Reliability of Metal 3D Printing with Respect to the Marginal Fit of Fixed Dental Prostheses: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Study Selection and Data Extraction
2.4. Quality Assessment
2.5. Data Analyses
3. Results
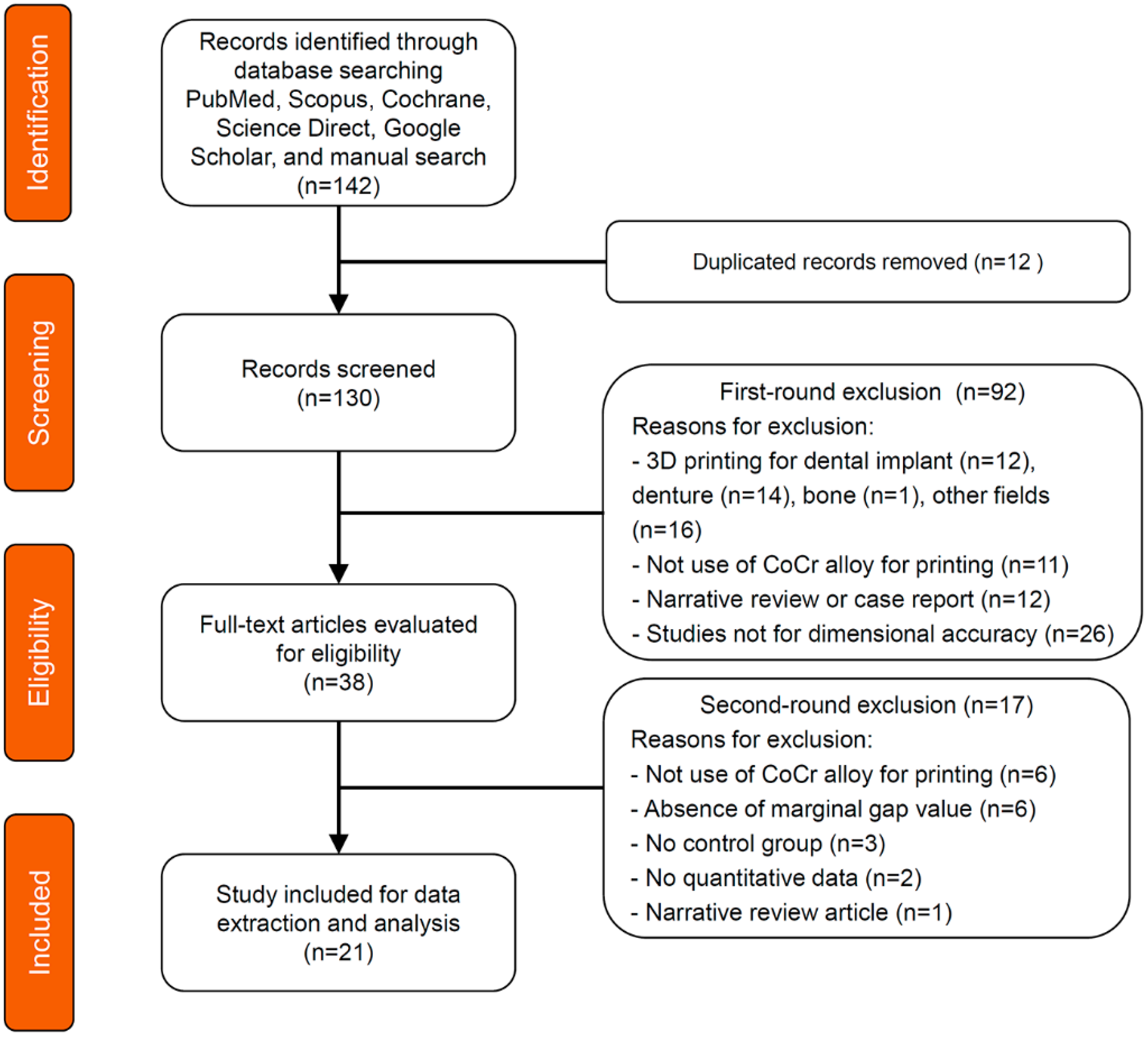
3.1. Search Results
3.2. Characteristics of the Included Studies
3.3. Quality Assessment and Applicability Concerns
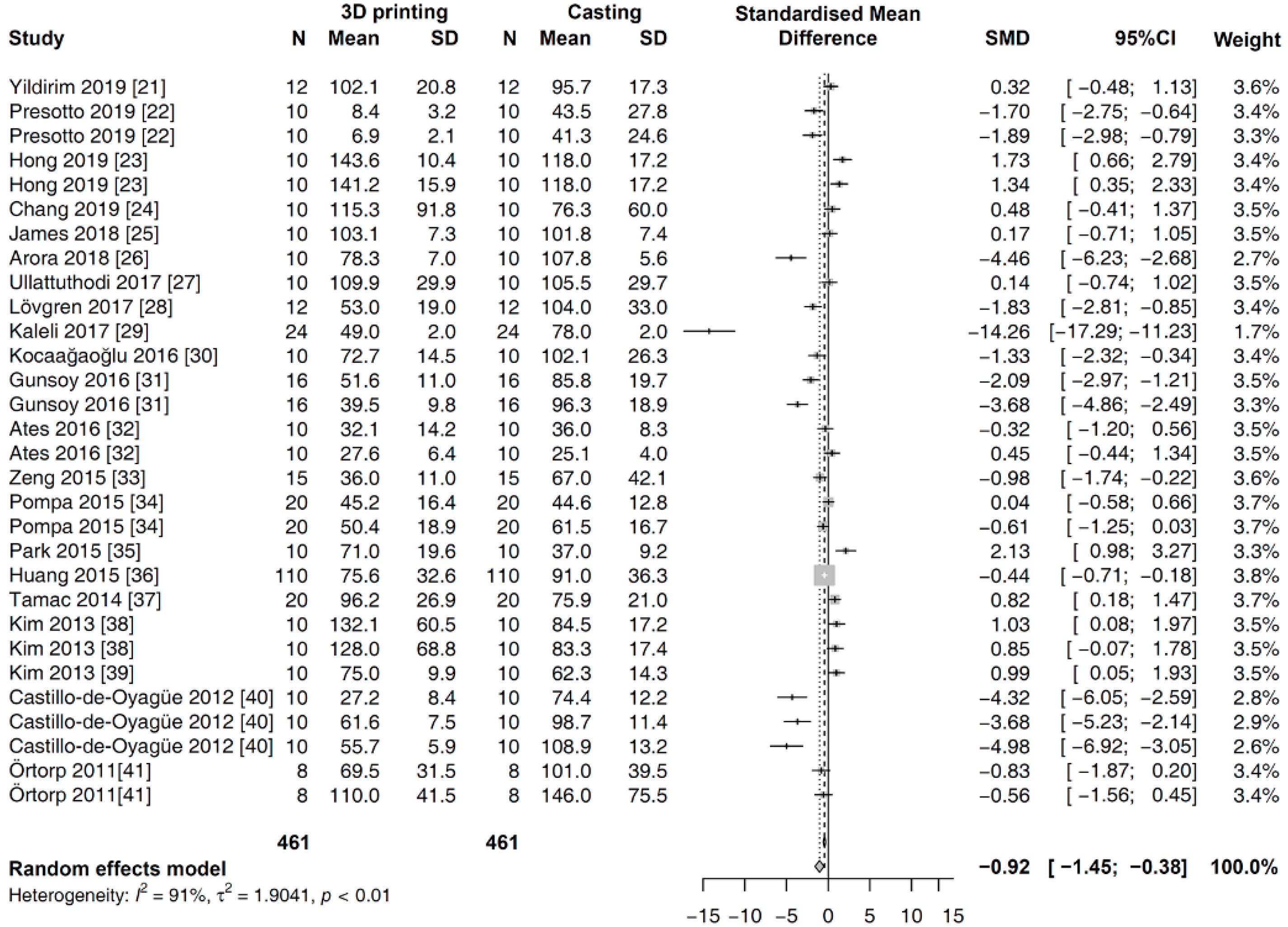
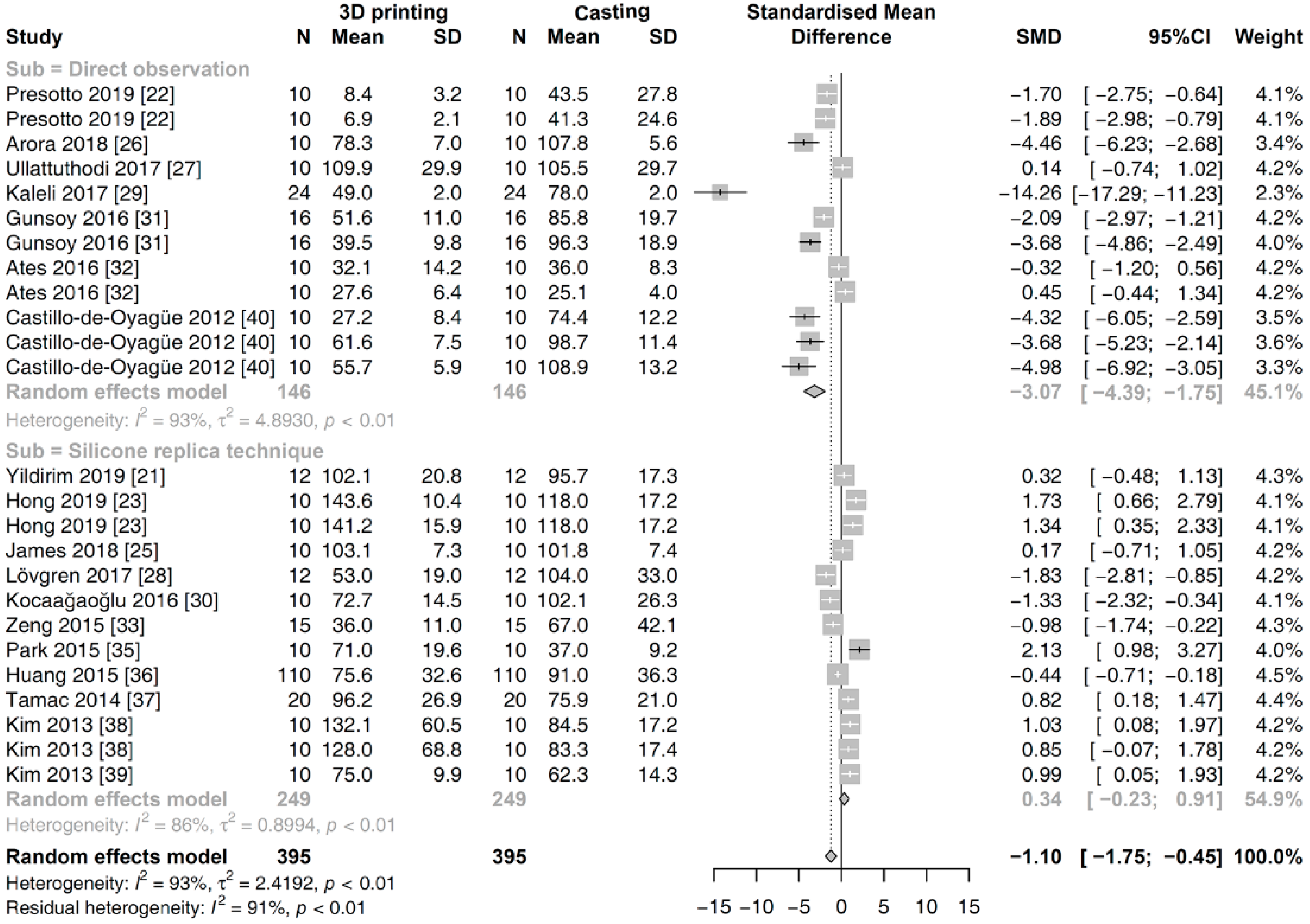
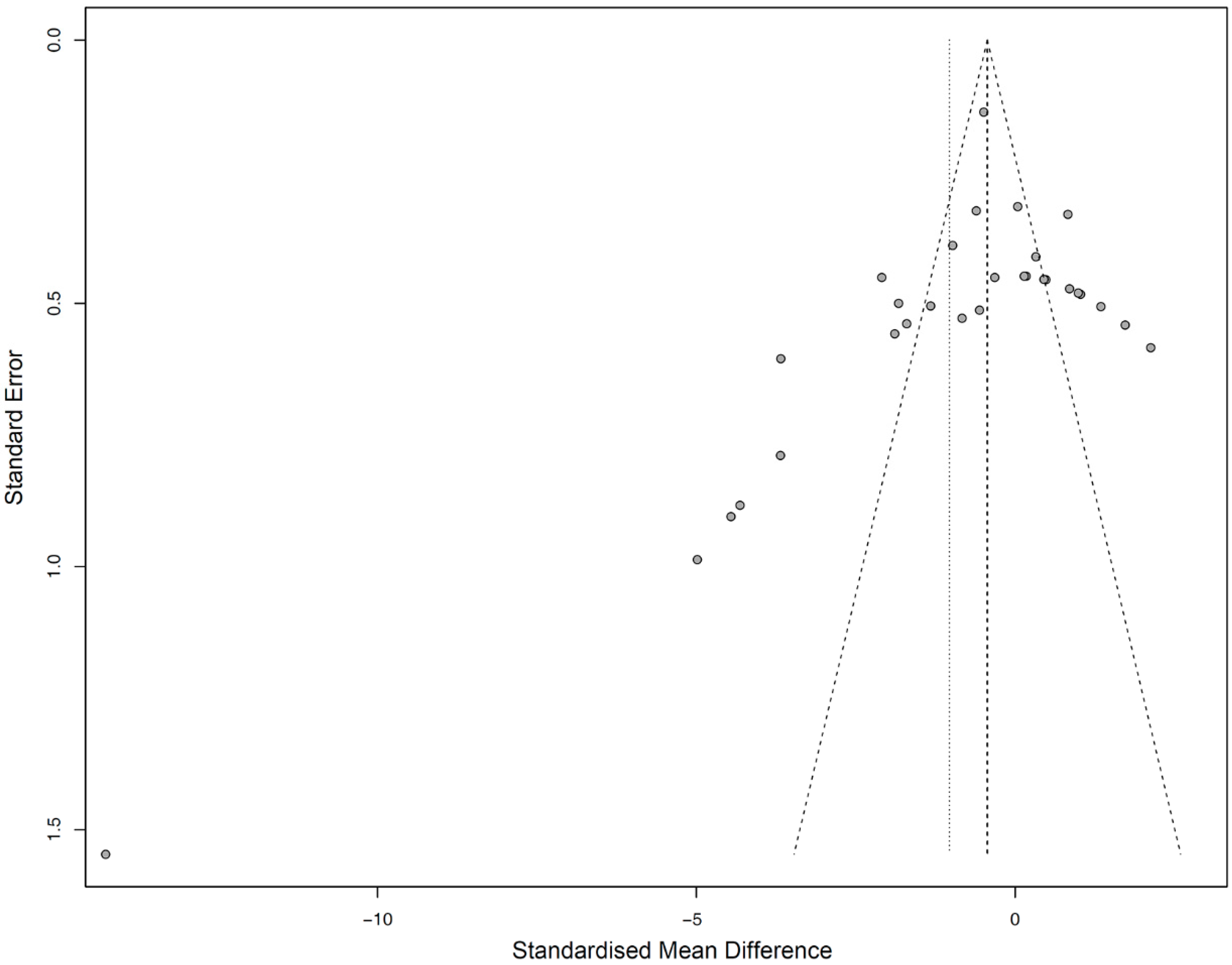
3.4. Meta-Analysis
4. Discussion
5. Conclusions
- Metal 3D printing technologies are reliable for fabricating Co-Cr-based fixed dental prostheses with an accurate marginal fit, as compared to conventional casting methods.
- The difference in methodology for evaluating the marginal fit could influence the results in comparative studies.
- Further controlled laboratory and clinical studies with a detailed protocol that discloses all the manufacturing parameters are needed to statistically analyze the factors affecting the accuracy of 3D-printed prostheses.
Author Contributions
Funding
Conflicts of Interest
References
- Korner, M.E.H.; Lambán, M.P.; Albajez, J.A.; Santolaria, J.; Ng Corrales, L.D.C.; Royo, J. Systematic Literature Review: Integration of Additive Manufacturing and Industry 4.0. Metals 2020, 10, 1061. [Google Scholar] [CrossRef]
- Akhila, A.S.; Nandakishore, B.; Miriam, M.; Anil, S.K.; Abhinav, M.; Fares, A. Rapid prototyping: An innovative technique in prosthodontics. Int. J. Prev. Clin. Dent. Res. 2019, 6, 46. [Google Scholar] [CrossRef]
- Fan, D.; Li, Y.; Wang, X.; Zhu, T.; Wang, Q.; Cai, H.; Li, W.; Tian, Y.; Liu, Z. Progressive 3D Printing Technology and Its Application in Medical Materials. Front. Pharmacol. 2020, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lim, J.Y. Metal-ceramic bond strength of a cobalt chromium alloy for dental prosthetic restorations with a porous structure using metal 3D printing. Comput. Biol. Med. 2019, 112, 103364. [Google Scholar] [CrossRef] [PubMed]
- Razavykia, A.; Brusa, E.; Delprete, C.; Yavari, R. An Overview of Additive Manufacturing Technologies—A Review to Technical Synthesis in Numerical Study of Selective Laser Melting. Materials 2020, 13, 3895. [Google Scholar] [CrossRef]
- Revilla-León, M.; Meyer, M.J.; Özcan, M. Metal additive manufacturing technologies: Literature review of current status and prosthodontic applications. Int. J. Comput. Dent. 2019, 22, 55–67. [Google Scholar]
- Alharbi, N.; Wismeijer, D.; Osman, R.B. Additive Manufacturing Techniques in Prosthodontics: Where Do We Currently Stand? A Critical Review. Int. J. Prosthodont. 2017, 30, 474–484. [Google Scholar] [CrossRef]
- Revilla-León, M.; Sadeghpour, M.; Özcan, M. A Review of the Applications of Additive Manufacturing Technologies Used to Fabricate Metals in Implant Dentistry. J. Prosthodont. 2020, 29, 579–593. [Google Scholar] [CrossRef]
- Konieczny, B.; Szczesio-Wlodarczyk, A.; Sokolowski, J.; Bociong, K. Challenges of Co–Cr Alloy Additive Manufacturing Methods in Dentistry—The Current State of Knowledge (Systematic Review). Materials 2020, 13, 3524. [Google Scholar] [CrossRef]
- Koutsoukis, T.; Zinelis, S.; Eliades, G.; Al-Wazzan, K.; Al Rifaiy, M.; Al Jabbari, Y.S. Selective Laser Melting Technique of Co-Cr Dental Alloys: A Review of Structure and Properties and Comparative Analysis with Other Available Techniques. J. Prosthodont. 2015, 24, 303–312. [Google Scholar] [CrossRef]
- Wang, J.-H.; Ren, J.; Liu, W.; Wu, X.-Y.; Gao, M.-X.; Bai, P. Effect of Selective Laser Melting Process Parameters on Microstructure and Properties of Co-Cr Alloy. Materials 2018, 11, 1546. [Google Scholar] [CrossRef]
- Takaichi, A.; Suyalatu; Nakamoto, T.; Joko, N.; Nomura, N.; Tsutsumi, Y.; Migita, S.; Doi, H.; Kurosu, S.; Chiba, A.; et al. Microstructures and mechanical properties of Co–29Cr–6Mo alloy fabricated by selective laser melting process for dental applications. J. Mech. Behav. Biomed. Mater. 2013, 21, 67–76. [Google Scholar] [CrossRef]
- Svanborg, P.; Hjalmarsson, L. A systematic review on the accuracy of manufacturing techniques for cobalt chromium fixed dental prostheses. Biomater. Investig. Dent. 2020, 7, 31–40. [Google Scholar] [CrossRef]
- Nascimento, C.D.; Ikeda, L.N.; Pita, M.S.; E Silva, R.C.P.; Pedrazzi, V.; de Albuquerque Junior, R.F.; Ribeiro, R.F. Marginal fit and microbial leakage along the implant-abutment interface of fixed partial prostheses: An in vitro analysis using Checkerboard DNA-DNA hybridization. J. Prosthet. Dent. 2015, 114, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, P.H.O.; Valle, A.L.D.; De Carvalho, R.M.; De Goes, M.F.; Pegoraro, L.F. Correlation between margin fit and microleakage in complete crowns cemented with three luting agents. J. Appl. Oral Sci. 2008, 16, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Phillips, B.; X, H.; J, L.; D, D.-F. Faculty Opinions recommendation of Evaluation of PICO as a knowledge representation for clinical questions. Fac. Opin. Post-Publ. Peer Rev. Biomed. Lit. 2007, 2006, 359–363. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M.M. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2020. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis; Wiley: Hoboken, NJ, USA, 2009; pp. 311–319. [Google Scholar]
- Yildirim, B.; Paken, G. Evaluation of the Marginal and Internal Fit of Implant-Supported Metal Copings Fabricated with 3 Different Techniques: An In Vitro Study. J. Prosthodont. 2019, 28, 315–320. [Google Scholar] [CrossRef]
- Presotto, A.G.C.; Barão, V.A.R.; Bhering, C.L.B.; Mesquita, M.F. Dimensional precision of implant-supported frameworks fabricated by 3D printing. J. Prosthet. Dent. 2019, 122, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-H.; Min, B.K.; Lee, D.-H.; Kwon, T.-Y. Marginal fit of metal-ceramic crowns fabricated by using a casting and two selective laser melting processes before and after ceramic firing. J. Prosthet. Dent. 2019, 122, 475–481. [Google Scholar] [CrossRef]
- Chang, H.-S.; Peng, Y.-T.; Hung, W.-L.; Hsu, M.-L. Evaluation of marginal adaptation of Co-Cr-Mo metal crowns fabricated by traditional method and computer-aided technologies. J. Dent. Sci. 2019, 14, 288–294. [Google Scholar] [CrossRef] [PubMed]
- James, A.E.; Umamaheswari, B.; Lakshmi, C.B.S. Comparative Evaluation of Marginal Accuracy of Metal Copings Fabricated using Direct Metal Laser Sintering, Computer-Aided Milling, Ringless Casting, and Traditional Casting Techniques: An In vitro Study. Contemp. Clin. Dent. 2018, 9, 421–426. [Google Scholar] [CrossRef]
- Yadav, A.; Arora, A.; Upadhyaya, V.; Jain, P.; Verma, M. Comparison of marginal and internal adaptation of copings fabricated from three different fabrication techniques: An in vitro study. J. Indian Prosthodont. Soc. 2018, 18, 102. [Google Scholar] [CrossRef] [PubMed]
- Ullattuthodi, S.; Cherian, K.P.; Anandkumar, R.; Nambiar, M.S. Marginal and internal fit of cobalt-chromium copings fabricated using the conventional and the direct metal laser sintering techniques: A comparative in vitro study. J. Indian Prosthodont. Soc. 2017, 17, 373–380. [Google Scholar] [CrossRef]
- Lövgren, N.; Roxner, R.; Klemendz, S.; Larsson, C. Effect of production method on surface roughness, marginal and internal fit, and retention of cobalt-chromium single crowns. J. Prosthet. Dent. 2017, 118, 95–101. [Google Scholar] [CrossRef]
- Kaleli, N.; Saraç, D. Influence of porcelain firing and cementation on the marginal adaptation of metal-ceramic restorations prepared by different methods. J. Prosthet. Dent. 2016, 117, 656–661. [Google Scholar] [CrossRef]
- Kocaağaoğlu, H.; Kılınç, H.I.; Albayrak, H.; Kara, M. In vitro evaluation of marginal, axial, and occlusal discrepancies in metal ceramic restorations produced with new technologies. J. Prosthet. Dent. 2016, 116, 368–374. [Google Scholar] [CrossRef]
- Gunsoy, S.; Ulusoy, M. Evaluation of marginal/internal fit of chrome-cobalt crowns: Direct laser metal sintering versus computer-aided design and computer-aided manufacturing. Niger. J. Clin. Pr. 2016, 19, 636–644. [Google Scholar] [CrossRef]
- Ates, S.M.; Duymus, Z.Y. Influence of Tooth Preparation Design on Fitting Accuracy of CAD-CAM Based Restorations. J. Esthet. Restor. Dent. 2016, 28, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Zhang, Y.; Liu, Z.; Wei, B. Effects of repeated firing on the marginal accuracy of Co-Cr copings fabricated by selective laser melting. J. Prosthet. Dent. 2015, 113, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Pompa, G.; Di Carlo, S.; De Angelis, F.; Cristalli, M.P.; Annibali, S. Comparison of Conventional Methods and Laser-Assisted Rapid Prototyping for Manufacturing Fixed Dental Prostheses: An In Vitro Study. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Park, J.-K.; Lee, W.-S.; Kim, H.-Y.; Kim, W.-C.; Kim, J.-H. Accuracy evaluation of metal copings fabricated by computer-aided milling and direct metal laser sintering systems. J. Adv. Prosthodont. 2015, 7, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhang, L.; Zhu, J.; Zhao, Y.; Zhang, X. Clinical Marginal and Internal Fit of Crowns Fabricated Using Different CAD/CAM Technologies. J. Prosthodont. 2014, 24, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Tamac, E.; Toksavul, S.; Toman, M. Clinical marginal and internal adaptation of CAD/CAM milling, laser sintering, and cast metal ceramic crowns. J. Prosthet. Dent. 2014, 112, 909–913. [Google Scholar] [CrossRef]
- Kim, K.-B.; Kim, W.-C.; Kim, H.-Y.; Kim, J.-H. An evaluation of marginal fit of three-unit fixed dental prostheses fabricated by direct metal laser sintering system. Dent. Mater. 2013, 29, e91–e96. [Google Scholar] [CrossRef]
- Kim, K.-B.; Kim, J.-H.; Kim, W.-C.; Kim, H.-Y.; Kim, J.-H. Evaluation of the marginal and internal gap of metal-ceramic crown fabricated with a selective laser sintering technology: Two- and three-dimensional replica techniques. J. Adv. Prosthodont. 2013, 5, 179–186. [Google Scholar] [CrossRef]
- Castillo-Oyagüe, R.; Turrión, A.S.; Lozano, J.F.L.; Albaladejo, A.; Torres-Lagares, D.; Montero, J.; Suárez-García, M.-J. Vertical misfit of laser-sintered and vacuum-cast implant-supported crown copings luted with definitive and temporary luting agents. Med. Oral Patol. Oral Cir. Bucal 2012, 17, e610–e617. [Google Scholar] [CrossRef]
- Örtorp, A.; Jönsson, D.; Mouhsen, A.; Von Steyern, P.V. The fit of cobalt–chromium three-unit fixed dental prostheses fabricated with four different techniques: A comparative in vitro study. Dent. Mater. 2011, 27, 356–363. [Google Scholar] [CrossRef]
- Nawafleh, N.A.; Mack, F.; Evans, J.; Mackay, J.; Hatamleh, M.M. Accuracy and Reliability of Methods to Measure Marginal Adaptation of Crowns and FDPs: A Literature Review. J. Prosthodont. 2013, 22, 419–428. [Google Scholar] [CrossRef]
- Lee, D.-H. Digital approach to assessing the 3-dimensional misfit of fixed dental prostheses. J. Prosthet. Dent. 2016, 116, 836–839. [Google Scholar] [CrossRef] [PubMed]
- Schlenz, M.A.; Vogler, J.A.H.; Schmidt, A.; Rehmann, P.; Wöstmann, B. Chairside measurement of the marginal and internal fit of crowns: A new intraoral scan–based approach. Clin. Oral Investig. 2019, 24, 2459–2468. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.-N.; Lee, K.E.; Ha, J.-H.; Lee, D.-H. Effects of image and education on the precision of the measurement method for evaluating prosthesis misfit. J. Prosthet. Dent. 2018, 119, 600–605. [Google Scholar] [CrossRef]
- Barrios-Muriel, J.; Romero-Sánchez, F.; Alonso, F.J.; Salgado, D.R. Advances in Orthotic and Prosthetic Manufacturing: A Technology Review. Materials 2020, 13, 295. [Google Scholar] [CrossRef]
- Oropallo, W.; Piegl, L.A. Ten challenges in 3D printing. Eng. Comput. 2015, 32, 135–148. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, X.; Zhu, J.; Zhang, X. Clinical marginal and internal fit of metal ceramic crowns fabricated with a selective laser melting technology. J. Prosthet. Dent. 2015, 113, 623–627. [Google Scholar] [CrossRef]
- Franz, H.; Plöchl, L.; Schimansky, F.-P. Recent advances of titanium alloy powder production by ceramic-free inert gas atomization. In Proceedings of the 24th Annual International Titanium Associaction Conference Titatnium 2008, Las Vesgas, NV, USA, 21–24 September 2008. [Google Scholar]
- Gu, D.; Meiners, W.; Wissenbach, K.; Poprawe, R. Laser additive manufacturing of metallic components: Materials, processes and mechanisms. Int. Mater. Rev. 2012, 57, 133–164. [Google Scholar] [CrossRef]
- Murr, L.E.; Gaytan, S.M.; Ramirez, D.A.; Martinez, E.; Hernandez, J.; Amato, K.N.; Shindo, P.W.; Medina, F.R.; Wicker, R.B. Metal Fabrication by Additive Manufacturing Using Laser and Electron Beam Melting Technologies. J. Mater. Sci. Technol. 2012, 28, 1–14. [Google Scholar] [CrossRef]
- Gusarov, A.V.; Yadroitsev, I.; Bertrand, P.; Smurov, I. Model of Radiation and Heat Transfer in Laser-Powder Interaction Zone at Selective Laser Melting. J. Heat Transf. 2009, 131, 072101. [Google Scholar] [CrossRef]
- Heeling, T.; Cloots, M.; Wegener, K. Melt pool simulation for the evaluation of process parameters in selective laser melting. Addit. Manuf. 2017, 14, 116–125. [Google Scholar] [CrossRef]
- Meier, C.; Penny, R.W.; Zou, Y.; Gibbs, J.S.; Hart, A.J. Thermophysical Phenomena in Metal Additive Manufacturing by Selective Laser Melting: Fundamentals, Modeling, Simulation, and Experimentation. Annu. Rev. Heat Transf. 2017, 20, 241–316. [Google Scholar] [CrossRef]
- Mitteramskogler, G.; Gmeiner, R.; Felzmann, R.; Gruber, S.; Hofstetter, C.; Stampfl, J.; Ebert, J.; Wachter, W.; Laubersheimer, J. Light curing strategies for lithography-based additive manufacturing of customized ceramics. Addit. Manuf. 2014, 110–118. [Google Scholar] [CrossRef]
- Wilkes, J.; Hagedorn, Y.; Meiners, W.; Wissenbach, K. Additive manufacturing of ZrO2-Al2O3ceramic components by selective laser melting. Rapid Prototyp. J. 2013, 19, 51–57. [Google Scholar] [CrossRef]
- Candotto, V.; Gabrione, F.; Oberti, L.; Lento, D.; Severino, M. The role of implant-abutment connection in preventing bacterial leakage: A review. J. Biol. Regul Homeost. Agents. 2019, 33, 129–134. [Google Scholar] [PubMed]


| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Study Design | Study Design |
| In vitro study | Case report |
| In vivo study | Animal studies |
| Clinical trial | Narrative review |
| Comparative study | Only charts and questionnaires |
| Evaluation study | No control group |
| Contents | Contents |
| Assessing marginal fit | Assessing only the whole or internal fit |
| Complete-coverage prostheses | Direct restorations and partial-coverage prostheses |
| 3D printing using cobalt-chromium alloy | 3D printing of dental implants, removable dentures, and artificial bones 3D printing using titanium and gold alloys |
| 3D Printing | Prosthesis | Measurement | ||||||
|---|---|---|---|---|---|---|---|---|
| Study | Device | Material | Ab | Type | Space | Gap | Way | N |
| Chang 2019 [24] | EOSINT M270, EOS | Wirobond C+, Bego | T | S | 30 | MG | CD | 10 |
| Yildirim 2019 [21] | EOSINT M270, EOS | EOS CoCr SP2, EOS | I | S | 25 | MG | SR | 12 |
| James 2018 [25] | EOSINT M270, EOS | EOS CoCr SP2, EOS | T | S | 25 | MG | SR | 10 |
| Ullattuthodi 2017 [27] | EOSINT M270, EOS | N/P | T | S | 50 | MG | DO | 10 |
| Kocaağaoğlu 2016 [30] | EOSINT M270, EOS | EOS CoCr SP2, EOS | T | S | 30 | MG | SR | 10 |
| Park 2015 [35] | EOSINT M270, EOS | EOS CoCr SP2, EOS | T | S | 25 | MG | SR | 10 |
| Tamac 2014 [37] | EOSINT M270, EOS | EOS CoCr SP2, EOS | T | S | 30 | MG | SR | 20 |
| Kim 2013 [39] | EOSINT M270, EOS | EOS CoCr SP2, EOS | T | S | 30 | MG | SR | 10 |
| Kaleli 2017 [29] | EOSINT M270, EOS | Keramit N/P-S, Nobil Metal | T | M | 20 | MG | DO | 24 |
| Kim 2013 [38] | EOSINT M270, EOS | EOS CoCr SP2, EOS | T | M | 30 | MG, AMD | SR | 10 |
| Hong 2019 [23] | M1, CL | Remanium Star CL, CL | I | S | N/P | MG, AMD | SR | 20 |
| Gunsoy 2016 [31] | M1, CL | N/P | T | S | 50 | MG | DO | 16 |
| Lövgren 2017 [28] | Mlab cusing, CL | N/P | T | S | 50 | MG | SR | 12 |
| Presotto 2019 [22] | Mlab cusing, CL | Remanium Star CL, CL | I | M | N/P | MG | DO | 10 |
| Ates 2016 [32] | N/P, CL | N/P, Dentaurum | T | S | 30 | MG | DO | 10 |
| Huang 2015 [36] | Medifacturing System, Bego | Wirobond C+, Bego | T | S | 70 | MG | SR | 110 |
| Zeng 2015 [33] | Medifacturing System, Bego | Wirobond C+, Bego | T | S | N/P | MG | SR | 15 |
| Arora 2018 [26] | Pro X, 100DP | N/P | T | S | N/P | MG | DO | 10 |
| Pompa 2015 [34] | N/P, DeguDent | Starloy LS, DeguDent | T | M | 20 | MG | MS | 20 |
| Castillo-de-Oyagüe 2012 [40] | PM100 Dental, Phenix System | ST2724G, Sint-Tech | T | S | N/P | MG | DO | 10 |
| Örtorp 2011 [41] | N/P, Biomain AB | N/P | T | M | 50 | MG | MS | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, S.; Hong, M.-H.; Lee, H.; Lee, C.-H.; Hong, M.; Lee, J.; Lee, D.-H. Reliability of Metal 3D Printing with Respect to the Marginal Fit of Fixed Dental Prostheses: A Systematic Review and Meta-Analysis. Materials 2020, 13, 4781. https://doi.org/10.3390/ma13214781
Bae S, Hong M-H, Lee H, Lee C-H, Hong M, Lee J, Lee D-H. Reliability of Metal 3D Printing with Respect to the Marginal Fit of Fixed Dental Prostheses: A Systematic Review and Meta-Analysis. Materials. 2020; 13(21):4781. https://doi.org/10.3390/ma13214781
Chicago/Turabian StyleBae, Soohyun, Min-Ho Hong, Hyunwoo Lee, Cheong-Hee Lee, Mihee Hong, Jaesik Lee, and Du-Hyeong Lee. 2020. "Reliability of Metal 3D Printing with Respect to the Marginal Fit of Fixed Dental Prostheses: A Systematic Review and Meta-Analysis" Materials 13, no. 21: 4781. https://doi.org/10.3390/ma13214781
APA StyleBae, S., Hong, M.-H., Lee, H., Lee, C.-H., Hong, M., Lee, J., & Lee, D.-H. (2020). Reliability of Metal 3D Printing with Respect to the Marginal Fit of Fixed Dental Prostheses: A Systematic Review and Meta-Analysis. Materials, 13(21), 4781. https://doi.org/10.3390/ma13214781





