First-Principle Studies on the Mechanical and Electronic Properties of AlxNiyZrz (x = 1~3, y = 1~2, z = 1~6) Alloy under Pressure
Abstract
1. Introduction
2. Computational Method and Theory
3. Results and Discussion
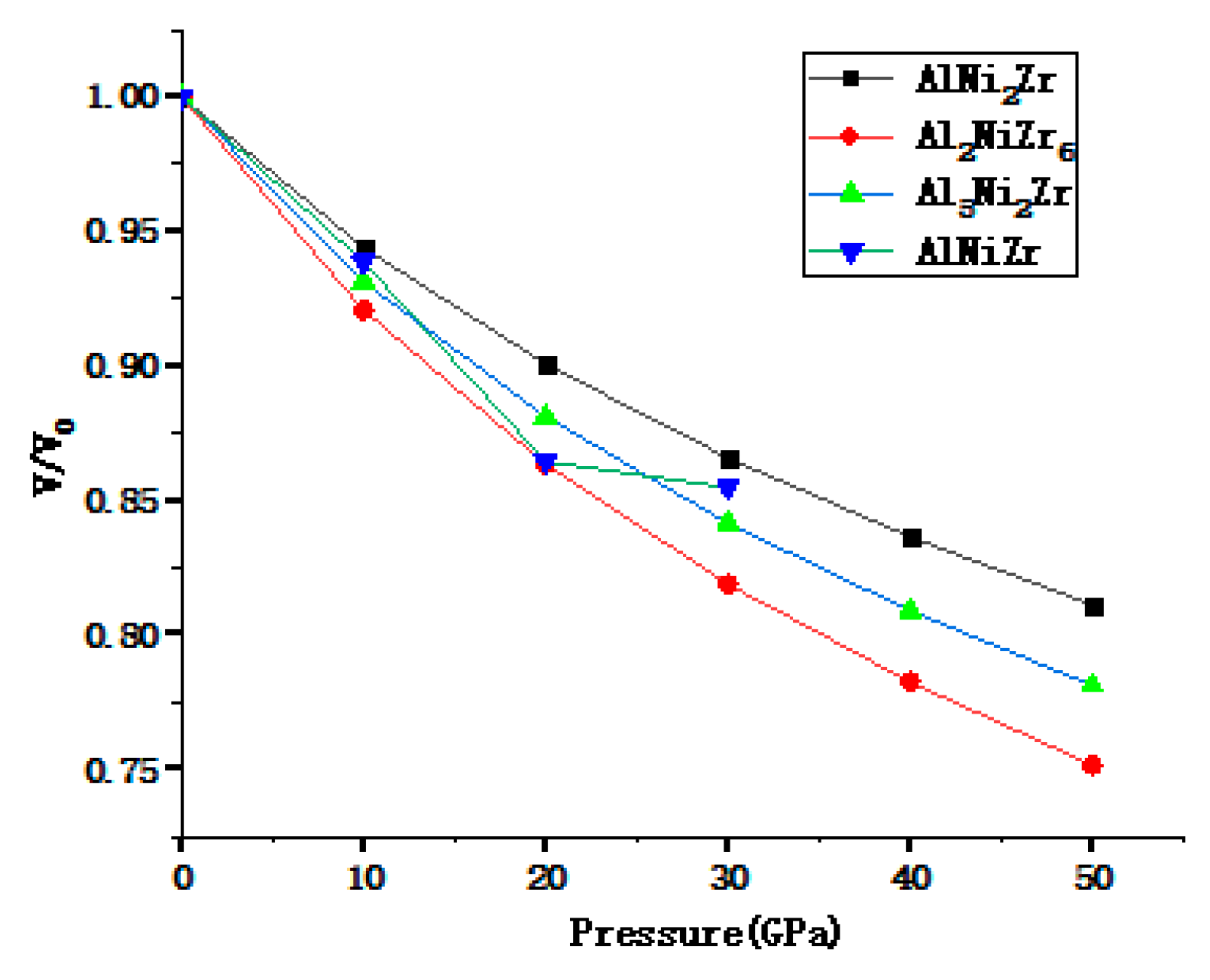
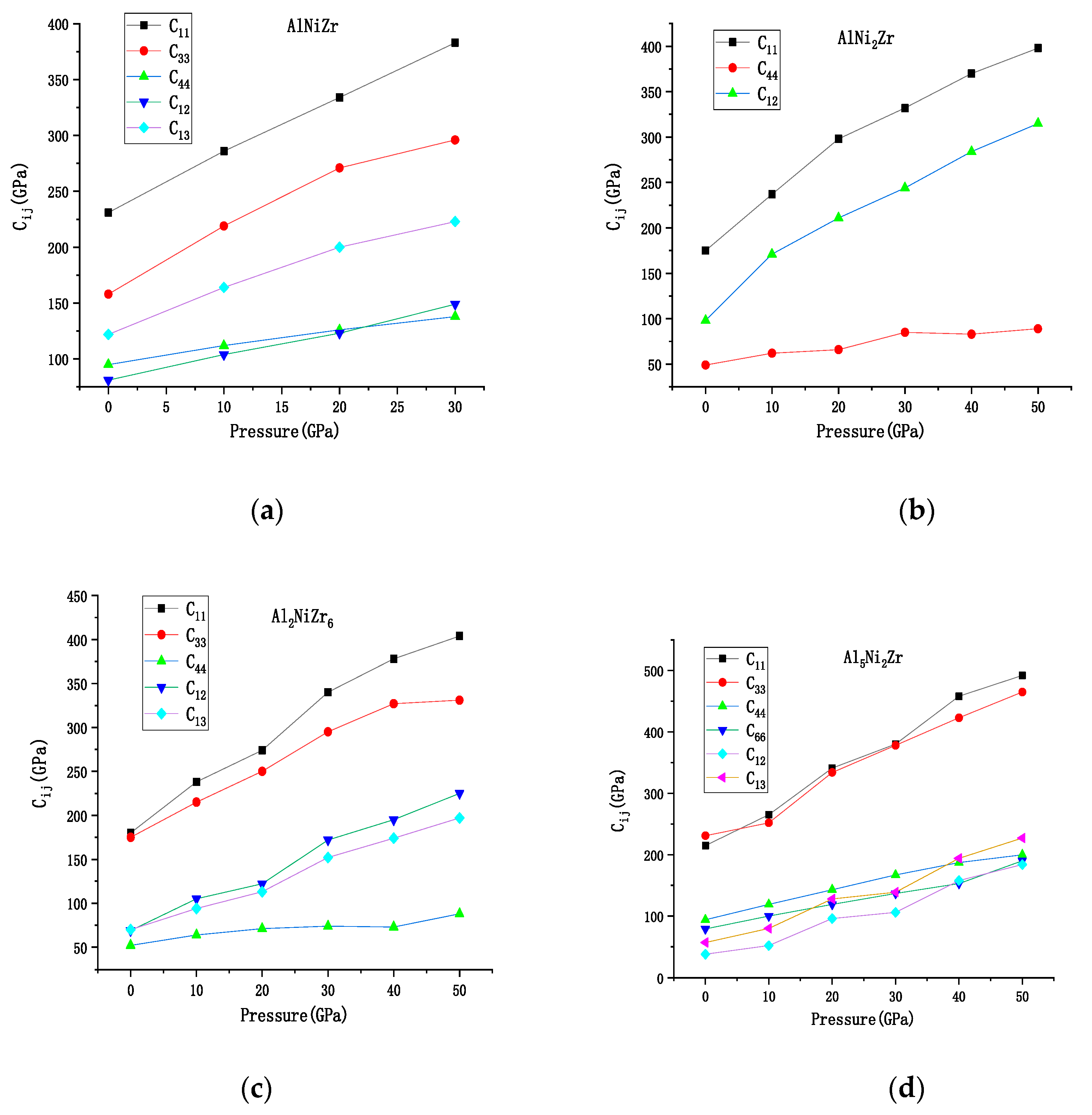
3.1. The Elastic Properties under Pressure
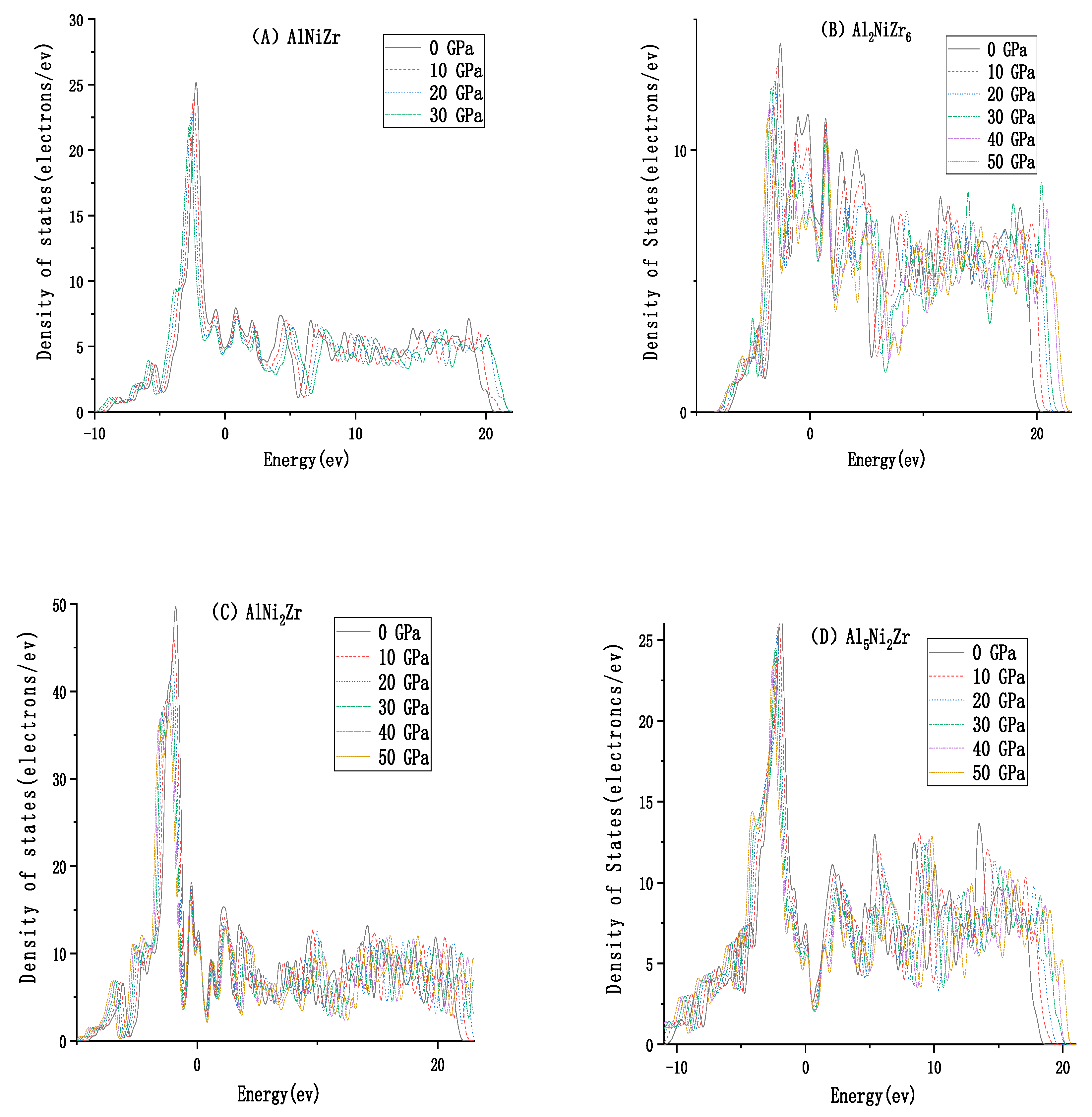
3.2. The Electronic Properties
3.3. Superconducting Properties
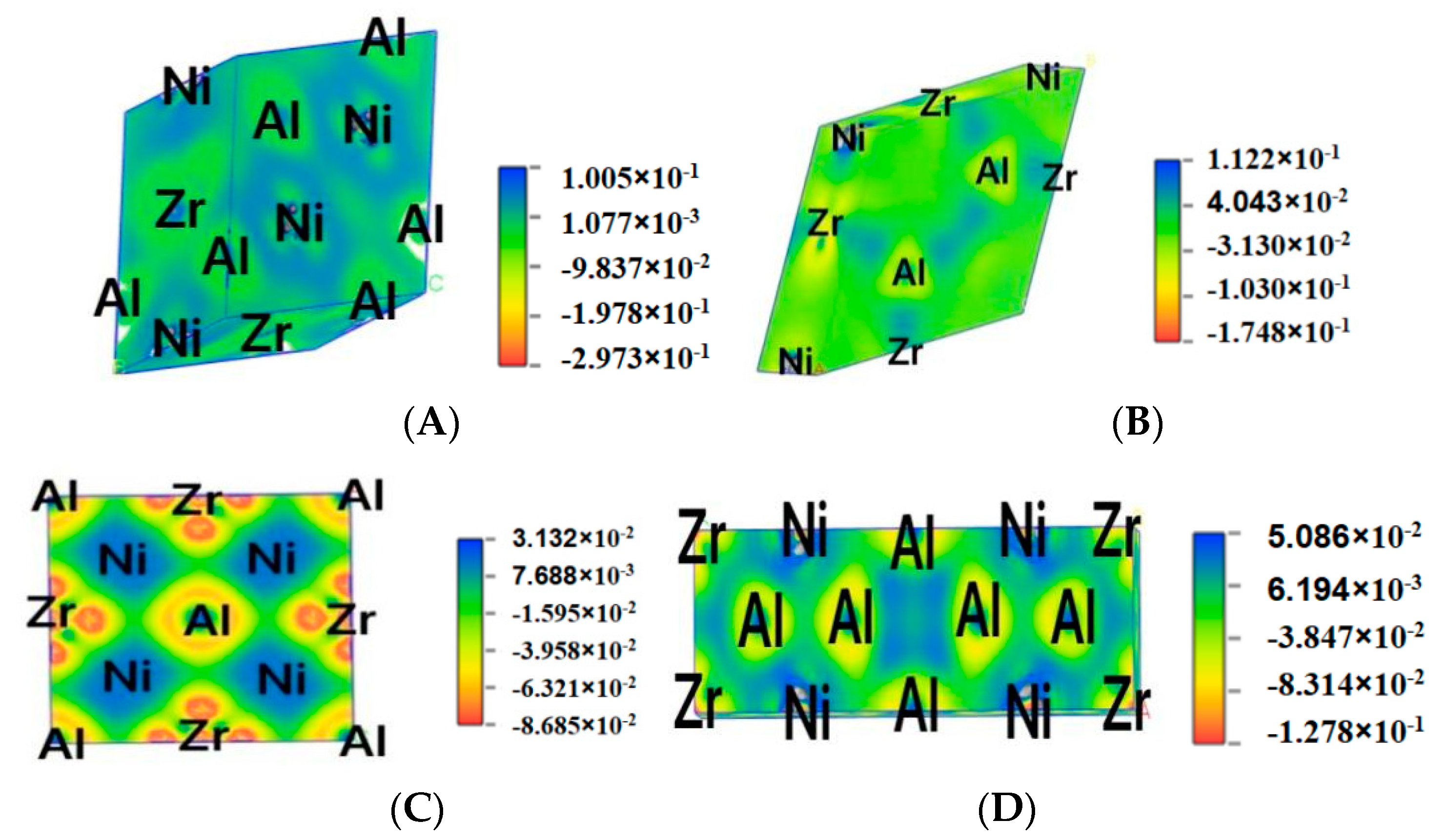
3.4. Difference Charge Density
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, D.; Wen, B.; Melnik, R. First-principles studies of Al-Ni intermetallic compounds. J. Solid State Chem. 2009, 182, 2664–2669. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.K.; Chen, L.Q. Thermodynamic properties of Al, Ni, NiAl and Ni3Al from first-principles calculations. Acta Mater. 2004, 52, 2665–2671. [Google Scholar]
- Xu, C.; Chen, D. Computer Simulation: A Tool for Researching the Basic Physical Properties of NiAl and Ni3Al. Adv. Mat. Res. 2014, 989–994, 216–219. [Google Scholar]
- Yu, S.; Wang, C.Y.; Yu, T. Self-diffusion in the intermetallic compounds NiAl and Ni3Al: An embedded atom method study. Phys. B 2007, 396, 138–144. [Google Scholar] [CrossRef]
- George, E.P.; Liu, C.T.; Pope, D.P. Environmental embrittlement: The major cause of room-temperature brittleness in polycrystalline Ni3Al. Scripta Metall. Mater. 1992, 27, 365–370. [Google Scholar] [CrossRef]
- George, E.P.; Liu, C.T.; Pope, D.P. Intrinsic ductility and environmental embrittlement of binary Ni3Al. Scripta Metall. Mater. 1993, 28, 857–862. [Google Scholar] [CrossRef]
- Chang, T.T.; Pan, Y.C.; Chuang, T.H. The oxidation behavior of Ni3AlZr alloys with various zirconium contents. J. Alloys Compd. 1996, 243, 126–132. [Google Scholar] [CrossRef]
- Lee, D.B.; Santella, M.L. High temperature oxidation of Ni3Al alloy containing Cr, Zr, Mo, and B. Mater. Sci. Eng. A 2004, 374, 217–223. [Google Scholar] [CrossRef]
- Kim, D.E.; Shang, S.L.; Liu, Z.K. Effects of alloying elements on elastic properties of Ni by first-principles calculations. Comput. Mater. Sci. 2009, 47, 254–260. [Google Scholar] [CrossRef]
- Kim, D.E.; Shang, S.L.; Liu, Z.K. Effects of alloying elements on thermal expansions of γ-Ni and γ′-Ni3Al by first-principles calculations. Acta Mater. 2012, 60, 1846–1856. [Google Scholar] [CrossRef]
- Zhang, C.M.; Guo, Y.Q.; Yuan, Z.P. Effect of octadeca carbon fatty acids on microbial fermentation, methanogenesis and microbial flora in vitro. Anim. Feed Sci. Technol. 2008, 146, 259–269. [Google Scholar] [CrossRef]
- Ball, J.; Zeumer, B.; Gottstein, G. Large strain deformation of Ni3Al+B: IV. The effect of Zr and Fe additions. Intermetallics 1995, 3, 209–219. [Google Scholar] [CrossRef]
- Li, Y.F.; Guo, J.T.; Zhou, L.Z. Effect of recrystallization on room-temperature mechanical properties of Zr-doped Ni3Al alloy. Mater. Lett. 2004, 58, 1853–1856. [Google Scholar] [CrossRef]
- Motejadded, H.B.; Soltanieh, M.; Rastegari, S. Dissolution Mechanism of a Zr Rich Structure in a Ni3Al Base Alloy. J. Mater. Sci. Technol. 2011, 27, 885–892. [Google Scholar] [CrossRef]
- Li, Y.F.; Guo, J.T.; Shen, Y.F. Influence of recrystallization and environment on tensile behavior of cold-rolled Ni3Al(Zr)alloys. Trans. Nonferrous Met. Soc. China 2006, 16, 368–375. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, W.; Guo, J.; Hou, J.; Li, X.; Huang, R.; Ma, X.; Zhang, Q. The First-principles Study on the Occupation Behavior and the Ductility Mechanism of Zr in Ni-Ni3Al System with Lattice Misfit. J. Mater. Sci. Technol. 2014, 30, 517–522. [Google Scholar] [CrossRef]
- Li, H.Q.; Yang, Y.S.; Tong, W.H. Gibbs energy calculation of liquid Zr-Al-Ni and Zr-Al-Cu-Ni alloys with clusters. J. Mater. Sci. 2007, 42, 4060–4065. [Google Scholar] [CrossRef]
- Li, H.Q.; Yang, Y.S.; Tong, W.H. Calculation of the solid-liquid interfacial energy for Zr-Ni-Al and Zr-Ni-Al-Cu alloys based on the non-structural approach. Model. Simul. Mater. Sci. Eng. 2006, 14, 1095–1103. [Google Scholar] [CrossRef]
- Liu, Q.J.; Liu, Z.T.; Feng, L.P. First-Principles Calculations of Structural, Elastic and Electronic Properties of Tetragonal HfO2 under Pressure. Commun. Theor. Phys. 2011, 56, 779–784. [Google Scholar] [CrossRef]
- Gonze, X.; Beuken, J.M.; Caracas, R. First-principles computation of material properties: The ABINIT software project. Comp. Mater. Sci. 2002, 25, 478–492. [Google Scholar] [CrossRef]
- Shang, S.L.; Wang, Y.; Kim, D.E. First-principles thermodynamics from phonon and Debye model: Application to Ni and Ni3Al. Comp. Mater. Sci. 2010, 47, 1040–1048. [Google Scholar] [CrossRef]
- Wang, B.T.; Shi, H.; Li, W.D. First-principles study of ground-state properties and high pressure behavior of ThO2. J. Nucl. Mater. 2010, 399, 181–188. [Google Scholar] [CrossRef]
- Payne, M.C.; Arias, T.A.; Joannopoulos, J.D. Iterative minimization techniques for ab initio total-energy calculations: Molecular dynamics and conjugate gradients. Rev. Mod. Phys. 1992, 64, 1045–1097. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X. Necessary and sufficient elastic stability conditions in various crystal systems. Phys. Rev. B 2014, 90, 224104. [Google Scholar] [CrossRef]
- Haines, J.; Leger, J.M.; Bocquillon, G. Synthesis and design of superhardmaterials. Annu. Rev. Mater. Sci. 2001, 31, 1–23. [Google Scholar] [CrossRef]
- Ji, Y.Q.; Yuan, X.L. Elastic Properties and Electronic Properties of MxNy (M = Ti, Zr) from First Principles Calculations. Materials 2018, 11, 1640. [Google Scholar] [CrossRef]
- Zha, C.S.; Mao, H.K.; Hemley, R.J. Elasticity of MgO and a primary pressure scale to 55 GPa. Proc. Natl. Acad. Sci. USA 2000, 97, 13494–13499. [Google Scholar] [CrossRef]
- Sinko, G.V.; Smirnov, N.A. Ab initio calculations of elastic constants and thermodynamic properties of bcc, fcc, and hcp Al crystals under pressure. J. Phys. Condens. Matter 2002, 14, 6989–7005. [Google Scholar]
- Yip, S.; Li, J.; Tang, M.J.; Wang, J.H. Mechanistic aspects and atomic-level consequences of elastic instabilities in homogeneous crystals. Mater. Sci. Eng. A 2001, 317, 236–240. [Google Scholar] [CrossRef]
- Xue, M.; Yuan, X.; Zhong, C.; Wan, P. First principles calculations on elastic, thermodynamic and electronic properties of Co2Zr and Co2Ti at high temperature and pressure. Appl. Sci. 2020, 10, 2097. [Google Scholar] [CrossRef]
- Reuss, A. Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle. J. Appl. Math. Mech. 1929, 9, 49–58. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. A 1952, 65, 349–354. [Google Scholar] [CrossRef]
- Li, X.Z.; Liu, L.; Ding, C.L. First-principles investigations on mechanical stability and elastic properties of hexagonal tungsten dinitride under pressure. Mater. Chem. Phys. 2011, 130, 14–19. [Google Scholar] [CrossRef]
- Born, M. On the stability of crystal lattices. I. Math. Proc. Camb. Philos. Soc. 1940, 36, 160–172. [Google Scholar] [CrossRef]
- Ghebouli, B.; Fatmi, M.; Ghebouli, M.A.; Choutri, H.; Louail, L.; Chihi, T.; Bouhemadou, A.; Bin-Omran, S. Theoretical study of the structural, elastic, electronic and optical properties of XCaF3 (X = K and Rb). Solid State Sci. 2015, 43, 9–14. [Google Scholar] [CrossRef]
- Chen, X.Q.; Niu, H.; Li, D. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Haddadi, K.; Bouhemadou, A.; Louail, L. Structural and elastic properties under pressure effect of the cubic antiperovskite compounds ANCa3 (A = P, As, Sb, and Bi). Phys. Lett. A 2009, 373, 1777–1781. [Google Scholar] [CrossRef]
- Bannikov, V.V.; Shein, I.R.; Ivanovskii, A.L. Ab initio Predictions of Stability and Electronic Properties of Cubic Rhodium Carbides RhCx as Dependent on Carbon Content. Phys. Stat. Solidi Rapid Res. Lett. 2009, 3, 218–220. [Google Scholar] [CrossRef]
- Yuan, X.L.; Xue, M.A.; Chen, W.; An, T.Q.; Cheng, Y. Investigations on the structural, elastic and electronic properties of the orthorhombic Zirconium–Nickel alloy under different pressure. Comput. Mater. Sci. 2012, 65, 127–132. [Google Scholar] [CrossRef]
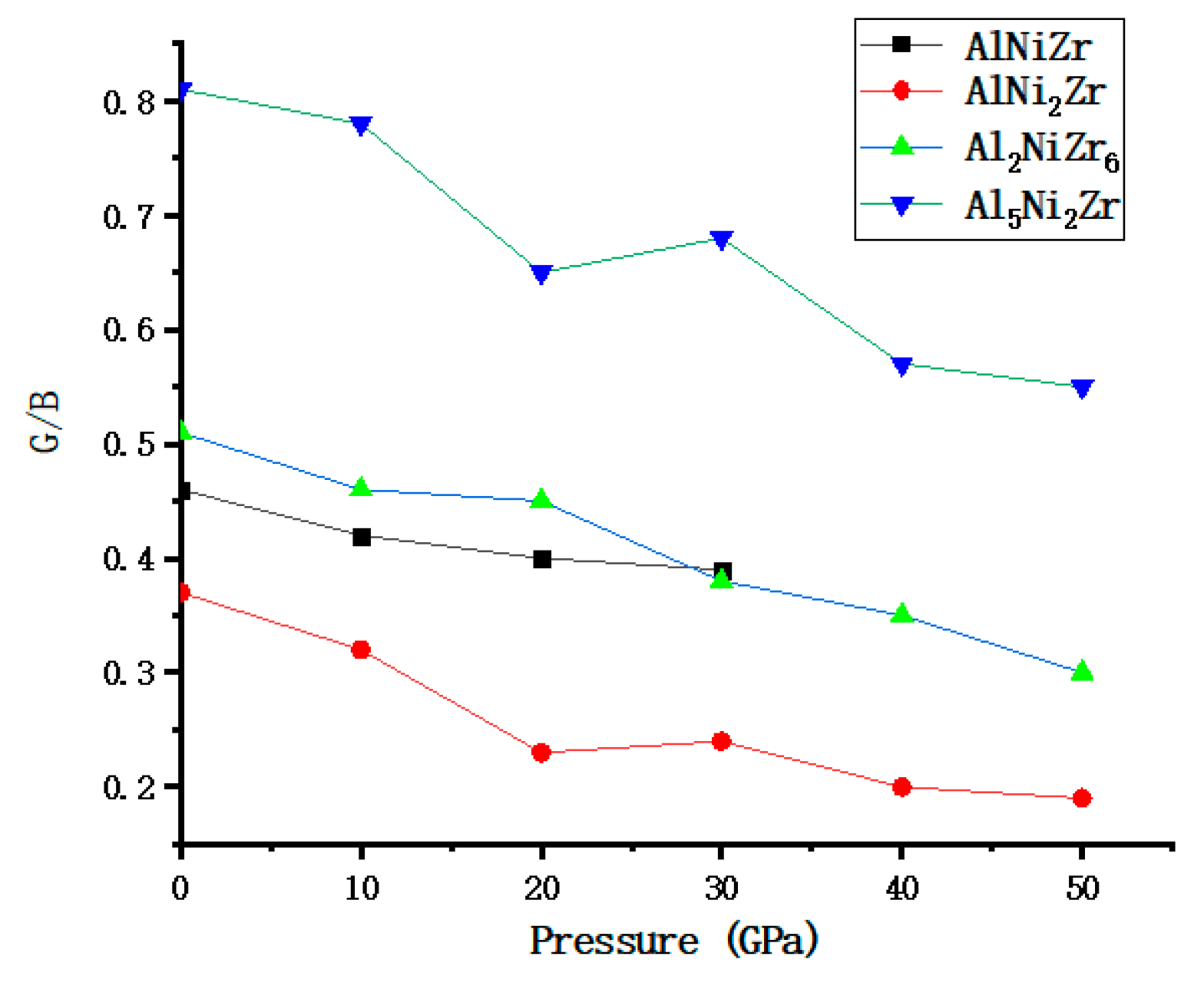

| Physical Quantities | G | B | E | G/B | σ | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P (GPa) | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 |
| 0 | 64 | 45 | 54 | 87 | 140 | 123 | 106 | 107 | 167 | 120 | 137 | 205 | 0.46 | 0.37 | 0.51 | 0.81 | 0.30 | 0.34 | 0.28 | 0.18 |
| 10 | 77 | 48 | 65 | 105 | 184 | 133 | 142 | 134 | 203 | 111 | 170 | 249 | 0.42 | 0.32 | 0.46 | 0.78 | 0.32 | 0.36 | 0.29 | 0.19 |
| 20 | 88 | 56 | 74 | 124 | 220 | 240 | 165 | 191 | 233 | 155 | 192 | 306 | 0.40 | 0.23 | 0.45 | 0.65 | 0.32 | 0.39 | 0.31 | 0.23 |
| 30 | 98 | 65 | 80 | 143 | 250 | 274 | 213 | 212 | 261 | 181 | 213 | 350 | 0.39 | 0.24 | 0.38 | 0.68 | 0.33 | 0.39 | 0.33 | 0.22 |
| 40 | … | 64 | 83 | 155 | … | 313 | 240 | 270 | … | 179 | 223 | 392 | … | 0.20 | 0.35 | 0.57 | … | 0.41 | 0.35 | 0.26 |
| 50 | … | 65 | 88 | 168 | … | 343 | 262 | 303 | … | 184 | 236 | 426 | … | 0.19 | 0.30 | 0.55 | … | 0.41 | 0.35 | 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, X.; Li, W.; Wan, P.; Xue, M.-A. First-Principle Studies on the Mechanical and Electronic Properties of AlxNiyZrz (x = 1~3, y = 1~2, z = 1~6) Alloy under Pressure. Materials 2020, 13, 4972. https://doi.org/10.3390/ma13214972
Yuan X, Li W, Wan P, Xue M-A. First-Principle Studies on the Mechanical and Electronic Properties of AlxNiyZrz (x = 1~3, y = 1~2, z = 1~6) Alloy under Pressure. Materials. 2020; 13(21):4972. https://doi.org/10.3390/ma13214972
Chicago/Turabian StyleYuan, Xiaoli, Weikang Li, Peng Wan, and Mi-An Xue. 2020. "First-Principle Studies on the Mechanical and Electronic Properties of AlxNiyZrz (x = 1~3, y = 1~2, z = 1~6) Alloy under Pressure" Materials 13, no. 21: 4972. https://doi.org/10.3390/ma13214972
APA StyleYuan, X., Li, W., Wan, P., & Xue, M.-A. (2020). First-Principle Studies on the Mechanical and Electronic Properties of AlxNiyZrz (x = 1~3, y = 1~2, z = 1~6) Alloy under Pressure. Materials, 13(21), 4972. https://doi.org/10.3390/ma13214972





