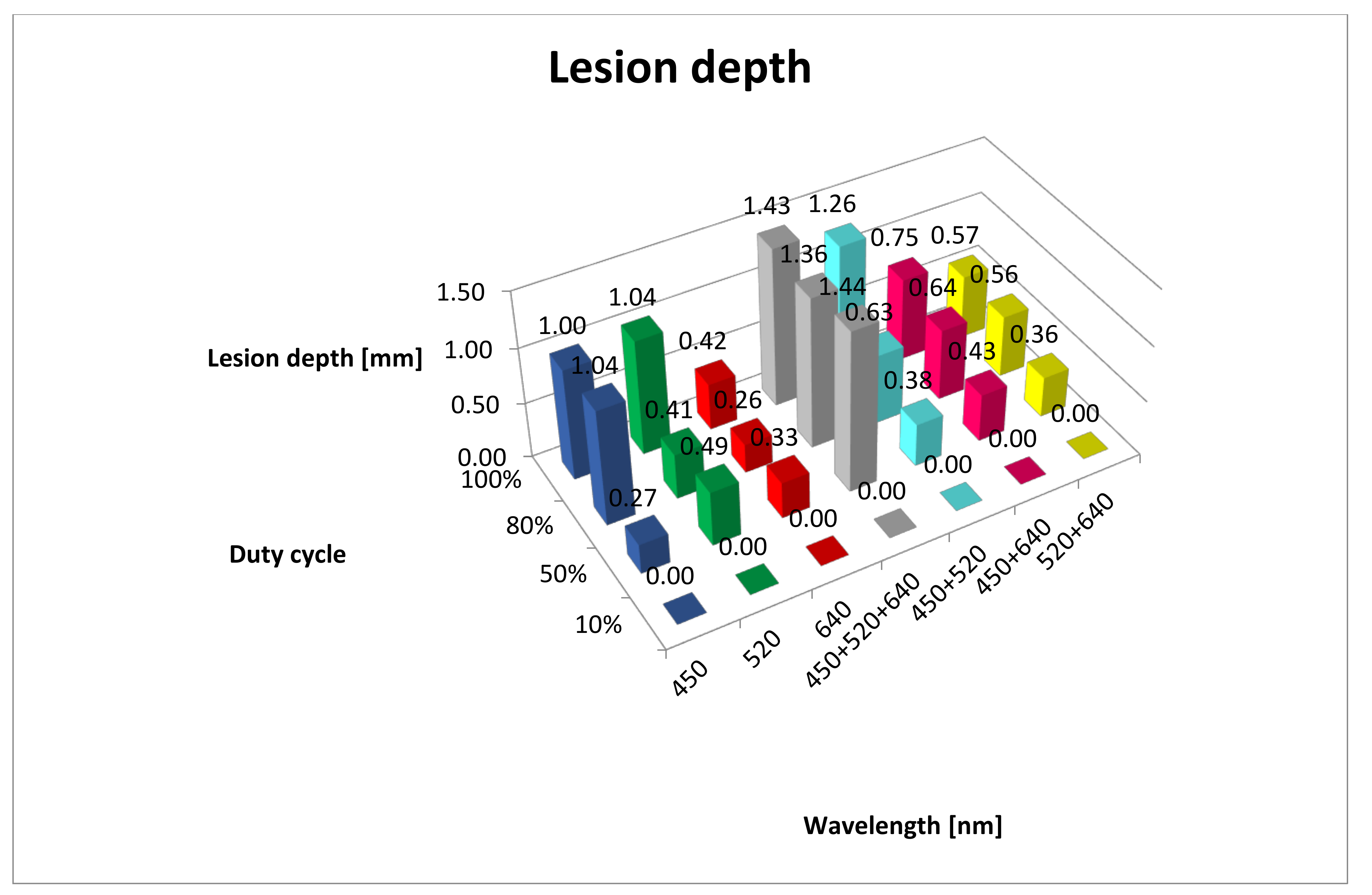
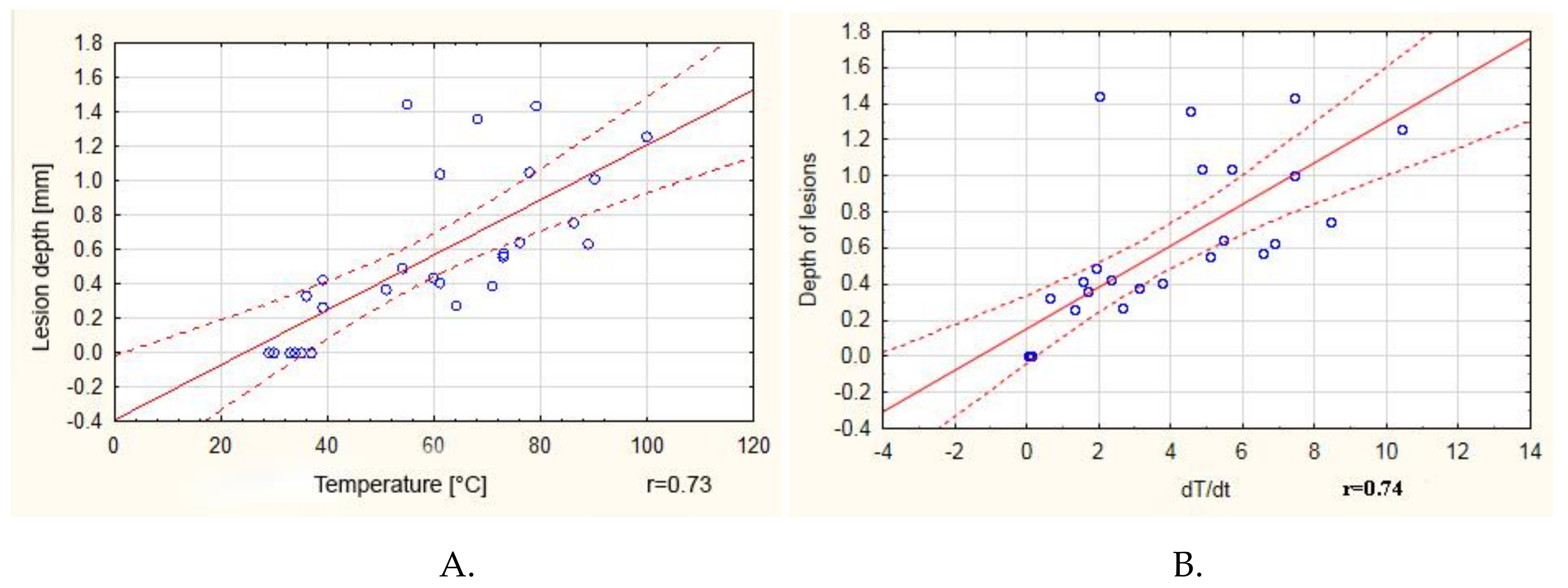
| Wavelength/ Duty Cycle (%) | dT/dt (°C/s) | SD | Max Temp (°C) | SD | Lesion Depth (mm) | SD |
|---|---|---|---|---|---|---|
| B10% | 0.11 | 0.53 | 35.00 | 0.58 | 0.00 | 0.00 |
| G10% | 0.06 | 0.24 | 31.00 | 0.00 | 0.00 | 0.00 |
| R10% | 0.03 | 0.24 | 29.00 | 0.00 | 0.00 | 0.00 |
| RGB10% | 0.09 | 0.28 | 33.00 | 0.00 | 0.00 | 0.00 |
| BG10% | 0.14 | 0.39 | 37.00 | 0.00 | 0.00 | 0.00 |
| BR10% | 0.10 | 0.39 | 34.00 | 0.00 | 0.00 | 0.00 |
| GR10% | 0.03 | 0.17 | 29.00 | 0.00 | 0.00 | 0.00 |
| B50% | 2.64 | 2.85 | 64.00 | 1.00 | 0.27 | 0.05 |
| G50% | 1.93 | 2.08 | 54.00 | 0.58 | 0.49 | 0.02 |
| R50% | 0.64 | 0.69 | 36.00 | 0.58 | 0.33 | 0.06 |
| RGB50% | 2.00 | 2.15 | 55.00 | 0.58 | 1.44 | 0.02 |
| BG50% | 3.14 | 3.38 | 71.00 | 1.00 | 0.38 | 0.03 |
| BR50% | 2.36 | 2.54 | 60.00 | 1.00 | 0.43 | 0.02 |
| GR50% | 1.71 | 1.85 | 51.00 | 0.58 | 0.36 | 0.05 |
| B80% | 5.67 | 4.24 | 78.00 | 2.52 | 1.04 | 0.06 |
| G80% | 3.78 | 2.82 | 61.00 | 3.00 | 0.41 | 0.05 |
| R80% | 1.33 | 0.76 | 39.00 | 1.00 | 0.26 | 0.12 |
| RGB80% | 4.56 | 3.44 | 68.00 | 2.52 | 1.36 | 0.06 |
| BG80% | 6.89 | 6.69 | 89.00 | 2.08 | 0.63 | 0.10 |
| BR80% | 5.44 | 4.82 | 76.00 | 1.73 | 0.64 | 0.06 |
| GR80% | 5.11 | 3.06 | 73.00 | 5.51 | 0.56 | 0.07 |
| B100% | 9.00 | 6.66 | 90.00 | 4.04 | 1.00 | 0.24 |
| G100% | 4.86 | 2.66 | 61.00 | 3.51 | 1.04 | 0.07 |
| R100% | 1.57 | 0.41 | 38.00 | 2.00 | 0.42 | 0.02 |
| RGB100% | 7.43 | 6.41 | 79.00 | 6.00 | 1.43 | 0.05 |
| BG100% | 10.43 | 6.18 | 100.00 | 8.74 | 1.26 | 0.04 |
| BR100% | 8.43 | 4.62 | 86.00 | 5.51 | 0.75 | 0.04 |
| GR100% | 6.57 | 2.42 | 73.00 | 6.03 | 0.57 | 0.08 |
| Maximum Temperature | |||||||
|---|---|---|---|---|---|---|---|
| Wavelength/ duty cycle | B10% | G10% | R10% | W10% | BG10% | BR10% | GR10% |
| B10% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.002189 | 0.000000 | |
| G10% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| R10% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 1.000000 | |
| RGB10% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000064 | 0.000000 | |
| BG10% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| BR10% | 0.002189 | 0.000000 | 0.000000 | 0.000064 | 0.000000 | 0.000000 | |
| GR10% | 0.000000 | 0.000000 | 1.000000 | 0.000000 | 0.000000 | 0.000000 | |
| Wavelength/ duty cycle | B50% | G50% | R50% | W50% | BG50% | BR50% | GR50% |
| B50% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000022 | 0.000000 | |
| G50% | 0.000000 | 0.000000 | 0.141872 | 0.000000 | 0.000001 | 0.000137 | |
| R50% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| RGB50% | 0.000000 | 0.141872 | 0.000000 | 0.000000 | 0.000009 | 0.000009 | |
| BG50% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| BR50% | 0.000022 | 0.000001 | 0.000000 | 0.000009 | 0.000000 | 0.000000 | |
| GR50% | 0.000000 | 0.000137 | 0.000000 | 0.000009 | 0.000000 | 0.000000 | |
| Wavelength/ Duty cycle | B80% | G80% | R80% | W80% | BG80% | BR80% | GR80% |
| B80% | 0.000034 | 0.000000 | 0.000941 | 0.000192 | 0.586834 | 0.418096 | |
| G80% | 0.000034 | 0.000000 | 0.092173 | 0.000000 | 0.000090 | 0.000149 | |
| R80% | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | 0.000000 | |
| RGB80% | 0.000941 | 0.092173 | 0.000000 | 0.000000 | 0.002811 | 0.004885 | |
| BG80% | 0.000192 | 0.000000 | 0.000000 | 0.000000 | 0.000070 | 0.000043 | |
| BR80% | 0.586834 | 0.000090 | 0.000000 | 0.002811 | 0.000070 | 0.784989 | |
| GR80% | 0.418096 | 0.000149 | 0.000000 | 0.004885 | 0.000043 | 0.784989 | |
| Wavelength/ duty cycle | B100% | G100% | R100% | W100% | BG100% | BR100% | GR100% |
| B100% | 0.000017 | 0.000000 | 0.010143 | 0.180197 | 0.227531 | 0.000837 | |
| G100% | 0.000017 | 0.000269 | 0.004185 | 0.000002 | 0.000155 | 0.049241 | |
| R100% | 0.000000 | 0.000269 | 0.000001 | 0.000000 | 0.000000 | 0.000006 | |
| RGB100% | 0.010143 | 0.004185 | 0.000001 | 0.000628 | 0.109788 | 0.227531 | |
| BG100% | 0.180197 | 0.000002 | 0.000000 | 0.000628 | 0.018204 | 0.000061 | |
| BR100% | 0.227531 | 0.000155 | 0.000000 | 0.109788 | 0.018204 | 0.010143 | |
| GR100% | 0.000837 | 0.049241 | 0.000006 | 0.227531 | 0.000061 | 0.010143 | |
| dT/dt | |||||||
|---|---|---|---|---|---|---|---|
| Wavelength/ duty cycle (%) | B10% | G10% | R10% | RGB10% | BG10% | BR10% | GR10% |
| B10% | 0.307940 | 0.126436 | 0.610064 | 0.610064 | 0.798710 | 0.123345 | |
| G10% | 0.307940 | 0.610064 | 0.610064 | 0.126436 | 0.444364 | 0.603765 | |
| R10% | 0.126436 | 0.610064 | 0.307940 | 0.041767 | 0.202643 | 0.994248 | |
| RGB10% | 0.610064 | 0.610064 | 0.307940 | 0.307940 | 0.798710 | 0.302842 | |
| BG10% | 0.610064 | 0.126436 | 0.041767 | 0.307940 | 0.444364 | 0.040348 | |
| BR10% | 0.798710 | 0.444364 | 0.202643 | 0.798710 | 0.444364 | 0.198530 | |
| GR10% | 0.123345 | 0.603765 | 0.994248 | 0.302842 | 0.040348 | 0.198530 | |
| Wavelength/ duty cycle (%) | B50% | G50% | R50% | RGB50% | BG50% | BR50% | GR50% |
| B50% | 0.372804 | 0.013920 | 0.422296 | 0.532259 | 0.720979 | 0.247329 | |
| G50% | 0.372804 | 0.110389 | 0.928830 | 0.131326 | 0.592311 | 0.788773 | |
| R50% | 0.013920 | 0.110389 | 0.092220 | 0.002316 | 0.034243 | 0.182460 | |
| RGB50% | 0.422296 | 0.928830 | 0.092220 | 0.155274 | 0.655344 | 0.720979 | |
| BG50% | 0.532259 | 0.131326 | 0.002316 | 0.155274 | 0.327132 | 0.076570 | |
| BR50% | 0.720979 | 0.592311 | 0.034243 | 0.655344 | 0.327132 | 0.422296 | |
| GR50% | 0.247329 | 0.788773 | 0.182460 | 0.720979 | 0.076570 | 0.422296 | |
| Wavelength/ duty cycle (%) | B80% | G80% | R80% | RGB80% | BG80% | BR80% | GR80% |
| B80% | 0.347996 | 0.034168 | 0.579935 | 0.542755 | 0.911741 | 0.781760 | |
| G80% | 0.347996 | 0.225781 | 0.698232 | 0.124672 | 0.407219 | 0.506835 | |
| R80% | 0.034168 | 0.225781 | 0.112041 | 0.007317 | 0.044070 | 0.063549 | |
| RGB80% | 0.579935 | 0.698232 | 0.112041 | 0.247309 | 0.657763 | 0.781760 | |
| BG80% | 0.542755 | 0.124672 | 0.007317 | 0.247309 | 0.472238 | 0.376871 | |
| BR80% | 0.911741 | 0.407219 | 0.044070 | 0.657763 | 0.472238 | 0.867958 | |
| GR80% | 0.781760 | 0.506835 | 0.063549 | 0.781760 | 0.376871 | 0.867958 | |
| Wavelength/ duty cycle (%) | B100% | G100% | R100% | RGB100% | BG100% | BR100% | GR100% |
| B100% | 0.161044 | 0.014214 | 0.591242 | 0.625293 | 0.844939 | 0.407680 | |
| G100% | 0.161044 | 0.264239 | 0.380892 | 0.061831 | 0.225558 | 0.558100 | |
| R100% | 0.014214 | 0.264239 | 0.050103 | 0.003950 | 0.022914 | 0.092439 | |
| RGB100% | 0.591242 | 0.380892 | 0.050103 | 0.307440 | 0.732273 | 0.769303 | |
| BG100% | 0.625293 | 0.061831 | 0.003950 | 0.307440 | 0.494752 | 0.191235 | |
| BR100% | 0.844939 | 0.225558 | 0.022914 | 0.732273 | 0.494752 | 0.525921 | |
| GR100% | 0.407680 | 0.558100 | 0.092439 | 0.769303 | 0.191235 | 0.525921 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jurczyszyn, K.; Trzeciakowski, W.; Woźniak, Z.; Ziółkowski, P.; Trafalski, M. Assessment of Effects of Laser Light Combining Three Wavelengths (450, 520 and 640 nm) on Temperature Increase and Depth of Tissue Lesions in an Ex Vivo Study. Materials 2020, 13, 5340. https://doi.org/10.3390/ma13235340
Jurczyszyn K, Trzeciakowski W, Woźniak Z, Ziółkowski P, Trafalski M. Assessment of Effects of Laser Light Combining Three Wavelengths (450, 520 and 640 nm) on Temperature Increase and Depth of Tissue Lesions in an Ex Vivo Study. Materials. 2020; 13(23):5340. https://doi.org/10.3390/ma13235340
Chicago/Turabian StyleJurczyszyn, Kamil, Witold Trzeciakowski, Zdzisław Woźniak, Piotr Ziółkowski, and Mateusz Trafalski. 2020. "Assessment of Effects of Laser Light Combining Three Wavelengths (450, 520 and 640 nm) on Temperature Increase and Depth of Tissue Lesions in an Ex Vivo Study" Materials 13, no. 23: 5340. https://doi.org/10.3390/ma13235340
APA StyleJurczyszyn, K., Trzeciakowski, W., Woźniak, Z., Ziółkowski, P., & Trafalski, M. (2020). Assessment of Effects of Laser Light Combining Three Wavelengths (450, 520 and 640 nm) on Temperature Increase and Depth of Tissue Lesions in an Ex Vivo Study. Materials, 13(23), 5340. https://doi.org/10.3390/ma13235340







