Low Temperature Sealing of Anodized Aluminum Alloy for Enhancing Corrosion Resistance
Abstract
1. Introduction
2. Materials and Methods
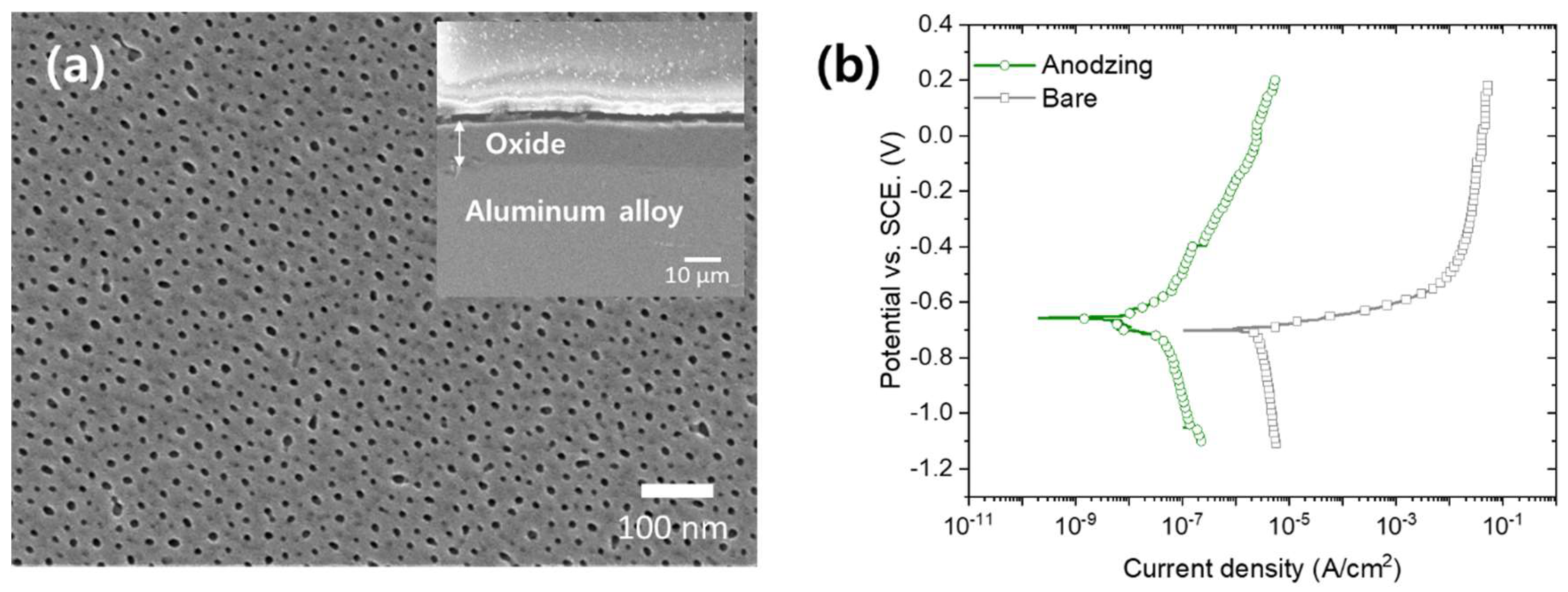
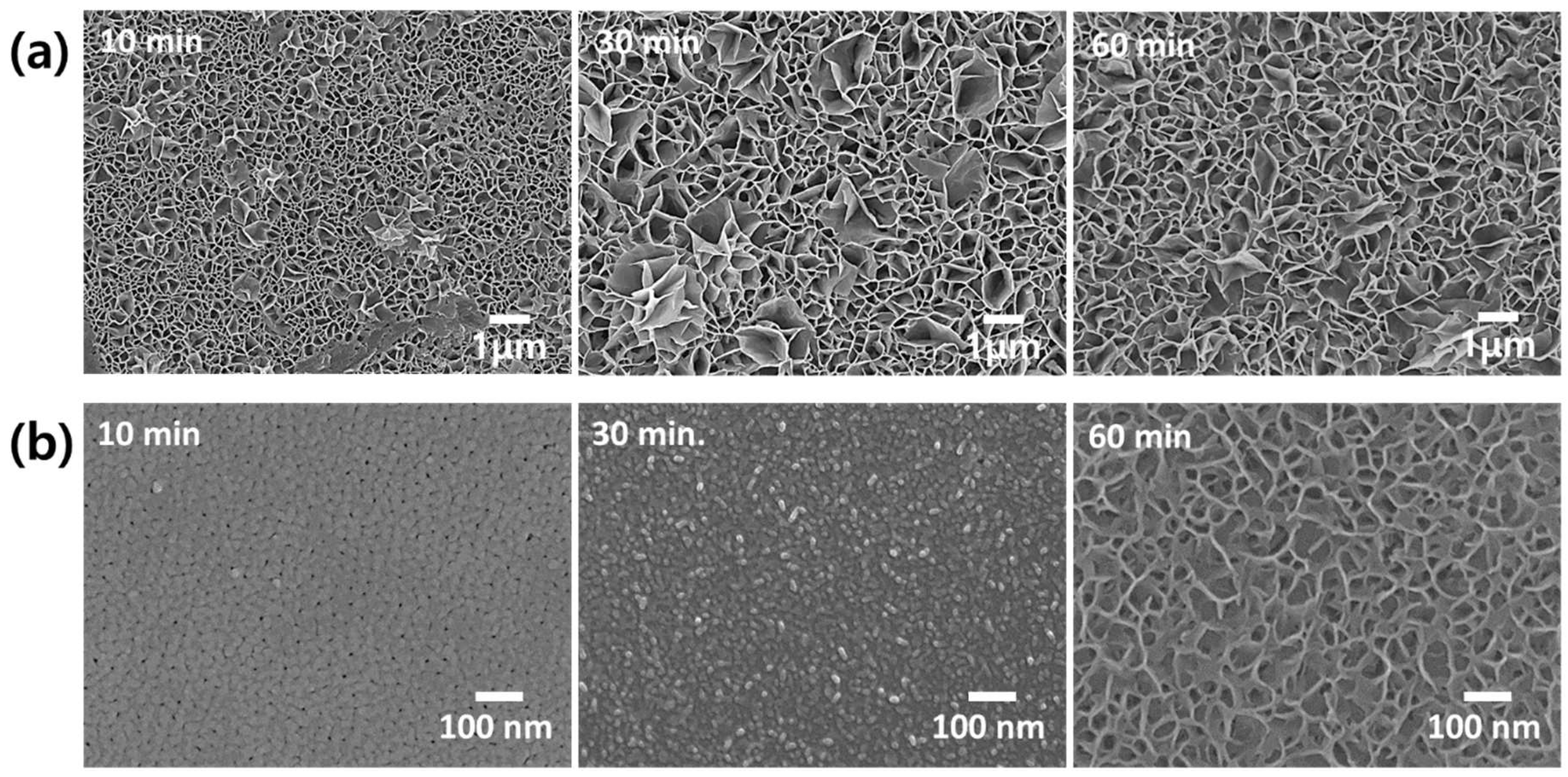
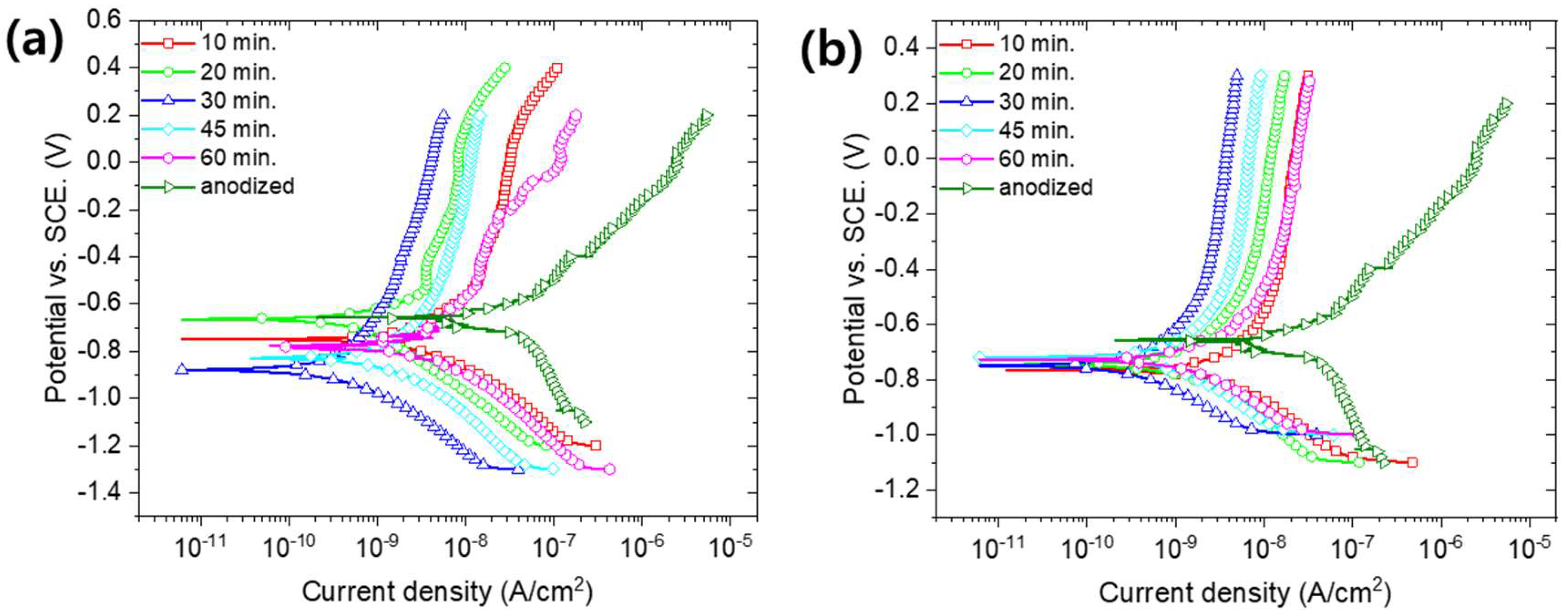
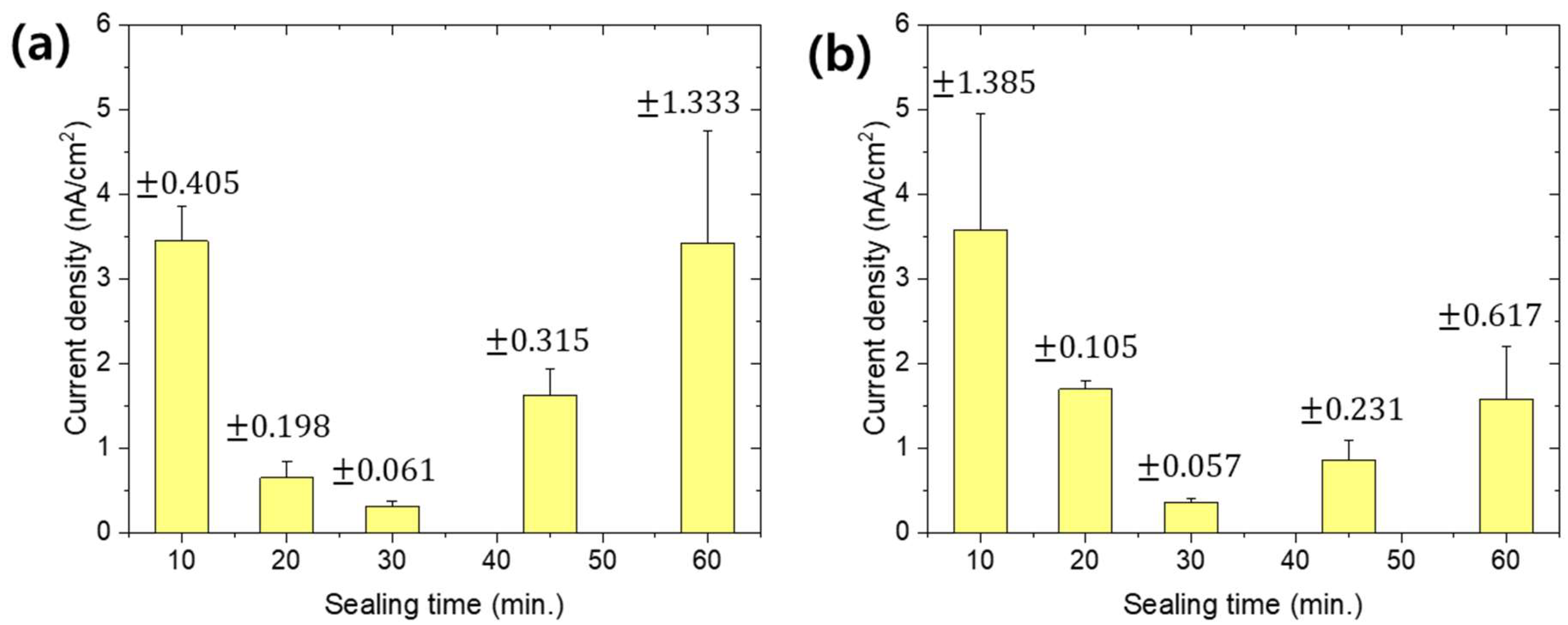
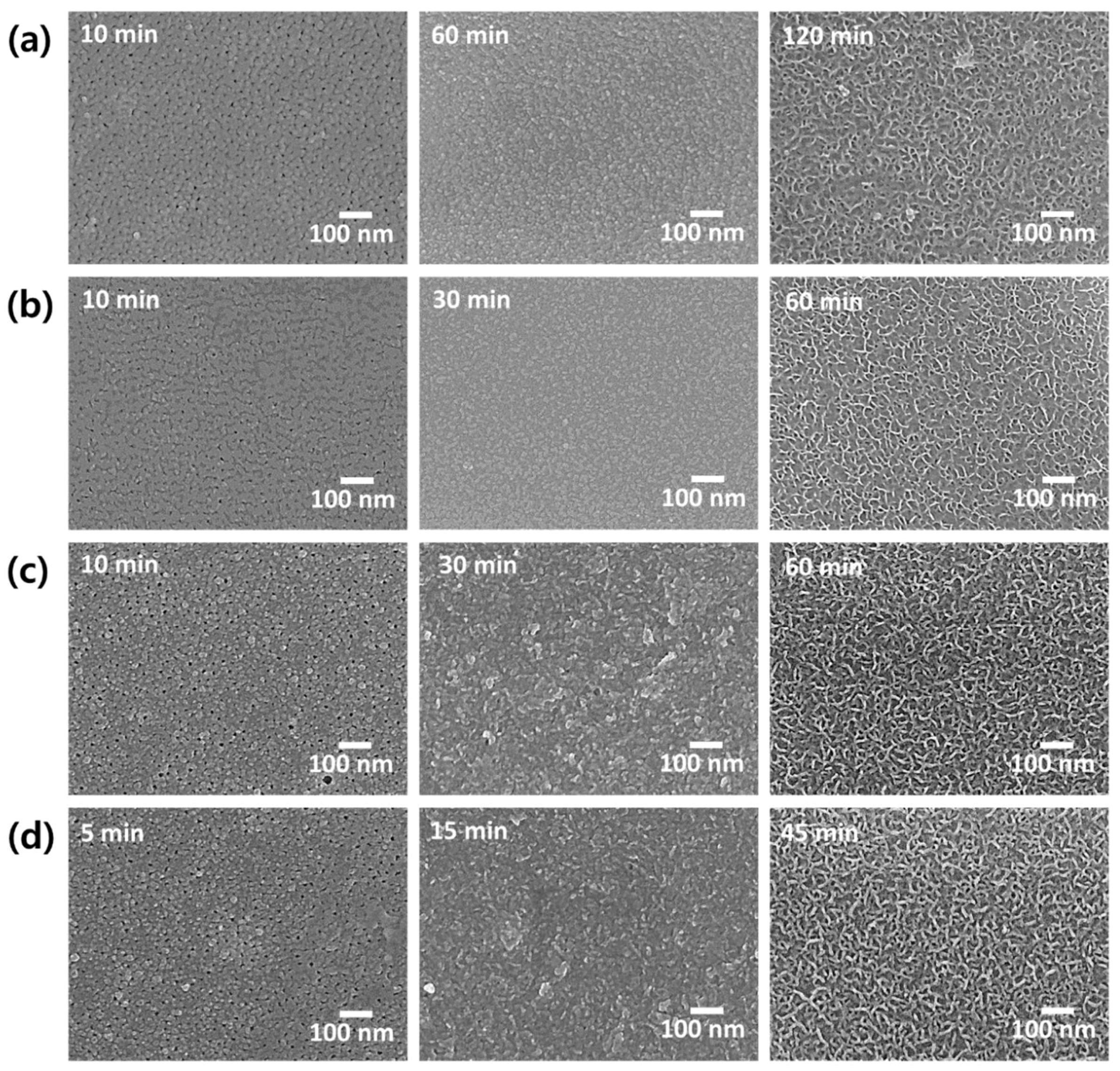
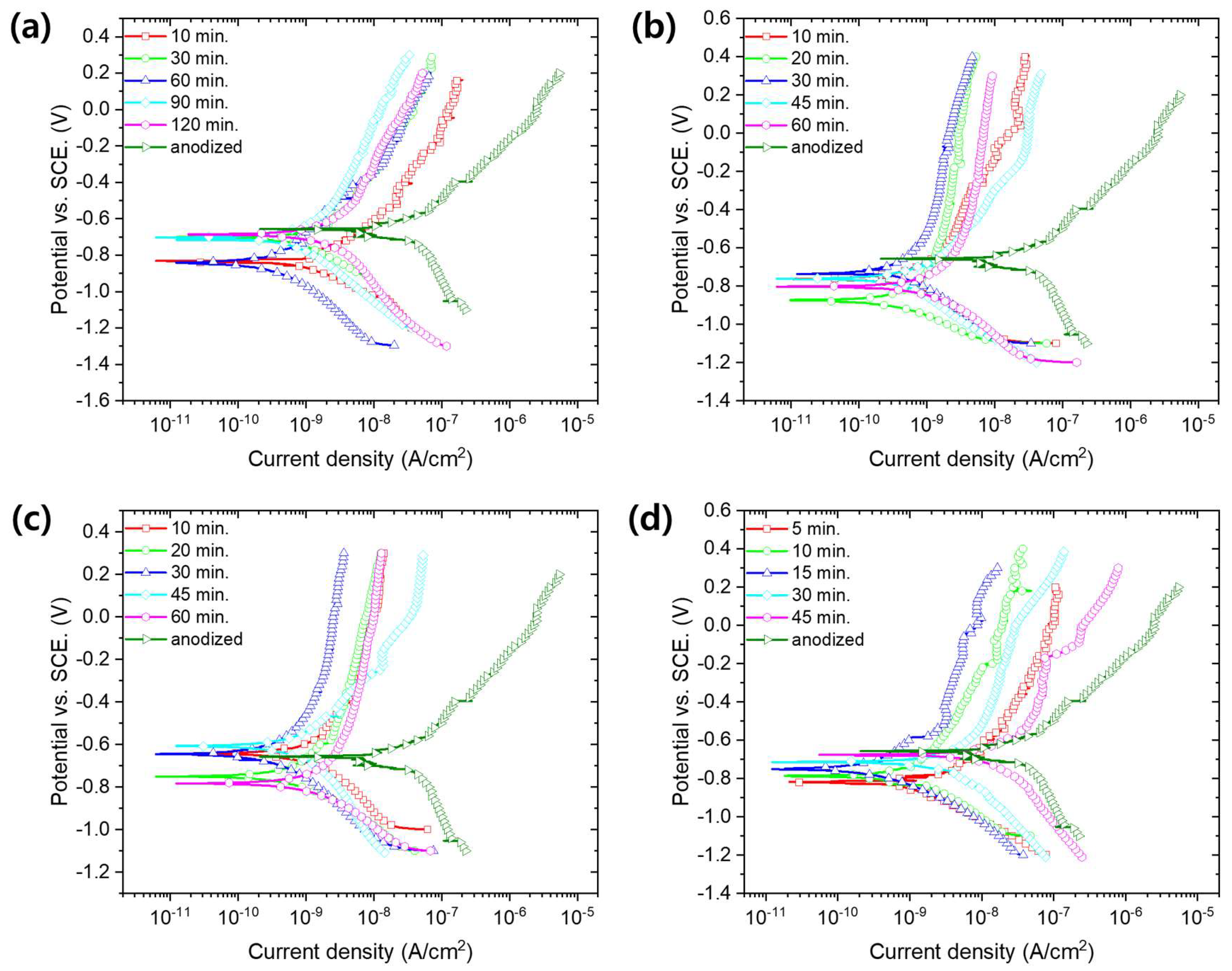
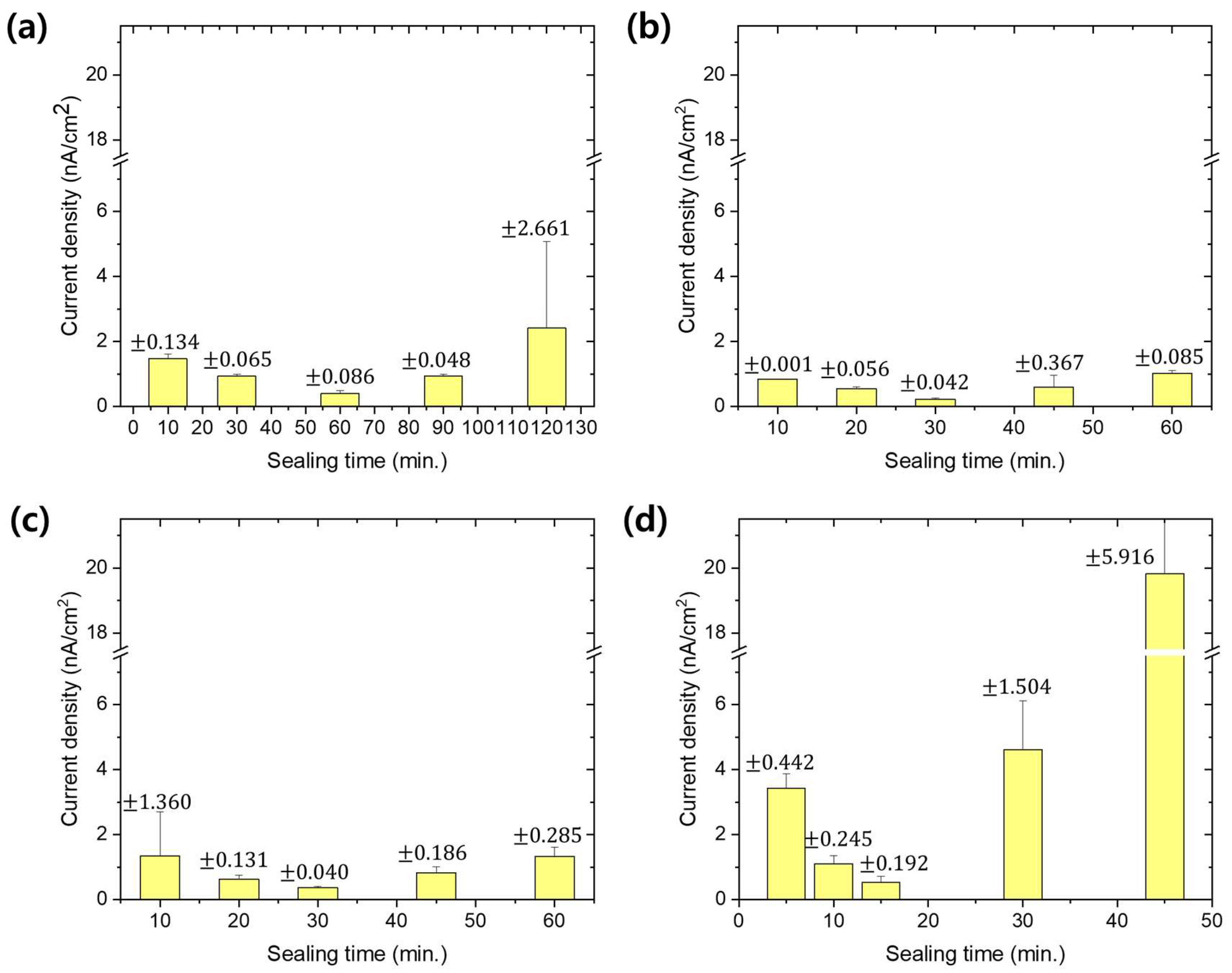
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shih, H.-H.; Tzou, S.-L. Study of anodic oxidation of aluminum in mixed acid using a pulsed current. Surf. Coat. Technol. 2000, 124, 278–285. [Google Scholar] [CrossRef]
- González, J.; Morcillo, M.; Escudero, E.; López, V.; Otero, E. Atmospheric corrosion of bare and anodized aluminium in a wide range of environmental conditions. Part I: Visual observations and gravimetric results. Surf. Coat. Technol. 2002, 153, 225–234. [Google Scholar] [CrossRef]
- López, V.; González, J.; Otero, E.; Escudero, E.; Morcillo, M. Atmospheric corrosion of bare and anodised aluminium in a wide range of environmental conditions. Part II: Electrochemical responses. Surf. Coat. Technol. 2002, 153, 235–244. [Google Scholar] [CrossRef]
- Aerts, T.; Dimogerontakis, T.; De Graeve, I.; Fransaer, J.; Terryn, H. Influence of the anodizing temperature on the porosity and the mechanical properties of the porous anodic oxide film. Surf. Coat. Technol. 2007, 201, 7310–7317. [Google Scholar] [CrossRef]
- Wang, F.; Liu, J.; Li, Y.; Wang, Y. Novel composite nanofilm of electropolymerization and self-assembling on AA5052 surface as anticorrosion coating. J. Appl. Polym. Sci. 2011, 123, 2906–2910. [Google Scholar] [CrossRef]
- Hakimizad, A.; Raeissi, K.; Ashrafizadeh, F. A comparative study of corrosion performance of sealed anodized layers of conventionally colored and interference-colored aluminium. Surf. Coat. Technol. 2012, 206, 4628–4633. [Google Scholar] [CrossRef]
- Moutarlier, V.; Gigandet, M.; Normand, B.; Pagetti, J. EIS characterisation of anodic films formed on 2024 aluminium alloy, in sulphuric acid containing molybdate or permanganate species. Corros. Sci. 2005, 47, 937–951. [Google Scholar] [CrossRef]
- Leth-Olsen, H.; Nisancioglu, K. Filiform Corrosion Morphologies on Painted Aluminum Alloy 3105 Coil Material. Corrosion 1997, 53, 705–717. [Google Scholar] [CrossRef]
- Hu, R.-G.; Zhang, S.; Bu, J.-F.; Lin, C.-J.; Song, G.-L. Recent progress in corrosion protection of magnesium alloys by organic coatings. Prog. Org. Coat. 2012, 73, 129–141. [Google Scholar] [CrossRef]
- Pathak, S.; Khanna, A. Investigation of anti-corrosion behavior of waterborne organosilane–polyester coatings for AA6011 aluminum alloy. Prog. Org. Coat. 2009, 65, 288–294. [Google Scholar] [CrossRef]
- Dong, C.; Sheng, H.; An, Y.; Li, X.; Xiao, K.; Cheng, Y. Corrosion of 7A04 aluminum alloy under defected epoxy coating studied by localized electrochemical impedance spectroscopy. Prog. Org. Coat. 2010, 67, 269–273. [Google Scholar] [CrossRef]
- Ni, L.; Chemtob, A.; Croutxé-Barghorn, C.; Moreau, N.; Bouder, T.; Chanfreau, S.; Pébère, N. Direct-to-metal UV-cured hybrid coating for the corrosion protection of aircraft aluminium alloy. Corros. Sci. 2014, 89, 242–249. [Google Scholar] [CrossRef]
- Leivo, E.; Vippola, M.S.; Sorsa, P.P.A.; Vuoristo, P.; Mäntyla, T.A. Wear and corrosion properties of plasma sprayed Al2O3 and Cr2O3 coatings sealed by aluminum phosphates. J. Therm. Spray Technol. 1997, 6, 205–210. [Google Scholar] [CrossRef]
- Knuuttila, J.; Sorsa, P.; Mäntylä, T. Sealing of thermal spray coatings by impregnation. J. Therm. Spray Technol. 1999, 8, 249–257. [Google Scholar] [CrossRef]
- Uozato, S.; Nakata, K.; Ushio, M. Corrosion and wear behaviors of ferrous powder thermal spray coatings on aluminum alloy. Surf. Coat. Technol. 2003, 169, 691–694. [Google Scholar] [CrossRef]
- Magnani, M.; Suegama, P.; Espallargas, N.; Dosta, S.; Fugivara, C.; Guilemany, J.; Benedetti, A. Influence of HVOF parameters on the corrosion and wear resistance of WC-Co coatings sprayed on AA7050 T7. Surf. Coat. Technol. 2008, 202, 4746–4757. [Google Scholar] [CrossRef]
- Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Electrodeposition of silane films on aluminum alloys for corrosion protection. Prog. Org. Coat. 2007, 58, 265–271. [Google Scholar] [CrossRef]
- Ghanbari, S.; Mahboubi, F. Corrosion resistance of electrodeposited Ni–Al composite coatings on the aluminum substrate. Mater. Des. 2011, 32, 1859–1864. [Google Scholar] [CrossRef]
- Hu, J.-M.; Liu, L.; Zhang, J.-Q.; Cao, C.-N. Effects of electrodeposition potential on the corrosion properties of bis-1,2-[triethoxysilyl] ethane films on aluminum alloy. Electrochimica Acta 2006, 51, 3944–3949. [Google Scholar] [CrossRef]
- Wu, L.-K.; Liu, L.; Li, J.; Hu, J.-M.; Zhang, J.-Q.; Cao, C.-N. Electrodeposition of cerium (III)-modified bis-[triethoxysilypropyl]tetra-sulphide films on AA2024-T3 (aluminum alloy) for corrosion protection. Surf. Coat. Technol. 2010, 204, 3920–3926. [Google Scholar] [CrossRef]
- Xingwen, Y.; Chunan, C.; Zhiming, Y.; Derui, Z.; Zhongda, Y. Study of double layer rare earth metal conversion coating on aluminum alloy LY12. Corros. Sci. 2001, 43, 1283–1294. [Google Scholar] [CrossRef]
- Yang, X.; Tallman, D.; Gelling, V.; Bierwagen, G.; Kasten, L.; Berg, J. Use of a sol–gel conversion coating for aluminum corrosion protection. Surf. Coat. Technol. 2001, 140, 44–50. [Google Scholar] [CrossRef]
- Zheludkevich, M.; Serra, R.; Montemor, M.; Yasakau, K.A.; Salvado, I.M.M.; Ferreira, M.G.S. Nanostructured sol–gel coatings doped with cerium nitrate as pre-treatments for AA2024-T3. Electrochimica Acta 2005, 51, 208–217. [Google Scholar] [CrossRef]
- Schem, M.; Schmidt, T.F.; Gerwann, J.; Wittmar, M.; Veith, M.; Thompson, G.; Molchan, I.; Hashimoto, T.; Skeldon, P.; Phani, A.; et al. CeO2-filled sol–gel coatings for corrosion protection of AA2024-T3 aluminium alloy. Corros. Sci. 2009, 51, 2304–2315. [Google Scholar] [CrossRef]
- Whelan, M.; Cassidy, J.; Duffy, B. Sol–gel sealing characteristics for corrosion resistance of anodised aluminium. Surf. Coat. Technol. 2013, 235, 86–96. [Google Scholar] [CrossRef]
- Diggle, J.W.; Downie, T.C.; Goulding, C.W. A Study of the Formation and Dissolution of Porous Anodic Oxide Films on Aluminum: Behavior of the Porous Layer. J. Electrochem. Soc. 1969, 116, 1347. [Google Scholar] [CrossRef]
- Li, F.; Zhang, L.; Metzger, R.M. On the Growth of Highly Ordered Pores in Anodized Aluminum Oxide. Chem. Mater. 1998, 10, 2470–2480. [Google Scholar] [CrossRef]
- Bai, A.; Hu, C.-C.; Yang, Y.-F.; Lin, C.-C. Pore diameter control of anodic aluminum oxide with ordered array of nanopores. Electrochimica Acta 2008, 53, 2258–2264. [Google Scholar] [CrossRef]
- Hoar, T.; Wood, G. The sealing of porous anodic oxide films on aluminium. Electrochimica Acta 1962, 7, 333–353. [Google Scholar] [CrossRef]
- Mansfeld, F.; Chen, C.; Breslin, C.B.; Dull, D. Sealing of Anodized Aluminum Alloys with Rare Earth Metal Salt Solutions. J. Electrochem. Soc. 1998, 145, 2792–2798. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Bojar, Z. Synthesis of anodic aluminum oxide (AAO) at relatively high temperatures. Study of the influence of anodization conditions on the alumina structural features. Surf. Coat. Technol. 2011, 206, 265–272. [Google Scholar] [CrossRef]
- Lee, J.; Jung, U.; Kim, W.; Chung, W. Effects of residual water in the pores of aluminum anodic oxide layers prior to sealing on corrosion resistance. Appl. Surf. Sci. 2013, 283, 941–946. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, W.; Yu, J.; Xue, Q. Influence of the Self-Sealing Layer on the Corrosion of Anodic Aluminum Oxide Films. ACS Appl. Nano Mater. 2018, 1, 5142–5147. [Google Scholar] [CrossRef]
- Gonzalez, J.; Lopez, V.; Otero, E.; Bautista, A.; Lizarbe, R.; Barba, C.; Baldonedo, J. Overaging of sealed and unsealed aluminium oxide films. Corros. Sci. 1997, 39, 1109–1118. [Google Scholar] [CrossRef]
- Hao, L.; Cheng, B.R. Sealing processes of anodic coatings—Past, present, and future. Met. Finish. 2000, 98, 8–18. [Google Scholar] [CrossRef]
- Hu, N.; Dong, X.; He, X.; Browning, J.F.; Schaefer, D.W. Effect of sealing on the morphology of anodized aluminum oxide. Corros. Sci. 2015, 97, 17–24. [Google Scholar] [CrossRef]
- García-Alonso, M.C.; Escudero, M.L.; González-Carrasco, J.L. Corrosion, Evaluation of the scale integrity in the Al2O3/MA956 system at different polarisation by using EIS. Mater. Corros. 2001, 52, 524–530. [Google Scholar] [CrossRef]
- Lopez, V.; Gonzalez, J.; Bautista, A.; Otero, E.; Lizarbe, R.; Bautista, A. The response of anodized materials sealed in acetate-containing baths to atmospheric exposure. Corros. Sci. 1998, 40, 693–704. [Google Scholar] [CrossRef]
- Otero, E.; Lopez, V.; Gonzalez, J.A. Aging of Cold-Sealed Aluminum Oxide Films at Room Temperature and at 50 C. Plat. Surf. Finish 1996, 83, 50–54. [Google Scholar]
- Kalantary, M.R.; Gabe, D.R.; Ross, D.H. A model for the mechanism of nickel fluoride cold sealing of anodized aluminium. J. Appl. Electrochem. 1992, 22, 268–276. [Google Scholar] [CrossRef]
- Kalantary, M.R.; Gabe, D.R.; Ross, D.H. Sealing of electrolytically formed porous films of aluminum by nickel fluoride process. Methods 1993, 1, 2. [Google Scholar]
- Zuo, Y.; Zhao, P.-H.; Zhao, J.-M. The influences of sealing methods on corrosion behavior of anodized aluminum alloys in NaCl solutions. Surf. Coat. Technol. 2003, 166, 237–242. [Google Scholar] [CrossRef]
- Mansfeld, F. Tafel Slopes and Corrosion Rates from Polarization Resistance Measurements. Corrosion 1973, 29, 397–402. [Google Scholar] [CrossRef]
| Name | Composition | Ni2+ (g/L) | Immersion Time (min) | Temperature (°C) |
|---|---|---|---|---|
| NAHS 1 | Ni(CH3CO2)2∙4H2O: 5.5 g/L (0.221 M) H3BO3: 8.2 g/L (0.133 M) | 1.3 | 10, 20, 30, 45, 60 | 95 |
| NFCS 2 | NiF2∙4H2O: 4.35 g/L (0.026 M) | 1.5 | 10, 20, 30, 45, 60 | 25 |
| MSS1 3 | Ni(CH3CO2)2∙4H2O: 2.14 g/L (0.009 M) NH4F: 0.64 g/L (0.017 M) | 0.5 | 10, 30, 60, 90, 120 | 25 |
| MSS2 3 | Ni(CH3CO2)2∙4H2O: 4.28 g/L (0.017 M) NH4F: 1.28 g/L (0.035 M) | 1.0 | 10, 20, 30, 45, 60 | 25 |
| MSS3 3 | Ni(CH3CO2)2∙4H2O: 6.42 g/L (0.026 M) NH4F: 1.92 g/L (0.052 M) | 1.5 | 10, 20, 30, 45, 60 | 25 |
| MSS4 3 | Ni(CH3CO2)2∙4H2O: 12.84 g/L (0.052 M) NH4F: 3.84 g/L (0.104 M) | 3.0 | 5, 10, 15, 30, 45 | 25 |
| Name | Al | O | Ni | F |
|---|---|---|---|---|
| As Anodized | 36.6 ± 1.4 | 63.2 ± 3.0 | 0.22 ± 0.1 | 0.1 ± 0.1 |
| NAHS | 26.4 ± 1.9 | 71.0 ± 2.3 | 2.63 ± 1.1 | – |
| NFCS | 22.3 ± 1.7 | 69.0 ± 2.1 | 1.53 ± 0.8 | 7.2 ± 1.2 |
| Name | Al | O | Ni | F |
|---|---|---|---|---|
| MSS1 | 28.1 ± 3.5 | 61.3 ± 3.7 | 2.2 ± 0.7 | 8.4 ± 1.2 |
| MSS2 | 25.9 ± 2.2 | 62.8 ± 4.0 | 3.3 ± 1.1 | 8.0 ± 1.3 |
| MSS3 | 23.7 ± 3.0 | 64.3 ± 3.3 | 5.0 ± 1.9 | 7.1 ± 1.8 |
| MSS4 | 21.4 ± 2.7 | 66.5 ± 4.5 | 6.0 ± 1.5 | 6.2 ± 2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jo, H.; Lee, S.; Kim, D.; Lee, J. Low Temperature Sealing of Anodized Aluminum Alloy for Enhancing Corrosion Resistance. Materials 2020, 13, 4904. https://doi.org/10.3390/ma13214904
Jo H, Lee S, Kim D, Lee J. Low Temperature Sealing of Anodized Aluminum Alloy for Enhancing Corrosion Resistance. Materials. 2020; 13(21):4904. https://doi.org/10.3390/ma13214904
Chicago/Turabian StyleJo, Hyunbin, Soomin Lee, Donghyun Kim, and Junghoon Lee. 2020. "Low Temperature Sealing of Anodized Aluminum Alloy for Enhancing Corrosion Resistance" Materials 13, no. 21: 4904. https://doi.org/10.3390/ma13214904
APA StyleJo, H., Lee, S., Kim, D., & Lee, J. (2020). Low Temperature Sealing of Anodized Aluminum Alloy for Enhancing Corrosion Resistance. Materials, 13(21), 4904. https://doi.org/10.3390/ma13214904





