Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Samples Preparation and Sintering Treatments
2.3. Characterizations of Raw Materials
2.4. Samples Characterizations after Sintering Treatment
3. Results and Discussion
3.1. Raw Materials Characterizations
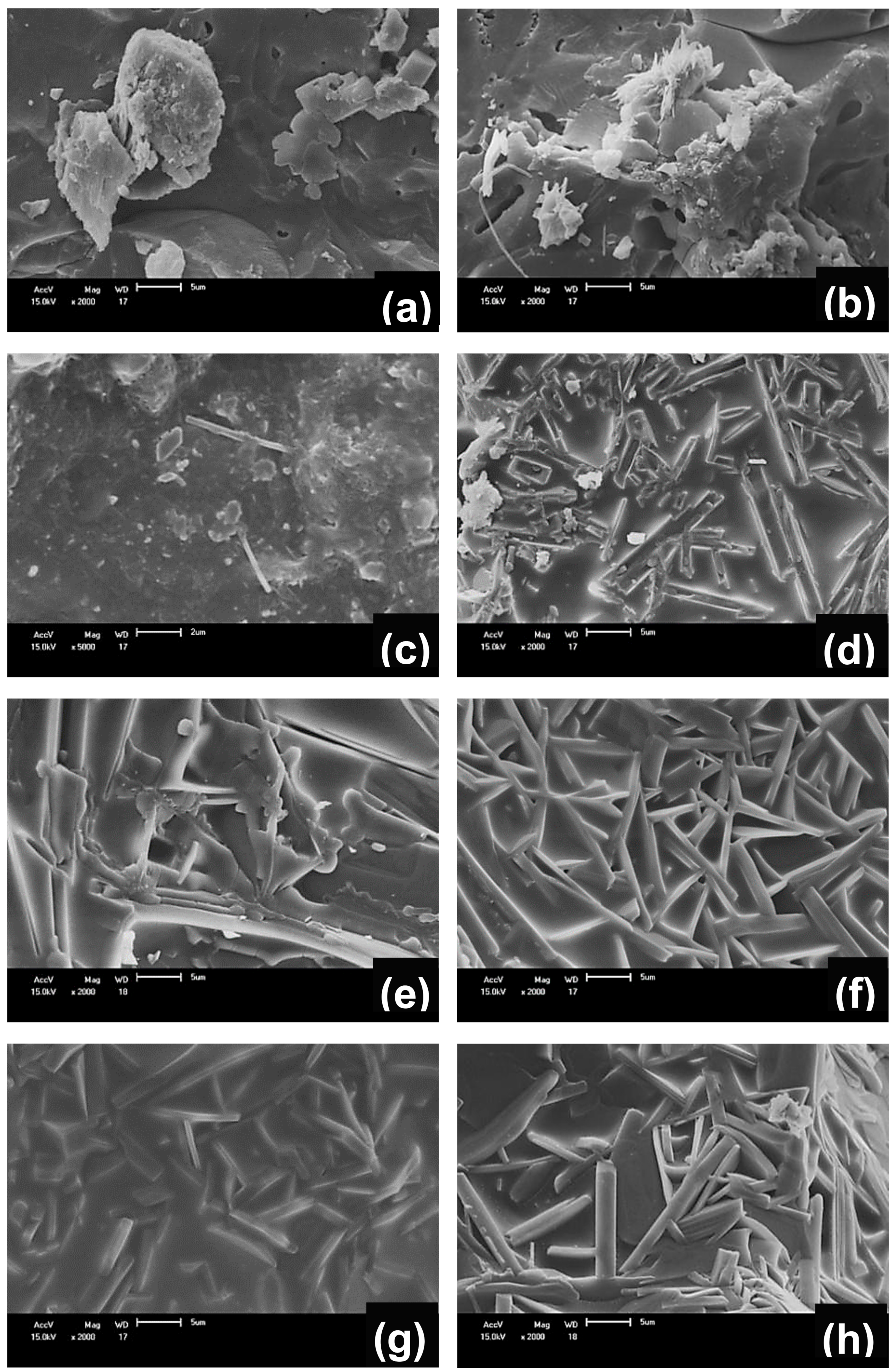
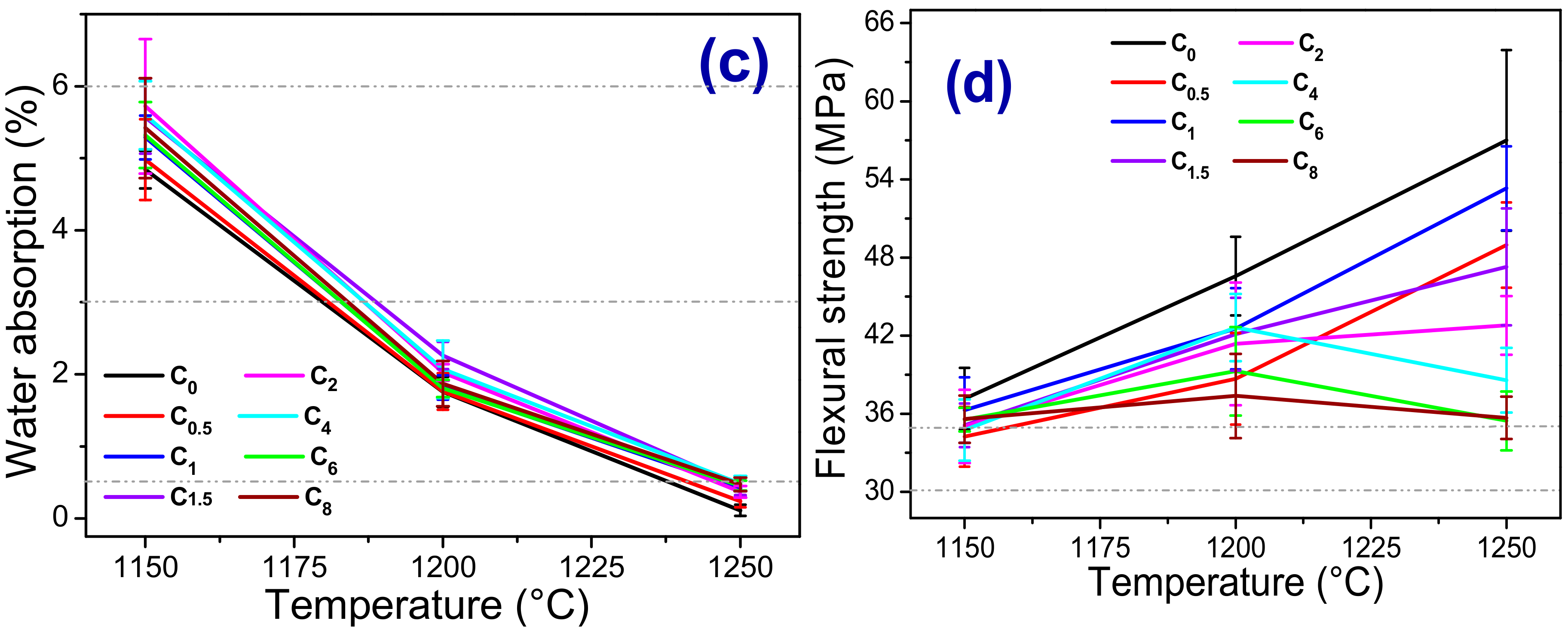
3.2. Crystalline Phases, Microstructural and Physicomechanical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hällström, L.P.B.; Alakangas, L.; Martinsson, O. Scheelite weathering and tungsten (W) mobility in historical oxidic-sulfidic skarn tailings at Yxsjöberg, Sweden. Environ. Sci. Pollut. Res. 2020, 27, 6180–6192. [Google Scholar] [CrossRef]
- Leal-Ayala, D.R.; Allwood, J.M.; Petavratzi, E.; Brown, T.J.; Gunn, G. Mapping the global flow of tungsten to identify key material efficiency and supply security opportunities. Resour. Conserv. Recycl. 2015, 103, 19–28. [Google Scholar] [CrossRef]
- Gong, L.; Chen, S.; Zhang, W.; Xie, F.; Xie, W.; Chen, J. Photoconductive characteristic in individual WO 3-x nanowire. J. Nanosci. Nanotechnol. 2011, 11, 10976–10980. [Google Scholar] [CrossRef]
- Vemuri, R.S.; Engelhard, M.H.; Ramana, C.V. Correlation between surface chemistry, density, and band gap in nanocrystalline WO 3 thin films. ACS Appl. Mater. Interfaces 2012, 4, 1371–1377. [Google Scholar] [CrossRef]
- Leffler, P.E.; Kazantzis, G. Tungsten. Handb. Toxicol. Met. Fourth Ed. 2015, 1, 1297–1306. [Google Scholar] [CrossRef]
- Beaudoin, G.; Pitre, D. Stable isotope geochemistry of the Archean Val-d’Or (Canada) orogenic gold vein field. Miner. Depos. 2005, 40, 59–75. [Google Scholar] [CrossRef]
- Mueller, A.G. The Savage Lode magnesian skarn in the Marvel Loch gold–silver mine, Southern Cross greenstone belt, Western Australia. Part 1: Structural setting, petrography, and geochemistry. Can. J. Earth Sci. 1991, 28, 659–685. [Google Scholar]
- Graupner, T.; Niedermann, S.; Rhede, D.; Kempe, U.; Seltmann, R.; Williams, C.T.; Klemd, R. Multiple sources for mineralizing fluids in the Charmitan gold(-tungsten) mineralization (Uzbekistan). Miner. Depos. 2010, 45, 667–682. [Google Scholar] [CrossRef]
- Medeiros, B.A.; Neves, G.A.; Barbosa, N.P.; Menezes, R.R.; Ferreira, H.C. Mechanical properties of mortar produced with the replacement of natural sand by scheelite residue. Ceramica 2019, 65, 443–451. [Google Scholar] [CrossRef]
- De Medeiros, P.S.S.; Lira, H.D.L.; Rodriguez, M.A.; Menezes, R.R.; Neves, G.D.A.; Santana, L.N.D.L. Incorporation of quartzite waste in mixtures used to prepare sanitary ware. J. Mater. Res. Technol. 2019, 8, 2148–2156. [Google Scholar] [CrossRef]
- Da Silva, V.J.; de Almeida, E.P.; Gonçalves, W.P.; da Nóbrega, R.B.; de Araújo Neves, G.; de Lucena Lira, H.; Menezes, R.R.; de Lima Santana, L.N. Mineralogical and dielectric properties of mullite and cordierite ceramics produced using wastes. Ceram. Int. 2019, 45, 4692–4699. [Google Scholar] [CrossRef]
- Souza, M.H.O.; Neta, M.L.X.F.; Barros, S.V.A.; Dantas, G.C.B.; Neves, G.A.; Cartaxo, J.M.; Pimentel, P.M. Influência do tipo de cura no comportamento mecânico de argamassas confeccionadas com areia de scheelita. Rev. Eletrônica De Mater. E Process. 2019, 14, 91–94. [Google Scholar]
- ISO 13006:2018 Ceramic Tiles—Definitions, Classification, Characteristics and Marking, ISO/TC 189 Ceramic Tile; International Organization for Standardization: Geneva, Switzerland, 2018.
- Fernandes, J.V.; Rodrigues, A.M.; Menezes, R.R.; Neves, G.A. Adsorption of Anionic Dye on the Acid-Functionalized Bentonite. Materials 2020, 13, 3600. [Google Scholar] [CrossRef]
- Fischer, J.; Stawarczyk, B.; Hämmerle, C.H.F. Flexural strength of veneering ceramics for zirconia. J. Dent. 2008, 36, 316–321. [Google Scholar] [CrossRef]
- Ma, B.; Li, Y.; Liu, G.; Liang, D. Preparation and properties of Al2O3-MgAl2O4 ceramic foams. Ceram. Int. 2015, 41, 3237–3244. [Google Scholar] [CrossRef]
- Pereira da Costa, F.; Rodrigues da Silva Morais, C.; Rodrigues, A.M. Sustainable glass-ceramic foams manufactured from waste glass bottles and bentonite. Ceram. Int. 2020, 46, 17957–17961. [Google Scholar] [CrossRef]
- Xu, X.; Lao, X.; Wu, J.; Zhang, Y.; Xu, X.; Li, K. Microstructural evolution, phase transformation, and variations in physical properties of coal series kaolin powder compact during firing. Appl. Clay Sci. 2015, 115, 76–86. [Google Scholar] [CrossRef]
- Alves, H.P.A.; Silva, J.B.; Campos, L.F.A.; Torres, S.M.; Dutra, R.P.S.; Macedo, D.A. Preparation of mullite based ceramics from clay–kaolin waste mixtures. Ceram. Int. 2016, 42, 19086–19090. [Google Scholar] [CrossRef]
- Mahmoudi, S.; Bennour, A.; Meguebli, A.; Srasra, E.; Zargouni, F. Characterization and traditional ceramic application of clays from the Douiret region in South Tunisia. Appl. Clay Sci. 2016, 127, 78–87. [Google Scholar] [CrossRef]
- De Aza, A.H.; Turrillas, X.; Rodriguez, M.A.; Duran, T.; Pena, P. Time-resolved powder neutron diffraction study of the phase transformation sequence of kaolinite to mullite. J. Eur. Ceram. Soc. 2014, 34, 1409–1421. [Google Scholar] [CrossRef]
- Nzeukou, A.; Fagel, N.; Njoya, A.; Beyala Kamgangde, V.; Eko Medjod, R.; Chinje Meloc, U. Mineralogy and physico-chemical properties of alluvial clays from Sanaga valley (Center, Cameroon): Suitability for ceramic application. Appl. Clay Sci. 2013, 83, 238–243. [Google Scholar] [CrossRef]
- Chen, C.Y.; Tuan, W.H. The processing of kaolin powder compact. Ceram. Int. 2001, 27, 795–800. [Google Scholar] [CrossRef]
- Costa, F.P.d.; Morais, C.R.d.S.; Pinto, H.C.; Rodrigues, A.M. Microstructure and physico-mechanical properties of Al2O3-doped sustainable glass-ceramic foams. Mater. Chem. Phys. 2020, 256, 123612. [Google Scholar] [CrossRef]
- Issaoui, M.; Limousy, L.; Lebeau, B.; Bouaziz, J.; Fourati, M. Design and characterization of flat membrane supports elaborated from kaolin and aluminum powders. C. R. Chim. 2016, 19, 496–504. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, L.; Luo, N. Elastic modulus evolution of rocks under heating–cooling cycles. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Ismaiel Saraya, M.E.-S.; Rokbaa, H.H.A.E.-L. Formation and Stabilization of Vaterite Calcium Carbonate by Using Natural Polysaccharide. Adv. Nanopart. 2017, 6, 158–182. [Google Scholar] [CrossRef]
- Da Silva, V.J.; da Silva, M.F.; Gonçalves, W.P.; de Menezes, R.R.; Araújo Neves, G.d.; Lucena Lira, H.d.; de Lima Santana, L.N. Porous mullite blocks with compositions containing kaolin and alumina waste. Ceram. Int. 2016, 42, 15471–15478. [Google Scholar] [CrossRef]
- Moulton, B.J.A.; Rodrigues, A.M.; Sampaio, D.V.; Silva, L.D.; Cunha, T.R.; Zanotto, E.D.; Pizani, P.S. The origin of the unusual DSC peaks of supercooled barium disilicate liquid. CrystEngComm 2019, 21, 2768–2778. [Google Scholar] [CrossRef]
- Santana, L.N.L.; Gomes, J.; Menezes, R.R.; Neves, G.A.; Lira, H.L.; Segadães, A.M. Microstructure development in clays upon heat treatment: Kinetics and equilibrium. Appl. Clay Sci. 2017, 135, 325–332. [Google Scholar] [CrossRef]
- Chin, C.L.; Ahmad, Z.A.; Sow, S.S. Relationship between the thermal behaviour of the clays and their mineralogical and chemical composition: Example of Ipoh, Kuala Rompin and Mersing (Malaysia). Appl. Clay Sci. 2017, 143, 327–335. [Google Scholar] [CrossRef]
- Tai, W.P.; Kimura, K.; Jinnai, K. A new approach to anorthite porcelain bodies using nonplastic raw materials. J. Eur. Ceram. Soc. 2002, 22, 463–470. [Google Scholar] [CrossRef]
- Pal, M.; Das, S.; Das, S.K. Anorthite porcelain: Synthesis, phase and microstructural evolution. Bull. Mater. Sci. 2015, 38, 551–555. [Google Scholar] [CrossRef]
- Silva, R.H.L.; Neves, G.A.; Ferreira, H.C.; Santana, L.N.L.; Nóbrega, A.C.V.; Menezes, R.R. Use of diopside in ceramic masses for sanitary ware. Cerâmica 2019, 65, 1–12. [Google Scholar] [CrossRef]
- Martín-Márquez, J.; Rincón, J.M.; Romero, M. Mullite development on firing in porcelain stoneware bodies. J. Eur. Ceram. Soc. 2010, 30, 1599–1607. [Google Scholar] [CrossRef]
- Kumar, P.H.; Srivastava, A.; Kumar, V.; Singh, V.K. Implementation of industrial waste ferrochrome slag in conventional and low cement castables: Effect of calcined alumina. J. Asian Ceram. Soc. 2014, 2, 371–379. [Google Scholar] [CrossRef]
- Piva, D.H.; Piva, R.H.; Venturini, J.; Morelli, M.R.; Bergmann, C.P. Microestrutura, fases cristalinas e propriedades elétricas de porcelanas aluminosas contendo diferentes concentrações de Fe2O3 sinterizadas em atmosfera redutora e oxidante. Ceramica 2015, 61, 374–382. [Google Scholar] [CrossRef][Green Version]
- Bennour, A.; Mahmoudi, S.; Srasra, E.; Boussen, S.; Htira, N. Composition, firing behavior and ceramic properties of the Sejnène clays (Northwest Tunisia). Appl. Clay Sci. 2015, 115, 30–38. [Google Scholar] [CrossRef]
- ISO 10545-3:2018 Ceramic Tiles—Part 3: Determination of Water Absorption, Apparent Porosity, Apparent Relative Density and Bulk Density; International Organization for Standardization: Geneva, Switzerland, 2018.
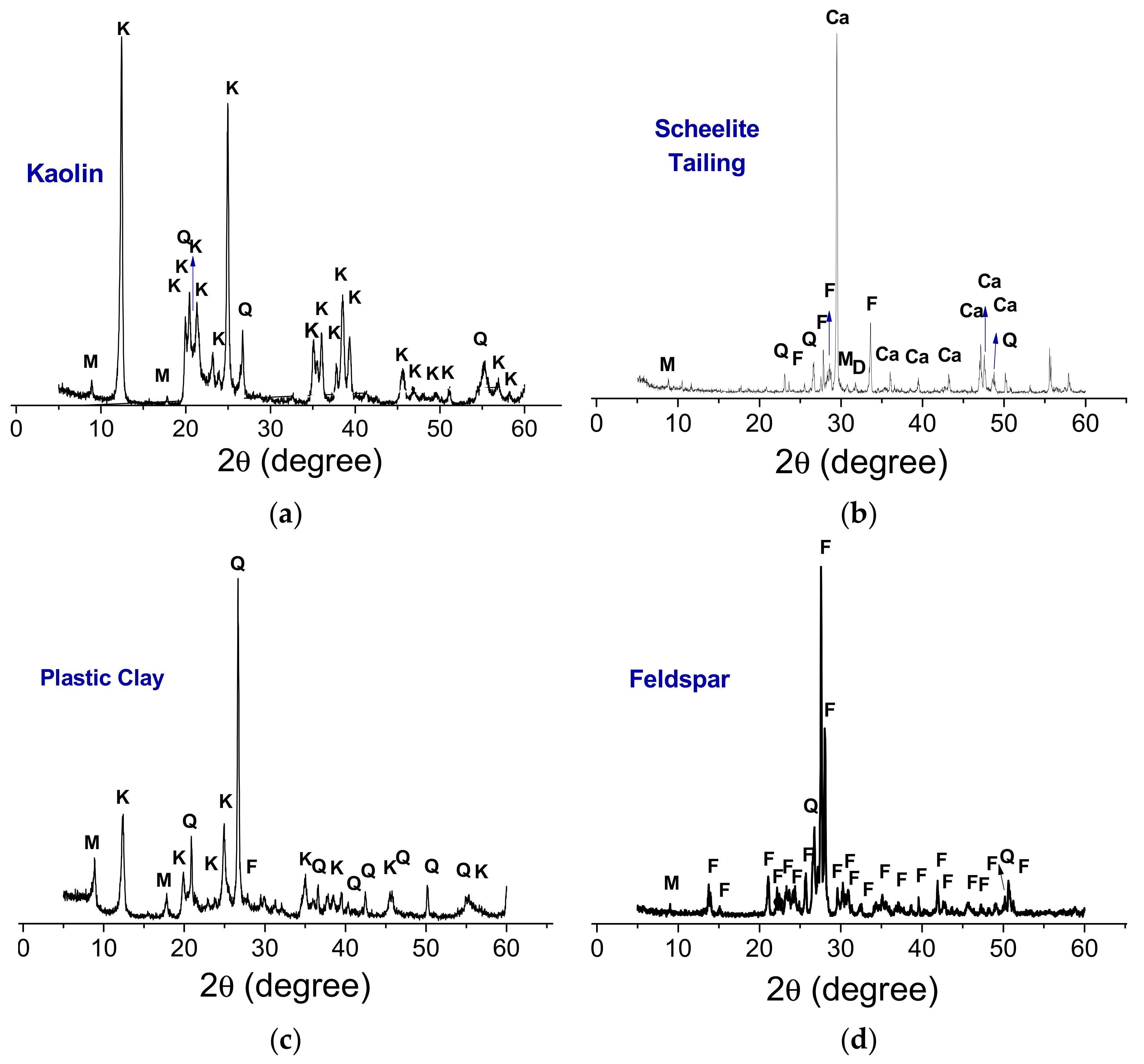
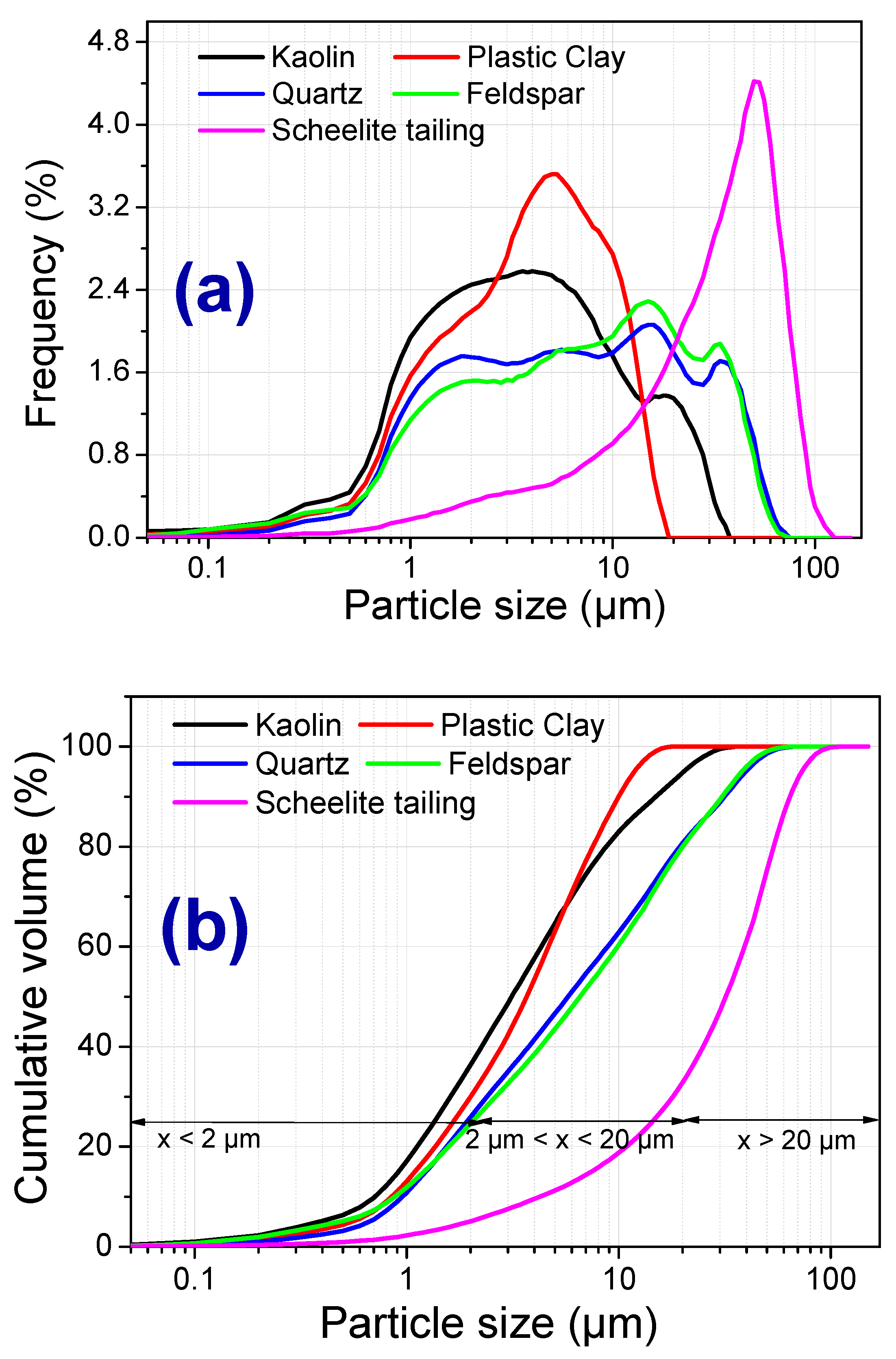

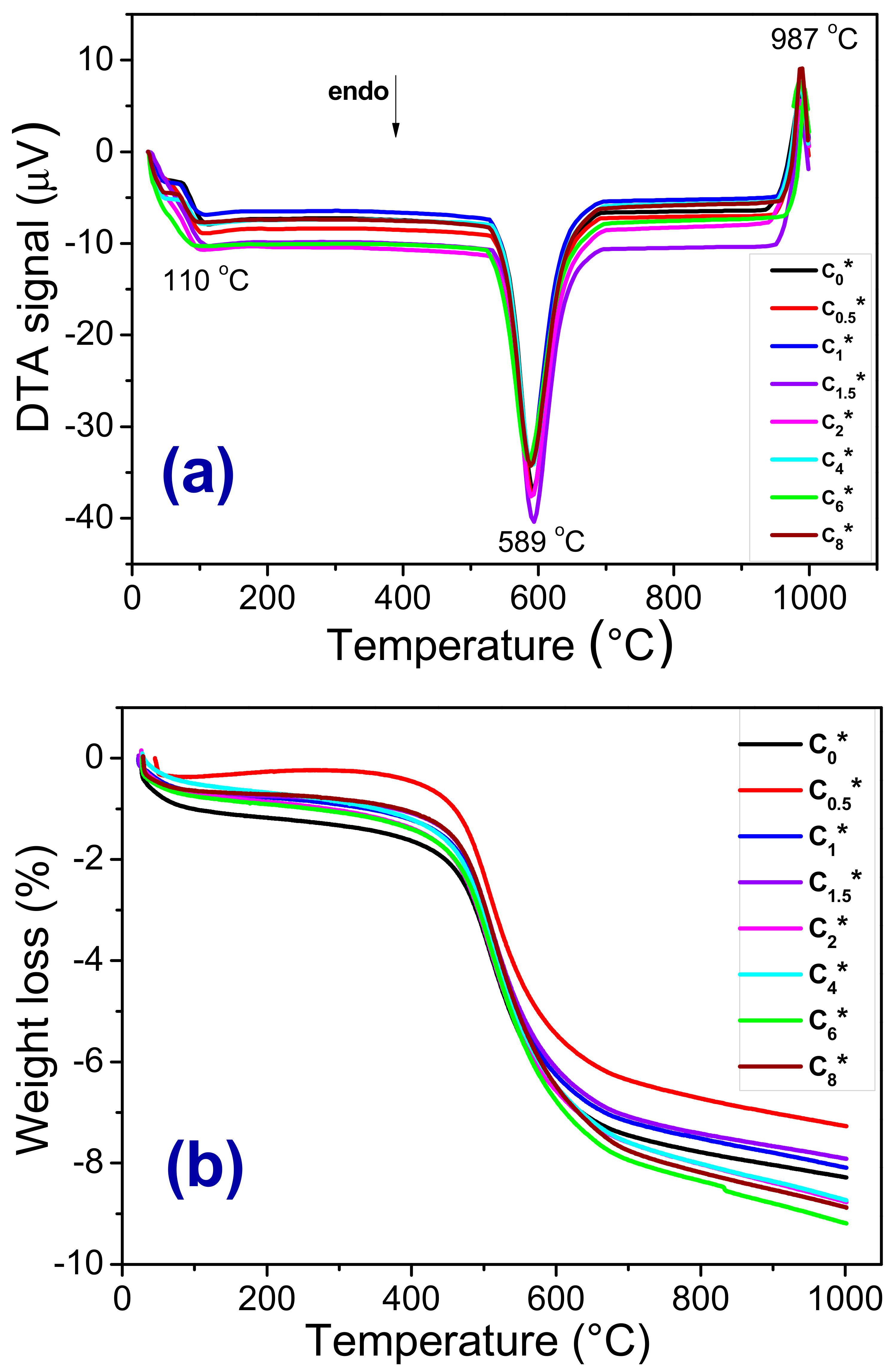
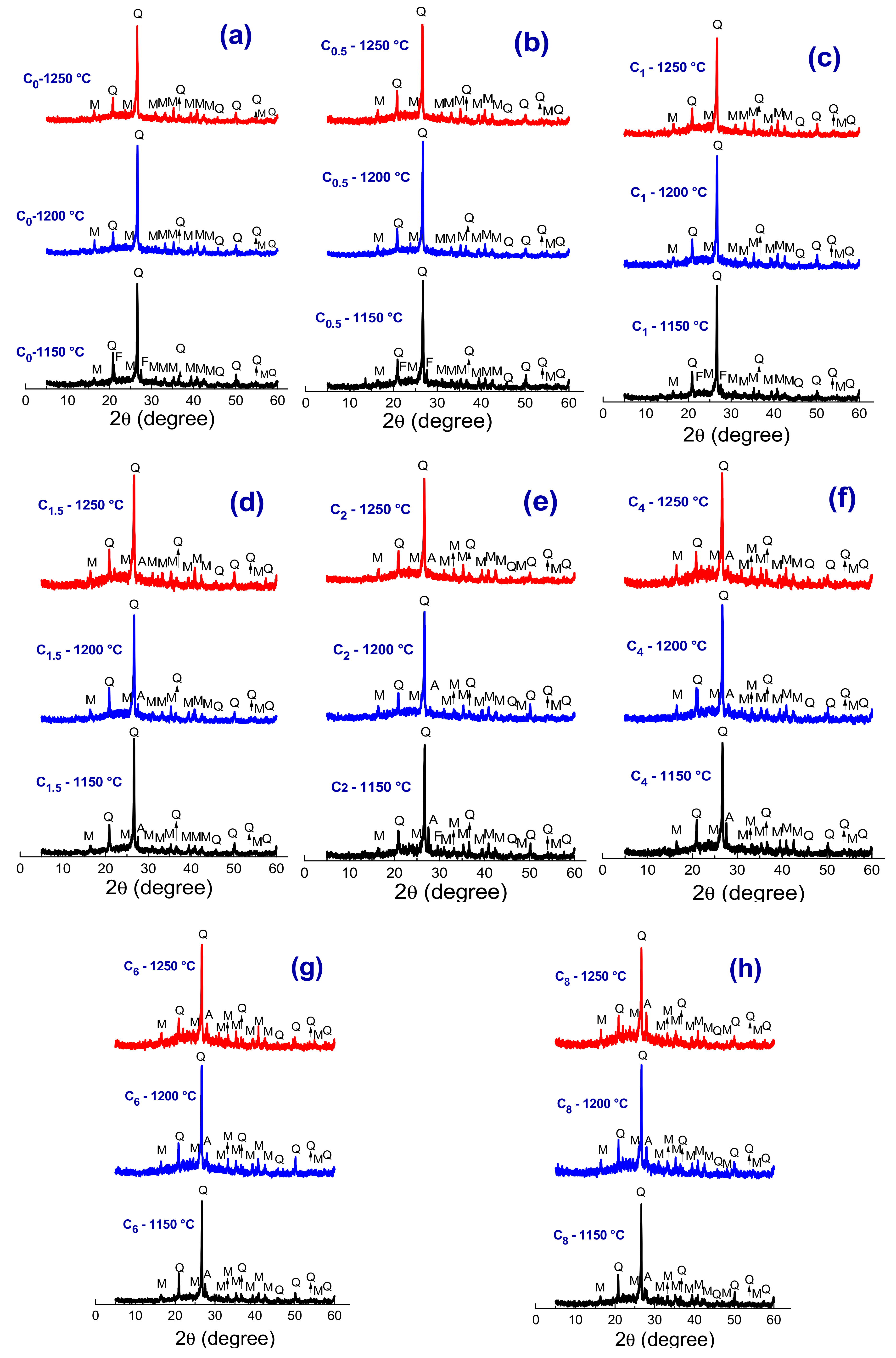


| Compositions | Raw Materials | ||||
|---|---|---|---|---|---|
| Kaolin | Plastic Clay | Quartz | Feldspar | Scheelite Tailings | |
| C0 | 27 | 29 | 11 | 33.0 | - |
| C0.5 | 27 | 29 | 11 | 32.5 | 0.5 |
| C1 | 27 | 29 | 11 | 32.0 | 1.0 |
| C1.5 | 27 | 29 | 11 | 31.5 | 1.5 |
| C2 | 27 | 29 | 11 | 31.0 | 2.0 |
| C4 | 27 | 29 | 11 | 29.0 | 4.0 |
| C6 | 27 | 29 | 11 | 27.0 | 6.0 |
| C8 | 27 | 29 | 11 | 25.0 | 8.0 |
| Raw Materials | Oxides (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | K2O | MgO | CaO | Na2O | Others | LF 1 | |
| Kaolin | 45.7 | 39.4 | 0.5 | 0.9 | - | - | - | 0.2 | 13.3 |
| Plastic clay | 54.5 | 27.4 | 2.6 | 4.0 | 1.5 | 0.8 | - | 1.4 | 7.8 |
| Quartz | 95.0 | 2.4 | 0.2 | 0.1 | - | - | - | 0.3 | 2.0 |
| Feldspar | 62.0 | 19.5 | - | 12.1 | - | - | 2.4 | 2.4 | 1.6 |
| Scheelite tailing | 20.7 | 7.0 | 6.8 | 0.4 | 2.6 | 44.6 | - | 2.1 | 15.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueirêdo, J.M.R.d.; Costa, F.P.d.; Fernandes, J.V.; Rodrigues, A.M.; Neves, G.d.A.; Menezes, R.R.; Santana, L.N.d.L. Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware. Materials 2020, 13, 5122. https://doi.org/10.3390/ma13225122
Figueirêdo JMRd, Costa FPd, Fernandes JV, Rodrigues AM, Neves GdA, Menezes RR, Santana LNdL. Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware. Materials. 2020; 13(22):5122. https://doi.org/10.3390/ma13225122
Chicago/Turabian StyleFigueirêdo, Julliana Marques R. de, Fabiana Pereira da Costa, Jucielle Veras Fernandes, Alisson Mendes Rodrigues, Gelmires de Araújo Neves, Romualdo Rodrigues Menezes, and Lisiane Navarro de Lima Santana. 2020. "Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware" Materials 13, no. 22: 5122. https://doi.org/10.3390/ma13225122
APA StyleFigueirêdo, J. M. R. d., Costa, F. P. d., Fernandes, J. V., Rodrigues, A. M., Neves, G. d. A., Menezes, R. R., & Santana, L. N. d. L. (2020). Development of Scheelite Tailings-Based Ceramic Formulations with the Potential to Manufacture Porcelain Tiles, Semi-Stoneware and Stoneware. Materials, 13(22), 5122. https://doi.org/10.3390/ma13225122








