3.1. Densification and Phase Analysis
The apparent density results measured by the hydrostatic method is presented in
Figure 3. The obtained data shows that dense materials can be obtained only with the addition of up to 8 vol.% of hexagonal boron nitride, which is higher than the literature data [
13]. A further increase of h-BN content led to a decrease in the material density, which results from porous agglomerates of this phase (
Figure 4). The density measurement error increases with the h-BN content, which is connected with the h-BN distribution, water adsorption to agglomerates, and a possible absence of h-BN after the HP graphite foil removal. The existence of hexagonal boron nitride agglomerates can lead to a decrease of the material friction coefficient [
21,
23]. Such agglomerates can strongly adsorb a large amount of water from the atmosphere, which was visible on the dilatometric curve changes as a negative jump on the curve. Such material required an additional time in the vacuum chamber in order to remove the adsorbed water before the final CTE analysis, which is described in a further part of this paper.
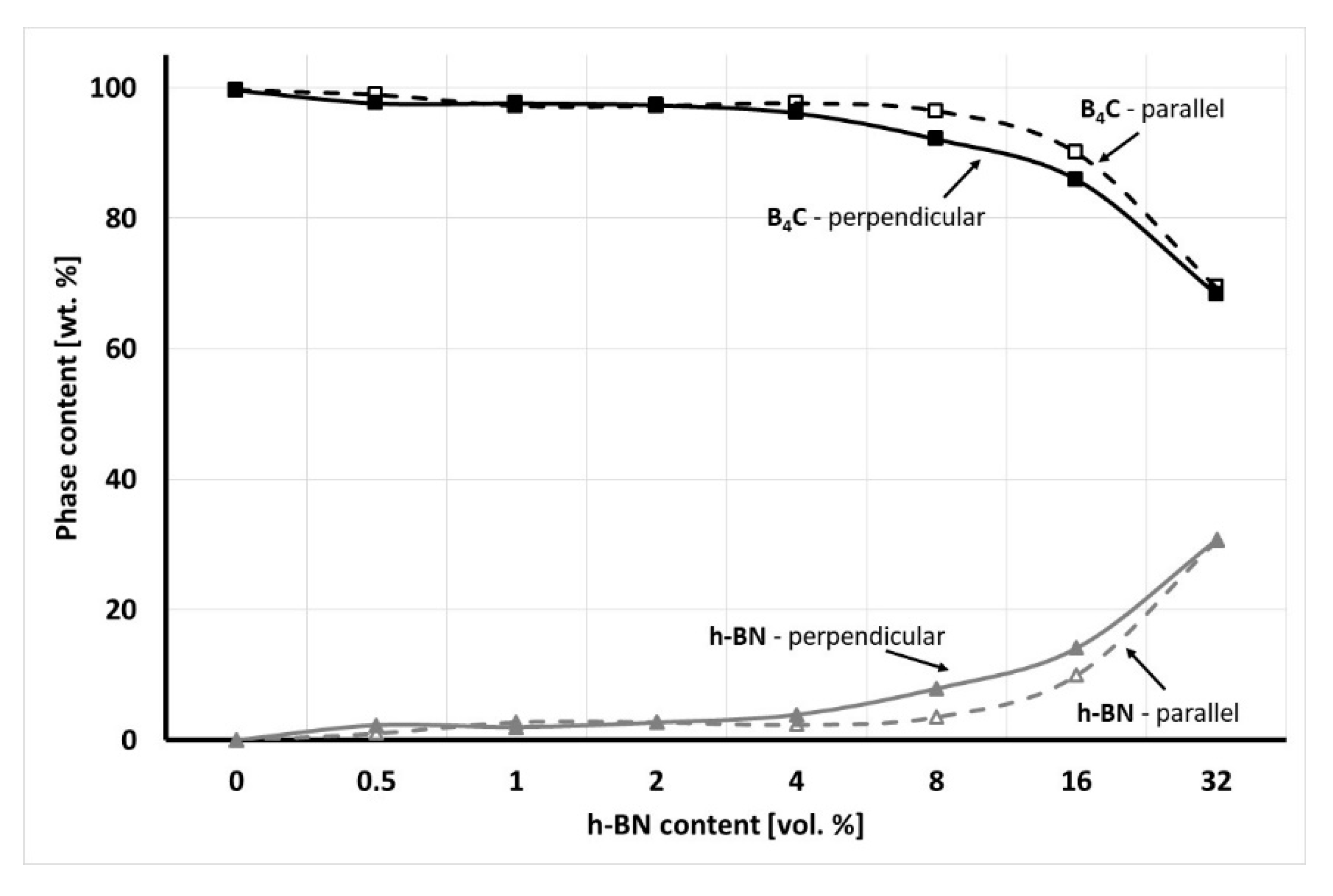
Due to the uniaxial pressure applied during the hot-pressing process, there should be a difference in the XRD/Rietveld analysis measured in various material directions. XRD peak intensities of selected material phases show various values in different material directions, which have a translation into their content. The result of the phase composition analysis made in perpendicular (in-plane) and parallel (out-of-plane) direction to the pressing axis is presented in
Figure 5.
The XRD/Rietveld analysis showed that for the hot-pressed material with up to 2 vol.% there is almost no difference in the composite phase composition in relation to the material direction. The change of the selected phase concentration was noticeable for composites with 8–16 vol.% of hexagonal boron nitride due to the 2D phase orientation in the material as a result of applied pressure. For the highest content of h-BN solid lubricant, there was almost no difference due to the high content of even oriented h-BN agglomerates. In the case of a perpendicular direction, 1% of B
2O
3 was present in the samples with 32 vol.% of h-BN. It formed due to the oxidation of h-BN agglomerates. Some of the boron oxide can be present on the initial B
4C powders grain surface, which cannot be detected due to its low amount. It can happen, for example, during the cutting or polishing process of the sample for XRD analysis. Also small quantity of tungsten borides were found with the used analysis detection level, about 0.4% in pure material and 1 vol.% h-BN composite. The W
xB
y tungsten boride is a result of the reaction between boron carbide and tungsten carbide impurities. The identification of the phase in the material microstructure was made by the SEM/EDS analysis and presented in
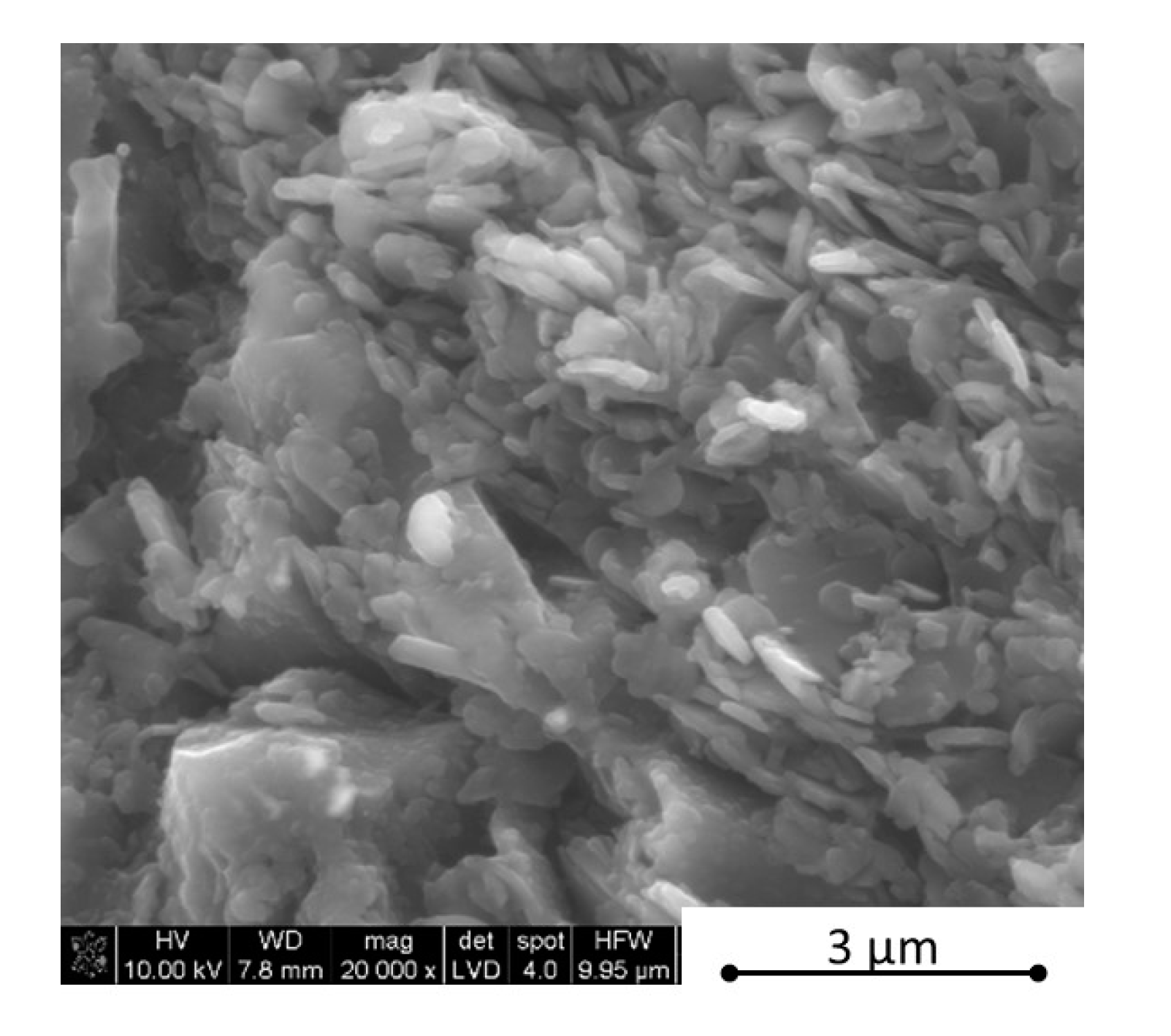
Figure 6. The EDS analysis was made on the h-BN agglomerate in order to get a good element count. The dark grey colour belongs to boron carbide and the light grey to hexagonal boron nitride.
3.3. Thermal Properties and Thermal Stability
The determination of thermal conductivity by the direct thermal diffusivity analysis on a LFA apparatus cannot be made without specific heat data, linear material dimension changes, and density changes by dilatometry. The calculated specific heat on the base of thermodynamic data [
22] and the calculated density on the base of dilatometric measurements are shown in
Figure 12 and
Figure 13. The density changes were calculated on the base of the thermal expansion coefficient measured in various material directions, which is presented in
Figure 14 for the technical linear CTE and in
Figure 15 for the physical linear CTE. The conducted measurements and calculations showed a slight decrease of material density with the increased temperature, which has a negligible influence on the thermal conductivity analysis. For future purposes, the mathematical functions were fitted to the experimental data and collected in
Table 2.
Figure 14 shows a similar behavior of density changes for all of the manufactured polycrystalline materials.
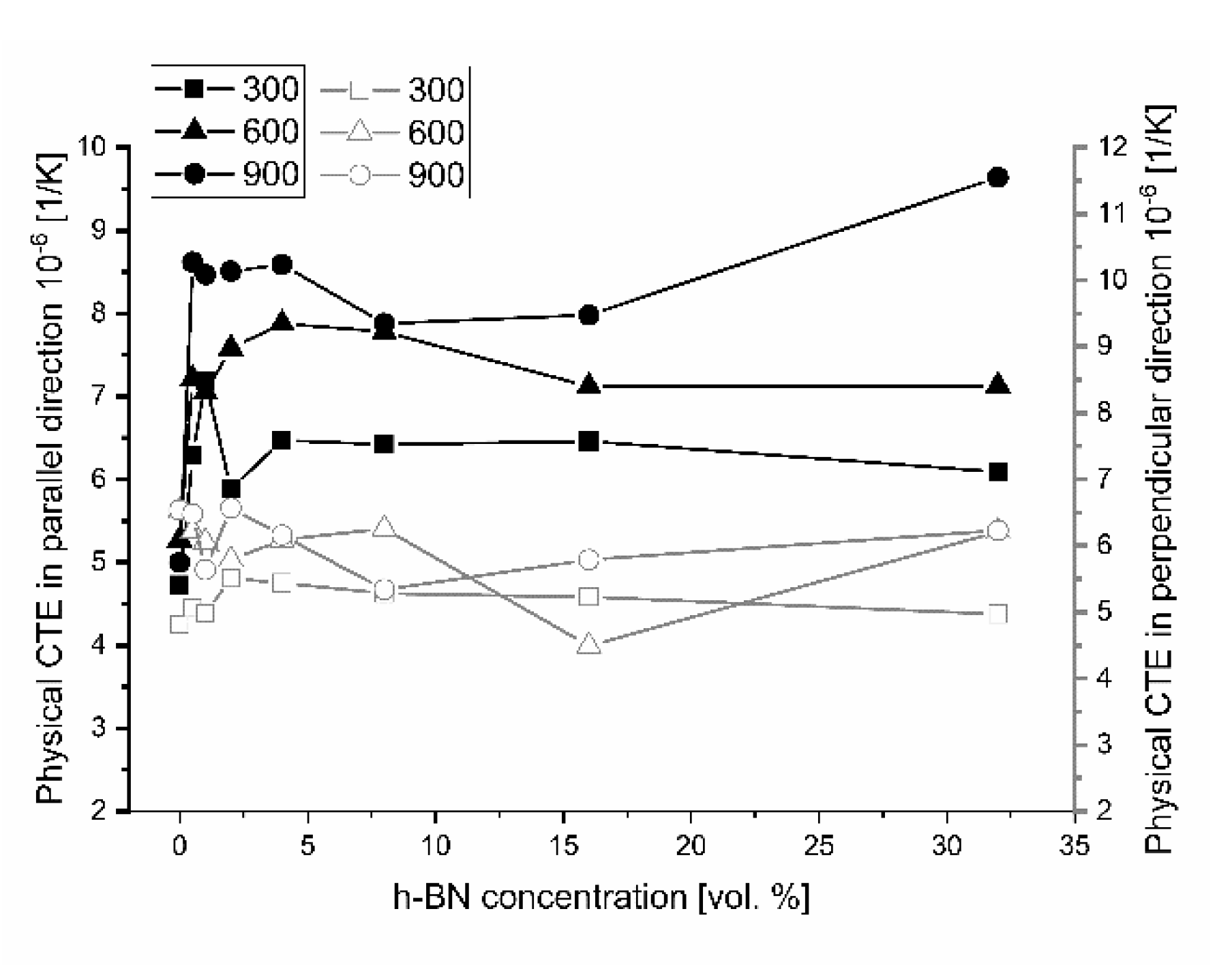
The results of thermal expansion coefficient shown in
Figure 14 and
Figure 15 indicate high anisotropy material linear changes versus material direction. A sudden increase of the technical and physical CTE value in the perpendicular direction begins for 0.5 vol.% of the h-BN content in the composite. A higher addition of h-BN does not significantly change the technical CTE. For the parallel direction to the pressing axis, it stays on the level of 5.4 × 10
−6 1/K for 25–300 °C measurement range, 5.9 × 10
−6 1/K for 25–600 °C measurement range, and 6.3 × 10
−6 1/K for 25–900 °C measurement range. For the perpendicular direction to the pressing axis, it is as follows: 4.2 × 10
−6 1/K for 25–300 °C measurement range, 4.9 × 10
−6 1/K for 25–600 °C measurement range, and 5.3 × 10
−6 1/K for 25–900 °C measurement range. The dilatometric analysis showed the material linear changes fluctuation in the case of physical CTE, which varied in relation to the h-BN content and especially the high temperatures visible for 32 vol.% h-BN at 900 °C. The calculated data of the technical and physical CTE anisotropy are illustrated in
Figure 16 and
Figure 17. For high temperatures, the physical CTE anisotropy stays on the level of 50%. For lower temperatures, it is mostly between 20% and 30% (
Figure 17). For the technical CTE, all the measured temperature ranges are between 20% and 30% (
Figure 16).
In the literature, there is no excessive information concerning the influence of 0–32 vol.% h-BN and the applied pressure of the hot-pressing process on the anisotropy of heat transport versus temperature in the boron carbide composite matrix. This is why the examples of thermal diffusivity data measured as a function of temperature and material direction are presented in
Figure 18 and
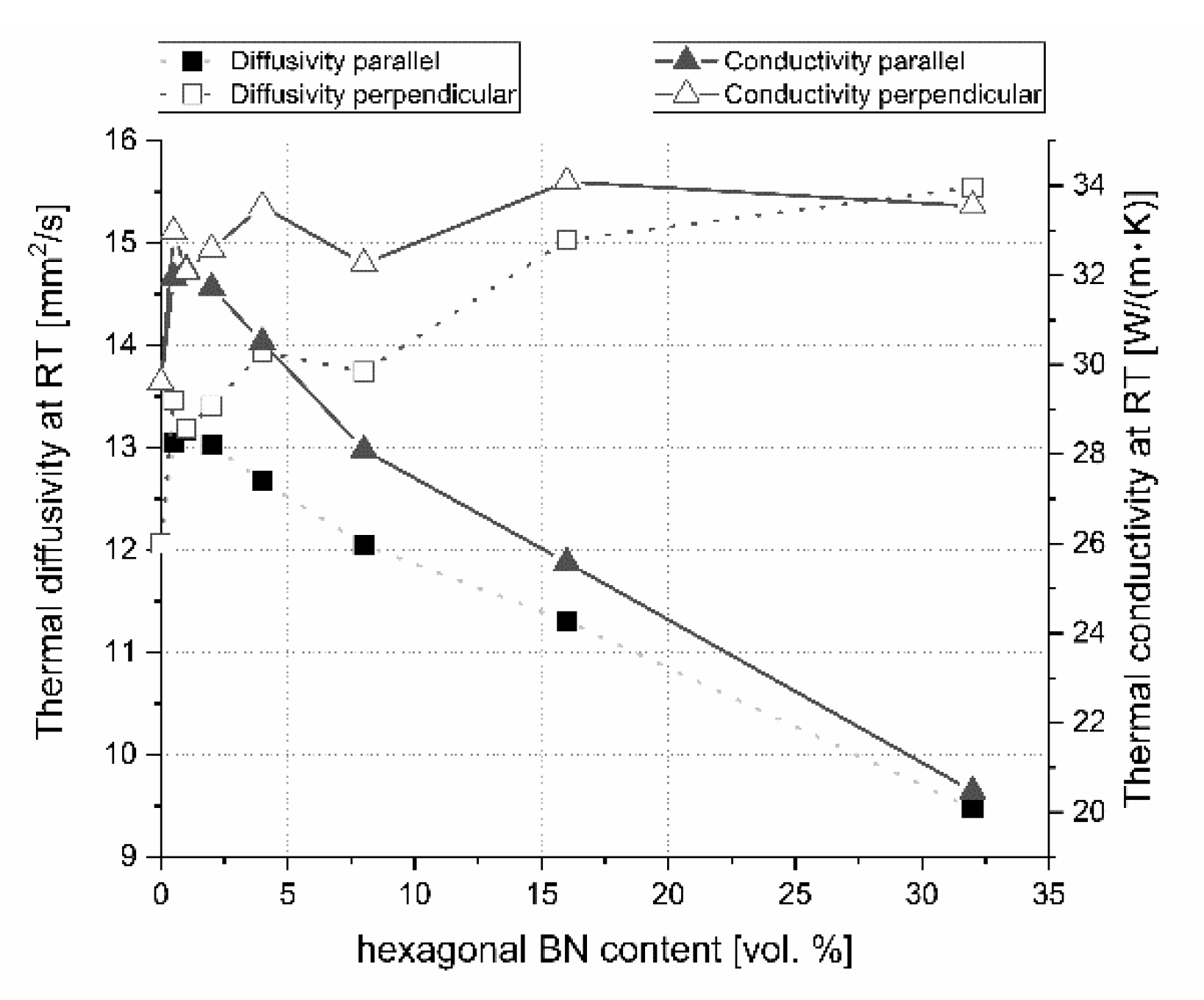
Figure 19. For lower contents of h-BN, there is a slight increase of heat transfer due to the high material densification above 99%, low content of flat defects (interphase boundary), and second 2D high conductive phase and low material anisotropy (
Figure 11). A similar situation occurs when using a low quantity of graphene powder (GNP), where a small amount of 2D particles leads to an increase of thermal conductivity [
23]. In the case of parallel direction to the pressing axis (
Figure 18), at lower temperatures diffusivity decreases with the h-BN addition higher than 4 vol.%. It is caused by a higher material porosity connected mostly with h-BN agglomerates, increasing the concentration of flat defects (fractures in
Figure 9 and
Figure 10) and orientation of 2D particles. The thermal conductivity of a platelet shaped h-BN is above 200 W/(m∙K) in the plane direction and around 2 W/(m∙K) through the plane direction [
24]. The authors of [
24] confirmed that in the plane orientation of h-BN, larger thermal properties than in the perpendicular direction make the 2D particle plane. They also showed that very small particles and large agglomerates tend to orient less, therefore, it can also have an influence on thermal properties, which is its value in our case in perpendicular direction to the pressing axis. The scattering of phonon at boundaries of the studied samples will cause the heat transfer to decrease mainly in perpendicular direction to the pressing axis due to the material anisotropy. For higher temperatures (900 °C) all curves reach values between 2.3 and 2.6 mm
2/s. The results of thermal diffusivity measured in perpendicular direction show higher values for the higher h-BN content in a whole range of temperatures. This parameter decreased with the temperature more slightly than in the parallel direction. At 900 °C, increasing the h-BN content thermal diffusivity changes from 2.4 to 3.8 mm
2/s is visible in
Figure 19.
Concerning the pure nonstoichiometric boron carbide, the electronic transport causing polarons and phonon a play role in thermal conductivity and it was explained in NASA papers [
25]. In our paper, we describe the B
4C/h-BN composites thermal conductivity calculated on the base of thermal diffusivity, material density, and specific heat changes. Results of this parameter are illustrated in
Figure 20 for the parallel measurement direction to the pressing axis (out-of-plane) and in
Figure 21 for the perpendicular measurement direction to the pressing axis (in-plane). Due to specific heat in most of the manufactured composites, thermal conductivity increases slightly in the 25–50 °C range. The increase of thermal conductivity is caused by high conductive 2D particles, high material densification, and lower 2D agglomerates content. For higher temperatures, there is a decrease of this property possibly due to phonon-phonon scattering (
Figure 20 and
Figure 21). The addition of a low amount of h-BN allows obtaining slightly about 7% higher thermal properties in parallel direction to the pressing axis than for the reference sample of 29.6 W/(m·K). In the pressing direction, the higher decrease in thermal properties of this material with a temperature rise is also visible for composites with a higher h-BN content above 8 vol.%, which is marked by empty chart markers (
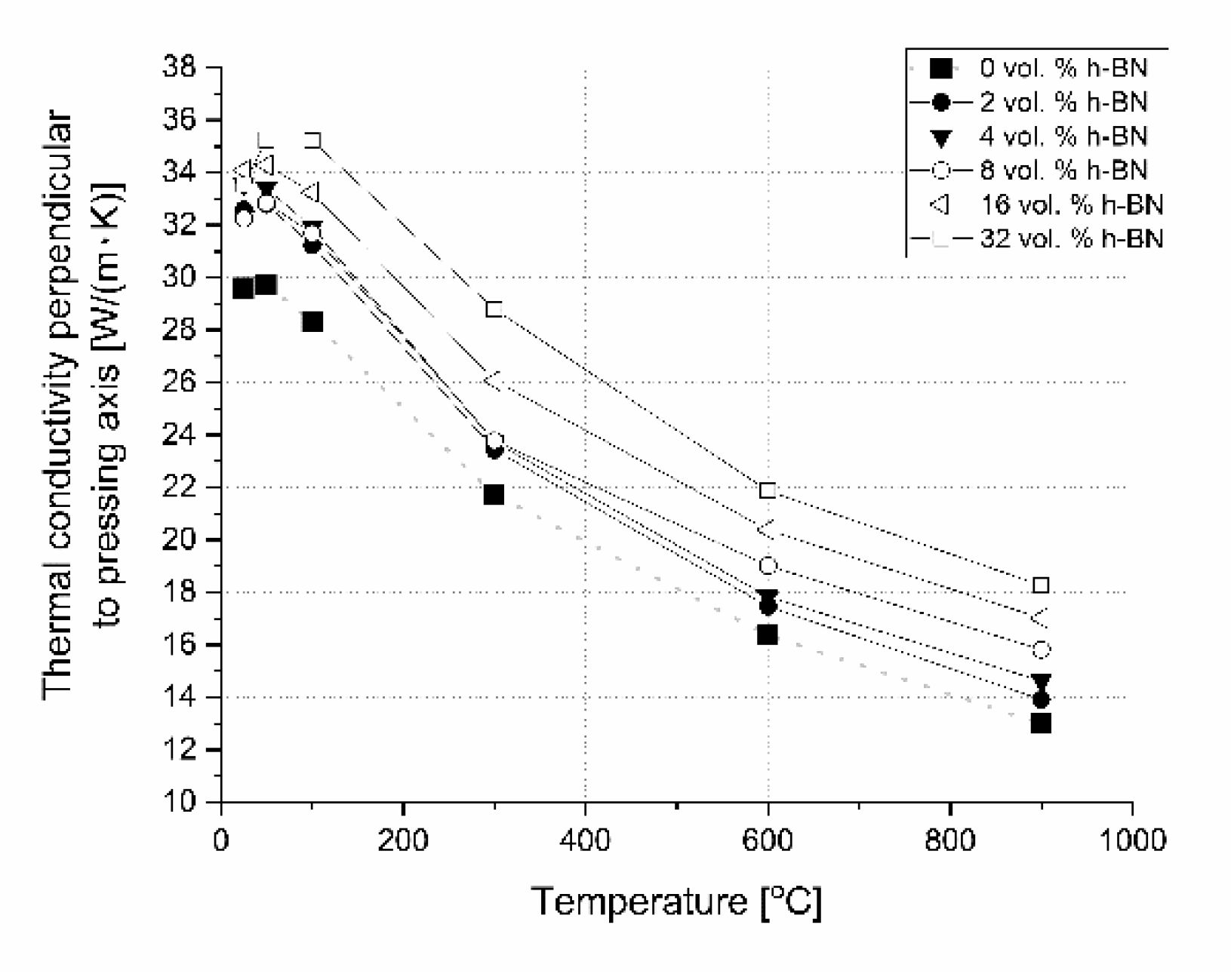
Figure 20). Here, we have additional phonon scattering at boundaries and higher material porosity, which influence the lower heat transfer especially in the pressing direction. The thermal conductivity at 900 °C in this material direction is in the level of 11.3–13.8 W/(m·K)—the highest value is for 2 vol.% h-BN addition and the lowest for 32 vol.% of this phase. Due to the high material densities (
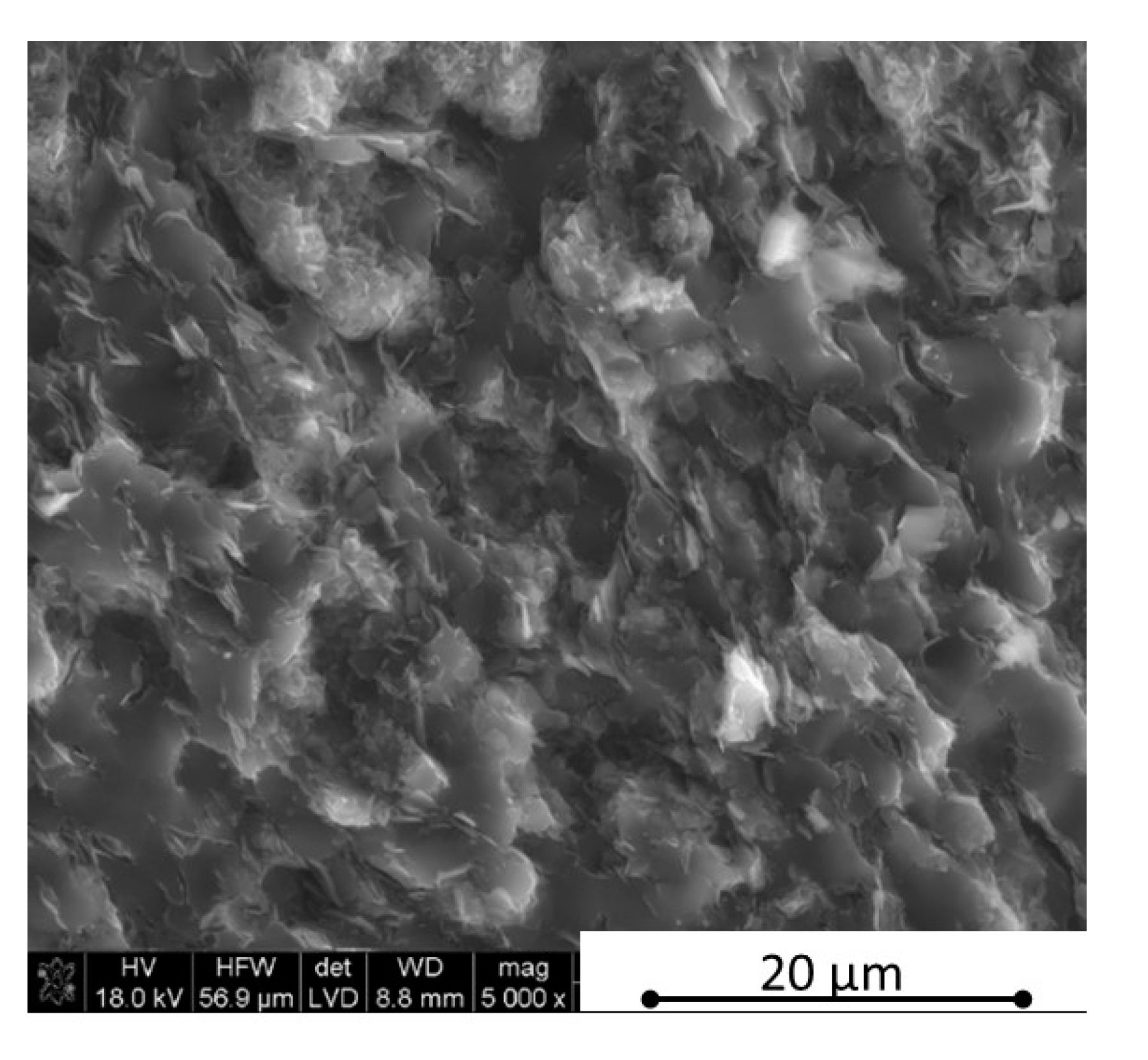
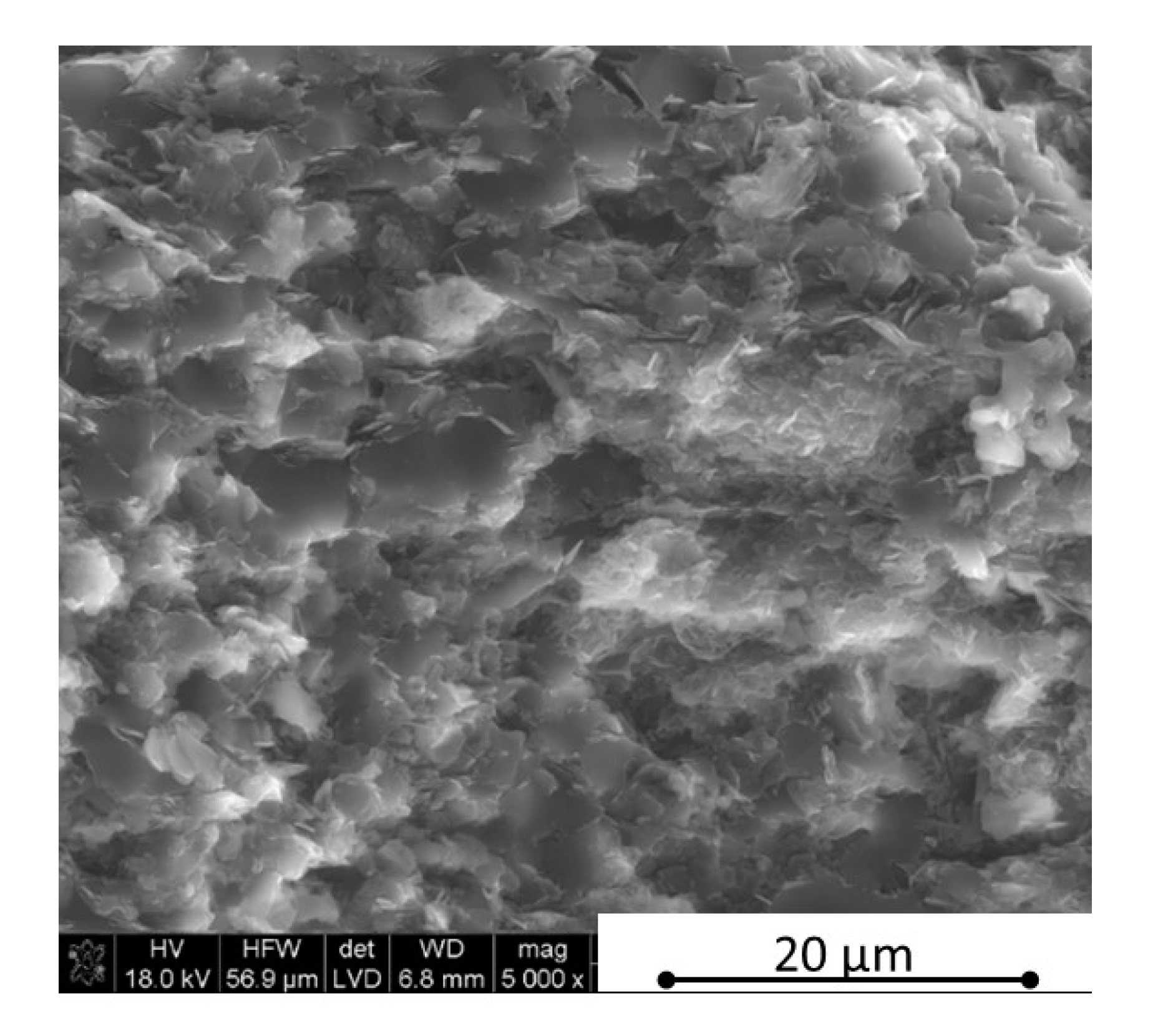
Figure 3), in the case of low h-BN concentrations the decrease of thermal properties in low temperatures can be explained by the finer microstructure following the h-BN addition, therefore, increasing flat defects (usually 2D particles are limiting the grain growth of matrix during the hot-pressing process). The fine material microstructure is presented in
Figure 9 and
Figure 10. For higher temperatures, the phonon-phonon scattering and phase orientation mostly influence thermal conductivity. In thermal conductivity, the thermal barrier existing on the B
4C/h-BN interface is very important, which was noticed by Ruh [
13]. He stated that the mismatch in the elastic modulus of B
4C and h-BN phases will cause phonon scattering. He also discussed that the microcracking or interracial adhesion can form a thermal barrier—which is in our case during the 2D particle orientation. Moreover, he added that Niihara discussed a possible thermal barrier on an example of Si
3N
4-BN composites. In our case, the higher amount of h-BN gives significantly lower thermal properties values, which is not only connected with an h-BN single particles/agglomerates orientation, but also higher porosity (lower density—
Figure 3) existing in h-BN agglomerates. In the case of perpendicular direction, thermal conductivity values increase with the addition of a h-BN solid lubricant, which is visible for low temperatures up to 100 °C (
Figure 21), but for higher temperatures they stay much higher than the reference sample. It can be explained by the h-BN particle orientation in perpendicular direction to the pressing axis (also confirmed by ultrasonic measurements) and agglomerates that the shape is elongated in this direction. The literature says that removing agglomerates or limiting their size can give a higher anisotropy, resulting in larger values of thermal properties in the 2D particle orientation [
24], which can be a task for future research. In this direction, thermal conductivities at 900 °C stay above 13 W/(m K) reaching 18.3 W/(m·K) for the case of the highest h-BN concentration. The decrease of thermal properties in perpendicular direction in the temperature function is less aggressive than in the parallel case. For a future investigation, the fitted mathematical functions of thermal conductivity are given in
Table 3.
The material properties can change as a result of: Oxygen contamination in argon protective gas, high temperature, long material treatment, and laser beam processing during the LFA measurement. This is the reason why two extreme sample compositions of the reference boron carbide polycrystalline material and one with 32 vol.% h-BN were taken under thermal conductivity measurements during heating and cooling steps. Results of this cyclic experiment are presented in
Figure 22 and
Figure 23. The obtained results show that there is almost no influence of laser processing, temperature, and gas purity on the thermal properties of boron carbide based polycrystalline materials with and without the h-BN dispersed phase. The material is thermally stable in a protective atmosphere.
For the onset of laser processing description, the heat transfer properties at room temperature are very important (
Figure 24). During the process, material properties at higher temperatures become much more interesting. The change of thermal diffusivity and conductivity at 900 °C is illustrated in
Figure 25, which is the approximate temperature that stays around the laser-treated place for a short time after the interaction between the laser and sample.
The addition of hexagonal boron nitride will lead to an anisotropy of the thermal conductivity/diffusivity as a result of the dispersed particles and pores orientation in the material microstructure. For room temperature (RT) conditions in the pressing axis direction, an increase of h-BN particles will cause a strong decrease in thermal properties especially in the case of its higher h-BN content. A different situation occurs in the perpendicular direction where the heat transfer is faster. A similar situation can be met in high temperatures. There is a large thermal properties anisotropy at low and high temperature which increased with the higher h-BN content in the composites, as clearly visible in
Figure 24 and
Figure 25. This situation can have an influence on the material treatment, which is presented in the next part of this paper.
3.4. Subtractive Laser Processing
There is some information in the literature about ceramic laser machining mostly in a pulse mode and also in a continuous wave mode. The available example of an 8 mm thick ceramic tails laser cutting was shown by Black and Chua [
26] in 1997. Their results confirmed that the pulse and continuous (CW) wave condition needs the multi-pass process, while in the case of CW, it was 60 passes, which is around 150 µm depth of a single cut. They were working with both the laser mode and CO
2 laser. For alumina it is 200 to 400 µm, and for a good quality silicon carbide it is up to 300 µm in the laser pulse mode using the Yb:KGW laser (city, country) [
27]. The laser ceramic treatment depth was show also in publication [
28]. However, there is no information concerning the laser cutting of anisotropic ceramic composites. In this study, the laser treated materials were taken into the laser cutting process using a standard cutting laser head, argon flow, and continuous wave working mode. The process was done and analyzed in two different material directions and for the material roughness (R
a) it was much lower and much higher than the wave length. For grinded materials with R
a around 2.69 µm, the observations are shown in
Figure 26,
Figure 27,
Figure 28,
Figure 29,
Figure 30 and
Figure 31 and for polished composites with R
a around 0.56 µm, the observations are presented in
Figure 32,
Figure 33,
Figure 34,
Figure 35,
Figure 36 and
Figure 37. A too low material roughness will lead to a light reflection. In this case, it gives a slightly better material treatment than in the case of a minimum of 2 times higher roughness towards the used laser wave length. In the case of a too high material (R
a), some energy absorbed by surface unevenness tends to generate some heat that is not involved in the material processing. Therefore, a too rough surface makes the effect of laser processing slightly lower in the perpendicular material direction than in the case of polished surface for a high amount of h-BN. For low concentrations of introduced hexagonal boron nitrides, there are visible cracks (
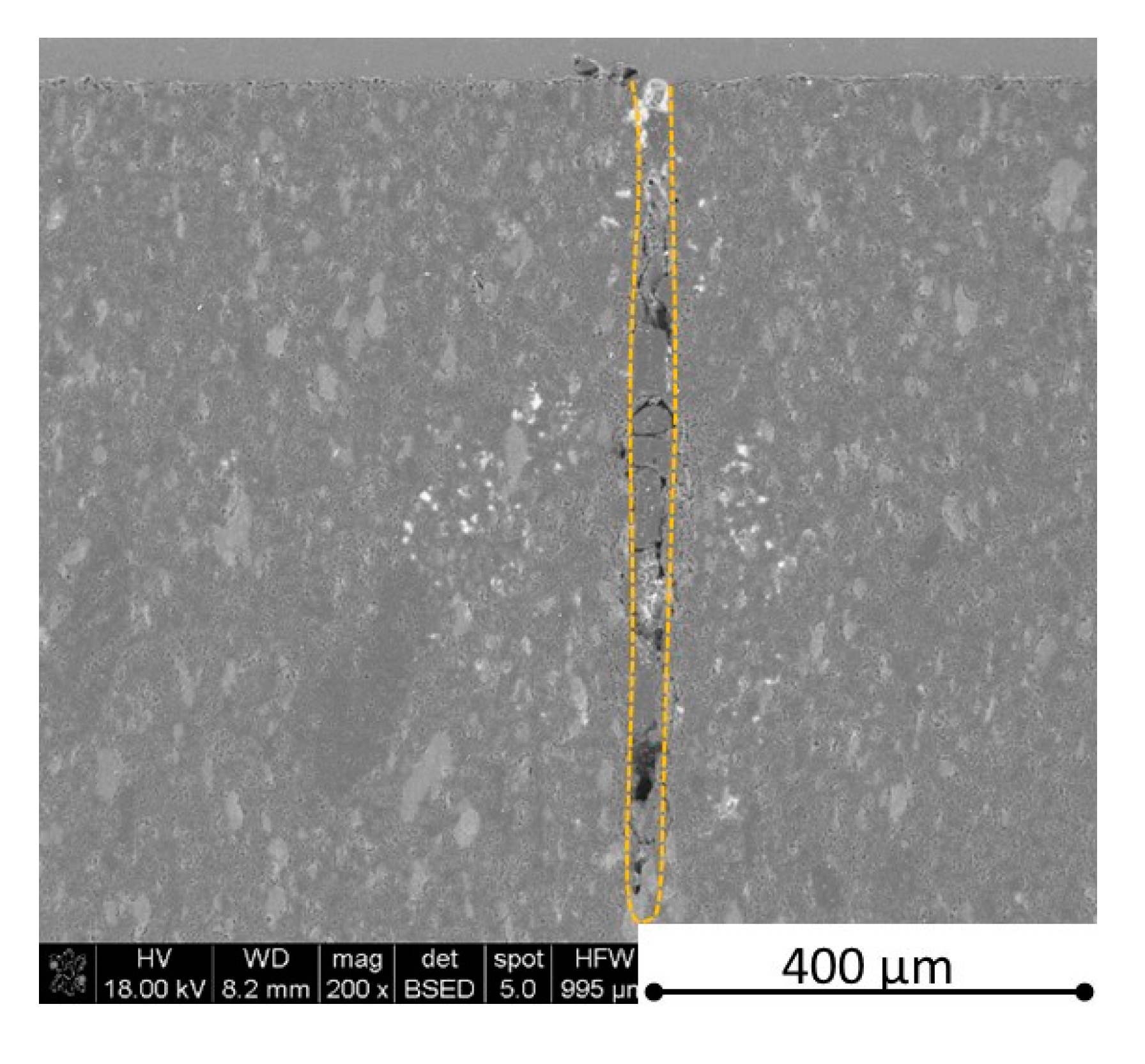
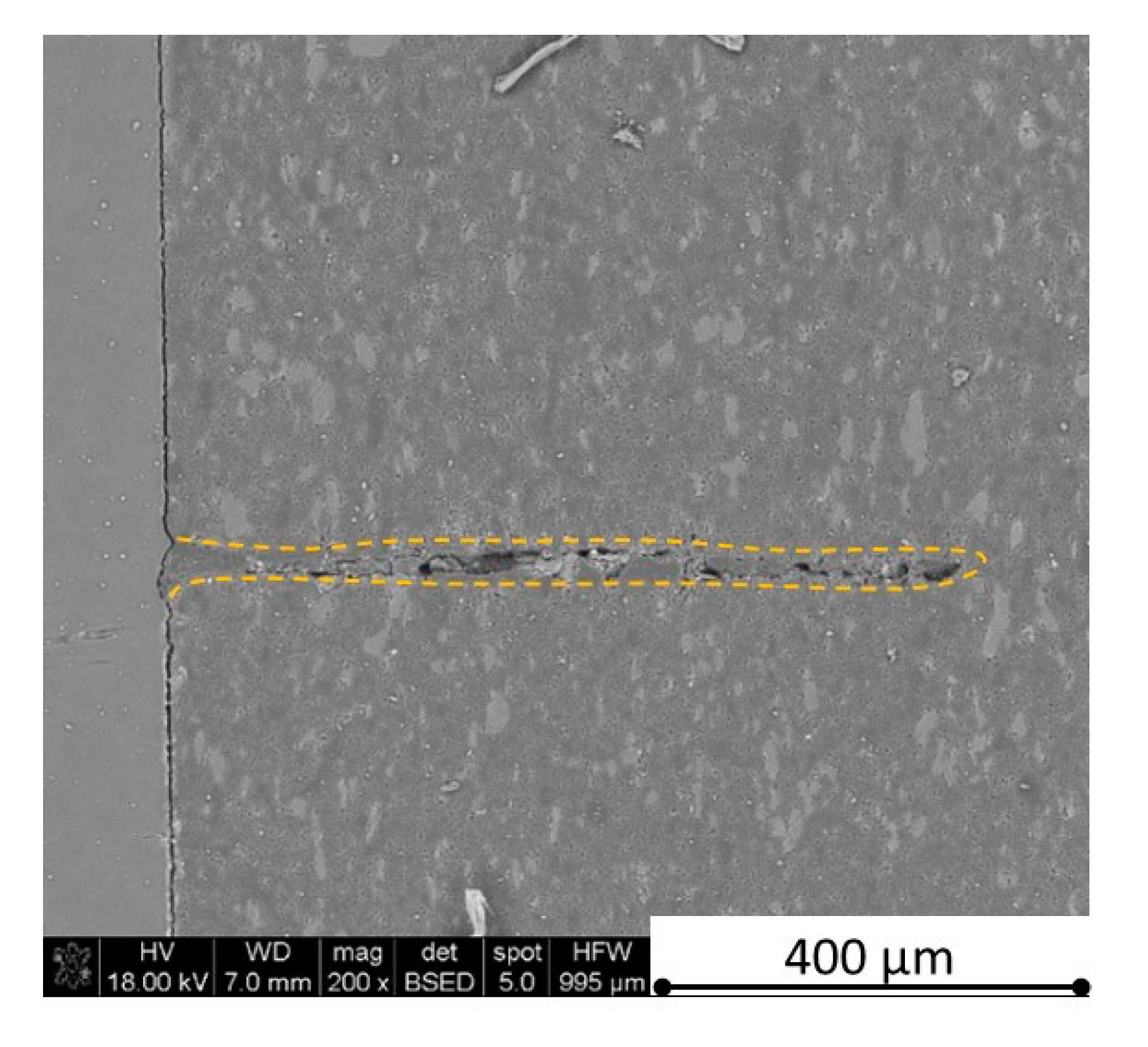
Figure 28,
Figure 29,
Figure 31 and
Figure 35 ) or materials which are almost destroyed (
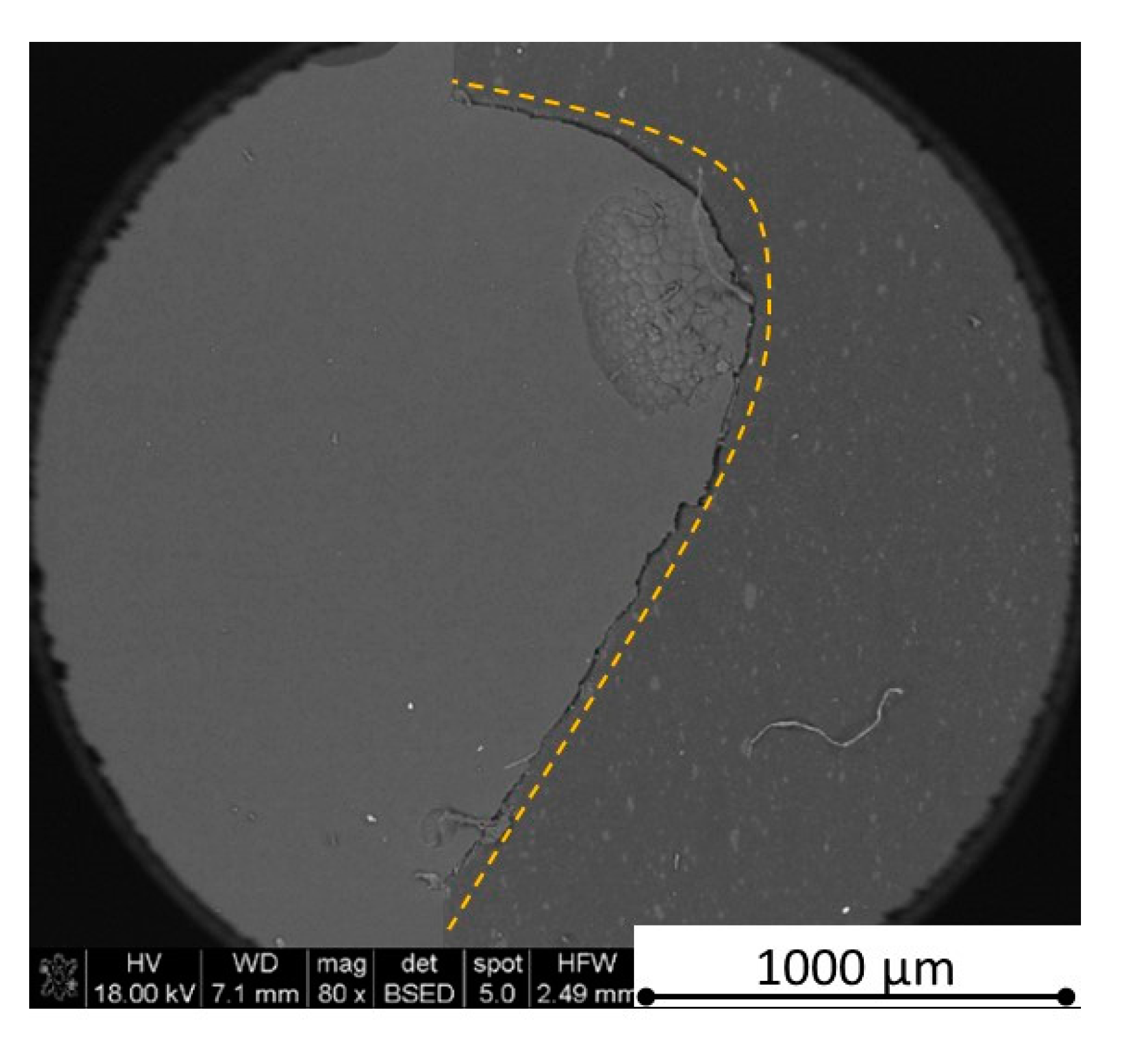
Figure 27 and
Figure 34). Lots of cracks appear in the remelted areas (
Figure 28). Cracks and material destruction during laser processing can appear as an effect of a large CTE difference between boron carbide and hexagonal boron nitride single phases (
Figure 14,
Figure 15,
Figure 16 and
Figure 17). From the authors’ experience, similar cracks can possibly appear as an effect of h-BN decomposition and released nitrogen overpressure or in the case of residual water adsorbed in h-BN agglomerates.
The increase in h-BN content above 8 vol.% causes the change of the laser cut shape, which is presented in
Figure 30 and
Figure 31. For higher h-BN concentrations, the thermal anisotropy and h-BN agglomerates of the located porosity have a significant influence in laser processing. In perpendicular direction to the pressing axis (in-plane), the thermal diffusivity/conductivity is about 60% higher than in the pressing axis direction. It causes a heat transfer limitation in the out-of-plane direction and results in only 250–320 microns of processing depth for the rough surface sample. In this case, the ablation width is about 100 microns due to the heat transfer to the zone sides (
Figure 32). There are no large cracks most likely due to the higher material porosity (
Figure 3). Moreover, in the case of the low roughness polished sample with 32 vol.% h-BN, narrow, crack free, and deep laser cuts were observed (
Figure 36 and
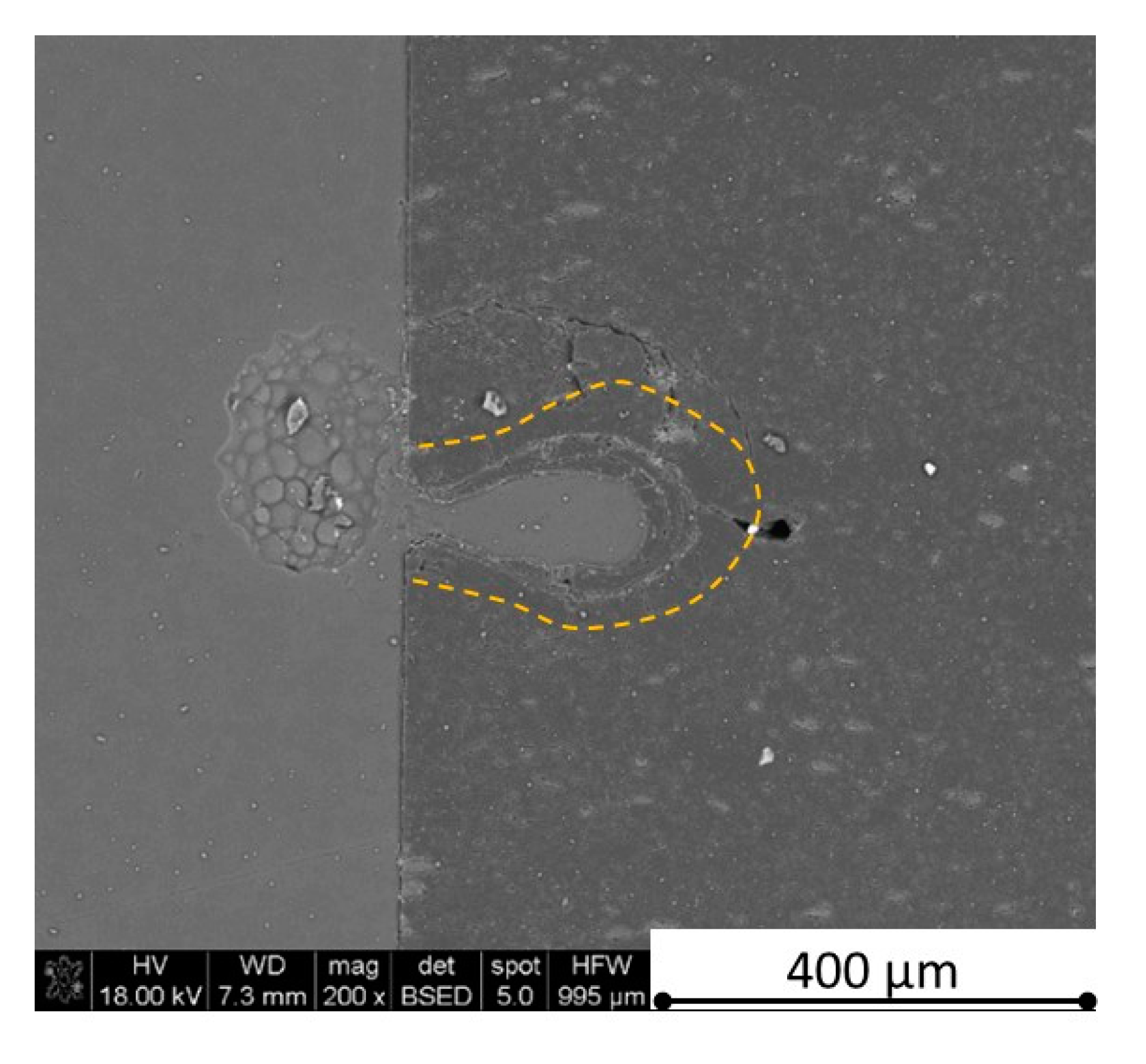
Figure 37). Due to the argon flow some material stays inside the cut, but it can be removed by use of short pulse laser processing and a vacuum or under pressure conditions. In most of the laser treatment cases, the anisotropy of thermal properties leads to deeper and narrower cuts. For a low concentration of h-BN, cuts have the shape of a droplet.
The anisotropy of thermal properties, h-BN content, and material roughness also has an influence on the width end depth of the material cut. Comparing samples containing 0, 8, and 32 vol.% of h-BN in the case of the material with high surface roughness which is higher than laser wavelength, the cut depth changes in following way: in parallel direction to pressing axis: 320 µm for reference sample, 260 µm for 8 vol.% h-BN and 310 µm for 32 vol.%. Therefore, in this direction, the cut depth is typical as for the ceramic materials. It changes in perpendicular direction: 360 µm for reference, 400 µm for 8 vol.% h-BN, and 720 µm for 32 vol.% h-BN. The width of the cut in the case of pure boron carbide can reach 200 microns in the case of material destruction. For 8% h-BN, the width of the cut is around 100 microns in the pressing direction and 70 microns in the perpendicular direction. For 32%, the width of the cut is around 145 microns in the pressing direction and 40 microns in the perpendicular direction. By analyzing the dimension of the laser process ceramics with a surface roughness below the beam length, the reference sample shows the cut around 300 µm in both material directions. For the high polished surface in the case of 8 vol.% h-BN addition, the measured depth is around 230 µm and the width is 75 microns in perpendicular direction to the pressing axis, therefore, it is similar to the pressing direction. An interesting situation was noticed in the case of 32 vol.% of 2D particles. In this case, for the well-polished surface the depth is 150 µm higher than the perpendicular direction (610 µm). The difference is also visible in the width, where 60 µm is for the pressing direction and 40 µm for the perpendicular direction to the pressing axis. Here, it can be suggested that the reflectivity and energy absorption rate should also be studied in the future.
Concerning the chemical phenomena taking place during laser processing, not only the decomposition of h-BN (about 2500 °C) should be taken under attention, but also the melting point of pure stoichiometric boron carbide (2450 °C) and eutectics in the system B
4C-C (2375 °C) and B
4C-B (2075 °C) [
29]. The confirmation of liquid phase and boron carbide formation in the cut tip is observed in
Figure 32 and revealed by the EDS analysis showing only boron and carbon elements. The recrystallized phase is also visible in
Figure 38.