Preparation and Study of Sulfonated Co-Polynaphthoyleneimide Proton-Exchange Membrane for a H2/Air Fuel Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Co-PNIS with ODAS/MDAC = 70/30 Synthesis
2.2. Conductivity Measurements
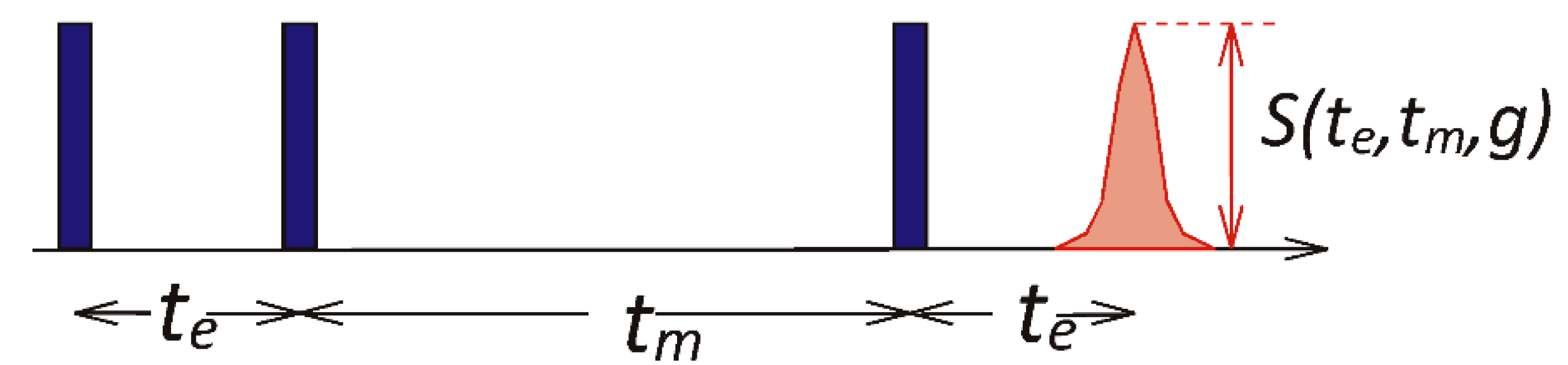
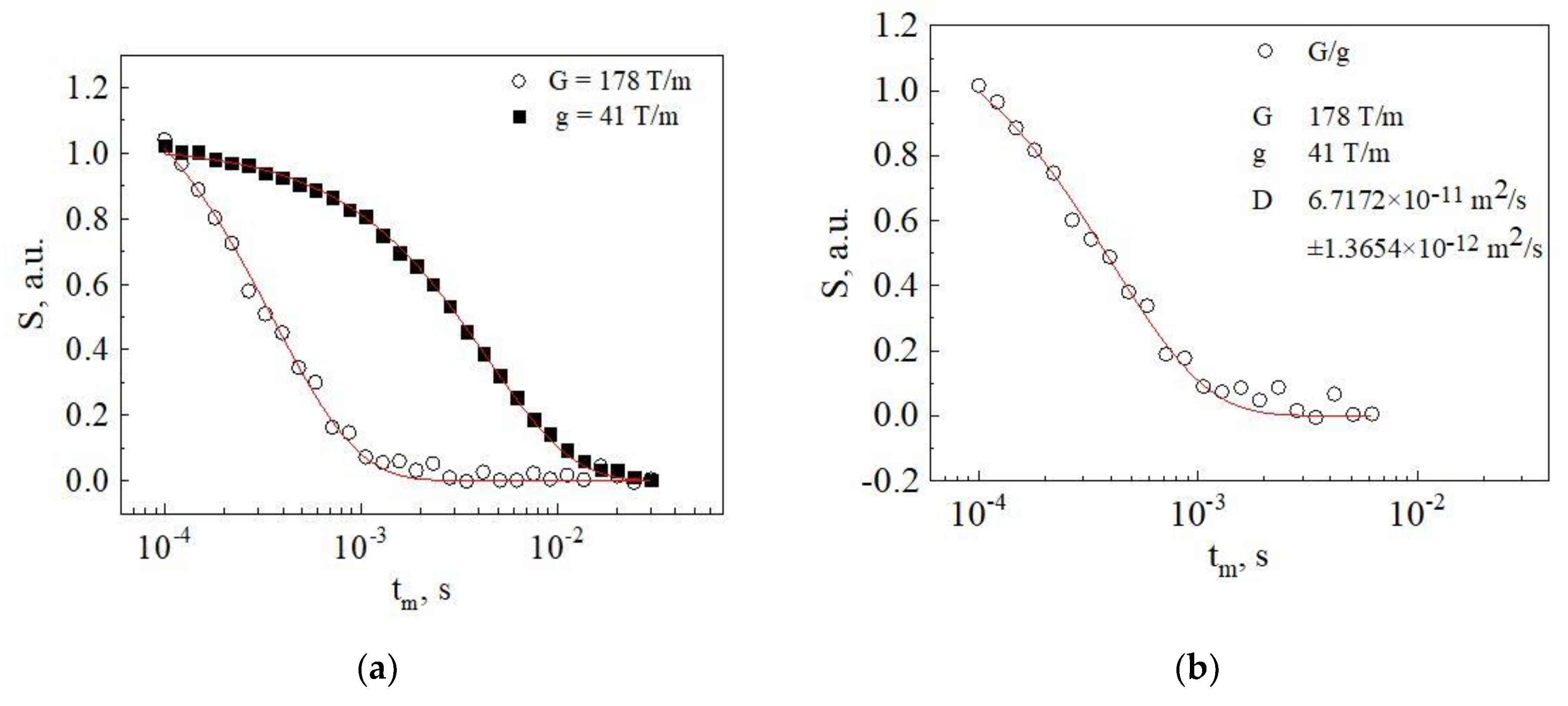
2.3. Diffusion Measurements
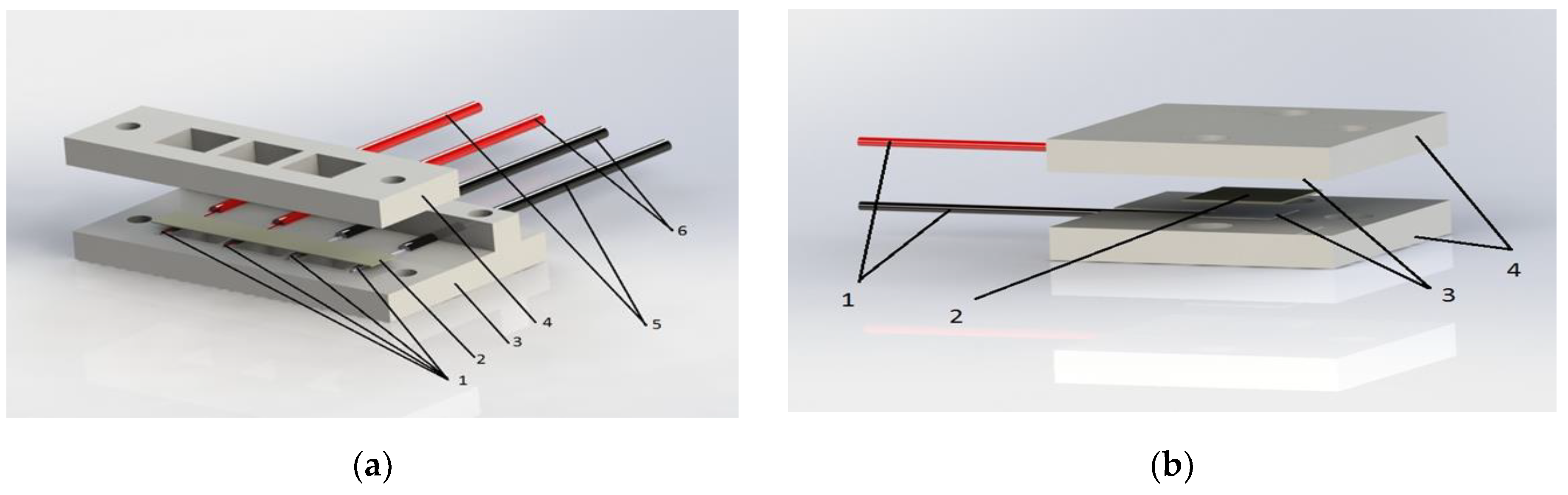
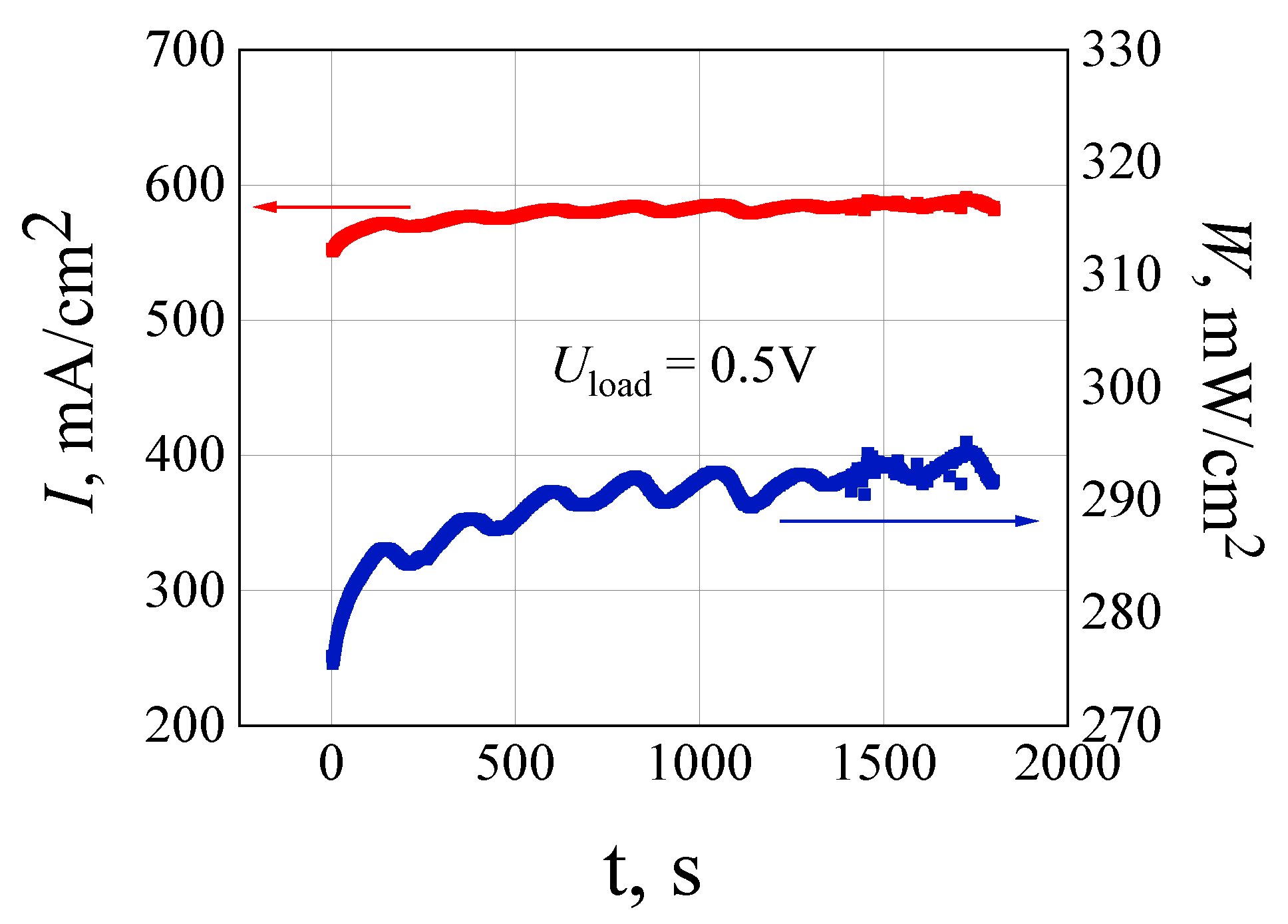
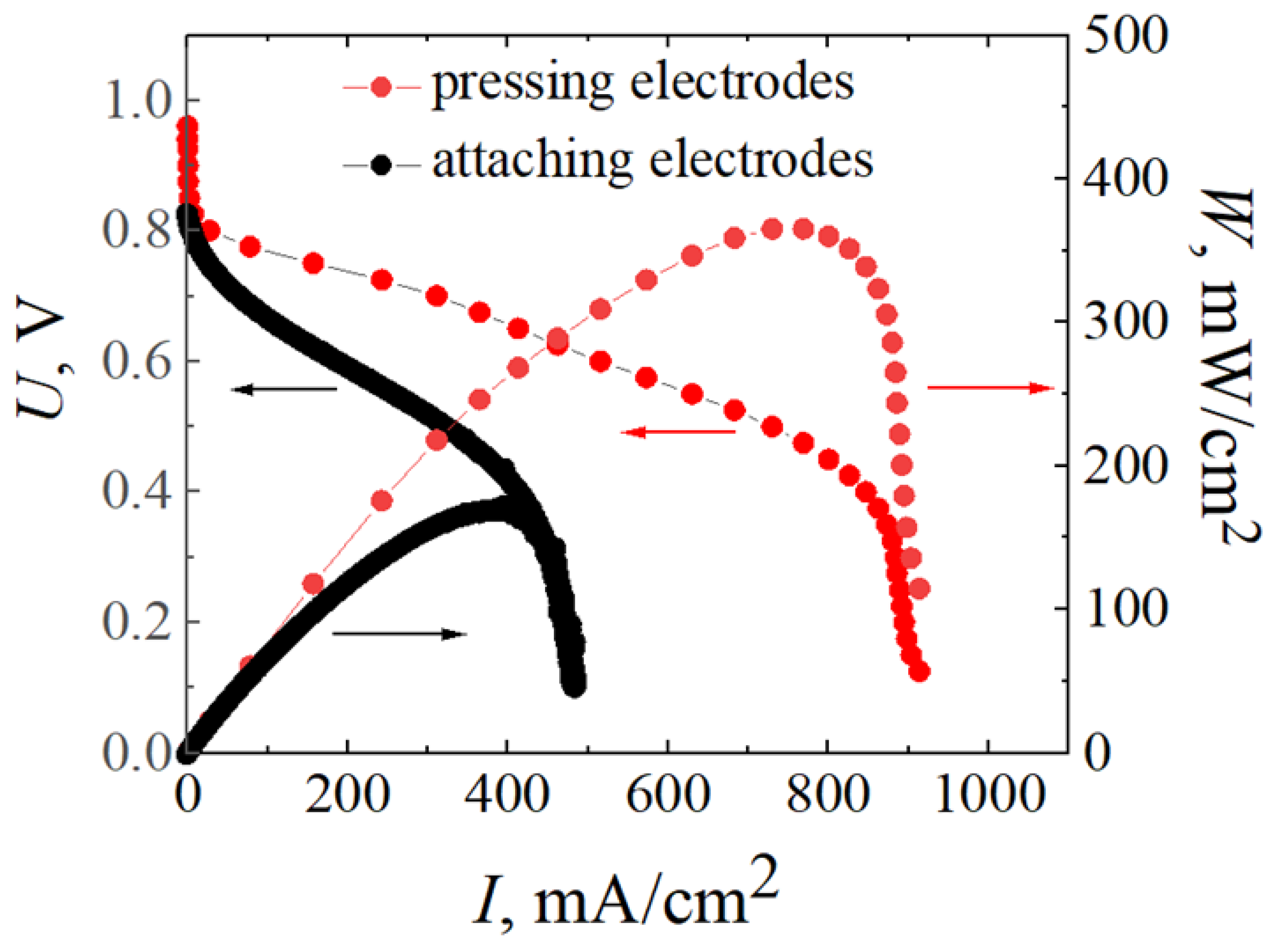
2.4. MEAs Fabrications and Investigations of Their Voltage-Current Characteristics
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Viral, M.J.; Smith, C. Review and analysis of PEM fuel cell design and manufacturing. J. Power Sources 2003, 114, 132–153. [Google Scholar]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. Handbook of Fuel Cells, 1st ed.; Wiley, J., Ed.; Sons Ltd.: Chichester, UK, 2003; Volume 1, pp. 41–45. [Google Scholar]
- Mauritz, K.A.; Moore, R.B. State of Understanding of Nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef]
- Vielstich, W.; Lamm, A.; Gasteiger, H.A. Handbook of Fuel Cells, 1st ed.; Wiley, J., Ed.; Sons Ltd.: Chichester, UK, 2009; Volume 5, pp. 24–31. [Google Scholar]
- Dobrovol’skii, Y.A.; Volkov, E.V.; Pisareva, A.V.; Fedotov, Y.A.; Likhachev, D.Y.; Rusanov, A.L. Proton-Exchange Membranes for Hydrogen-Air Fuel Cells. Russ. J. Gen. Chem. 2007, 77, 766–777. [Google Scholar] [CrossRef]
- O’Hayre, R.; Prinz, F.B. The air/platinum/Nafion triple-phase boundary: Characteristics, scaling, and implications for fuel cells. J. Electrochem. Soc. 2004, 151, 756–762. [Google Scholar] [CrossRef]
- Wu, H.W. A Review of Recent Development: Transport and performance modeling of PEM fuel cells. Appl. Energy 2016, 165, 81–106. [Google Scholar] [CrossRef]
- Berg, P. Reaction Kinetics at the Triple-Phase Boundary in PEM Fuel Cells. J. Fuel Cell Sci. Technol. 2008, 2, 021007. [Google Scholar] [CrossRef]
- Connolly, D.J.; Gresham, W.F. Fluorocarbon Vinyl Ether Polymers. U.S. Patent 3,282,875, 1 November 1966. [Google Scholar]
- Marestin, C.; Gebel, G.; Diat, O.; Mercier, R. Sulfonated Polyimides. Adv. Polym. Sci. 2008, 216, 185. [Google Scholar]
- Timofeeva, G.I.; Ponomarev, I.I.; Khokhlov, A.R.; Mercier, R.; Sillion, B. Synthesis and investigation of new water soluble sulfonated rigid-rod polynaphthoyleneimide. Macromol. Symp. 1996, 106, 345. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Nikolskii, O.G.; Volkova, Y.A.; Zakharov, A.V. New Rigid-Chain Copoly(naphthoylenelmidobenzimidazoles) and Their Films. Polym. Sci. Ser. A 1994, 36, 1185. [Google Scholar]
- Kabasawa, A.; Saito, J.; Miyatake, K.; Uchida, H.; Watanabe, M. Durability of a novel sulfonated polyimide membrane in polymer electrolyte fuel cell operation. Electrochim. Acta 2009, 54, 2754–2760. [Google Scholar] [CrossRef]
- Genies, C.; Mercier, R.; Sillion, B.; Petiaud, R.; Cornet, N.; Gebel, G.; Pineri, M. Stability study of sulfonated phthalic and naphthalenic polyimide structures in aqueous medium. Polymer 2001, 42, 5097. [Google Scholar] [CrossRef]
- Lavrenko, P.; Pogodina, N.; Ponomarev, I.; Okatova, O.; Evlampieva, N. Stability and degradation of poly(naphthoyleneimidobenzimidazole). Polym. Degrad. Stab. 1994, 43, 379–384. [Google Scholar] [CrossRef]
- Lavrenko, P.; Pogodina, N.; Ponomarev, I.; Okatova, O.; Evlampieva, N. Vysokomolekulyarnye soedineniya. Pol. Sci. USSR 1994, 36, 26–30. [Google Scholar]
- Fang, J.; Guo, X.; Harada, S.; Watari, T.; Tanaka, K.; Kita, H.; Okamoto, K. Novel Sulfonated Polyimides as Polyelectrolytes for Fuel Cell Application. Synthesis, Proton Conductivity, and Water Stability of Polyimides from 4,4’-Diaminodiphenyl Ether-2,2’-disulfonic Acid. Macromolecules 2002, 35, 9022–9028. [Google Scholar] [CrossRef]
- Ponomarev, I.I.; Nikolsky, O.G. Polyimides and Other High-Temperature Polymers; Elsevier: Amsterdam, The Netherlands, 1991; p. 207. [Google Scholar]
- Ponomarev, I.I.; Grinberg, V.A.; Emets, V.V.; Maiorova, N.A.; Zharinova, M.Y.; Volkova, Y.A.; Razorenov, D.Y.; Skupov, K.M.; Nizhnikovskii, E.A. Development of methanol–air fuel cells with membrane materials based on new sulfonated polyheteroarylenes. Russ. J. Electrochem. 2016, 52, 525–532. [Google Scholar] [CrossRef]
- Emets, V.V.; Ponomarev, I.I.; Grinberg, V.A.; Mayorova, N.A.; Zharinova, M.Y.; Volkova, Y.A.; Nizhnikovskii, E.A.; Skupov, K.M.; Razorenov, D.Y.; Andreev, V.N.; et al. Development of hydrogen-air fuel cells with membranes based on sulfonated polyheteroarylenes. Russ. J. Electrochem. 2017, 53, 86–91. [Google Scholar] [CrossRef]
- Chang, F.I.; Fujara, B.; Geil, G.; Hinze, H.; Sillescu, A. New perspectives of NMR in ultrahigh static magnetic field gradients. J. Non-Cryst. Solid 1994, 674, 172–174. [Google Scholar] [CrossRef]
- William, S.P. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion. In Concepts in Magnetic Resonance. Part 1. Basic Theory; John Wiley & Sons, Inc: Hoboken, NJ, USA, 1997; Volume 9, pp. 299–336. [Google Scholar]
- Mugtasimova, R.; Melnikov, A.P.; Galitskaya, E.A.; Kashin, A.M.; Dobrovolskiy, Y.A.; Don, G.M.; Likhomanov, V.S.; Sivak, A.V.; Sinitsyn, V.V. Fabrication of Aquivion-type membranes and optimization of their elastic and transport characteristics. Ionics 2018, 24, 3897–3903. [Google Scholar] [CrossRef]
- Pavlov, V.I.; Gerasimova, E.V.; Zolotukhina, E.V. Degradation of Pt/C electrocatalysts with different morphologies in low-temperature polymer membrane fuel cells. Nanotechnol. Russ. 2016, 11, 44–49. [Google Scholar] [CrossRef]
- Galitskaya, E.A.; Gerasimova, E.V.; Dobrovol’skii, Y.A.; Don, G.M.; Afanas’ev, A.S.; Levchenko, A.V.; Sivak, A.V.; Sinitsyn, V.V. Pulsed Activation of a Fuel Cell on the Basis of a Proton-Conducting Polymer Membrane. Tech. Phys. Lett. 2018, 44, 570–573. [Google Scholar] [CrossRef]
- Kusoglu, A.; Weber, A.Z. New Insights into Perfluorinated Sulfonic-Acid Ionomers. Chem. Rev. 2017, 117, 987–1104. [Google Scholar] [CrossRef]
- Van Nguyen, T.; Mack, W. Knobbe A liquid water management strategy for PEM fuel cell stacks. J. Power Sources 2003, 114, 70–79. [Google Scholar] [CrossRef]
- Kaspar, B.; Letterio, P.M.; Wittkopf, J.A.; Gong, K.; Gu, S.; Yan, Y. ManipulatingWater in High-Performance Hydroxide Exchange Membrane Fuel Cells through Asymmetric Humidification and Wetproofing. J. Electrochem. Soc. 2015, 162, 483–488. [Google Scholar] [CrossRef]
- Yan, Q.; Toghiani, H.; Wu, J. Investigation of water transport through membrane in a PEM fuel cell by water balance experiments. J. Power Sources 2006, 158, 316–325. [Google Scholar] [CrossRef]
- Privalov, A.F.; Galitskaya, E.; Sinitsyn, V.; Vogel, M. Isotope Effect on Diffusion in Nafion Studied by NMR Diffusometry. Appl. Magn. Reson. 2019, 51, 145–153. [Google Scholar] [CrossRef]
- Galitskaya, E.; Privalov, A.F.; Weigler, M.; Vogel, M.; Kashin, A.; Ryzhkin, M.; Sinitsyn, V. NMR diffusion studies of proton-exchange membranes in wide temperature range. J. Membr. Sci. 2020, 596, 117691. [Google Scholar] [CrossRef]
- Chernyak, A.V.; Vasiliev, S.G.; Avilova, I.A.; Volkov, V.I. Hydration and Water Molecules Mobility in Acid Form of Nafion Membrane Studied by 1 H NMR Techniques. Appl. Magn. Reson. 2019, 50, 677–693. [Google Scholar] [CrossRef]
- Giuseppe, F.; Brunello, M.; Lee, R.W.; Ji, S.G.; Choi, I.; Jang, S.S. Effect of temperature on structure and water transport of hydrated sulfonated poly(ether ether ketone): A molecular dynamics simulation approach. J. Renew. Sustain. Energy 2011, 3, 043111. [Google Scholar]
- El-kharouf, A.; Chandan, A.; Hattenberger, M.; Pollet, B.G. Proton exchange membrane fuel cell degradation and testing: Review. J. Energy Inst. 2012, 85, 188–200. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Q.; Zhou, X.; Wang, J.; Tang, Y. Degradation characteristics of membrane electrode assembly under drive cycle test protocol. Int. J. Green Energy 2019, 16, 789–795. [Google Scholar] [CrossRef]

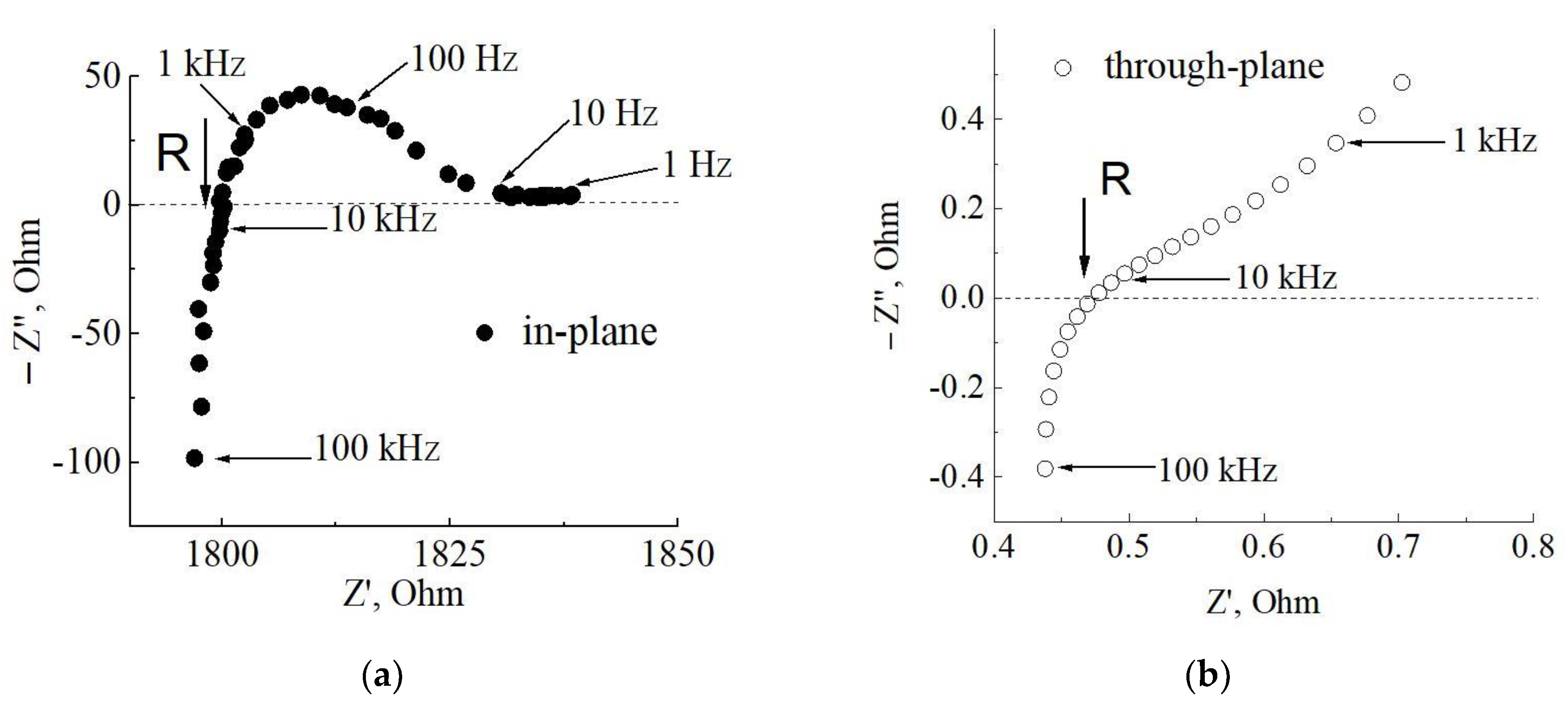

| Membrane Thickness | In-Plane Geometry of Measurements | Through-Plane Geometry of Measurements | ||
|---|---|---|---|---|
| d (μm) | R, Ohm | σ, mS/cm | R, Ohm | σ, mS/cm |
| 58 | 2120 | 102 | 0.47 | 60 |
| 64 | 1924 | 101 | 0.38 | 69 |
| 72 | 1810 | 96 | 0.53 | 54 |
| 80 | 1774 | 88 | 0.58 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavorotnaya, U.M.; Ponomarev, I.I.; Volkova, Y.A.; Modestov, A.D.; Andreev, V.N.; Privalov, A.F.; Vogel, M.; Sinitsyn, V.V. Preparation and Study of Sulfonated Co-Polynaphthoyleneimide Proton-Exchange Membrane for a H2/Air Fuel Cell. Materials 2020, 13, 5297. https://doi.org/10.3390/ma13225297
Zavorotnaya UM, Ponomarev II, Volkova YA, Modestov AD, Andreev VN, Privalov AF, Vogel M, Sinitsyn VV. Preparation and Study of Sulfonated Co-Polynaphthoyleneimide Proton-Exchange Membrane for a H2/Air Fuel Cell. Materials. 2020; 13(22):5297. https://doi.org/10.3390/ma13225297
Chicago/Turabian StyleZavorotnaya, Ulyana M., Igor I. Ponomarev, Yulia A. Volkova, Alexander D. Modestov, Vladimir N. Andreev, Alexei F. Privalov, Michael Vogel, and Vitaly V. Sinitsyn. 2020. "Preparation and Study of Sulfonated Co-Polynaphthoyleneimide Proton-Exchange Membrane for a H2/Air Fuel Cell" Materials 13, no. 22: 5297. https://doi.org/10.3390/ma13225297
APA StyleZavorotnaya, U. M., Ponomarev, I. I., Volkova, Y. A., Modestov, A. D., Andreev, V. N., Privalov, A. F., Vogel, M., & Sinitsyn, V. V. (2020). Preparation and Study of Sulfonated Co-Polynaphthoyleneimide Proton-Exchange Membrane for a H2/Air Fuel Cell. Materials, 13(22), 5297. https://doi.org/10.3390/ma13225297







