A Novel, Nontoxic and Scalable Process to Produce Lipidic Vehicles
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Lipidic Vehicle Preparation through the MHM
2.2.2. Lyophilization and Reconstitution
2.2.3. Incorporation of Curcumin
2.2.4. Light Scattering Techniques
2.2.5. Statistical Analysis
3. Results
3.1. Physicochemical Properties and Colloidal Stability of the Prepared Lipidic Vehicles
3.2. Physicochemical Properties of Lyophilized Lipidic Vehicles after Reconstitution
3.3. Physicochemical Properties and Colloidal Stability of Lipidic Vehicles with Incorporated Curcumin
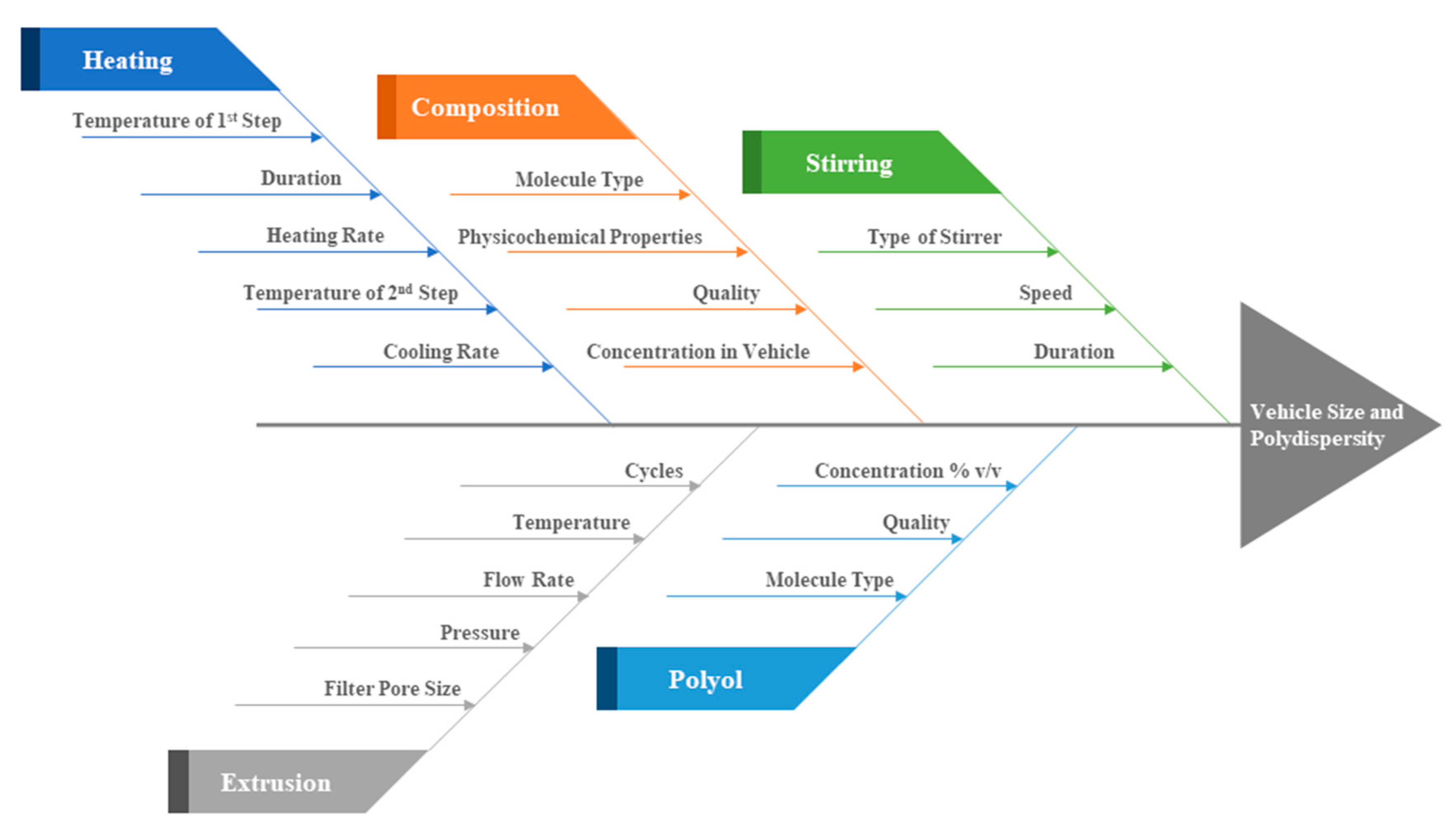
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Papahadjopoulos, D.; Watkins, J.C. Phospholipid model membrane: Permeability properties of hydrated liquid crystals. Biochim. Biophys. Acta 1967, 135, 639–652. [Google Scholar] [CrossRef]
- Milhaud, J. New insights into water-phospholipid model membrane interactions. Biochim. Biophys. Acta 2004, 1663, 19–51. [Google Scholar] [CrossRef] [PubMed]
- Lasic, D.D.; Joannic, R.; Keller, B.C.; Frederik, P.M.; Auvray, L. Spontaneous vesiculation. Adv. Colloid Interface Sci. 2001, 89, 337–349. [Google Scholar] [CrossRef]
- Demetzos, C. Pharmaceutical Nanotechnology: Fundamentals and Practical Applications, 1st ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Guida, V. Thermodynamics and kinetics of vesicles formation processes. Adv. Colloid Interface Sci. 2010, 161, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Puglia, C.; Bonina, F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Expert Opin. Drug Deliv. 2012, 9, 429–441. [Google Scholar] [CrossRef]
- Israelachvili, J.N.; Mitchell, D.J.; Ninham, B.W. Theory of self-assembly of hydrocarbon amphiphiles into micelles and bilayers. J. Chem. Soc. Far. Trans. 2 Mol. Chem. Phys. 1976, 72, 1525–1568. [Google Scholar] [CrossRef]
- Nagarajan, R. Molecular Packing Parameter and Surfactant Self-Assembly: The Neglected Role of the Surfactant Tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in cosmetics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Ishida, T. Liposomal Delivery systems: Design Optimization and Current Applications. Biol. Pharm. Bull. 2017, 40, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Moses, J.A.; Anandharamakrishnan, C. Encapsulation of Nutraceutical Ingredeints in Liposomes and their Potential for Cancer Treatment. Nutr. Cancer 2018, 70, 1184–1198. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Cuo, N.; Teng, Y.; Mengd, X.; Sun, H.; Li, S.; Yu, P.; Galons, H. Composistion design and medical applications of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Allen, T.M.; Gabizon, A.; Mayhew, E.; Matthay, K.; Huang, S.K.; Lee, K.D.; Woodle, M.C.; Lasic, D.D.; Redemann, C. Sterically stabilized liposomes: Improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc. Natl. Acad. Sci. USA 1991, 88, 11460–11464. [Google Scholar] [CrossRef]
- Riaz, M. Liposome Preparation Methods. Pak J. Pharm. Sci. 1996, 19, 65–77. [Google Scholar]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef]
- Worsham, R.D.; Thomas, V.; Farid, S.S. Potential of Continuous Manufacturing for Liposomal Drug Products. Biotechnol. J. 2019, 14, e1700740. [Google Scholar] [CrossRef]
- Naziris, N.; Pippa, A.G.; Demetzos, C. Process for the Production of Lipidic. Vehicles. Patent EP3533442A1, 28 February 2018. [Google Scholar]
- Mozafari, M.R.; Reed, C.J.; Rostron, C. Cytotoxicity evaluation of anionic nanoliposomes and nanolipoplexes prepared by the heating method without employing volatile solvents and detergents. Pharmazie 2007, 62, 205–209. [Google Scholar]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.I.; Yeh, M.K. Clinical development of liposome-based drugs: Formulation, characterization, and therapeutic efficacy. Int. J. Nanomed. 2012, 7, 49–60. [Google Scholar]
- Mozafari, M.R. Nanoliposomes: Preparation and analysis. Methods Mol. Biol. 2010, 605, 29–50. [Google Scholar]
- Villasmil-Sánchez, S.; Drhimeur, W.; Ospino, S.C.; Rabasco Alvarez, A.M.; González-Rodríguez, M.L. Positively and negatively charged liposomes as carriers for transdermal delivery of sumatriptan: In vitro characterization. Drug Dev. Ind. Pharm. 2010, 36, 666–675. [Google Scholar] [CrossRef]
- Papagiannaros, A.; Hatziantoniou, S.; Dimas, K.; Papaioannou, G.T.; Demetzos, C. A liposomal formulation of doxorubicin, composed of hexadecylphosphocholine (HePC): Physicochemical characterization and cytotoxic activity against human cancer cell lines. Biomed. Pharm. 2006, 60, 36–42. [Google Scholar] [CrossRef]
- Mattheolabakis, G.; Nie, T.; Constantinides, P.P.; Rigas, B. Sterically stabilized liposomes incorporating the novel anticancer agent phospho-ibuprofen (MDC-917): Preparation, characterization, and in vitro/in vivo evaluation. Pharm. Res. 2012, 29, 1435–1443. [Google Scholar] [CrossRef]
- Wei, X.; Cohen, R.; Barenholz, Y. Insights into composition/structure/function relationships of Doxil® gained from “high-sensitivity” differential scanning calorimetry. Eur. J. Pharm. Biopharm. 2016, 104, 260–270. [Google Scholar] [CrossRef]
- Bruggemann, E.P.; Melchior, D.L. Alterations in the organization of phosphatidylcholine/CHOL bilayers by tetrahydrocannabinol. J. Biol. Chem. 1983, 258, 8298–8303. [Google Scholar]
- Rajendran, V.; Rohra, S.; Raza, M.; Hasan, G.M.; Dutt, S.; Ghosh, P.C. SA Liposomal Delivery of Monensin in Combination with Free Artemisinin Eliminates Blood Stages of Plasmodium falciparum in Culture and P. berghei Infection in Murine Malaria. Antimicrob. Agents Chemother. 2015, 60, 1304–1318. [Google Scholar] [CrossRef]
- Naziris, N.; Pippa, N.; Stellas, D.; Chrysostomou, V.; Pispas, S.; Demetzos, C.; Libera, M.; Trzebicka, B. Development and Evaluation of Stimuli-Responsive Chimeric Nanostructures. AAPS Pharmscitech. 2018, 19, 2971–2989. [Google Scholar] [CrossRef] [PubMed]
- Naziris, N.; Pippa, N.; Meristoudi, A.; Pispas, S.; Demetzos, C. Design and development of pH-responsive HSPC:C12H25-PAA chimeric liposomes. J. Liposome Res. 2017, 27, 108–117. [Google Scholar] [CrossRef]
- Ong, S.G.; Chitneni, M.; Lee, K.S.; Ming, L.C.; Yuen, K.H. Evaluation of Extrusion Technique for Nanosizing Liposomes. Pharmaceutics 2016, 8, 36. [Google Scholar] [CrossRef]
- Shah, V.M.; Nguyen, D.X.; Patel, P.; Cote, B.; Al-Fatease, A.; Pham, Y.; Huynh, M.G.; Woo, Y.; Alani, A.W. Liposomes produced by microfluidics and extrusion: A comparison for scale-up purposes. Nanomedicine 2019, 18, 146–156. [Google Scholar] [CrossRef]
- Stark, B.; Pabst, G.; Prassl, R. Long-term stability of sterically stabilized liposomes by freezing and freeze-drying: Effects of cryoprotectants on structure. Eur. J. Pharm. Sci. 2010, 41, 546–555. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6, E92. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Tommonaro, G. Curcumin and Cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “Curecumin”: From kitchen to clinic. Biochem. Pharm. 2008, 75, 787–809. [Google Scholar] [CrossRef]
- Feng, T.; Wei, Y.; Lee, R.J.; Zhao, L. Liposomal curcumin and its application in cancer. Int. J. Nanomed. 2017, 12, 6027–6044. [Google Scholar] [CrossRef]
- Pandelidou, M.; Dimas, K.; Georgopoulos, A.; Hatziantoniou, S.; Demetzos, C. Preparation and characterization of lyophilised egg PC liposomes incorporating curcumin and evaluation of its activity against colorectal cancer cell lines. J. Nanosci. Nanotechnol. 2011, 11, 1259–1266. [Google Scholar] [CrossRef]
- García-Sáez, A.J.; Schwille, P. Stability of lipid domains. FEBS Lett. 2010, 584, 1653–1658. [Google Scholar] [CrossRef]
- Daraee, H.; Etemadi, A.; Kouhi, M.; Alimirzalu, S.; Akbarzadeh, A. Application of liposomes in medicine and drug delivery. Artif. Cells Nanomed. Biotechnol. 2016, 44, 381–391. [Google Scholar] [CrossRef] [PubMed]
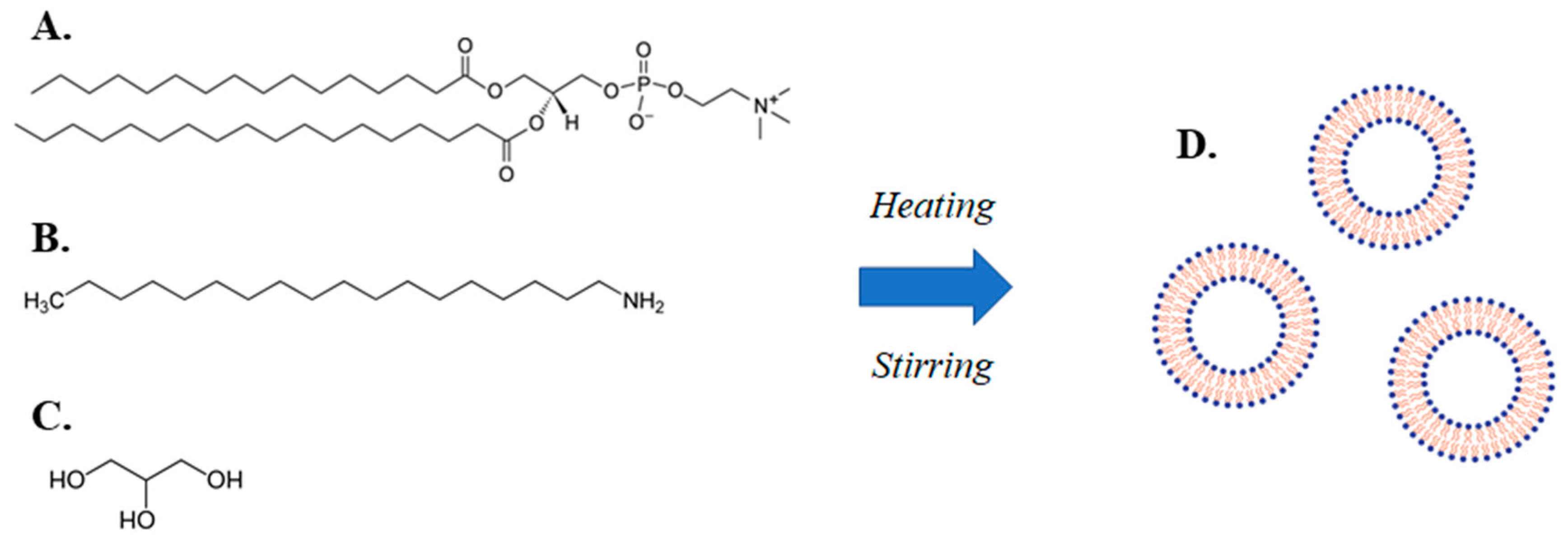
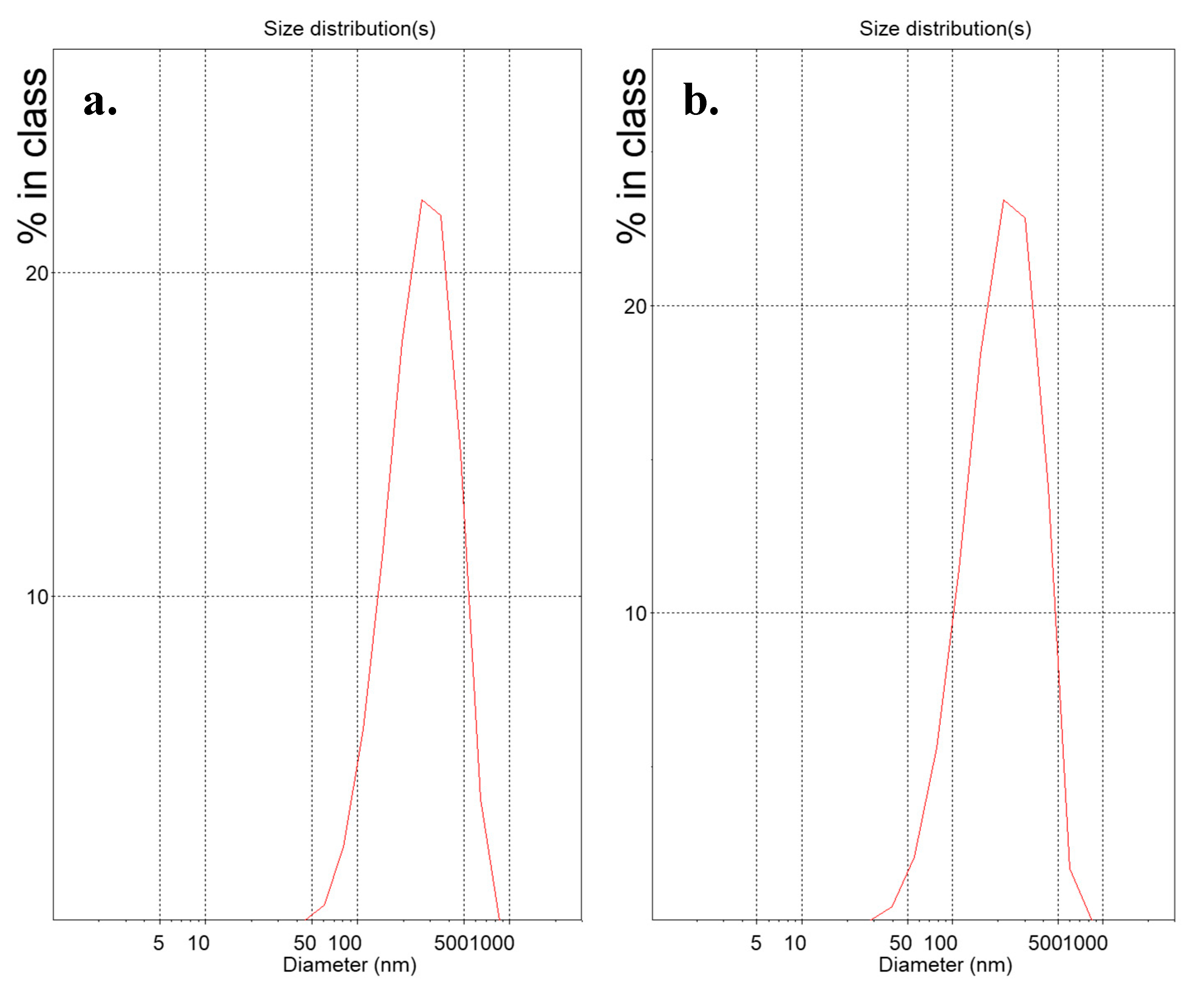
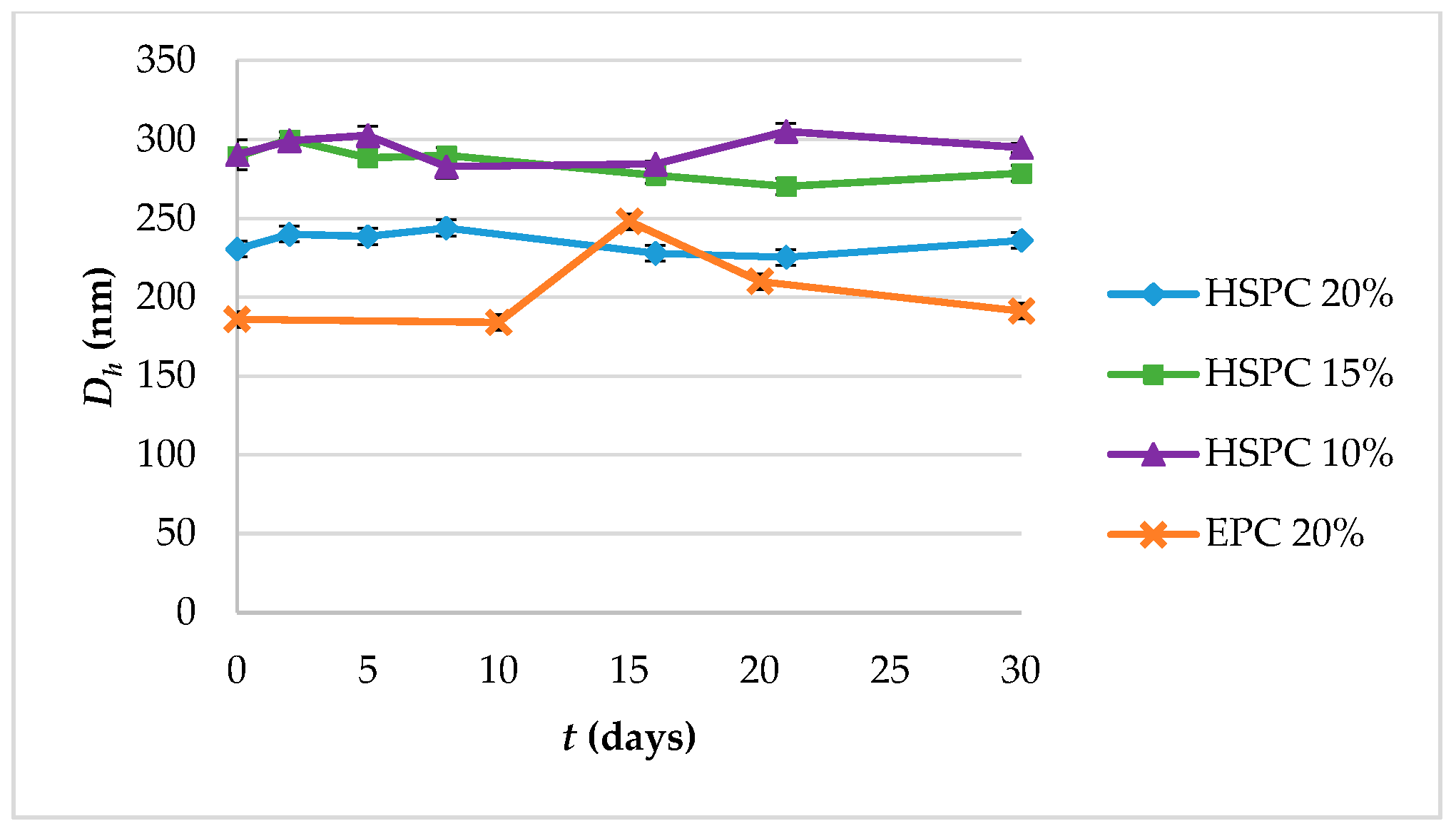
| System | Molar Ratio | Glycerine Concentration (% v/v) | Hours at 90 °C | Dh1 (nm) | SD 2 | PDI 3 | SD | z-pot 4 (mV) | SD |
|---|---|---|---|---|---|---|---|---|---|
| HSPC:SA | 9:0.25 | 20% | 1 | 275.5 | 4.1 | 0.349 | 0.003 | - | - |
| HSPC:SA | 9:0.25 | 20% | 2 | 230.5 | 4.6 | 0.272 | 0.017 | 51.5 | 2.9 |
| HSPC:SA | 9:0.25 | 15% | 1 | 366.8 | 10.8 | 0.616 | 0.078 | - | - |
| HSPC:SA | 9:0.25 | 15% | 2 | 289.2 | 10.0 | 0.432 | 0.016 | 52.8 | 2.2 |
| HSPC:SA | 9:0.25 | 10% | 1 | 346.9 | 9.4 | 0.567 | 0.080 | - | - |
| HSPC:SA | 9:0.25 | 10% | 2 | 290.4 | 9.7 | 0.484 | 0.055 | 54.5 | 0.6 |
| EPC:CHOL:SA | 9:0.5:0.25 | 20% | 2 | 272.2 | 13.9 | 0.602 | 0.026 | - | - |
| EPC:CHOL:SA | 9:1.8:0.25 | 20% | 2 | 186.0 | 4.8 | 0.382 | 0.007 | - | - |
| System | Molar Ratio | Glycerine Concentration (% v/v) | Dh1 (nm) | SD 2 | PDI 3 | SD | z-pot 4 (mV) | SD |
|---|---|---|---|---|---|---|---|---|
| HSPC:SA | 9:0.25 | 20% | 228.3 | 1.6 | 0.294 | 0.021 | 42.3 | 0.7 |
| HSPC:SA | 9:0.25 | 15% | 304.8 | 1.3 | 0.559 | 0.016 | 49.2 | 1.9 |
| System | Molar Ratio | Lipid Concentration (mg/mL) | Glycerine Concentration(% v/v) | Dh1 (nm) | SD 2 | PDI 3 | SD |
|---|---|---|---|---|---|---|---|
| HSPC:SA:CUR | 9:0.25:0.8 | 10 | 20% | 277.9 | 5.3 | 0.468 | 0.056 |
| HSPC:SA:CUR | 9:0.25:1 | 33 | 5% | 459.3 | 11.5 | 0.842 | 0.032 |
| HSPC:SA:CUR | 9:0.25:1 | 50 | 10% | 539.4 | 5.8 | 0.706 | 0.126 |
| System | Preparation Method | Molar Ratio | Dh1 (nm) | SD 2 | PDI 3 | SD | Source |
|---|---|---|---|---|---|---|---|
| EPC:CH:SA | MHM | 9:0.5:0.25 | 272.2 | 13.9 | 0.602 | 0.026 | Present |
| EPC:CH:SA | MHM | 9:1.8:0.25 | 186.0 | 4.8 | 0.382 | 0.007 | |
| EPC:CH:SA | TLE 5 | 6.6:10.3:3.71 | 408.6 | 279.1 | 0.56 | 0.01 | 24 |
| EPC:CH:SA | TLE | 6.6:10.3:7.42 | 311.4 | 154.6 | 0.25 | 0.09 | |
| EPC:CH:SA | TLE | 6.6:10.3:11.13 | 348.7 | 100.3 | 0.28 | 0.24 | |
| EPC:CH:SA | REV | 6.6:10.3:3.71 | 313.5 | 97.8 | 0.30 | 0.15 | |
| EPC:CH:SA | REV | 6.6:10.3:7.42 | 376.5 | 160.7 | 0.18 | 0.17 | |
| EPC:CH:SA | REV | 6.6:10.3:11.13 | 603.7 | 180.5 | 0.34 | 0.31 | |
| EPC:CH:SA | FAT 6 | 6.6:10.3:3.71 | 360.0 | 220.3 | 0.48 | 0.12 | |
| EPC:CH:SA | FAT | 6.6:10.3:7.42 | 351.1 | 121.8 | 0.31 | 0.15 | |
| EPC:CH:SA | FAT | 6.6:10.3:11.13 | 317.1 | 232.8 | 0.26 | 0.14 | |
| EPC:SA | REV 4 | 10:0.1 | 91.3 | 0.7 | - | - | 25 |
| EPC:SA | TFH 7 | 95:5 | 131 | 8 | 0.082 | 0.08 | 26 |
| EPC:SA | TFH | 90:10 | 127 | 2 | 0.098 | 0.01 | |
| EPC:SA | TFH | 85:15 | 123 | 3 | 0.112 | 0.01 |
| System | Preparation Method | Molar Ratio | Glycerine Concentration (% v/v) | Dh1 (nm) | SD 2 | PDI 3 | Source |
|---|---|---|---|---|---|---|---|
| HSPC:SA | MHM | 9:0.25 | 20% | 230.5 | 4.6 | 0.272 | Present |
| HSPC:SA:CUR | MHM | 9:0.25:0.8 | 20% | 277.9 | 5.3 | 0.468 | |
| EPC | TFH 4 | - | - | 123.5 | 9.9 | 0.100 | 39 |
| EPC:CUR | TFH | 14:1 | - | 108.0 | 8.9 | 0.146 |
| Production Method | Absence of Toxic Organic Solvents | Simple | Small and Monodisperse Vehicle Size | Does not Require Size-Reduction | Easy Scale-Up |
|---|---|---|---|---|---|
| Thin-film Hydration | - | ✓ | - | - | - |
| Reverse-phase Evaporation | - | ✓ | - | - | - |
| Solvent Injection | - | ✓ | - | - | ✓ |
| Detergent Depletion (Dialysis) | - | - | - | - | - |
| Supercritical Fluid | ✓ | - | - | - | ✓ |
| Modified Heating Method | ✓ | ✓ | ✓ | ✓ | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naziris, N.; Pippa, N.; Demetzos, C. A Novel, Nontoxic and Scalable Process to Produce Lipidic Vehicles. Materials 2020, 13, 5035. https://doi.org/10.3390/ma13215035
Naziris N, Pippa N, Demetzos C. A Novel, Nontoxic and Scalable Process to Produce Lipidic Vehicles. Materials. 2020; 13(21):5035. https://doi.org/10.3390/ma13215035
Chicago/Turabian StyleNaziris, Nikolaos, Natassa Pippa, and Costas Demetzos. 2020. "A Novel, Nontoxic and Scalable Process to Produce Lipidic Vehicles" Materials 13, no. 21: 5035. https://doi.org/10.3390/ma13215035
APA StyleNaziris, N., Pippa, N., & Demetzos, C. (2020). A Novel, Nontoxic and Scalable Process to Produce Lipidic Vehicles. Materials, 13(21), 5035. https://doi.org/10.3390/ma13215035







