1. Introduction
Interest in nanotechnology has increased significantly in recent decades, although the first research reports on the subject of nanoparticles were published in the 1950s [
1]. This rapidly-developing field of science is increasingly focused on issues related to nanomaterial fabrication, properties, and the use of nanoparticles with natural origins, including nanocellulose, which is often considered the “material of the future” [
2]. Cellulose is a biopolymer commonly found in nature, e.g., in plants, bacteria, or algae [
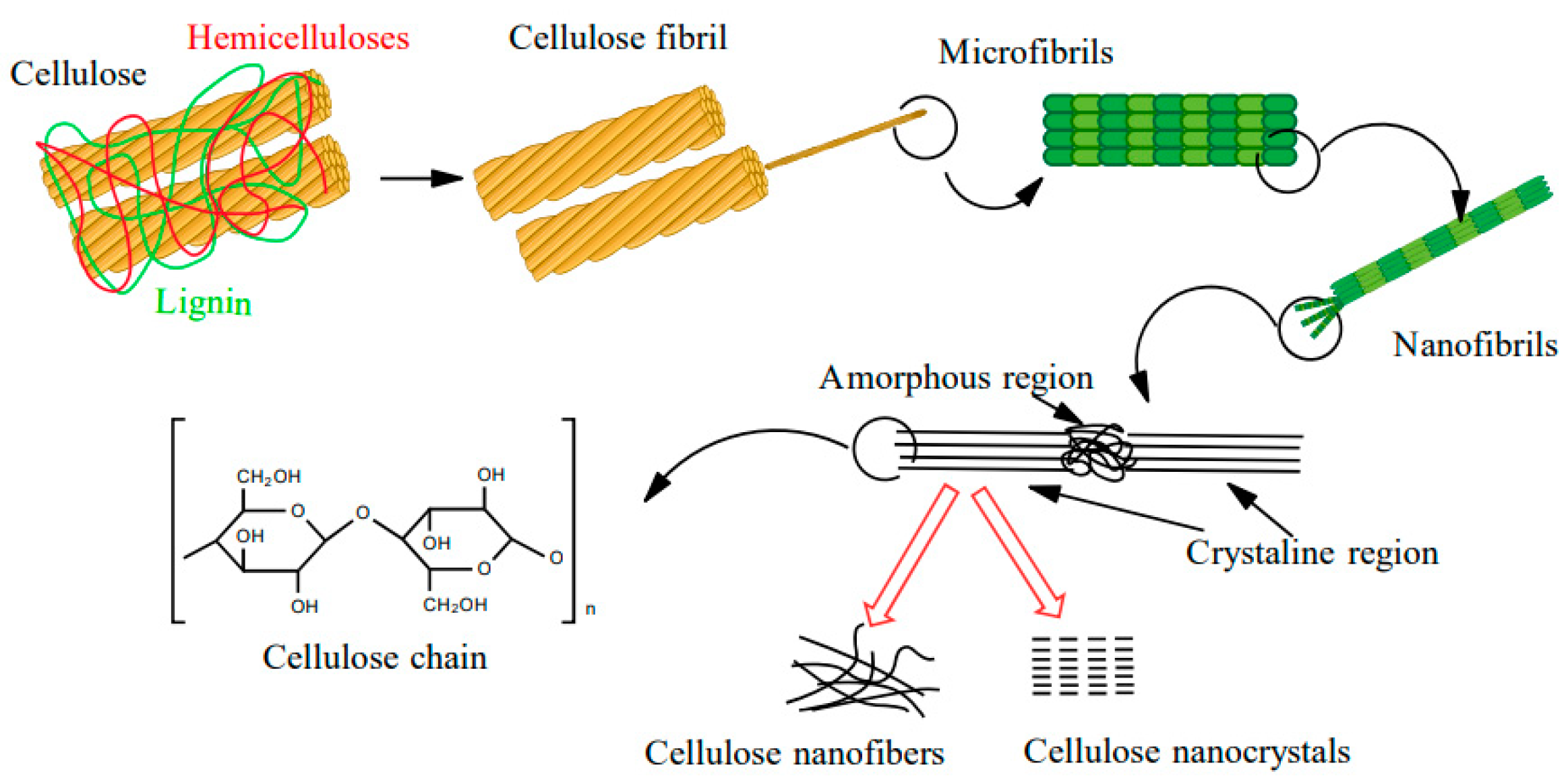
3]. The structural units of cellulose molecules are D-anhydro glucopyranose, which are linked by a β-1,4-glycosidic bond. In nature, cellulose typically fulfills a skeletal function in plant cell walls and exists there in the form of macrofibrils. Macrofibrils are made of microfibrils, which are, in turn, composed of nanofibrils. The lattermost are crystalline or amorphous nanoparticles. The crystalline regions of cellulose are strongly linked through hydrogen bonds between hydroxyl groups, thus making them difficult to separate (
Figure 1).
Nanocellulose (NC) is a biopolymer characterized by an organized structure, in which at least one cross-sectional dimension is on the nanometer scale, i.e., less than 100 nm. Three types of NC can be distinguished, namely nanocrystalline cellulose (CNC), nanofibrous cellulose (CNF), and bacterial cellulose (BC) [
4]. CNC, which is most commonly extracted from cellulose fibers via acid hydrolysis, resembles elongated crystalline rods, with a compact ordered structure that enables less elasticity than the structure of CNF [
5,
6,
7]. This material exhibits a low proportionality factor, and the diameter and length of a single “rod” varies from 2 to 20 nm and from 100 nm to several µm, respectively [
8,
9]. The degree of crystallinity of CNC is typically between 54–88% [
10]. Habibi et al. [
11] emphasized that the degree of crystallization, size distribution, and morphology depend on the starting material and the conditions by which the NC was obtained. In contrast, CNF consists of bundles of cellulose chains [
12] bound into long, flexible, tangled nanofibers ranging from 1–100 nm in length [
13], and they represent the smallest plant fiber structures. Similar to the macromolecular cellulose, CNF consists of alternating crystalline and amorphous regions [
5] and it is usually formed as a result of mechanical, chemical, or enzymatic treatment [
14].
NC can be fabricated using either a “bottom-up” or a “top-down” method. Bacterial nanocellulose is synthesized by the “bottom-up” method (i.e., forming larger units from smaller building blocks) during the fermentation of low-molecular weight sugars, with the assistance of
Acetobacter bacteria. In contrast, “top-down” methods focus on disintegrating natural fibers into smaller units through mechanical and/or chemical treatment [
15,
16]. The most prominent methods for obtaining NC by a “top-down” approach are mechanical (e.g., high-pressure homogenization, microfluidization, milling, cryogenic milling, or high-intensity ultrasonication), enzymatic, and chemical (e.g., using ionic liquids, acid-base treatment, acid hydrolysis, or oxidation).
High-intensity ultrasonication (HIUS) is one type of mechanical approach for fabricating NC. This treatment method involves separating the fibers from the cell wall using cavitation in an aqueous environment. When water molecules absorb the applied ultrasonic energy, microscopic gas bubbles form, grow, and implode on the surface of the cellulose. The resulting hydrodynamic forces cause defibrillation of the starting material, which may be pure cellulose, microcrystalline cellulose (MCC), pulp, banana peel, rice straw, or microfiber cellulose, etc. Studies have shown that the mixture of nano- and microfibrils obtained after an ultrasonic bath can reach dimensions of 20 nm up to several micrometers, which indicates that the product mixture contains some nanofibers which are isolable and some that are not separated from microfibrils. This method enables the production of filament aggregates with varying sizes. Depending on the characteristics of the starting material, an ultrasonic bath will affect the crystalline structure of the cellulose in different ways. For example, in the case of pure cellulose, the degree of crystallinity increased following the treatment, whereas in the case of MCC, the degree of crystallinity decreased, and in the case of the cellulose pulp, the degree of crystallinity remained constant [
17]. Wang and Cheng [
18] described how ultrasonic processing parameters impact the degree of cellulose defibrillation. They demonstrated that the degree of defibrillation increased with increases in both the device power and the process temperature. Additionally, a more concentrated cellulose suspension and a greater distance between the working vessel and the ultrasonic probe did not improve the defibrillation process. It was also determined that longer fibers were less prone to defibrillation than shorter ones. In addition, the combined use of HIUS and high-pressure homogenization (HPH) methods enhanced the defibrillation efficiency. Another combined approach paired HIUS with oxidation by the 2,2,6,6-tetramethylpiperidine-1-oxyl reagent (TEMPO), and achieved an NC yield of 71% [
19]. Alternatively, milling a sample of TEMPO pre-treated cellulose pulp produced NC in 90% yield. Oxidation with the TEMPO reagent also led to an additional increase in the NC yield obtained using the ultrasonic probe (100% efficiency) and the ultrasonic bath (50% efficiency). Chen et al. [
20] showed that the HIUS treatment of bamboo, wood, and wheat straw generated NC with increased cellulose crystallinity (60%) and a degradation temperature over 330 °C.
Although chemical techniques represent direct methods for extracting NC, they can also be applied in intermediate stages, such as during pre-treatment to promote further refinement of the raw material. The goal of implementing such intermediate stages is primarily to reduce the energy consumption of the mechanical processes used in the downstream stages of NC fabrication. In fact, the high energy consumption is a major disadvantage related to the process of obtaining NC. Applying a pre-treatment reduces the energy requirement from an average of 25,000 to 1000 kWh/ton of cellulose fiber [
21]. The pre-treatment usually involves treating cellulose with enzymes, ionic liquids, acids, or alkaline reagents, or subjecting cellulose to oxidation reactions (e.g., in the presence of TEMPO). The alkaline-acid treatment was most commonly used as a pre-treatment prior to a mechanical treatment in the three-step process of obtaining CNF [
6,
22,
23]. The first step of this method consisted of soaking the fibers in a solution of sodium hydroxide to hydrolyze them. The second step involved hydrolysis of the fibers using hydrochloride to dissolve hemicellulose, and the third step employed sodium hydroxide to break down the structure of the lignin and cleave the bonds between carbohydrates and lignin. Using such an alkaline-acid pre-treatment scheme, NC can be extracted from wheat straw and soybean husks [
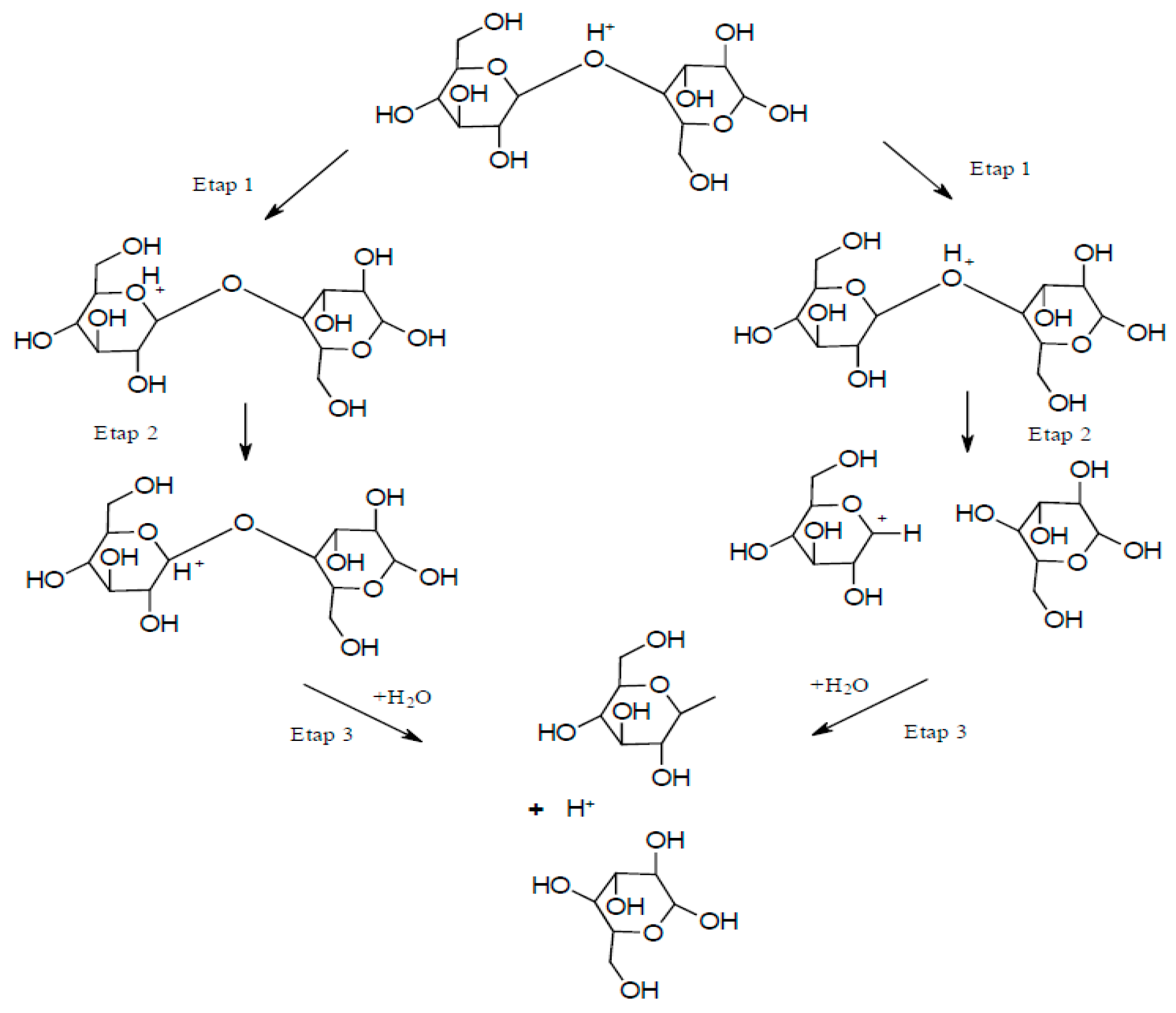
23]. The resulting fibrous material was characterized as NC, with cellulose fiber dimensions (on the cross-section) of 10–80 and 20–120 nm, respectively. Acid hydrolysis is the oldest method for obtaining CNC. The hydrolysis reaction rate depends on the temperature and the concentration of the acid used. During hydrolysis, amorphous areas decay and crystalline areas remain intact. The hydrolysis mechanism can be divided into three steps [
24]: (i) Proton binding to an oxygen atom of a glycosidic bond or an oxygen atom between two carbon atoms in an anhydro glucopyranose ring, (ii) proton transfer to the C1 atom leading to the formation of a carbocation and cleavage of the glycosidic bond, and (iii) release of simple sugars and regeneration of the proton due to the interaction between water and the carbocation (
Figure 2).
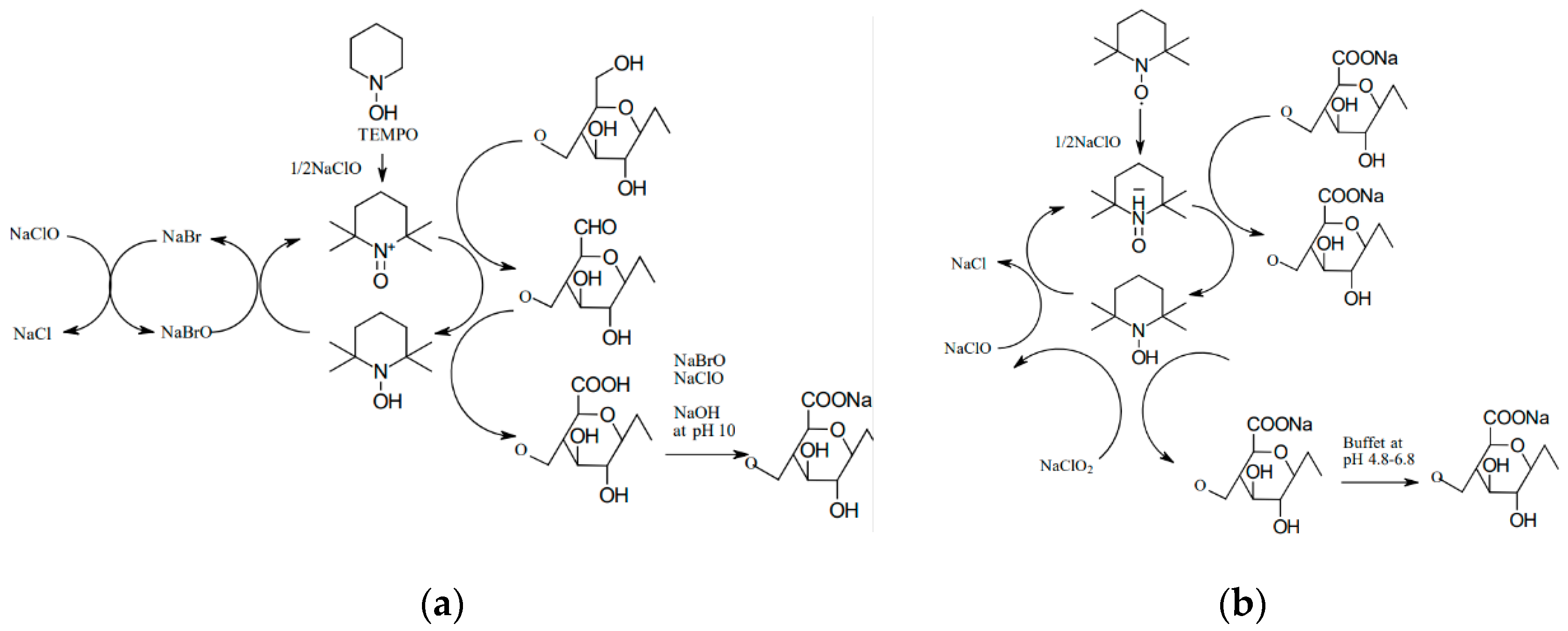
The so-called controlled hydrolysis is carried out in such a way to promote the formation of shortened polymer chains rather than a complete destruction of cellulose into simple sugars. A 60–65% aqueous solution of sulfuric acid is most commonly used for this purpose, and the reaction is conducted at 45–75 °C over a period of 40–70 min. A disadvantage of this method is that sulfone groups tend to persist in the resulting CNC, which leads to a low thermal stability. However, other acids, such as hydrochloric acid or hydrobromic acid, can be used, but they typically produce NC with a higher tendency to agglomerate. Combining acid and ultrasonic treatments led to a more than 20% increase in crystallinity relative to the starting material. The oxidation reaction with TEMPO in an aqueous solution has also been described extensively in the literature. During this reaction, secondary hydroxyl groups remain intact, while a carboxyl or aldehyde group is formed with a negative charge at the C6 position. The reaction can be carried out at room temperature and in an alkaline solution in the presence of NaClO, NaBr, and catalytic amounts of the TEMPO reagent [
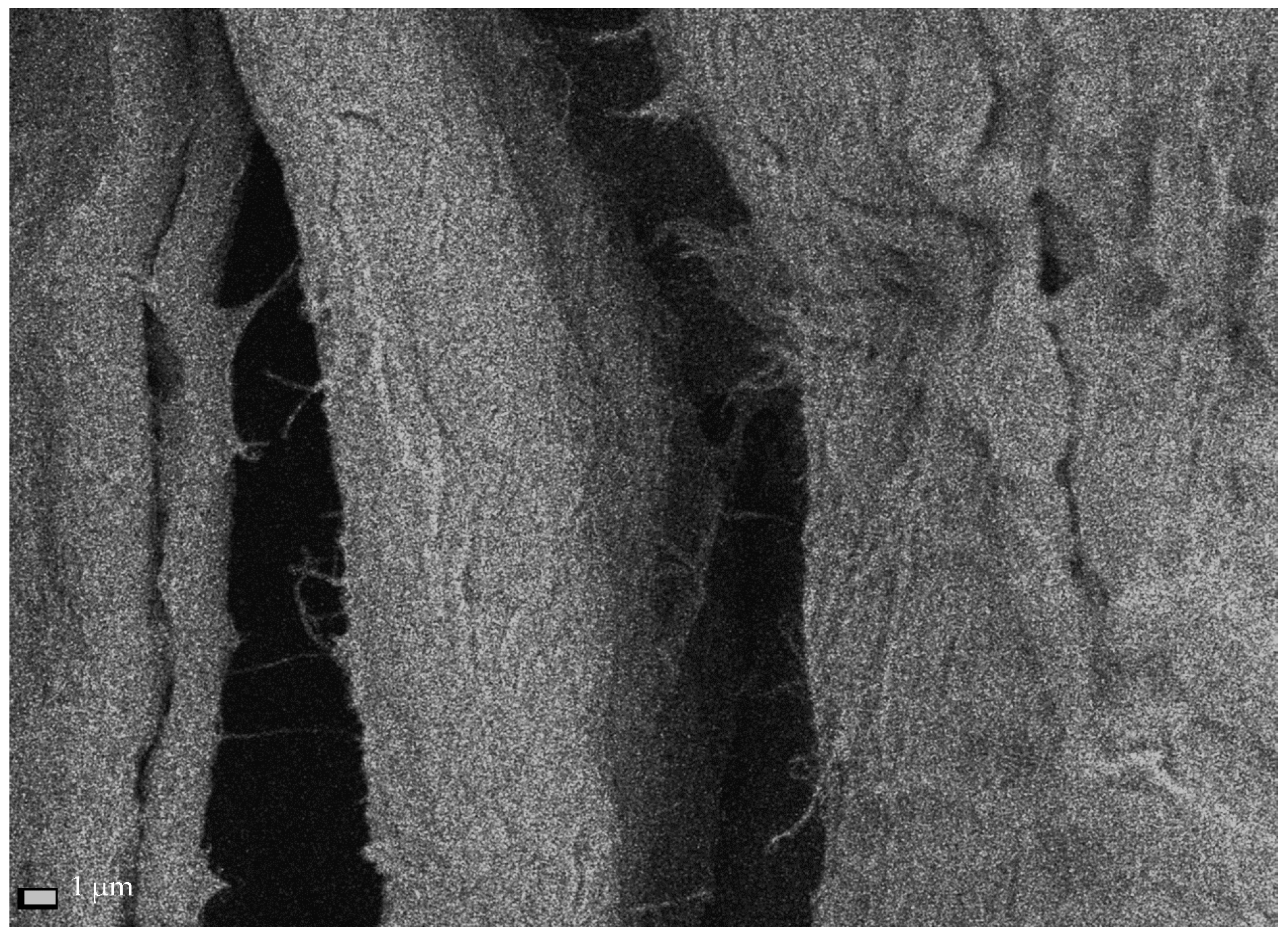
25]. It was discovered that when the process is conducted in an alkaline solution, aldehyde groups are formed, resulting in reduced thermal stability, hindered microfibrillation, and discoloration of the NC after drying (
Figure 3a). In contrast, conducting such a process in an acidic environment (pH 6.0–6.5) at a temperature of 50–60 °C increases the product’s thermal stability (
Figure 3b). In this case, no aldehyde groups are formed, and consequently, there is no uncontrolled cellulose depolymerization [
17,
25].
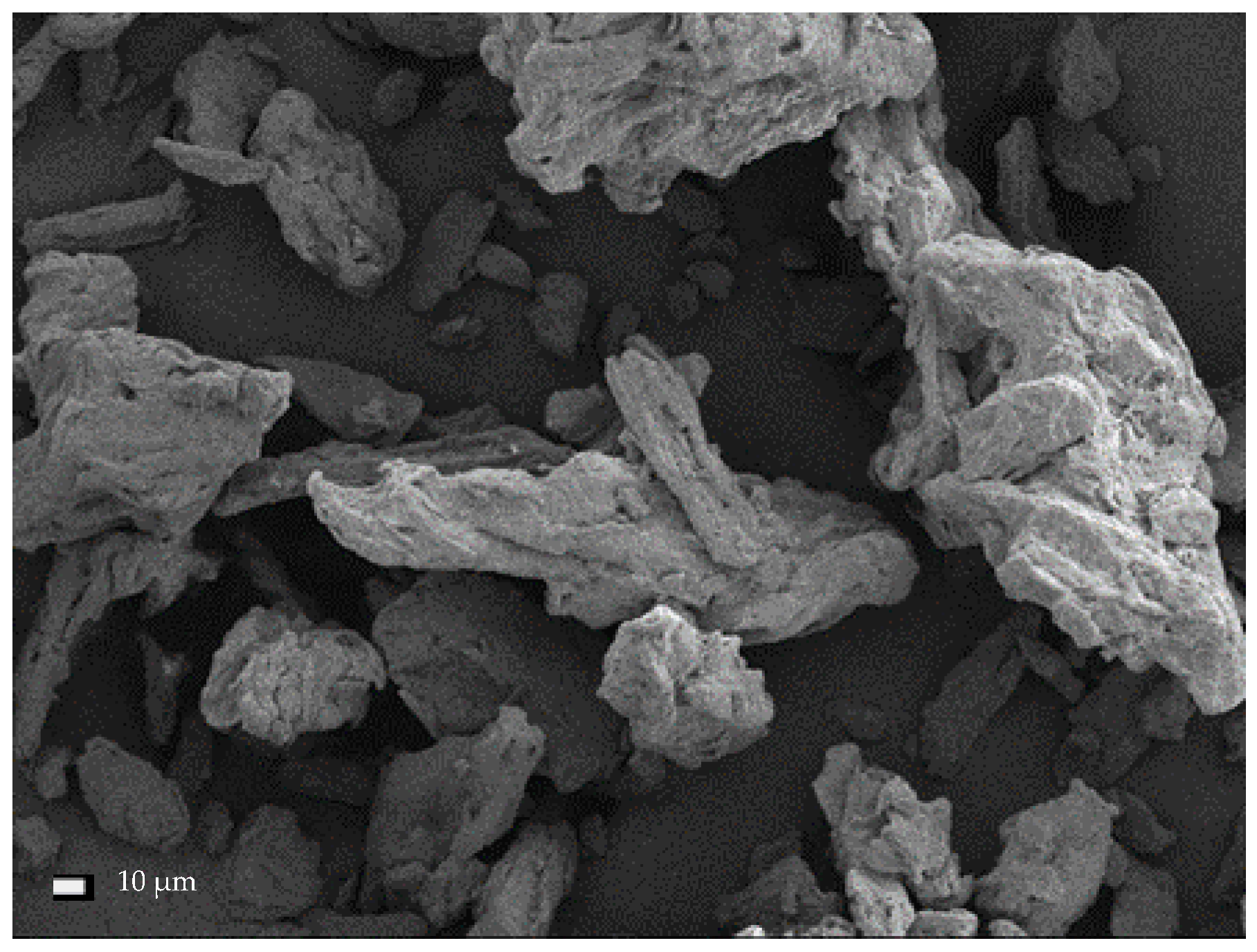
Liew et al. [
26] described the results of acid hydrolysis of cellulose (i.e., native cellulose) yielding CNC I. It is clear from the performed microimaging that the cellulose has been defibrillated and the size of its particles has been reduced. The authors observed the fragmentation of particles during preparation of CNC II from cellulose II (i.e., cellulose obtained by mercerization and regeneration of native cellulose). The particles were characterized by irregular shapes close to the spherical [
27,
28]. Kim et al. [
2] observed that the structure of nanocellulose fibers largely depends on their origin. This conclusion arose from comparing the structure of BC, MCC, and bamboo cellulose fibers.
In light of the literature analysis presented here, this work aims to evaluate the effects of hybrid cellulose treatments, specifically, chemical methods paired with medium frequency ultrasonication. The study employs two different starting materials (Södra Black R cellulose or microcrystalline cellulose), two types of chemical pre-treatments (acid hydrolysis or oxidation), and two sonication durations at 45 kHz (1 or 6 h). The NC products are characterized using scanning electron microscopy, and the impacts of treatment parameters are discussed.
2. Materials and Methods
2.1. Materials
Cellulose in the commercial form of Södra Black R and microcrystalline cellulose (Sigma-Aldrich, Darmstadt, Germany) were used as the starting materials for nanocellulose generation. The Södra Black R cellulose was a mixture of wood fibers from Picea abies (80%) and Pinus sylvestris (20%) and had the following performance parameters: Average fiber length (2050 µm); average fiber width (30.0 µm); coarseness (0.135 µg/m); brightness (89.5%); pH (4.8); and ash content (0.2%). The Södra Black R cellulose sheet had a very compact structure. Therefore, it was ground in a laboratory grinder (CHemLand, Stargard Szczecinski, Poland) and then fractionated using a 400 µm sieve to produce a homogeneous raw material.
2.2. Hydrolysis of Cellulose
The ground Södra Black R cellulose and microcrystalline cellulose (Sigma-Aldrich) were acid-treated using 65% sulphuric acid (Poch, CAS no. 7664-93-9, Gliwice, Poland) with a mass ratio of cellulose material to acid solution of 1:30. The fibers were dispersed in the acid by mixing with a magnetic stirrer (600 rpm) at 45 °C for 30 min. The stirring time was measured from the moment the desired process temperature was reached. To complete the reaction and neutralize the pulp, the raw material was rinsed 6 times with 100 mL of distilled water. The washed raw material was centrifuged using a laboratory centrifuge (2500 rpm, 20 min, Scilogex DMO412 Rocky Hill, CT, USA) and then decanted with the sediment solution (,. The treated pulp was dried in a laboratory dryer at 40 °C.
2.3. Cellulose Oxidation
The treatment with TEMPO (Pol-Aura, CAS no. 2564-83-2, Gliwice, Poland) was carried out by introducing cellulose into a saturated solution of the TEMPO reagent (9.7 g/dm3). In order to increase the pH of the solution (to pH > 8), the NaOH solution (Poch, CAS no. 1310-73-2, Gliwice, Poland) was added to the reaction mixture in a proportion of 0.1 g per 9 mL. The oxidation process took place during sonication in the ultrasonic cleaner (POLSONIC Palczyński Sp. J., Warszawa, Poland).
2.4. Cellulose Sonication
Sonication was carried out in tightly-closed 15 mL vials. Suspensions with a cellulose concentration of 1% (0.09 g of cellulose material per 9 g of liquid) were treated in a 45 kHz ultrasonic cleaner. The raw material was (i) placed in distilled water when no pre-treatment or acid treatment was involved, or (ii) placed in an alkaline solution saturated with TEMPO, depending on the desired experiment. The vials were placed in a POLSONIC SONIC-6D ultrasonic cleaner and subjected to sonication for a designated time period: Either for 60 min or for two cycles of 180 min each (i.e., 6 h total). The initial temperature of the bath was 18 °C, but during the process, it increased significantly (rising to approx. 60 °C after 3 h). In order to avoid excessive heating of the system, after the first 3 h of sonication, the process was interrupted for 24 h before continuing for the final 3 h.
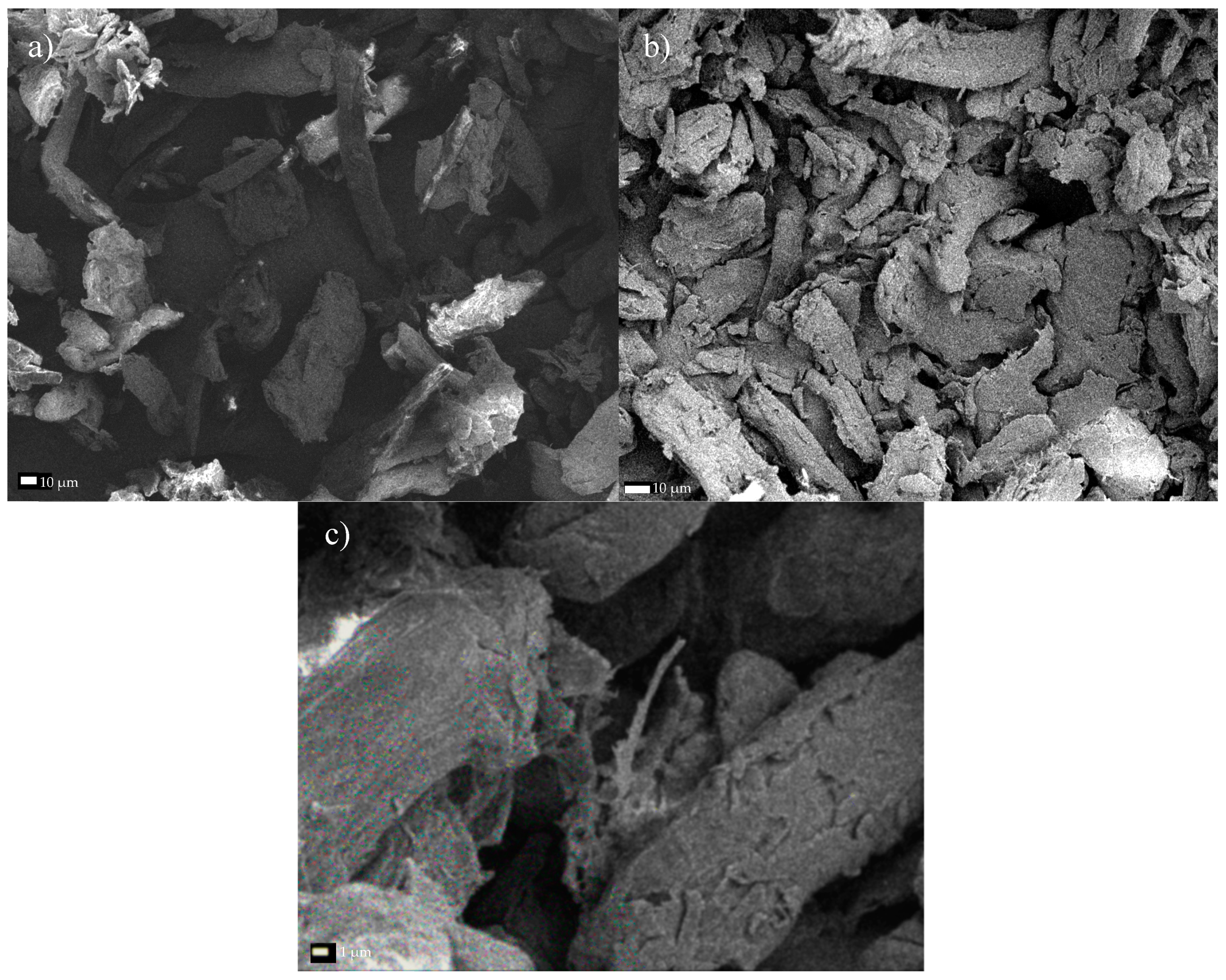
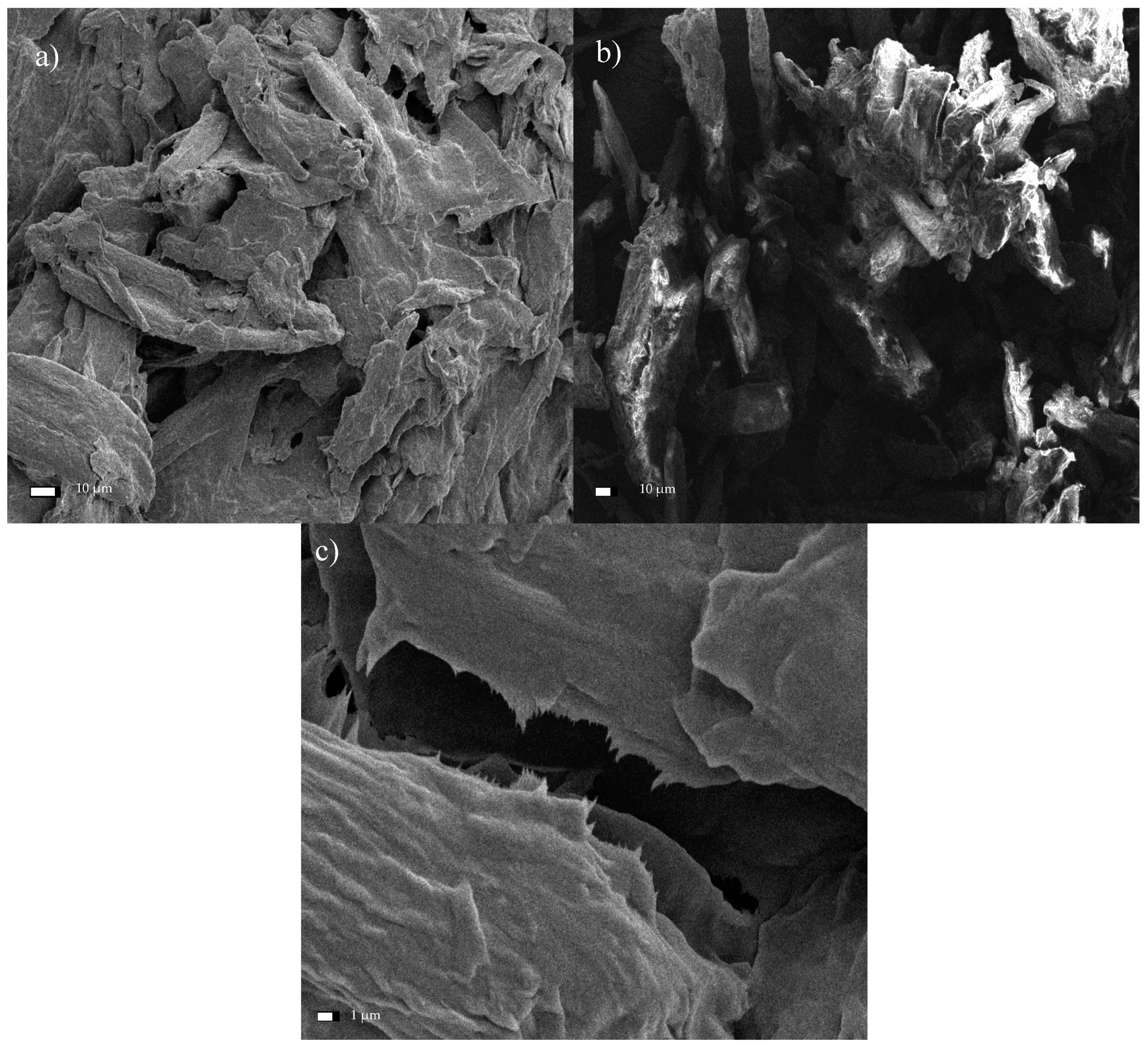
2.5. SEM Analysis
Direct observation of the microstructure morphology of cellulose before and after the chemical and mechanical treatment was examined by the SEM method. The samples were placed on a double sided adhesive carbon tape and coated with a gold layer of thickness of 10 nm. Images were taken with the use of JEOL JSM-7001F TTLS (JEOL Ltd., Tokio, Japan) scanning electron microscope applying the accelerating voltage of 5 kV, a working distance of about 10 mm (depending on the focus), and a secondary electron (SEI) detector. The sizes of cellulose fibers were manually measured using ImageJ® (an open source Java image processing program inspired by NIH Image) from 20 particles shown in three independent images using ImageJ®1.52v.
4. Conclusions
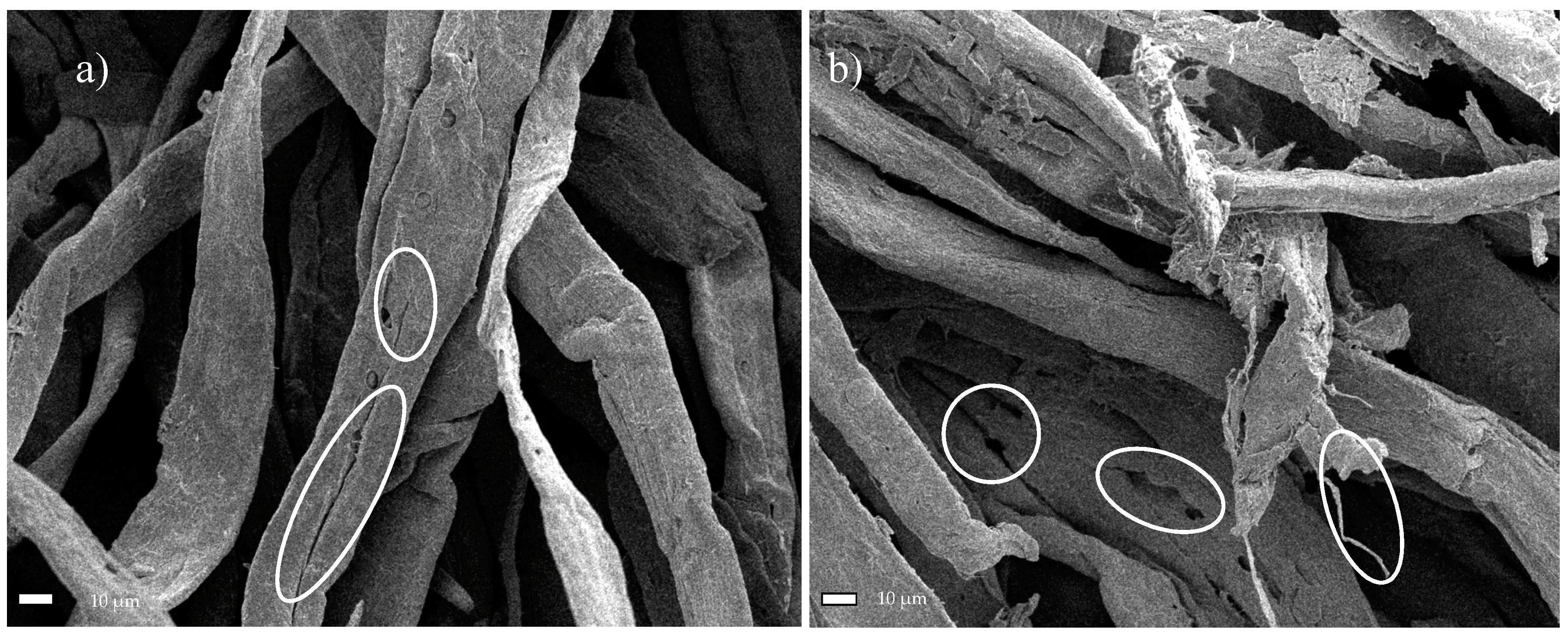
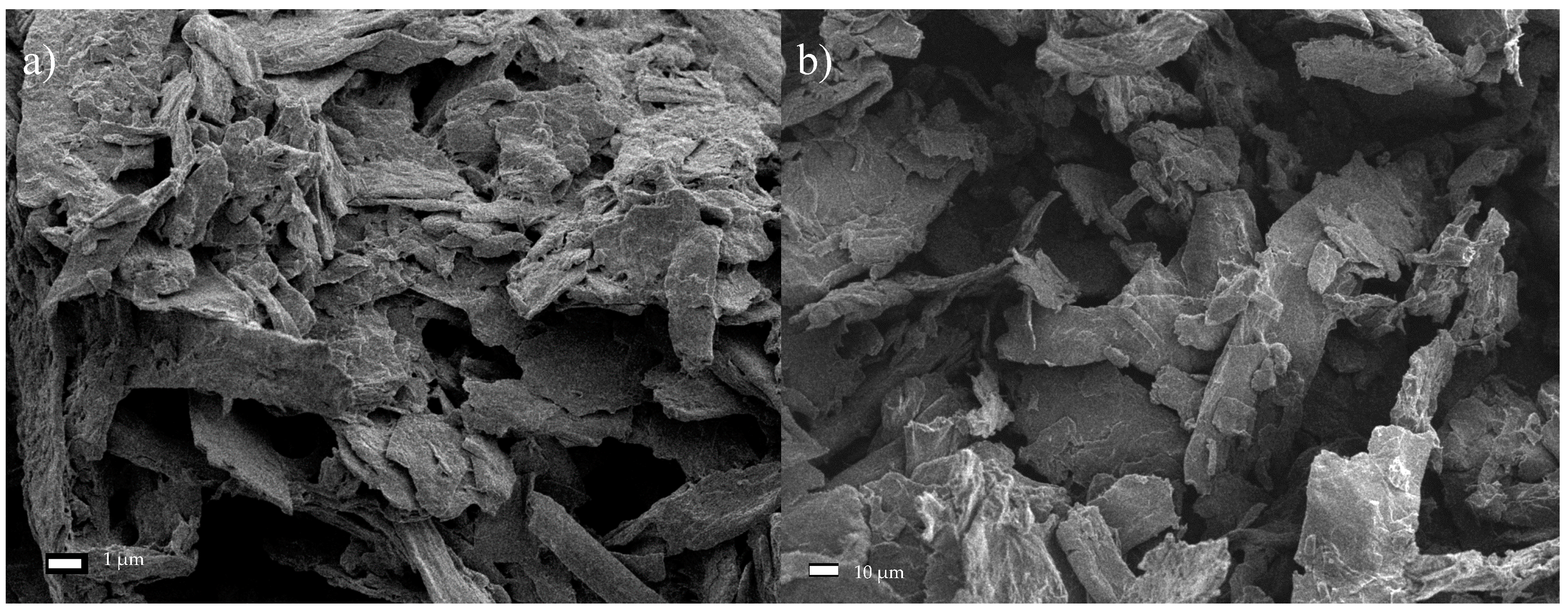
The performed experiments on the hydrolysis and oxidation of commercial and microcrystalline cellulose, combined with its exposure to the ultrasound, allowed assessing the structure of the obtained material through SEM analysis. First, it was found that the reduced fiber cross-sectional dimensions was the result of increased exposure of cellulose to the ultrasound. Specifically, subjecting both Södra Black R cellulose and MCC to the ultrasound for 6 h led to nanometer scale structures in the form of isolated fibers. Second, the TEMPO reagent accelerated the degradation process of the two cellulose varieties due to its oxidizing character. The resulting products had nanofibrous structures. Third, the degradation of cellulose through the combined action of sonication and TEMPO activity was gradual. Early effects of the treatment manifested in characteristic oval regions, where the initiation of surface fiber degradation took place. In the next stage, these areas showed further progression of longitudinal cracking in the fibers. Additionally, a significant degradation of the cellulose structure, creating a solid/crystalline structure from the fibrous system, occurred as a result of the acid treatment. The degradation of cellulose continued throughout the process of sonication, and increasing time led to the agglomeration of the cellulose particles. Ultimately, nanometer-sized particles were not observed following such treatment. Finally, products exhibited limited thermal stability due to the introduction of sulfone groups into the cellulose structure during the initial acid treatment. The change in the cellulose color was observed during the drying process, while preparing the material for SEM analysis confirmed this effect. The preliminary treatment of cellulose described in this paper, consisting of hybrid acid hydrolysis or oxidation in combination with medium-frequency sonication, clearly indicates the direction of further research on the reduction of the size of cellulose fibers. The novelty of this study was the demonstration of the beneficial effects of oxidation treatment and sonication. As a result, the dimensions of the cross-section of the fibers were reduced. Therefore, avoiding the agglomeration of the fibers, which was the case with the acid treatment. The hybrid method described herein based on the oxidation and sonication of Södra Black R or microcrystalline cellulose, could potentially be used for the in situ generation of cellulose nanofilms, for example, for the preservation or reinforcement of wooden or paper antique items. The authors still believe that the hybrid (i.e., chemical-physical or biological-physical) treatment of cellulose can give satisfactory results in terms of obtaining nanocellulose, which is why further research in the cellulose pre-treatment should focus on optimizing the chemical oxidizing agent in conjunction with the ultrasound.