A Self-Healing Hierarchical Fiber Hydrogel That Mimics ECM Structure
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
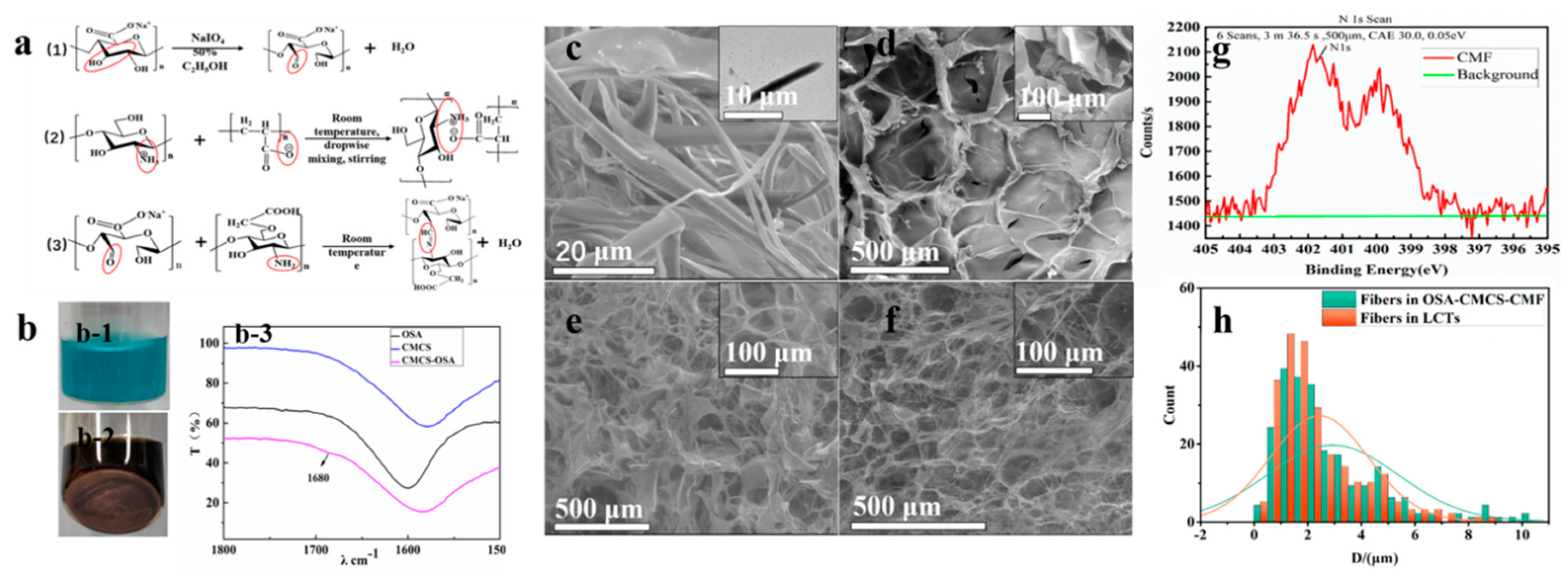
2.2.1. Alginate Oxidation
2.2.2. Preparation of Fibers
2.2.3. Preparation of Basic Hydrogel
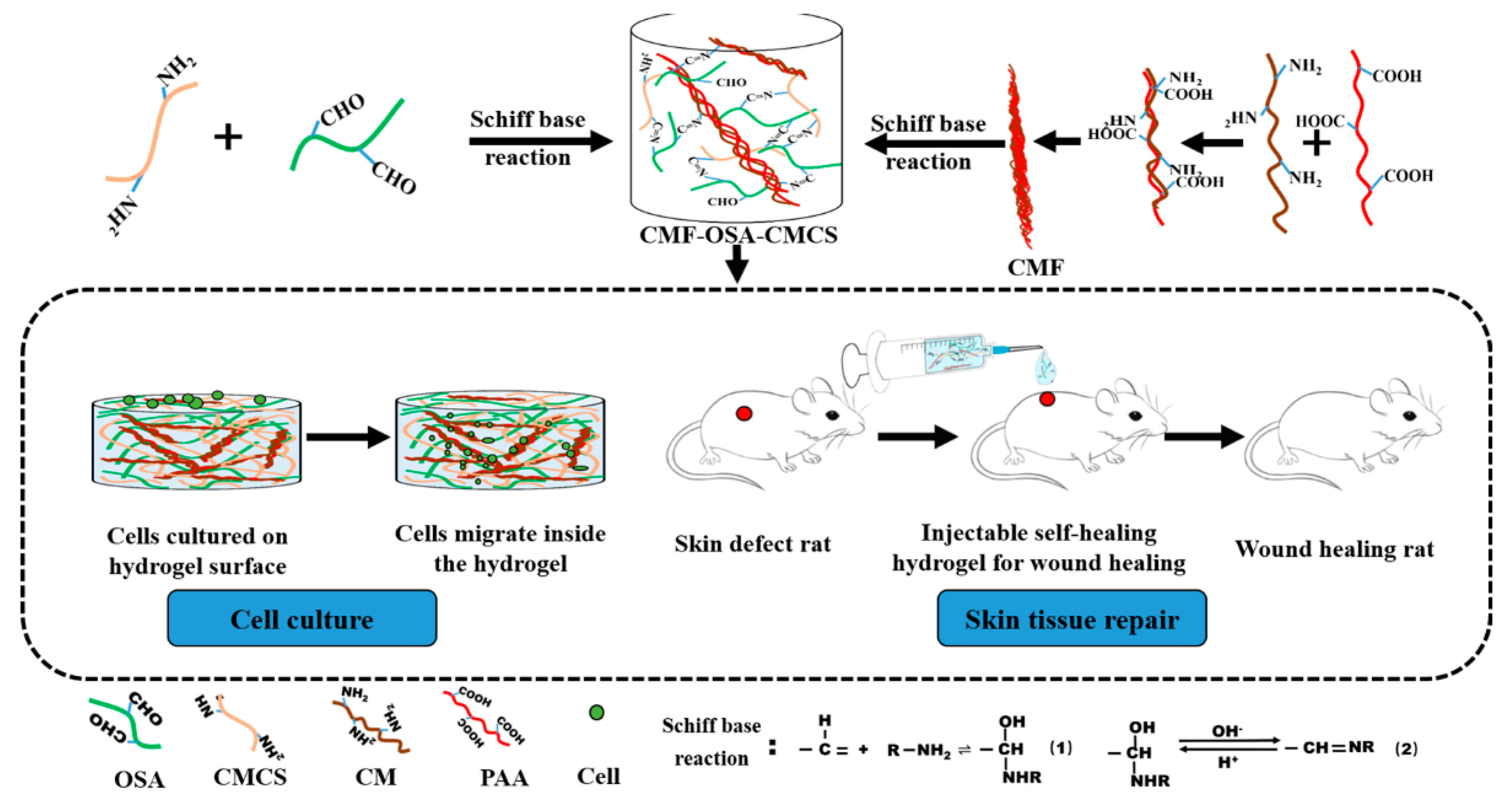
2.2.4. Preparation of Hierarchical Fiber Hydrogel
2.2.5. Fourier Transform Infrared Spectroscopy
2.2.6. Characterization of CMF Morphology and Surface Element Analysis
2.2.7. Environmental Scanning Electron Microscopy
2.2.8. Shear Force During Injection
2.2.9. Degradation Kinetics of Hydrogel
2.2.10. Self‑Healing of Hydrogel
2.2.11. In Vitro Cell Culture
2.2.12. Animal Experiments
3. Results
3.1. Morphological Characterization and Chemical Composition
3.2. Gelation Time
3.3. The Properties of OSA‑CMCS‑CMF
3.3.1. Shear Force Properties
3.3.2. Degradation Time Properties
3.3.3. Self‑Healing Properties
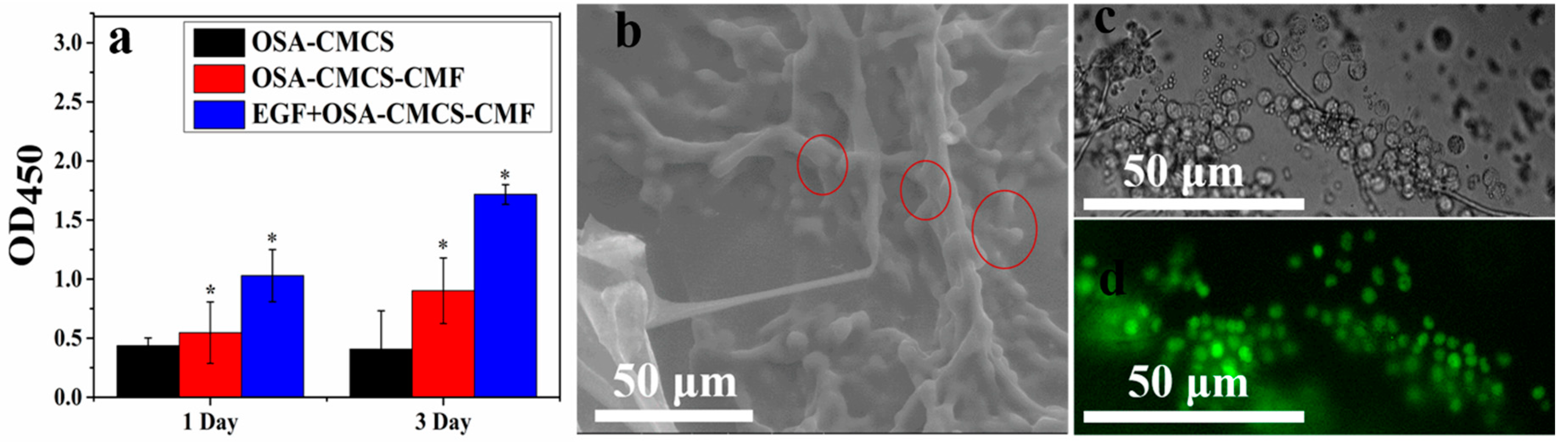
3.4. Cytocompatibility of OSA‑CMCS‑CMF
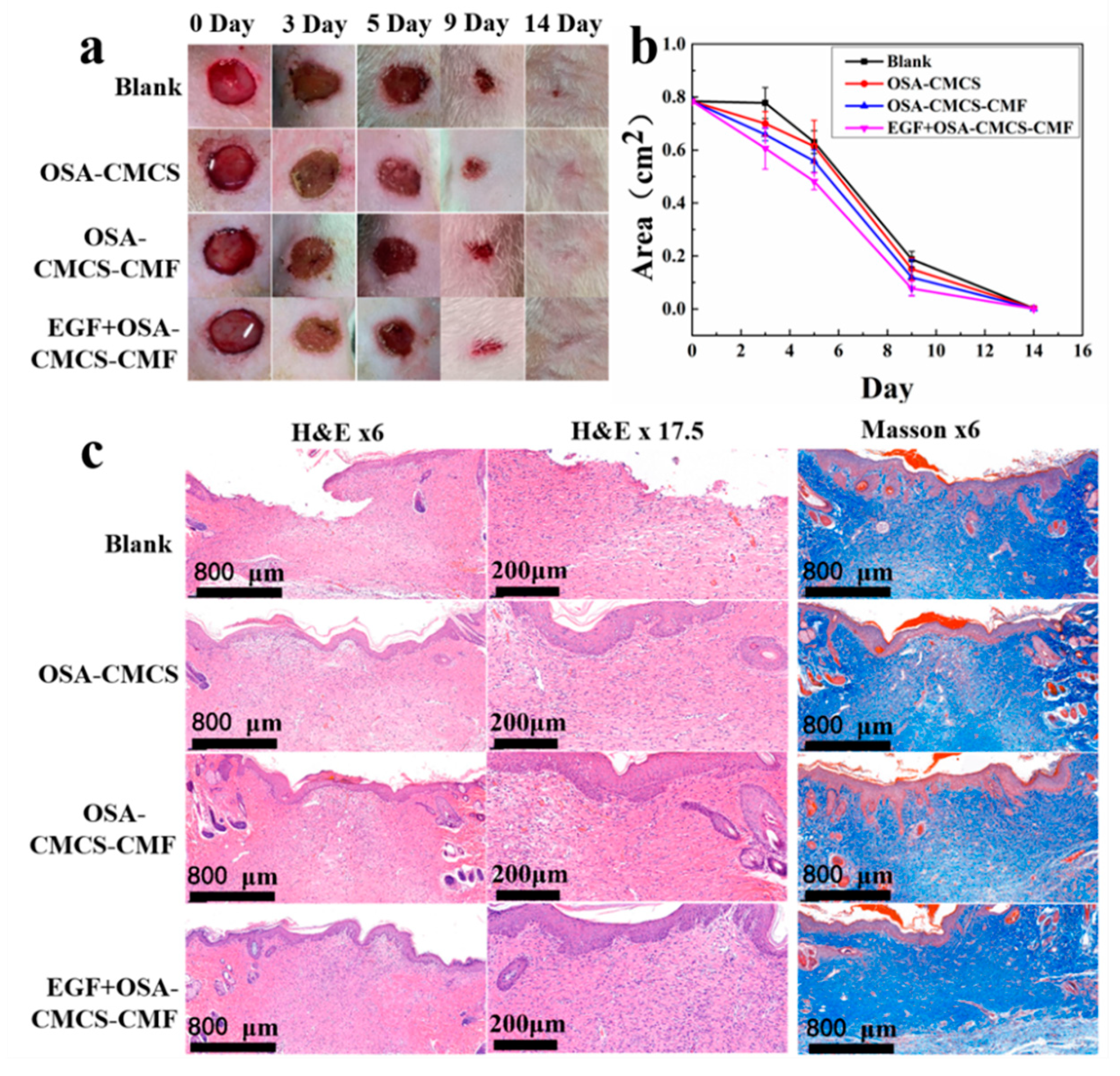
3.5. Skin Wound Repair
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langevin, H.M. Connective tissue: A body-wide signaling network? Med. Hypotheses 2006, 66, 1074–1077. [Google Scholar] [CrossRef]
- Feng, J.; Wang, F.; Han, X.; Ao, Z.; Sun, Q.; Hua, W.; Chen, P.; Jing, T.; Li, H.; Han, D. A “green pathway” different from simple diffusion in soft matter: Fast molecular transport within micro/nanoscale multiphase porous systems. Nano Res. 2014, 7, 434–442. [Google Scholar] [CrossRef]
- Mackintosh, F.C.; Käs, J.; Janmey, P.A. Elasticity of Semiflexible Biopolymer Networks. Phys. Rev. Lett. 1995, 75, 4425–4428. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Chen, C.S. Mechanotransduction in development: A growing role for contractility. Nat. Rev. Mol. Cell Biol. 2009, 10, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Dufort, C.C.; Paszek, M.J.; Weaver, V.M. Balancing forces: Architectural control of mechanotransduction. Nat. Rev. Mol. Cell Biol. 2011, 12, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Yuan, J.; Peng, C.; Li, Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014, 35, 2871–2882. [Google Scholar] [CrossRef]
- Cox, T.R.; Erler, J.T. Remodeling and homeostasis of the extracellular matrix: Implications for fibrotic diseases and cancer. Dis. Model. Mech. 2011, 4, 165–178. [Google Scholar] [CrossRef]
- Prince, E.; Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. Mater. 2019, 4, 99–115. [Google Scholar] [CrossRef]
- Hynes, R.O. The Extracellular Matrix: Not Just Pretty Fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef]
- Alam, N.; Goel, H.L.; Zarif, M.J.; Butterfield, J.E.; Perkins, H.M.; Sansoucy, B.G.; Sawyer, T.K.; Languino, L.R. The integrin—Growth factor receptor duet. J. Cell. Physiol. 2007, 213, 649–653. [Google Scholar] [CrossRef]
- Burdick, J.A.; Murphy, W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012, 3, 1269. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Seliktar, D. Designing Cell-Compatible Hydrogels for Biomedical Applications. Science 2012, 336, 1124–1128. [Google Scholar] [CrossRef] [PubMed]
- Timin, A.S.; Litvak, M.M.; Gorin, D.A.; Atochina-Vasserman, E.N.; Atochin, D.N.; Sukhorukov, G.B. Drug Delivery: Cell-Based Drug Delivery and Use of Nano-and Microcarriers for Cell Functionalization. Adv. Healthc. Mater. 2018, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Peppas, N.A.; Hilt, J.Z.; Khademhosseini, A.; Langer, R. Hydrogels in Biology and Medicine: From Molecular Principles to Bionanotechnology. Adv. Mater. 2006, 18, 1345–1360. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Yoshii, F. Hydrogels and their medical applications. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 1999, 151, 56–64. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Mehrali, M.; Thakur, A.; Pennisi, C.P.; Talebian, S.; Arpanaei, A.; Nikkhah, M.; Dolatshahi-Pirouz, A. Nanoreinforced Hydrogels for Tissue Engineering: Biomaterials that are Compatible with Load-Bearing and Electroactive Tissues. Adv. Mater. 2017, 29, 26. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, Q.; Shi, X.; Han, D. Hierarchical Hydrogel Composite Interfaces with Robust Mechanical Properties for Biomedical Applications. Adv. Mater. 2019, 31, e1804950. [Google Scholar] [CrossRef]
- Chau, M.; Sriskandha, S.E.; Thérien-Aubin, H.; Kumacheva, E. Supramolecular Nanofibrillar Polymer Hydrogels. In Advances in Polymer Science; Springer: Berlin, Germany, 2015; Volume 268, pp. 167–208. [Google Scholar]
- Blanazs, A.; Verber, R.; Mykhaylyk, O.O.; Ryan, A.J.; Heath, J.Z.; Douglas, C.W.I.; Armes, S.P. Sterilizable Gels from Thermoresponsive Block Copolymer Worms. J. Am. Chem. Soc. 2012, 134, 9741–9748. [Google Scholar] [CrossRef]
- Warren, N.J.; Rosselgong, J.; Madsen, J.; Armes, S.P.; Madsen, P.J. Disulfide-Functionalized Diblock Copolymer Worm Gels. Biomacromolecules 2015, 16, 2514–2521. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, M.A.; Hoffman, J.R.; De La Cruz, M.O.; Stupp, S.I. Tunable Mechanics of Peptide Nanofiber Gels. Langmuir 2010, 26, 3641–3647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Greenfield, M.A.; Mata, A.; Palmer, L.C.; Bitton, R.; Mantei, J.R.; Aparicio, C.; De La Cruz, M.O.; Stupp, S.I. A self-assembly pathway to aligned monodomain gels. Nat. Mater. 2010, 9, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.-S.; Su, C.-H.; Ho, W.-Y.; Huang, C.-C.; Chang, J.-C.; Chien, Y.-H.; Hung, S.-T.; Liau, M.-C.; Ho, H.-Y. Tumor targeting and MR imaging with lipophilic cyanine-mediated near-infrared responsive porous Gd silicate nanoparticles. Biomaterials 2013, 34, 5677–5688. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhao, X. A 3D model of ovarian cancer cell lines on peptide nanofiber scaffold to explore the cell–scaffold interaction and chemotherapeutic resistance of anticancer drugs. Int. J. Nanomed. 2011, 6, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Liu, S.; Zhang, Y.; Chi, Z.; Xu, J. A pH-responsive polymer based on dynamic imine bonds as a drug delivery material with pseudo target release behavior. Polym. Chem. 2018, 9, 878–884. [Google Scholar] [CrossRef]
- Balakrishnan, B.; Jayakrishnan, A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials 2005, 26, 3941–3951. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wang, J.-W.; Hon, M.-H. Polyion Complex Nanofibrous Structure Formed by Self-Assembly of Chitosan and Poly(acrylic acid). Macromol. Mater. Eng. 2006, 291, 123–127. [Google Scholar] [CrossRef]
- Lu, H.; Yuan, L.; Yu, X.; Wu, C.; He, D.; Deng, J. Recent advances of on-demand dissolution of hydrogel dressings. Burn. Trauma 2018, 6, 35. [Google Scholar] [CrossRef]
- Cordes, E.H.; Jencks, W.P. On the Mechanism of Schiff Base Formation and Hydrolysis. J. Am. Chem. Soc. 1962, 84, 832–837. [Google Scholar] [CrossRef]
- Rosales, A.M.; Anseth, K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016, 1, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Hu, N.; Cao, Y.; Ao, Z.; Han, X.; Zhang, Q.; Liu, W.; Liu, S.; Liao, F.; Han, N. Flow behavior of liquid metal in the connected fascial space: Intervaginal space injection in the rat wrist and mice with tumor. Nano Res. 2018, 11, 2265–2276. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Zhu, Y.; Zhang, Q.; Shi, X.; Liang, F.; Han, D. A Self-Healing Hierarchical Fiber Hydrogel That Mimics ECM Structure. Materials 2020, 13, 5277. https://doi.org/10.3390/ma13225277
Li K, Zhu Y, Zhang Q, Shi X, Liang F, Han D. A Self-Healing Hierarchical Fiber Hydrogel That Mimics ECM Structure. Materials. 2020; 13(22):5277. https://doi.org/10.3390/ma13225277
Chicago/Turabian StyleLi, Kai, Yuting Zhu, Qiang Zhang, Xiaoli Shi, Feng Liang, and Dong Han. 2020. "A Self-Healing Hierarchical Fiber Hydrogel That Mimics ECM Structure" Materials 13, no. 22: 5277. https://doi.org/10.3390/ma13225277
APA StyleLi, K., Zhu, Y., Zhang, Q., Shi, X., Liang, F., & Han, D. (2020). A Self-Healing Hierarchical Fiber Hydrogel That Mimics ECM Structure. Materials, 13(22), 5277. https://doi.org/10.3390/ma13225277



