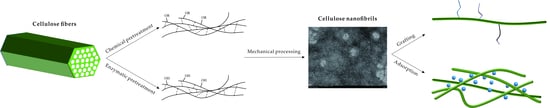
From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils
Abstract
1. Introduction
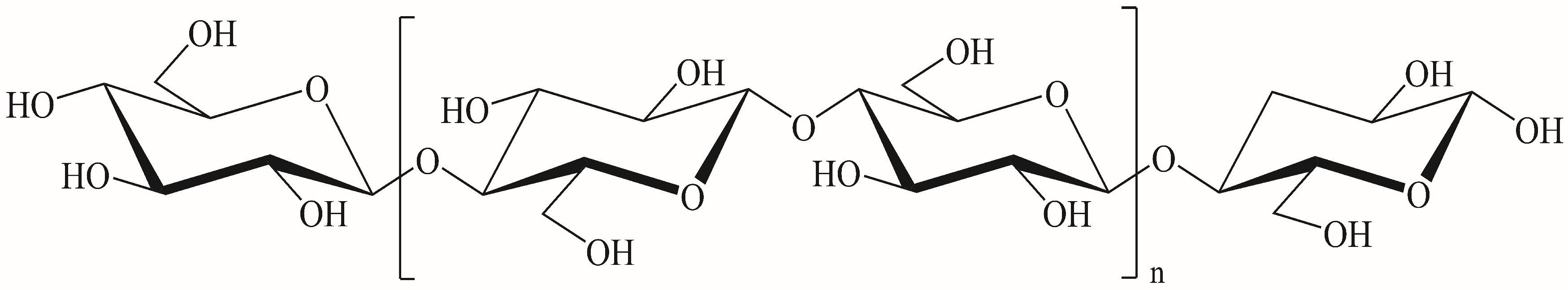
2. Cellulose Raw Materials and CNFs
3. Pretreatment of Cellulose
3.1. Cellulase Cutting Pretreatment
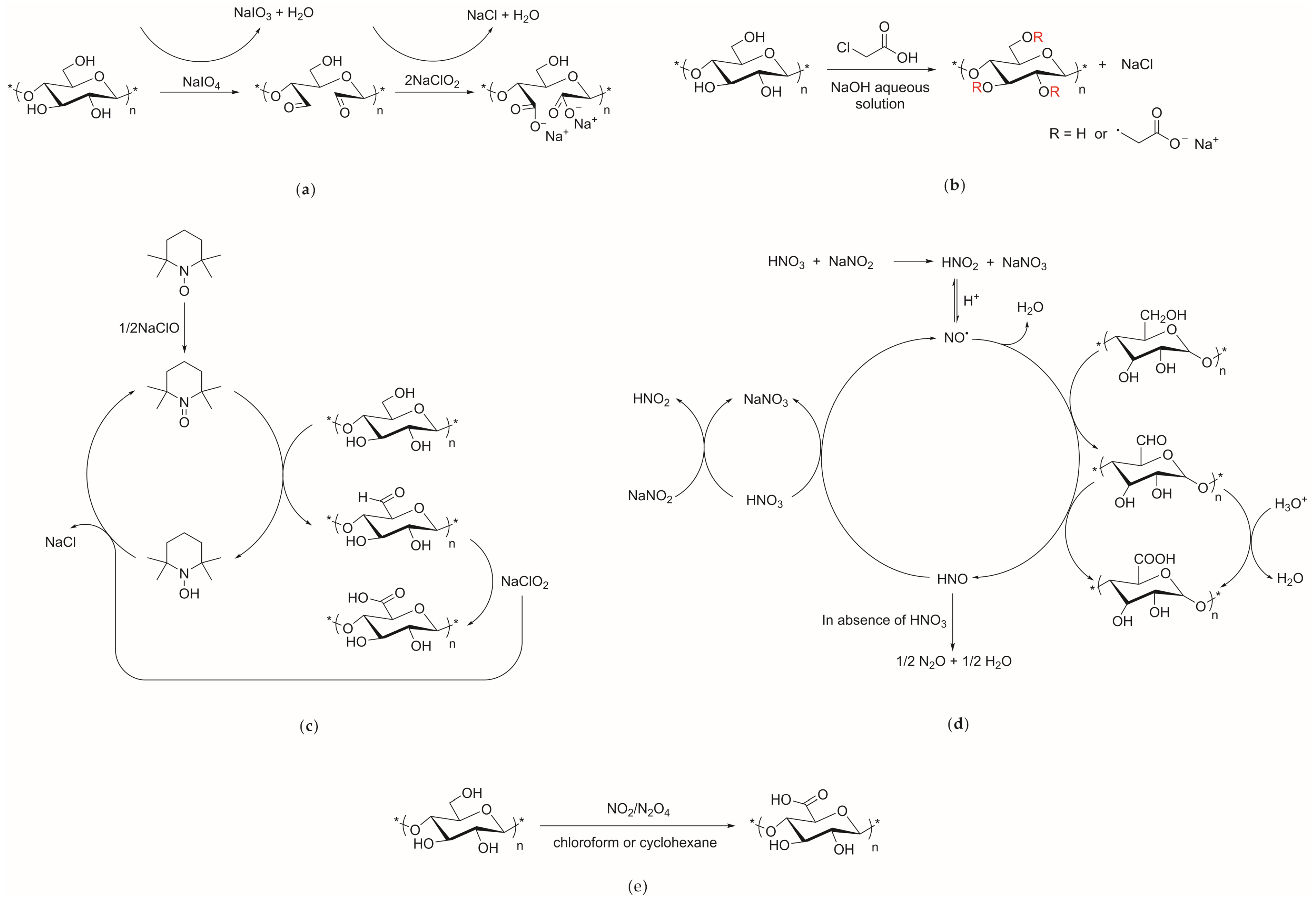
3.2. Chemical Pretreatment of Cellulose
3.2.1. TEMPO Oxidation
3.2.2. Carboxymethylation
3.2.3. Phosphorylation
3.2.4. Cationization
3.2.5. Periodate Oxidation
3.2.6. Supercritical Fluid Technology
4. CNFs Preparation
4.1. High Pressure Homogenization
4.2. Micro-Jet
4.3. Milling
4.4. Ultrasonic Treatment
4.5. Low Temperature Pressing
4.6. Steam Explosion
4.7. Electrospinning
4.8. Solvent Method
4.9. Ionic Liquid Method
5. Surface Modification of Nanofibrils
5.1. Surface Adsorption Modification
5.2. Graft Modification
5.2.1. Esterification
5.2.2. Acylation
5.2.3. Silanization
5.2.4. Polymer Grafting
6. CNFs Applications
7. Results and Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crop. Prod. 2016, 93, 2–25. [Google Scholar] [CrossRef]
- Höfte, H.; Gonneau, M.; Vernhettes, S. 2.22—Biosynthesis of Cellulose. In Comprehensive Glycoscience; Kamerling, H., Ed.; Elsevier: Oxford, UK, 2007; pp. 737–763. [Google Scholar] [CrossRef]
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Dias, Y.J.; Kolbasov, A.; Sinha-Ray, S.; Pourdeyhimi, B.; Yarin, A.L. Theoretical and experimental study of dissolution mechanism of cellulose. J. Mol. Liq. 2020, 312, 113450. [Google Scholar] [CrossRef]
- Lethesh, K.C.; Evjen, S.; Venkatraman, V.; Shah, S.N.; Fiksdahl, A. Highly efficient cellulose dissolution by alkaline ionic liquids. Carbohydr. Polym. 2020, 229, 115594. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindstrom, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Edit. 2011, 50, 5438–5466. [Google Scholar] [CrossRef]
- Mishra, R.K.; Sabu, A.; Tiwari, S.K. Materials chemistry and the futurist eco-friendly applications of nanocellulose: Status and prospect. J. Saudi Chem. Soc. 2018, 22, 949–978. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Grenda, K.; Arnold, J.; Gamelas, J.A.F.; Rasteiro, M.G. Environmentally friendly cellulose-based polyelectrolytes in wastewater treatment. Water Sci. Technol. 2017, 76, 1490–1499. [Google Scholar] [CrossRef]
- Rol, F.; Belgacem, M.N.; Gandini, A.; Bras, J. Recent advances in surface-modified cellulose nanofibrils. Prog. Polym. Sci. 2019, 88, 241–264. [Google Scholar] [CrossRef]
- Khalil, H.A.; Bhat, A.; Yusra, A.I. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979. [Google Scholar] [CrossRef]
- Diniz, J.M.B.F.; Gil, M.H.; Castro, J.A.A.M. Hornification?its origin and interpretation in wood pulps. Wood Sci. Technol. 2004, 37, 489–494. [Google Scholar] [CrossRef]
- Spinu, M.; Santos, N.; Le Moigne, N.; Navard, P. How does the never-dried state influence the swelling and dissolution of cellulose fibres in aqueous solvent? Cellulose 2010, 18, 247–256. [Google Scholar] [CrossRef]
- Yang, T.; Li, X.; Guo, Y.; Peng, S.; Liu, G.; Zhao, J. Effect of endoglucanases from different glycoside hydrolase families on enzymatic preparation of cellulose nanocrystal. Ind. Crop. Prod. 2020, 155, 112755. [Google Scholar] [CrossRef]
- Jia, L.; Qin, Y.; Wang, J.; Zhang, J. Lignin extracted by γ-valerolactone/water from corn stover improves cellulose enzymatic hydrolysis. Bioresour. Technol. 2020, 302, 122901. [Google Scholar] [CrossRef]
- Hasanin, M.S.; Mostafa, A.M.; Mwafy, E.A.; Darwesh, O.M. Eco-friendly cellulose nano fibers via first reported Egyptian Humicola fuscoatra Egyptia X4: Isolation and characterization. Environ. Nanotechnol. Monit. Manag. 2018, 10, 409–418. [Google Scholar] [CrossRef]
- Farro, E.G.S.; Leite, A.E.T.; Silva, I.A.; Filgueiras, J.G.; De Azevedo, E.R.; Polikarpov, I.; Nascimento, A.S. GH43 endo-arabinanase from Bacillus licheniformis: Structure, activity and unexpected synergistic effect on cellulose enzymatic hydrolysis. Int. J. Biol. Macromol. 2018, 117, 7–16. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, Y.; Chen, G.; Cai, M. Enhanced mechanical and hydrophobic properties of composite cassava starch films with stearic acid modified MCC (microcrystalline cellulose)/NCC (nanocellulose) as strength agent. Int. J. Biol. Macromol. 2020, 142, 846–854. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Mammadli, N.; Yildiz, U. Bioinspired modified nanocellulose adsorbent for enhanced boron recovery from aqueous media: Optimization, kinetics, thermodynamics and reusability study. J. Environ. Chem. Eng. 2019, 7, 103281. [Google Scholar] [CrossRef]
- Hokkanen, S.; Repo, E.; Sillanpää, M. Removal of heavy metals from aqueous solutions by succinic anhydride modified mercerized nanocellulose. Chem. Eng. J. 2013, 223, 40–47. [Google Scholar] [CrossRef]
- Pawcenis, D.; Chlebda, D.K.; Jędrzejczyk, R.J.; Leśniak, M.; Sitarz, M.; Łojewska, J. Preparation of silver nanoparticles using different fractions of TEMPO-oxidized nanocellulose. Eur. Polym. J. 2019, 116, 242–255. [Google Scholar] [CrossRef]
- Isogai, A.; Zhou, Y. Diverse nanocelluloses prepared from TEMPO-oxidized wood cellulose fibers: Nanonetworks, nanofibers, and nanocrystals. Curr. Opin. Solid State Mater. Sci. 2019, 23, 101–106. [Google Scholar] [CrossRef]
- Mendoza, D.J.; Hossain, L.; Browne, C.; Raghuwanshi, V.S.; Simon, G.P.; Garnier, G. Controlling the transparency and rheology of nanocellulose gels with the extent of carboxylation. Carbohydr. Polym. 2020, 245, 116566. [Google Scholar] [CrossRef]
- Lin, Q.; Zheng, Y.; Wang, G.; Shi, X.; Zhang, T.; Yu, J.; Sun, J. Protein adsorption behaviors of carboxymethylated bacterial cellulose membranes. Int. J. Biol. Macromol. 2015, 73, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Onyianta, A.J.; Castellano, M.; Dorris, M.; Williams, R.L.; Vicini, S. The effects of morpholine pre-treated and carboxymethylated cellulose nanofibrils on the properties of alginate-based hydrogels. Carbohydr. Polym. 2018, 198, 320–327. [Google Scholar] [CrossRef]
- Charreau, H.; Cavallo, E.; Foresti, M.L. Patents involving nanocellulose: Analysis of their evolution since 2010. Carbohydr. Polym. 2020, 237, 116039. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H. Fiber Characteristics and Fiber Atlas of China Papermaking Raw Materials, 1st ed.; China Light Industry Press: Beijing, China, 1999; pp. 3–126. [Google Scholar]
- Winandy, J.E. Relating wood chemistry and strength: Part II. Fundamental relationships between changes in wood chemistry and strength of wood. Wood Fiber Sci. 2017, 49, 2–11. [Google Scholar]
- Atalla, R.H.; Isogai, A. 6.16—Celluloses. In Comprehensive Natural Products II; Liu, H.-W., Mander, L., Eds.; Elsevier: Oxford, UK, 2010; pp. 493–539. [Google Scholar] [CrossRef]
- Pei, J.C. Phytofibrochemistry, 3rd ed.; Light Industry Press: Beijing, China, 2014; pp. 22–69. [Google Scholar]
- Eero, S. Wood Chemistry; Elsevier BV: Amsterdam, The Netherlands, 1993; pp. 15–112. [Google Scholar]
- Mishra, P.K.; Gregor, T.; Wimmer, R. Utilising Brewer’s Spent Grain as a Source of Cellulose Nanofibres Following Separation of Protein-based Biomass. BioResources 2017, 12, 107–116. [Google Scholar] [CrossRef]
- Thomas, P.; Duolikun, T.; Rumjit, N.P.; Moosavi, S.; Lai, C.W.; Bin Johan, M.R.; Fen, L.B. Comprehensive review on nanocellulose: Recent developments, challenges and future prospects. J. Mech. Behav. Biomed. Mater. 2020, 110, 103884. [Google Scholar] [CrossRef]
- Su, H.; Chong, Y.; Wang, J.; Long, D.; Qiao, W.; Ling, L. Nanocrystalline celluloses-assisted preparation of hierarchical carbon monoliths for hexavalent chromium removal. J. Colloid Interface Sci. 2018, 510, 77–85. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Erdem, A.; Yildiz, U.; Yıldız, U. A design optimization study on synthesized nanocrystalline cellulose, evaluation and surface modification as a potential biomaterial for prospective biomedical applications. Int. J. Biol. Macromol. 2018, 114, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Wu, G.; Alriksson, B.; Wang, W.; Hong, F.F.; Jönsson, L.J. Bioconversion of Waste Fiber Sludge to Bacterial Nanocellulose and Use for Reinforcement of CTMP Paper Sheets. Polymers 2017, 9, 458. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sharma, R.K.; Singh, A.P. Grafted cellulose: A bio-based polymer for durable applications. Polym. Bull. 2018, 75, 2213–2242. [Google Scholar] [CrossRef]
- Gupta, P.; Singh, B.; Agrawal, A.K.; Maji, P.K. Low density and high strength nanofibrillated cellulose aerogel for thermal insulation application. Mater. Des. 2018, 158, 224–236. [Google Scholar] [CrossRef]
- Magalhães, S.; Alves, L.; Medronho, B.; Fonseca, A.C.; Romano, A.; Coelho, J.F.J.; Norgren, M. Brief Overview on Bio-Based Adhesives and Sealants. Polymers 2019, 11, 1685. [Google Scholar] [CrossRef]
- Broxterman, S.E.; Schols, H.A. Interactions between pectin and cellulose in primary plant cell walls. Carbohydr. Polym. 2018, 192, 263–272. [Google Scholar] [CrossRef]
- Carvalho, A.F.A.; Neto, P.D.O.; Da Silva, D.F.; Pastore, G.M. Xylo-oligosaccharides from lignocellulosic materials: Chemical structure, health benefits and production by chemical and enzymatic hydrolysis. Food Res. Int. 2013, 51, 75–85. [Google Scholar] [CrossRef]
- Wang, R.; Xin, D.; Zhang, J. Inhibitory effects of vanillin, 4-hydroxybenzaldehyde and syringaldehyde on cellulases and xylanases. J. For. Eng. 2019, 4, 78–84. [Google Scholar]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, M.; Viikari, L. Xylans inhibit enzymatic hydrolysis of lignocellulosic materials by cellulases. Bioresour. Technol. 2012, 121, 8–12. [Google Scholar] [CrossRef]
- Pääkkö, M.; Ankerfors, M.; Kosonen, H.; Nykänen, A.; Ahola, S.; Österberg, M.; Ruokolainen, J.; Laine, J.; Larsson, P.T.; Ikkala, O.; et al. Enzymatic Hydrolysis Combined with Mechanical Shearing and High-Pressure Homogenization for Nanoscale Cellulose Fibrils and Strong Gels. Biomacromolecules 2007, 8, 1934–1941. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.; Berglund, L.; Lindström, T. An environmentally friendly method for enzyme-assisted preparation of microfibrillated cellulose (MFC) nanofibers. Eur. Polym. J. 2007, 43, 3434–3441. [Google Scholar] [CrossRef]
- Nechyporchuk, O.; Pignon, F.; Belgacem, M.N. Morphological properties of nanofibrillated cellulose produced using wet grinding as an ultimate fibrillation process. J. Mater. Sci. 2015, 50, 531–541. [Google Scholar] [CrossRef]
- Mou, H.; Huang, J.; Li, W.; Wu, X.; Liu, Y.; Fan, H. Study on the chemical modification of alkali lignin towards for cellulase adsorbent application. Int. J. Biol. Macromol. 2020, 149, 794–800. [Google Scholar] [CrossRef]
- Penttilä, P.A.; Várnai, A.; Pere, J.; Tammelin, T.; Salmén, L.; Siika-Aho, M.; Viikari, L.; Serimaa, R. Xylan as limiting factor in enzymatic hydrolysis of nanocellulose. Bioresour. Technol. 2013, 129, 135–141. [Google Scholar] [CrossRef]
- Long, L.; Tian, D.; Hu, J.; Wang, F.; Saddler, J.N. A xylanase-aided enzymatic pretreatment facilitates cellulose nanofibrillation. Bioresour. Technol. 2017, 243, 898–904. [Google Scholar] [CrossRef]
- Bian, H.; Dong, M.; Chen, L.; Zhou, X.; Ni, S.; Fang, G.; Dai, H. Comparison of mixed enzymatic pretreatment and post-treatment for enhancing the cellulose nanofibrillation efficiency. Bioresour. Technol. 2019, 293, 122171. [Google Scholar] [CrossRef]
- Isogai, T.; Saito, T.; Isogai, A. TEMPO Electromediated Oxidation of Some Polysaccharides Including Regenerated Cellulose Fiber. Biomacromolecules 2010, 11, 1593–1599. [Google Scholar] [CrossRef]
- Inamochi, T.; Funahashi, R.; Nakamura, Y.; Saito, T.; Isogai, A. Effect of coexisting salt on TEMPO-mediated oxidation of wood cellulose for preparation of nanocellulose. Cellulose 2017, 24, 4097–4101. [Google Scholar] [CrossRef]
- Saito, T.; Isogai, A. TEMPO-Mediated Oxidation of Native Cellulose. The Effect of Oxidation Conditions on Chemical and Crystal Structures of the Water-Insoluble Fractions. Biomacromolecules 2004, 5, 1983–1989. [Google Scholar] [CrossRef]
- Löbmann, K.; Svagan, A.J. Cellulose nanofibers as excipient for the delivery of poorly soluble drugs. Int. J. Pharm. 2017, 533, 285–297. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Isogai, A. Introduction of aldehyde groups on surfaces of native cellulose fibers by TEMPO-mediated oxidation. Colloids Surf. A Physicochem. Eng. Asp. 2006, 289, 219–225. [Google Scholar] [CrossRef]
- Tarrés, Q.; Delgado-Aguilar, M.; Pèlach, M.A.; González, I.; Boufi, S.; Mutjé, P. Remarkable increase of paper strength by combining enzymatic cellulose nanofibers in bulk and TEMPO-oxidized nanofibers as coating. Cellulose 2016, 23, 3939–3950. [Google Scholar] [CrossRef]
- Isogai, A.; Bergström, L. Preparation of cellulose nanofibers using green and sustainable chemistry. Curr. Opin. Green Sustain. Chem. 2018, 12, 15–21. [Google Scholar] [CrossRef]
- Isogai, A.; Hänninen, T.; Fujisawa, S.; Saito, T. Review: Catalytic oxidation of cellulose with nitroxyl radicals under aqueous conditions. Prog. Polym. Sci. 2018, 86, 122–148. [Google Scholar] [CrossRef]
- Shinoda, R.; Saito, T.; Okita, Y.; Isogai, A. Relationship between Length and Degree of Polymerization of TEMPO-Oxidized Cellulose Nanofibrils. Biomacromolecules 2012, 13, 842–849. [Google Scholar] [CrossRef]
- Saito, T.; Kimura, S.; Nishiyama, Y.; Isogai, A. Cellulose Nanofibers Prepared by TEMPO-Mediated Oxidation of Native Cellulose. Biomacromolecules 2007, 8, 2485–2491. [Google Scholar] [CrossRef]
- Zhou, Y.; Saito, T.; Bergström, L.; Isogai, A. Acid-Free Preparation of Cellulose Nanocrystals by TEMPO Oxidation and Subsequent Cavitation. Biomacromolecules 2018, 19, 633–639. [Google Scholar] [CrossRef]
- Daicho, K.; Saito, T.; Fujisawa, S.; Isogai, A. The Crystallinity of Nanocellulose: Dispersion-Induced Disordering of the Grain Boundary in Biologically Structured Cellulose. ACS Appl. Nano Mater. 2018, 1, 5774–5785. [Google Scholar] [CrossRef]
- Saito, T.; Hirota, M.; Tamura, N.; Kimura, S.; Fukuzumi, H.; Heux, L.; Isogai, A. Individualization of Nano-Sized Plant Cellulose Fibrils by Direct Surface Carboxylation Using TEMPO Catalyst under Neutral Conditions. Biomacromolecules 2009, 10, 1992–1996. [Google Scholar] [CrossRef]
- Aracri, E.; Vidal, T.; Ragauskas, A.J. Wet strength development in sisal cellulose fibers by effect of a laccase–TEMPO treatment. Carbohydr. Polym. 2011, 84, 1384–1390. [Google Scholar] [CrossRef]
- Aracri, E.; Valls, C.; Vidal, T. Paper strength improvement by oxidative modification of sisal cellulose fibers with laccase–TEMPO system: Influence of the process variables. Carbohydr. Polym. 2012, 88, 830–837. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Lourenço, A.F.; Ferreira, P.J.; Rasteiro, M.G.; Gamelas, J.A. Tuning rheology and aggregation behaviour of TEMPO-oxidised cellulose nanofibrils aqueous suspensions by addition of different acids. Carbohydr. Polym. 2020, 237, 116109. [Google Scholar] [CrossRef] [PubMed]
- Hirota, M.; Tamura, N.; Saito, T.; Isogai, A. Oxidation of regenerated cellulose with NaClO2 catalyzed by TEMPO and NaClO under acid-neutral conditions. Carbohydr. Polym. 2009, 78, 330–335. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, W.; Yu, J.; Fan, Y.; Ono, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by oxidation of α-chitin using the O2/laccase/TEMPO system. Carbohydr. Polym. 2018, 189, 178–183. [Google Scholar] [CrossRef]
- Bu, X.; Pei, J.; Zhang, F.; Liu, H.; Zhou, Z.; Zhen, X.; Wang, J.; Zhang, X.; Chan, H. The hydration mechanism and hydrogen bonding structure of 6-carboxylate chitooligosaccharides superabsorbent material prepared by laccase/TEMPO oxidation system. Carbohydr. Polym. 2018, 188, 151–158. [Google Scholar] [CrossRef]
- Ma, Z.; Ramakrishna, S. Electrospun regenerated cellulose nanofiber affinity membrane functionalized with protein A/G for IgG purification. J. Membr. Sci. 2008, 319, 23–28. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D.; Hedenqvist, M.; Ankerfors, M.; Lindström, T. Highly transparent films from carboxymethylated microfibrillated cellulose: The effect of multiple homogenization steps on key properties. J. Appl. Polym. Sci. 2011, 119, 2652–2660. [Google Scholar] [CrossRef]
- Aulin, C.; Ahola, S.; Josefsson, P.; Nishino, T.; Hirose, Y.; Osterberg, M.; Wågberg, L. Nanoscale Cellulose Films with Different Crystallinities and Mesostructures—Their Surface Properties and Interaction with Water. Langmuir 2009, 25, 7675–7685. [Google Scholar] [CrossRef]
- Stigsson, V.; Kloow, G.; Germgård, U. The influence of the solvent system used during manufacturing of CMC. Cellulose 2006, 13, 705–712. [Google Scholar] [CrossRef]
- Naderi, A.; Sundström, J.; Lindström, T.; Erlandsson, J. Enhancing the properties of carboxymethylated nanofibrillated cellulose by inclusion of water in the pretreatment process. Nord. Pulp Pap. Res. J. 2016, 31, 372–378. [Google Scholar] [CrossRef]
- Naderi, A.; Lindström, T.; Flodberg, G.; Sundström, J.; Junel, K.; Runebjörk, A.; Weise, C.F.; Erlandsson, J. Phosphorylated nanofibrillated cellulose: Production and properties. Nord. Pulp Pap. Res. J. 2016, 31, 20–29. [Google Scholar] [CrossRef]
- Ghanadpour, M.; Carosio, F.; Larsson, P.T.; Wågberg, L. Phosphorylated Cellulose Nanofibrils: A Renewable Nanomaterial for the Preparation of Intrinsically Flame-Retardant Materials. Biomacromolecules 2015, 16, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Ghanadpour, M.; Carosio, F.; Wågberg, L. Ultrastrong and flame-resistant freestanding films from nanocelluloses, self-assembled using a layer-by-layer approach. Appl. Mater. Today 2017, 9, 229–239. [Google Scholar] [CrossRef]
- Noguchi, Y.; Homma, I.; Matsubara, Y. Complete nanofibrillation of cellulose prepared by phosphorylation. Cellulose 2017, 24, 1295–1305. [Google Scholar] [CrossRef]
- Cai, X.; Riedl, B.; Ait-Kadi, A. Effect of surface-grafted ionic groups on the performance of cellulose-fiber-reinforced thermoplastic composites. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 2022–2032. [Google Scholar] [CrossRef]
- Littunen, K.; De Castro, J.S.; Samoylenko, A.; Xu, Q.; Quaggin, S.; Vainio, S.; Seppälä, J. Synthesis of cationized nanofibrillated cellulose and its antimicrobial properties. Eur. Polym. J. 2016, 75, 116–124. [Google Scholar] [CrossRef]
- Grenda, K.; Gamelas, J.A.F.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Evaluation of Anionic and Cationic Pulp-Based Flocculants With Diverse Lignin Contents for Application in Effluent Treatment From the Textile Industry: Flocculation Monitoring. Front. Chem. 2020, 8, 5. [Google Scholar] [CrossRef]
- Grenda, K.; Gamelas, J.A.F.; Arnold, J.; Cayre, O.J.; Rasteiro, M.G. Cationization of Eucalyptus wood waste pulps with diverse lignin contents for potential application in colored wastewater treatment. RSC Adv. 2019, 9, 34814–34826. [Google Scholar] [CrossRef]
- Odabas, N.; Amer, H.; Bacher, M.; Henniges, U.; Potthast, A.; Rosenau, T. Properties of Cellulosic Material after Cationization in Different Solvents. ACS Sustain. Chem. Eng. 2016, 4, 2295–2301. [Google Scholar] [CrossRef]
- Sehaqui, H.; Mautner, A.; De Larraya, U.P.; Pfenninger, N.; Tingaut, P.; Zimmermann, T. Cationic cellulose nanofibers from waste pulp residues and their nitrate, fluoride, sulphate and phosphate adsorption properties. Carbohydr. Polym. 2016, 135, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Chaker, A.; Boufi, S. Cationic nanofibrillar cellulose with high antibacterial properties. Carbohydr. Polym. 2015, 131, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zhang, J.; Gan, W.; Zhou, J.; Zhang, L. Flocculation Properties and Antimicrobial Activities of Quaternized Celluloses Synthesized in NaOH/Urea Aqueous Solution. Ind. Eng. Chem. Res. 2010, 49, 1242–1246. [Google Scholar] [CrossRef]
- Willberg-Keyriläinen, P.; Pitkänen, P.; Hulkko, J.; Asikainen, M.; Setälä, H. The effect of mixing and consistency on cellulose cationization. Heliyon 2019, 5, e01349. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Tao, H.; Bangal, P.R. Synthesis and Flocculation Behavior of Cationic Cellulose Prepared in a NaOH/Urea Aqueous Solution. Clean-Soil Air Water 2009, 37, 39–44. [Google Scholar] [CrossRef]
- Kristiansen, K.A.; Potthast, A.; Christensen, B.E. Periodate oxidation of polysaccharides for modification of chemical and physical properties. Carbohydr. Res. 2010, 345, 1264–1271. [Google Scholar] [CrossRef]
- Kim, U.-J.; Kuga, S.; Wada, M.; Okano, T.; Kondo, T. Periodate Oxidation of Crystalline Cellulose. Biomacromolecules 2000, 1, 488–492. [Google Scholar] [CrossRef]
- Liu, X.; Wang, L.; Song, X.; Song, H.; Zhao, J.R.; Wang, S. A kinetic model for oxidative degradation of bagasse pulp fiber by sodium periodate. Carbohydr. Polym. 2012, 90, 218–223. [Google Scholar] [CrossRef]
- Sirviö, J.; Liimatainen, H.; Niinimäki, J.; Hormi, O. Dialdehyde cellulose microfibers generated from wood pulp by milling-induced periodate oxidation. Carbohydr. Polym. 2011, 86, 260–265. [Google Scholar] [CrossRef]
- Plappert, S.F.; Liebner, F.W.; Konnerth, J.; Nedelec, J.-M. Anisotropic nanocellulose gel–membranes for drug delivery: Tailoring structure and interface by sequential periodate–chlorite oxidation. Carbohydr. Polym. 2019, 226, 115306. [Google Scholar] [CrossRef]
- Larsson, P.A.; Berglund, L.A.; Wågberg, L. Highly ductile fibres and sheets by core-shell structuring of the cellulose nanofibrils. Cellulose 2014, 21, 323–333. [Google Scholar] [CrossRef]
- Milovanovic, S.; Stamenic, M.; Markovic, D.; Ivanovic, J.; Zizovic, I. Supercritical impregnation of cellulose acetate with thymol. J. Supercrit. Fluids 2015, 97, 107–115. [Google Scholar] [CrossRef]
- Nanta, P.; Kasemwong, K.; Skolpap, W.; Shimoyama, Y. Influence of supercritical carbon dioxide treatment on the physicochemical properties of cellulose extracted from cassava pulp waste. J. Supercrit. Fluids 2019, 154, 104605. [Google Scholar] [CrossRef]
- Putrino, F.M.; Tedesco, M.; Bodini, R.B.; De Oliveira, A.L. Study of supercritical carbon dioxide pretreatment processes on green coconut fiber to enhance enzymatic hydrolysis of cellulose. Bioresour. Technol. 2020, 309, 123387. [Google Scholar] [CrossRef] [PubMed]
- Milovanovic, S.; Adamović, T.N.; Aksentijevic, K.; Misic, D.; Ivanovic, J.; Zizovic, I. Cellulose Acetate Based Material with Antibacterial Properties Created by Supercritical Solvent Impregnation. Int. J. Polym. Sci. 2017, 8762649. [Google Scholar] [CrossRef]
- Alves, L.; Medronho, B.; Antunes, F.E.; Topgaard, D.; Lindman, B. Dissolution state of cellulose in aqueous systems. 1. Alkaline solvents. Cellulose 2016, 23, 247–258. [Google Scholar] [CrossRef]
- Wagberg, L.; Decher, G.; Norgren, M.; Lindstrom, T.; Ankerfors, M.; Axnas, K. The Build-Up of Polyelectrolyte Multilayers of Microfibrillated Cellulose and Cationic Polyelectrolytes. Langmuir 2008, 24, 784–795. [Google Scholar] [CrossRef]
- Alinia, R.; Zabihi, S.; Esmaeilzadeh, F.; Kalajahi, J.F. Pretreatment of wheat straw by supercritical CO2 and its enzymatic hydrolysis for sugar production. Biosyst. Eng. 2010, 107, 61–66. [Google Scholar] [CrossRef]
- Nishino, T.; Kotera, M.; Suetsugu, M.; Murakami, H.; Urushihara, Y. Acetylation of plant cellulose fiber in supercritical carbon dioxide. Polymer 2011, 52, 830–836. [Google Scholar] [CrossRef]
- Nakagaito, A.; Yano, H. The effect of morphological changes from pulp fiber towards nano-scale fibrillated cellulose on the mechanical properties of high-strength plant fiber based composites. Appl. Phys. A 2004, 78, 547–552. [Google Scholar] [CrossRef]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and Application of High Pressure–Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef] [PubMed]
- Dinand, E.; Chanzy, H.; Vignon, M.R. Suspensions of cellulose microfibrils from sugar beet pulp. Food Hydrocoll. 1999, 13, 275–283. [Google Scholar] [CrossRef]
- Dufresne, A.; Vignon, M.R. Cellulose microfibrils from potato tuber cells: Processing and characterization of starch-cellulose microfibril composites. J. Appl. Polym. Sci. 2000, 76, 2080–2092. [Google Scholar] [CrossRef]
- Iwamoto, S.; Nakagaito, A.; Yano, H.; Nogi, M. Optically transparent composites reinforced with plant fiber-based nanofibers. Appl. Phys. A 2005, 81, 1109–1112. [Google Scholar] [CrossRef]
- Teo, H.L.; Wahab, R.A. Towards an eco-friendly deconstruction of agro-industrial biomass and preparation of renewable cellulose nanomaterials: A review. Int. J. Biol. Macromol. 2020, 161, 1414–1430. [Google Scholar] [CrossRef]
- Khalil, H.A.; Davoudpour, Y.; Islam, N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef]
- Yokota, S.; Kamada, K.; Sugiyama, A.; Kondo, T. Pickering emulsion stabilization by using amphiphilic cellulose nanofibrils prepared by aqueous counter collision. Carbohydr. Polym. 2019, 226, 115293. [Google Scholar] [CrossRef]
- Taipale, T.; Österberg, M.; Nykänen, A.; Ruokolainen, J.; Laine, J. Effect of microfibrillated cellulose and fines on the drainage of kraft pulp suspension and paper strength. Cellulose 2010, 17, 1005–1020. [Google Scholar] [CrossRef]
- Ling, S.; Chen, W.; Fan, Y.; Zheng, K.; Jin, K.; Yu, H.; Buehler, M.J.; Kaplan, D.L. Biopolymer nanofibrils: Structure, modeling, preparation, and applications. Prog. Polym. Sci. 2018, 85, 1–56. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; De Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127. [Google Scholar] [CrossRef]
- Jonoobi, M.; Mathew, A.P.; Oksman, K. Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind. Crop. Prod. 2012, 40, 232–238. [Google Scholar] [CrossRef]
- Spence, K.L.; Venditti, R.A.; Rojas, O.J.; Habibi, Y.; Pawlak, J.J. A comparative study of energy consumption and physical properties of microfibrillated cellulose produced by different processing methods. Cellulose 2011, 18, 1097–1111. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaille, J.Y.; Vignon, M.R. Mechanical behavior of sheets prepared from sugar beet cellulose microfibrils. J. Appl. Polym. Sci. 1997, 64, 1185–1194. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Liu, Y.; Chen, P.; Zhang, M.; Hai, Y. Individualization of cellulose nanofibers from wood using high-intensity ultrasonication combined with chemical pretreatments. Carbohydr. Polym. 2011, 83, 1804–1811. [Google Scholar] [CrossRef]
- Wang, S.; Cheng, Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. J. Appl. Polym. Sci. 2009, 113, 1270–1275. [Google Scholar] [CrossRef]
- Chen, W.; Abe, K.; Uetani, K.; Yu, H.; Liu, Y.; Yano, H. Individual cotton cellulose nanofibers: Pretreatment and fibrillation technique. Cellulose 2014, 21, 1517–1528. [Google Scholar] [CrossRef]
- Lee, H.V.; Hamid, S.B.A.; Zain, S.K. Conversion of Lignocellulosic Biomass to Nanocellulose: Structure and Chemical Process. Sci. World J. 2014, 1–20. [Google Scholar] [CrossRef]
- Wang, B.; Sain, M.; Oksman, K. Study of Structural Morphology of Hemp Fiber from the Micro to the Nanoscale. Appl. Compos. Mater. 2007, 14, 89–103. [Google Scholar] [CrossRef]
- Deepa, B.; Abraham, E.; Cherian, B.M.; Bismarck, A.; Blaker, J.J.; Pothan, L.A.; Leao, A.L.; De Souza, S.F.; Kottaisamy, M. Structure, morphology and thermal characteristics of banana nano fibers obtained by steam explosion. Bioresour. Technol. 2011, 102, 1988–1997. [Google Scholar] [CrossRef]
- Dizge, N.; Shaulsky, E.; Karanikola, V. Electrospun cellulose nanofibers for superhydrophobic and oleophobic membranes. J. Membr. Sci. 2019, 590, 117271. [Google Scholar] [CrossRef]
- Hamad, A.A.; Hassouna, M.S.; Shalaby, T.I.; Elkady, M.F.; Elkawi, M.A.A.; Hamad, H.A. Electrospun cellulose acetate nanofiber incorporated with hydroxyapatite for removal of heavy metals. Int. J. Biol. Macromol. 2020, 151, 1299–1313. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.W. Electrospinning Cellulose and Cellulose Derivatives. Polym. Rev. 2008, 48, 378–391. [Google Scholar] [CrossRef]
- Qi, H.; Sui, X.; Yuan, J.; Wei, Y.; Zhang, L. Electrospinning of Cellulose-Based Fibers From NaOH/Urea Aqueous System. Macromol. Mater. Eng. 2010, 295, 695–700. [Google Scholar] [CrossRef]
- Nelson, K.; Deng, Y. Encapsulation of Inorganic Particles with Nanostructured Cellulose. Macromol. Mater. Eng. 2007, 292, 1158–1163. [Google Scholar] [CrossRef]
- Oksman, K.; Mathew, A.; Bondeson, D.; Kvien, I. Manufacturing process of cellulose whiskers/polylactic acid nanocomposites. Compos. Sci. Technol. 2006, 66, 2776–2784. [Google Scholar] [CrossRef]
- Chhotaray, P.K.; Biswal, S.K.; Pandey, S. Development of novel hybrid ionic fluids for efficient CO2 capture and cellulose dissolution. J. Mol. Liq. 2020, 312, 113477. [Google Scholar] [CrossRef]
- Abushammala, H.; Mao, J. A Review on the Partial and Complete Dissolution and Fractionation of Wood and Lignocelluloses Using Imidazolium Ionic Liquids. Polymers 2020, 12, 195. [Google Scholar] [CrossRef]
- Xie, W.-H.; Hu, B.-B.; Zhang, X.-B.; Yi, X.-M.; Tan, W.-X.; Zhu, M.-J. Enhanced Enzymatic Digestibility of Sugarcane Bagasse Pretreated by Ionic Liquids. J. Biobased Mater. Bioenergy 2015, 9, 493–501. [Google Scholar] [CrossRef]
- Samsudin, N.A.; Low, F.W.; Yusoff, Y.; Shakeri, M.; Tan, X.Y.; Lai, C.W.; Asim, N.; Oon, C.; Newaz, K.S.; Tiong, S.K.; et al. Effect of temperature on synthesis of cellulose nanoparticles via ionic liquid hydrolysis process. J. Mol. Liq. 2020, 308, 113030. [Google Scholar] [CrossRef]
- Kilpeläinen, I.; Xie, H.; King, A.; Granstrom, M.; Heikkinen, S.; Argyropoulos, D.S. Dissolution of Wood in Ionic Liquids. J. Agric. Food Chem. 2007, 55, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Ahn, Y.; Hu, D.-H.; Hong, J.H.; Lee, S.H.; Kim, H.J.; Kim, H. Effect of co-solvent on the spinnability and properties of electrospun cellulose nanofiber. Carbohydr. Polym. 2012, 89, 340–345. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, G.; Murugesan, S.; Pushparaj, V.; Nalamasu, O.; Ajayan, P.M.; Linhardt, R.J. Preparation of Biopolymer Fibers by Electrospinning from Room Temperature Ionic Liquids. Biomacromolecules 2006, 7, 415–418. [Google Scholar] [CrossRef] [PubMed]
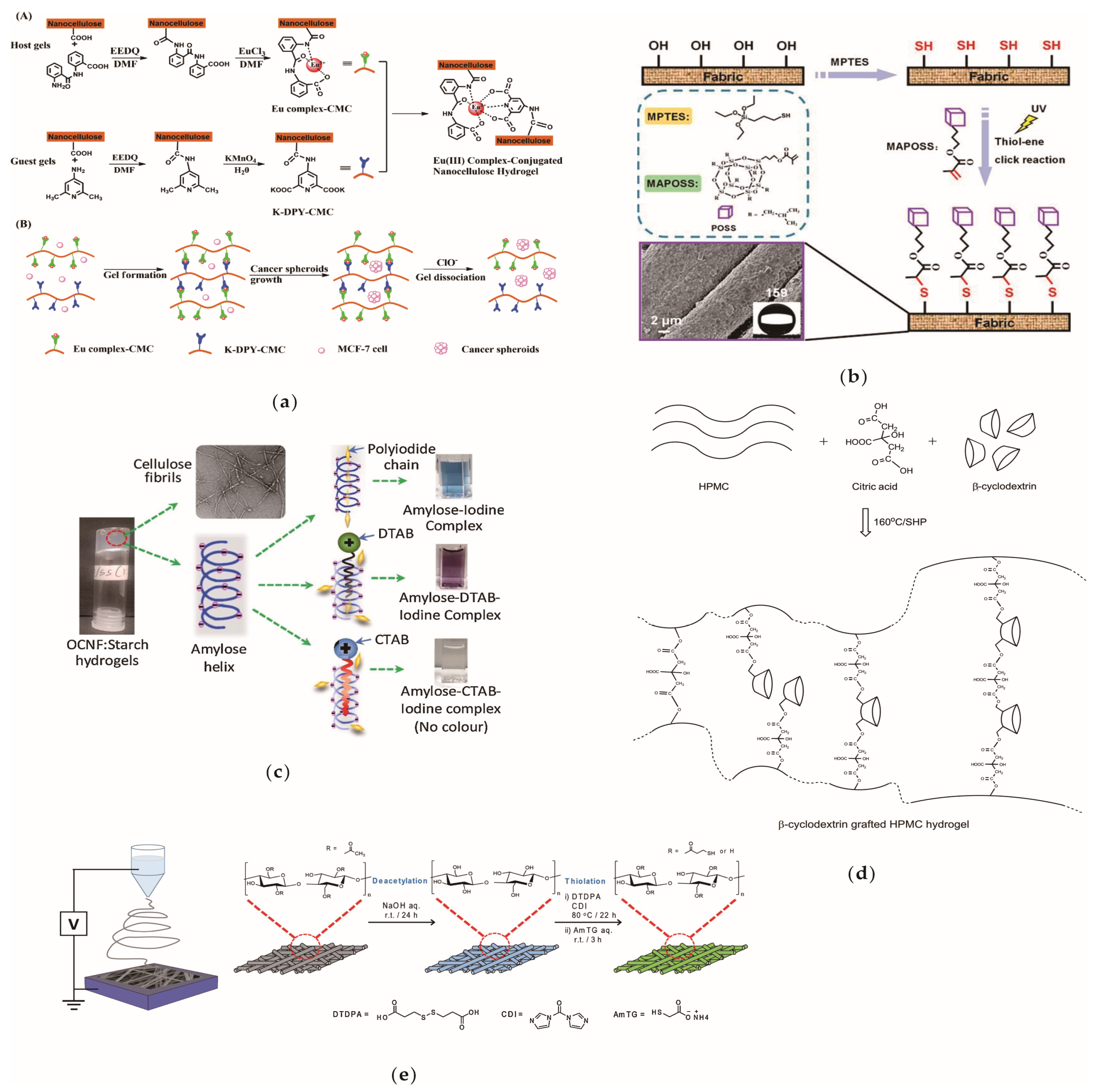
- Hai, J.; Zeng, X.; Zhu, Y.; Wang, B. Anions reversibly responsive luminescent nanocellulose hydrogels for cancer spheroids culture and release. Biomaterials 2019, 194, 161–170. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zeng, Y.; Zhou, C.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Pi, P. Facile generation of robust POSS-based superhydrophobic fabrics via thiol-ene click chemistry. Chem. Eng. J. 2018, 332, 150–159. [Google Scholar] [CrossRef]
- Hossain, K.M.Z.; Calabrese, V.; da Silva, M.A.; Bryant, S.J.; Schmitt, J.; Scott, J.L.; Edler, K.J. Cationic surfactants as a non-covalent linker for oxidised cellulose nanofibrils and starch-based hydrogels. Carbohyd. Polym. 2020, 233, 115816. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked cyclodextrin/hydroxypropylmethylcellulose hydrogel films for hydrophobic drug delivery. Int. J. Biol. Macromol. 2016, 93, 75–86. [Google Scholar] [CrossRef]
- Choi, H.Y.; Bae, J.H.; Hasegawa, Y.; An, S.; Kim, I.S.; Lee, H.; Kim, M. Thiol-functionalized cellulose nanofiber membranes for the effective adsorption of heavy metal ions in water. Carbohyd. Polym. 2020, 234, 115881. [Google Scholar] [CrossRef]
- Alves, L.; Ferraz, E.; Gamelas, J. Composites of nanofibrillated cellulose with clay minerals: A review. Adv. Colloid Interface Sci. 2019, 272, 101994. [Google Scholar] [CrossRef]
- Bao, X.; Dong, F.; Yu, Y.; Wang, Q.; Wang, P.; Fan, X.; Yuan, J. Green modification of cellulose-based natural materials by HRP-initiated controlled “graft from” polymerization. Int. J. Biol. Macromol. 2020, 164, 1237–1245. [Google Scholar] [CrossRef]
- Veronica Galvan, M.; Peresin, M.S.; Mocchiutti, P.; Granqvist, N.; Angel Zanuttini, M.; Tammelin, T. Effects of charge ratios of xylan-poly(allylamine hydrochloride) complexes on their adsorption onto different surfaces. Cellulose 2015, 22, 2955–2970. [Google Scholar] [CrossRef]
- Mocchiutti, P.; Schnell, C.N.; Rossi, G.D.; Peresin, M.S.; Zanuttini, M.A.; Galván, M.V. Cationic and anionic polyelectrolyte complexes of xylan and chitosan. Interaction with lignocellulosic surfaces. Carbohydr. Polym. 2016, 150, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Raj, P.; Varanasi, S.; Batchelor, W.; Garnier, G. Effect of cationic polyacrylamide on the processing and properties of nanocellulose films. J. Colloid Interface Sci. 2015, 447, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.C.T.; Freire, C.S.R.; Pinto, R.J.B.; Fernandes, S.C.M.; Neto, C.P.; Silvestre, A.J.D.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 2012, 19, 1425–1436. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Boufi, S. Porous material from cellulose nanofibrils coated with aluminum hydroxyde as an effective adsorbent for fluoride. J. Environ. Chem. Eng. 2020, 8, 103779. [Google Scholar] [CrossRef]
- Chimphango, A.F.; Görgens, J.F.; Van Zyl, W. In situ enzyme aided adsorption of soluble xylan biopolymers onto cellulosic material. Carbohydr. Polym. 2016, 143, 172–178. [Google Scholar] [CrossRef]
- Hatton, F.L.; Engström, J.; Forsling, J.; Malmström, E.; Carlmark, A. Biomimetic adsorption of zwitterionic–xyloglucan block copolymers to CNF: Towards tailored super-absorbing cellulose materials. RSC Adv. 2017, 7, 14947–14958. [Google Scholar] [CrossRef]
- Aulin, C.; Shchukarev, A.; Lindqvist, J.; Malmström, E.; Wågberg, L.; Lindström, T. Wetting kinetics of oil mixtures on fluorinated model cellulose surfaces. J. Colloid Interface Sci. 2008, 317, 556–567. [Google Scholar] [CrossRef]
- Mulyadi, A.; Deng, Y. Surface modification of cellulose nanofibrils by maleated styrene block copolymer and their composite reinforcement application. Cellulose 2016, 23, 519–528. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, H.; Yi, T.; Qi, M.; Xu, H.; Mo, Q.; Huang, C.-X.; Wang, S.; Liu, Y. Preparation and Properties of Cassava Residue Cellulose Nanofibril/Cassava Starch Composite Films. Nanomaterials 2020, 10, 755. [Google Scholar] [CrossRef]
- Forero-Doria, O.; Polo, E.; Marican, A.; Guzmán, L.; Venegas, B.; Vijayakumar, S.; Wehinger, S.; Guerrero, M.; Gallego, J.; Durán-Lara, E.F. Supramolecular hydrogels based on cellulose for sustained release of therapeutic substances with antimicrobial and wound healing properties. Carbohyd. Polym. 2020, 242, 116383. [Google Scholar] [CrossRef] [PubMed]
- Auvinen, V.-V.; Virtanen, J.; Merivaara, A.; Virtanen, V.; Laurén, P.; Tuukkanen, S.; Laaksonen, T. Modulating sustained drug release from nanocellulose hydrogel by adjusting the inner geometry of implantable capsules. J. Drug Deliv. Sci. Technol. 2020, 57, 101625. [Google Scholar] [CrossRef]
- Marani, P.L.; Bloisi, G.D.; Petri, D.F.S. Hydroxypropylmethyl cellulose films crosslinked with citric acid for control release of nicotine. Cellulose 2015, 22, 3907–3918. [Google Scholar] [CrossRef]
- Ghorpade, V.S.; Yadav, A.V.; Dias, R.J. Citric acid crosslinked β -cyclodextrin/carboxymethylcellulose hydrogel films for controlled delivery of poorly soluble drugs. Carbohydr. Polym. 2017, 164, 339–348. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Pickering stabilized nanocellulose-alginate: A diosgenin-mediated delivery of quinalizarin as a potent cyto-inhibitor in human lung/breast cancer cell lines. Mater. Sci. Eng. C 2020, 109, 110621. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef]
- Singh, M.; Kaushik, A.; Ahuja, D. Surface functionalization of nanofibrillated cellulose extracted from wheat straw: Effect of process parameters. Carbohydr. Polym. 2016, 150, 48–56. [Google Scholar] [CrossRef]
- Bodirlau, R.; Teaca, C.A. Fourier transform infrared spectroscopy and thermal analysis of lignocellulose fillers treated with organic anhydrides. Rom. J. Phys. 2009, 54, 93–104. [Google Scholar]
- Zepic, V.; Poljansek, I.; Oven, P.; Skapin, A.S.; Hancic, A. Effect of Drying Pretreatment on the Acetylation of Nanofibrillated Cellulose. BioResources 2015, 10, 8148–8167. [Google Scholar]
- Yetiş, F.; Liu, X.; Sampson, W.W.; Gong, H. Acetylation of lignin containing microfibrillated cellulose and its reinforcing effect for polylactic acid. Eur. Polym. J. 2020, 134, 109803. [Google Scholar] [CrossRef]
- Wang, W.; Liang, T.; Bai, H.; Dong, W.; Liu, X. All cellulose composites based on cellulose diacetate and nanofibrillated cellulose prepared by alkali treatment. Carbohydr. Polym. 2018, 179, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Bledzki, A.K.; Mamun, A.A.; Lucka-Gabor, M.; Gutowski, V.S. The effects of acetylation on properties of flax fibre and its polypropylene composites. Express Polym. Lett. 2008, 2, 413–422. [Google Scholar] [CrossRef]
- Bulota, M.; Kreitsmann, K.; Hughes, M.; Paltakari, J. Acetylated microfibrillated cellulose as a toughening agent in poly(lactic acid). J. Appl. Polym. Sci. 2012, 126, E448–E457. [Google Scholar] [CrossRef]
- Huang, L.; Zhao, H.; Xu, H.; An, S.; Li, C.; Huang, C.-X.; Wang, S.; Liu, Y.; Chen, J. Study of 4,4‘-Methylene Diisocyanate Phenyl Ester-Modified Cassava Residues/Polybutylene Succinate Biodegradable Composites: Preparation and Performance Research. Processes 2019, 7, 588. [Google Scholar] [CrossRef]
- Behera, P.K.; Mondal, P.; Singha, N.K. Self-Healable and Ultrahydrophobic Polyurethane-POSS Hybrids by Diels–Alder “Click” Reaction: A New Class of Coating Material. Macromolecules 2018, 51, 4770–4781. [Google Scholar] [CrossRef]
- Musikavanhu, B.; Hu, Z.; Dzapata, R.L.; Xu, Y.; Christie, P.; Guo, D.; Li, J. Facile method for the preparation of superhydrophobic cellulosic paper. Appl. Surf. Sci. 2019, 496, 143648. [Google Scholar] [CrossRef]
- Deng, Y.; Han, D.; Deng, Y.-Y.; Zhang, Q.; Chen, F.; Fu, Q. Facile one-step preparation of robust hydrophobic cotton fabrics by covalent bonding polyhedral oligomeric silsesquioxane for ultrafast oil/water separation. Chem. Eng. J. 2020, 379, 122391. [Google Scholar] [CrossRef]
- Cunha, A.G.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A.; Belgacem, M.N.; Chaussy, D.; Beneventi, D. Preparation of highly hydrophobic and lipophobic cellulose fibers by a straightforward gas–solid reaction. J. Colloid Interface Sci. 2010, 344, 588–595. [Google Scholar] [CrossRef]
- Yeo, J.-S.; Kim, O.Y.; Hwang, S.-H. The effect of chemical surface treatment on the fracture toughness of microfibrillated cellulose reinforced epoxy composites. J. Ind. Eng. Chem. 2017, 45, 301–306. [Google Scholar] [CrossRef]
- Peresin, M.S.; Kammiovirta, K.; Heikkinen, H.; Johansson, L.-S.; Vartiainen, J.; Setälä, H.; Österberg, M.; Tammelin, T. Understanding the mechanisms of oxygen diffusion through surface functionalized nanocellulose films. Carbohydr. Polym. 2017, 174, 309–317. [Google Scholar] [CrossRef]
- Andresen, M.; Johansson, L.-S.; Tanem, B.S.; Stenius, P. Properties and characterization of hydrophobized microfibrillated cellulose. Cellulose 2006, 13, 665–677. [Google Scholar] [CrossRef]
- Johansson, L.-S.; Tammelin, T.; Campbell, J.M.; Setälä, H.; Österberg, M. Experimental evidence on medium driven cellulose surface adaptation demonstrated using nanofibrillated cellulose. Soft Matter 2011, 7, 10917–10924. [Google Scholar] [CrossRef]
- Muiruri, J.K.; Liu, S.; Teo, W.S.; Kong, J.; He, C. Highly Biodegradable and Tough Polylactic Acid–Cellulose Nanocrystal Composite. ACS Sustain. Chem. Eng. 2017, 5, 3929–3937. [Google Scholar] [CrossRef]
- Dias, O.A.T.; Konar, S.; Leão, A.L.; Sain, M. Flexible electrically conductive films based on nanofibrillated cellulose and polythiophene prepared via oxidative polymerization. Carbohydr. Polym. 2019, 220, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Parit, M.; Du, H.; Zhang, X.; Prather, C.; Adams, M.; Jiang, Z. Polypyrrole and cellulose nanofiber based composite films with improved physical and electrical properties for electromagnetic shielding applications. Carbohydr. Polym. 2020, 240, 116304. [Google Scholar] [CrossRef]
- Mishra, P.K.; Ekielski, A.; Mukherjee, S.; Sahu, S.; Chowdhury, S.; Mishra, M.; Talegaonkar, S.; Siddiqui, L.; Mishra, H. Wood-Based Cellulose Nanofibrils: Haemocompatibility and Impact on the Development and Behaviour of Drosophila melanogaster. Biomolecules 2019, 9, 363. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Mousa, M.H.; Dong, Y.; Davies, I.J. Recent advances in bionanocomposites: Preparation, properties, and applications. Int. J. Polym. Mater. 2016, 65, 225–254. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Dong, L.; Yan, G.; Ren, S.; Zhang, X.; Lei, T. Platinum Nanoparticle Decorated Poly(diallyldimethylammonium chloride)/Cellulose Nanocrystal Nanohybrid for Electrochemical Sensing of Dopamine. J. Biobased Mater. Bioenergy 2018, 12, 519–524. [Google Scholar] [CrossRef]
- Ling, Z.; Xu, F.; Edwards, J.V.; Prevost, N.T.; Nam, S.; Condon, B.D.; French, A.D. Nanocellulose as a colorimetric biosensor for effective and facile detection of human neutrophil elastase. Carbohydr. Polym. 2019, 216, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Qian, B.; Uto, K.; Chen, J.; Liu, X.; Minari, T. Functional biomaterials towards flexible electronics and sensors. Biosens. Bioelectron. 2018, 119, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.A.; Davoudpour, Y.; Saurabh, C.K.; Hossain, S.; Adnan, A.S.; Dungani, R.; Tahir, P.M.; Sarker, Z.I.; Fazita, M.N.; Syakir, M.; et al. A review on nanocellulosic fibres as new material for sustainable packaging: Process and applications. Renew. Sustain. Energy Rev. 2016, 64, 823–836. [Google Scholar] [CrossRef]
- Li, F.; Biagioni, P.; Bollani, M.; Maccagnan, A.; Piergiovanni, L. Multi-functional coating of cellulose nanocrystals for flexible packaging applications. Cellulose 2013, 20, 2491–2504. [Google Scholar] [CrossRef]
- Azeredo, H.M.; Rosa, M.F.; Mattoso, L.H.C. Nanocellulose in bio-based food packaging applications. Ind. Crop. Prod. 2017, 97, 664–671. [Google Scholar] [CrossRef]
- Farooq, A.; Patoary, M.K.; Zhang, M.; Mussana, H.; Li, M.; Naeem, M.A.; Mushtaq, M.; Farooq, A.; Liu, L. Cellulose from sources to nanocellulose and an overview of synthesis and properties of nanocellulose/zinc oxide nanocomposite materials. Int. J. Biol. Macromol. 2020, 154, 1050–1073. [Google Scholar] [CrossRef]
- Homaeigohar, S.; Boccaccini, A.R. Antibacterial biohybrid nanofibers for wound dressings. Acta Biomater. 2020, 107, 25–49. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, H.; Wu, M.; Ma, J.; Lu, P. Bio-based antimicrobial packaging from sugarcane bagasse nanocellulose/nisin hybrid films. Int. J. Biol. Macromol. 2020, 161, 627–635. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, N.; Lin, X.; Nie, S. Emerging challenges in the thermal management of cellulose nanofibril-based supercapacitors, lithium-ion batteries and solar cells: A review. Carbohydr. Polym. 2020, 234, 115888. [Google Scholar] [CrossRef]
- Jose, J.; Thomas, V.; Vinod, V.; Abraham, R.; Abraham, S. Nanocellulose based functional materials for supercapacitor applications. J. Sci. Adv. Mater. Devices 2019, 4, 333–340. [Google Scholar] [CrossRef]
- Guo, R.; Zhang, L.; Lu, Y.; Zhang, X.; Yang, D. Research progress of nanocellulose for electrochemical energy storage: A review. J. Energy Chem. 2020, 51, 342–361. [Google Scholar] [CrossRef]
- Hou, M.; Xu, M.; Hu, Y.; Li, B. Nanocellulose incorporated graphene/polypyrrole film with a sandwich-like architecture for preparing flexible supercapacitor electrodes. Electrochim. Acta 2019, 313, 245–254. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices—A review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Agate, S.; Joyce, M.; Lucia, L.; Pal, L. Cellulose and nanocellulose-based flexible-hybrid printed electronics and conductive composites—A review. Carbohydr. Polym. 2018, 198, 249–260. [Google Scholar] [CrossRef]
- Costes, L.; Laoutid, F.; Brohez, S.; Dubois, P. Bio-based flame retardants: When nature meets fire protection. Mater. Sci. Eng. R Rep. 2017, 117, 1–25. [Google Scholar] [CrossRef]
- Guo, W.; Wang, X.; Zhang, P.; Liu, J.; Song, L.; Hu, Y. Nano-fibrillated cellulose-hydroxyapatite based composite foams with excellent fire resistance. Carbohydr. Polym. 2018, 195, 71–78. [Google Scholar] [CrossRef]
- Gebauer, D.; Oliynyk, V.; Salajkova, M.; Sort, J.; Zhou, Q.; Bergström, L.; Salazar-Alvarez, G. A transparent hybrid of nanocrystalline cellulose and amorphous calcium carbonate nanoparticles. Nanoscale 2011, 3, 3563–3566. [Google Scholar] [CrossRef]
- Ruiz-Palomero, C.; Soriano, M.L.; Valcárcel, M. Nanocellulose as analyte and analytical tool: Opportunities and challenges. TrAC Trends Anal. Chem. 2017, 87, 1–18. [Google Scholar] [CrossRef]
- Dumanli, A.G. Nanocellulose and its Composites for Biomedical Applications. Curr. Med. Chem. 2017, 24, 512–528. [Google Scholar] [CrossRef]
- Singla, R.; Soni, S.; Kulurkar, P.M.; Kumari, A.; Mahesh, S.; Patial, V.; Padwad, Y.S.; Yadav, S.K. In situ functionalized nanobiocomposites dressings of bamboo cellulose nanocrystals and silver nanoparticles for accelerated wound healing. Carbohydr. Polym. 2017, 155, 152–162. [Google Scholar] [CrossRef]
- Luzi, F.; Puglia, D.; Torre, L. 10—Natural fiber biodegradable composites and nanocomposites: A biomedical application. In Biomass, Biopolymer-Based Materials, and Bioenergy; Verma, D., Fortunati, E., Jain, S., Zhang, X., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 179–201. [Google Scholar] [CrossRef]
- Sampath Udeni Gunathilake, T.M.; Ching, Y.C.; Chuah, C.H.; Rahman, N.A.; Liou, N.-S. Recent advances in celluloses and their hybrids for stimuli-responsive drug delivery. Int. J. Biol. Macromol. 2020, 158, 670–688. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, J.; Mishra, H.; Mishra, P.K.; Wimmer, R.; Ahmad, F.J.; Talegaonkar, S. Cellulose nanofiber aerogel as a promising biomaterial for customized oral drug delivery. Int. J. Nanomed. 2017, 12. [Google Scholar] [CrossRef] [PubMed]
- Amiralian, N.; Mustapic, M.; Hossain, M.S.A.; Wang, C.; Konarova, M.; Tang, J.; Na, J.; Khan, A.; Rowan, A. Magnetic nanocellulose: A potential material for removal of dye from water. J. Hazard. Mater. 2020, 394, 122571. [Google Scholar] [CrossRef] [PubMed]
- Gennari, A.; Führ, A.J.; Volpato, G.; Volken de Souza, C.F. Magnetic cellulose: Versatile support for enzyme immobilization—A review. Carbohydr. Polym. 2020, 246, 116646. [Google Scholar] [CrossRef]
- Olsson, R.T.; Samir, M.A.S.A.; Salazar-Alvarez, G.; Belova, L.; Strom, V.; Berglund, L.A.; Ikkala, O.; Nogues, J.; Gedde, U.W. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates. Nat. Nanotechnol. 2010, 5, 584–588. [Google Scholar] [CrossRef]
- Singh, A.A.; Khan, M.J.; Ansari, M.A.; Farooqi, H.; Svedberg, A.; Karim, Z. Chapter 8—Nanocellulose and nanohydrogel matrices as sustainable biomass materials: Structure, properties, present status, and future prospects in construction and other engineering. In Sustainable Nanocellulose and Nanohydrogels from Natural Sources; Mohammad, F., Al-Lohedan, H.A., Jawaid, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 177–195. [Google Scholar] [CrossRef]
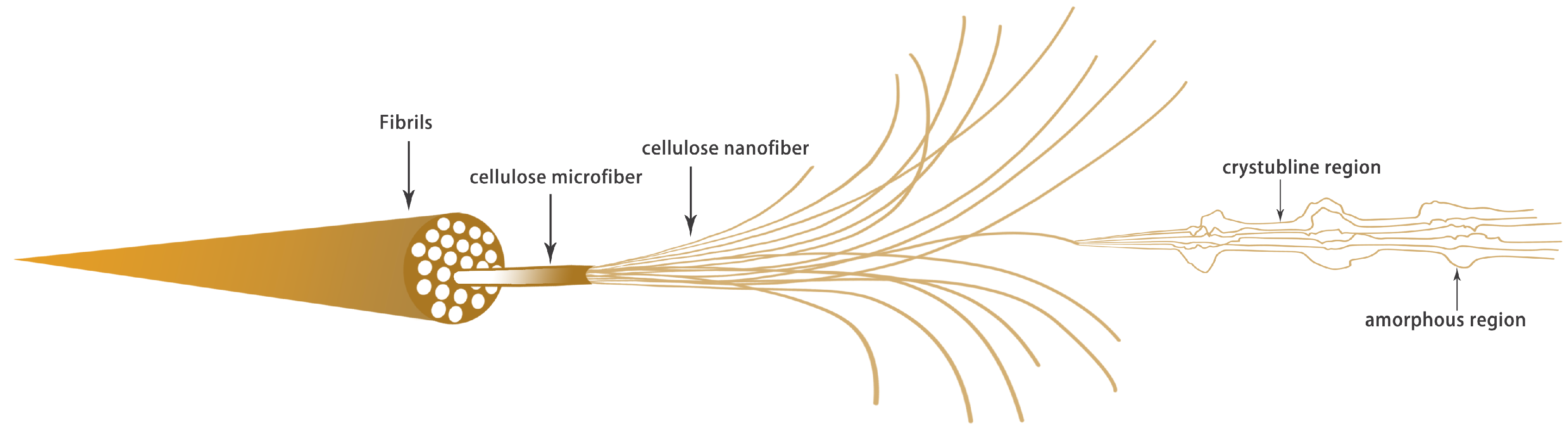
| Raw Material | Fiber Content (%) | Fiber Length (mm) | Fiber Diameter (µm) | Lignin Content (%) | References |
|---|---|---|---|---|---|
| Wood-Conifer | 40–53 | 2–5 | 30–70 | 25–35 | Wang et al. [28], Winandy et al. [29], Pei et al. [31], Eero et al. [32], Mishra et al. [33] |
| Wood-Hardwood | 41–44 | ≈1 | 14–20 | 20–25 | |
| Bast Fiber | 65–80 | – | 10–25 | 4–20 | |
| Herb | 35–55 | 1–2 | 10–30 | 15–25 | |
| Cotton Fiber | 95–97 | 25–65 | 12–38 | – | |
| Lint | 90–91 | 10–20 | <3 | ||
| Brewing Waste | 16–25 | – | – | 11–27 |
| TEMPO Oxidation System | pH | Temperature Reflex (°C) | Oxidation Yield (%) | References |
|---|---|---|---|---|
| TEMPO/NaClO/NaBr | 10 | Room Temperature | 75–98 | Saito et al. [57] |
| TEMPO/NaClO/NaClO2 | 7 | 60 | ≈100 | Saito et al. [65] |
| 4–acetamide–TEMPO/NaClO/NaClO2 | 3–7 | 40–60 | 40–70 | Hirota et al. [69] |
| 4–acetamide–TEMPO Dielectric Oxidation (0.5 V) | 6–8 | 91–98 | 91–98 | Isogai et al. [53] |
| Laccase / TEMPO or Amino TEMPO | 7 | 30 | – | Jiang et al. [70], Bu et al. [71] |
| TEMPO/NaClO/Na2 SO4 /NaBr | 10 | Room Temperature | >95 | Inamochi et al. [54] |
| Pretreatment Method | Effect on Fiber | Fiber Quality | Industrialization Prospects | References |
|---|---|---|---|---|
| Enzymatic Hydrolysis | DP Drops, CI Drops | High Degree of Fibrillation, no Functionalization | Excellent | Pääkkö et al. [46], Henriksson et al. [47], Nechyporchuk et al. [48] |
| TEMPO Oxidation | DP Drops, CI Drops | Functionalize Cellulose and Make the Surface of Cellulose Carry Charges | General | Isogai et al. [23], Tarres et al. [58], Shinoda et al. [61] |
| Carboxymethylation | DP Dropped Slightly, CI Basically Had no Effect | Functionalize Cellulose, which is Stronger than Enzymatically Degraded Fiber and Contains More Fiber Fragments | General | Wagberg et al. [102], Siró et al. [73], Naderi et al. [76] |
| Phosphorylation | DP Drops, CI Basically Has no Effect | Reinforce CNFs Fiber Strength, Impart Flame Retardancy to Fiber and Introduce Negative Charge | Excellent | Maryam et al. [79], Naderi et al. [77], Noguchi et al. [80] |
| Cationization | DP Drops, CI Basically Has no Effect | Improve Fiber Dispersion, can make CNFs Products have Good Antibacterial Properties or in Certain Cytotoxicity and introduce Positive Charge | Bad | Odabas et al. [85], Chaker et al. [87] Littunen et al. [82] |
| Periodate Oxidation | DP Drops, CI Drops | Excellent Dispersibility, Fiber Structure and Functionalization | Bad | Sven et al. [95], Larsson et al. [96], Sirviö et al. [94] |
| Supercritical Fluid Technology | DP Basically Has no Effect, CI Improve | Promote the Fibrillating of Fiber, Improve the Thermal Performance and Introduce Functional Groups Directly to the Surface of Cellulose | General | Stoja et al. [100], Roozbeh et al. [103], Takashi et al. [104] |
| Application Field | Reference |
|---|---|
| Composite Material | Siro et al. [182], Mousa et al. [183], Mathew et al. [184] |
| Sensor | Dong et al. [185], Ling et al. [186], Sun et al. [187] |
| Food Packaging | Abdul et al. [188], Nathalie et al. [9], Li et al. [189], Azeredo et al. [190] |
| Food Packaging | Amjad et al. [191], Shahin et al. [192], Yang et al. [193] |
| Capacitor | Zhang et al. [194], Jose et al. [195], Guoet al. [196], Hou et al. [197] |
| Conductive Material | Xu et al. [198], Agate et al. [199] |
| Fireproof Materials | Costes et al. [200], Ghanadpour et al. [79], Guo et al. [201], Gebauer et al. [202] |
| Chemical Substance Detection | Ruiz-Palomero et al. [203] |
| Medical | Dumanli et al. [204], Singla et al. [205], Luzi et al. [206], Sampath et al. [207], Bhandari et al. [208] |
| Magnetic Material | Amiralian et al. [209], Adriano et al. [210], Olsson et al. [211] |
| Engineering Building | Singh et al. [212] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, T.; Zhao, H.; Mo, Q.; Pan, D.; Liu, Y.; Huang, L.; Xu, H.; Hu, B.; Song, H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials 2020, 13, 5062. https://doi.org/10.3390/ma13225062
Yi T, Zhao H, Mo Q, Pan D, Liu Y, Huang L, Xu H, Hu B, Song H. From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials. 2020; 13(22):5062. https://doi.org/10.3390/ma13225062
Chicago/Turabian StyleYi, Tan, Hanyu Zhao, Qi Mo, Donglei Pan, Yang Liu, Lijie Huang, Hao Xu, Bao Hu, and Hainong Song. 2020. "From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils" Materials 13, no. 22: 5062. https://doi.org/10.3390/ma13225062
APA StyleYi, T., Zhao, H., Mo, Q., Pan, D., Liu, Y., Huang, L., Xu, H., Hu, B., & Song, H. (2020). From Cellulose to Cellulose Nanofibrils—A Comprehensive Review of the Preparation and Modification of Cellulose Nanofibrils. Materials, 13(22), 5062. https://doi.org/10.3390/ma13225062