The Use of Ultra-Small Fe3O4 Magnetic Nanoparticles for Hydrothermal Synthesis of Fe3+-Doped Titanate Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of TNTs
2.2. Modified TNTs
2.3. Photocatalytic Examination
2.4. Measuring Techniques
- -
- transmission electron microscopy (TEM) by using the Fei Tecnai G2 F20 S Twin transmission electron microscope (Hillsboro, OR, USA) was used,
- -
- EPR measurements by using the BRUKER E500 EPR spectrometer (Billerica, MA, USA) working at 9.4 GHz (X-band) with 100 kHz magnetic field modulation,
- -
- XRD measurements were performed using a Bruker D8 Advance (Billerica, MA, USA) with Johansson monochromator ( Å) and detector LynxEye.
- -
- dc magnetization was measured using a Quantum Design Magnetic Property Measurements System MPMS XL-7 (Quantum Design Inc, San Diego, CA, USA) with a superconducting quantum interference device magnetometer (SQUID).
3. Results and Discussion
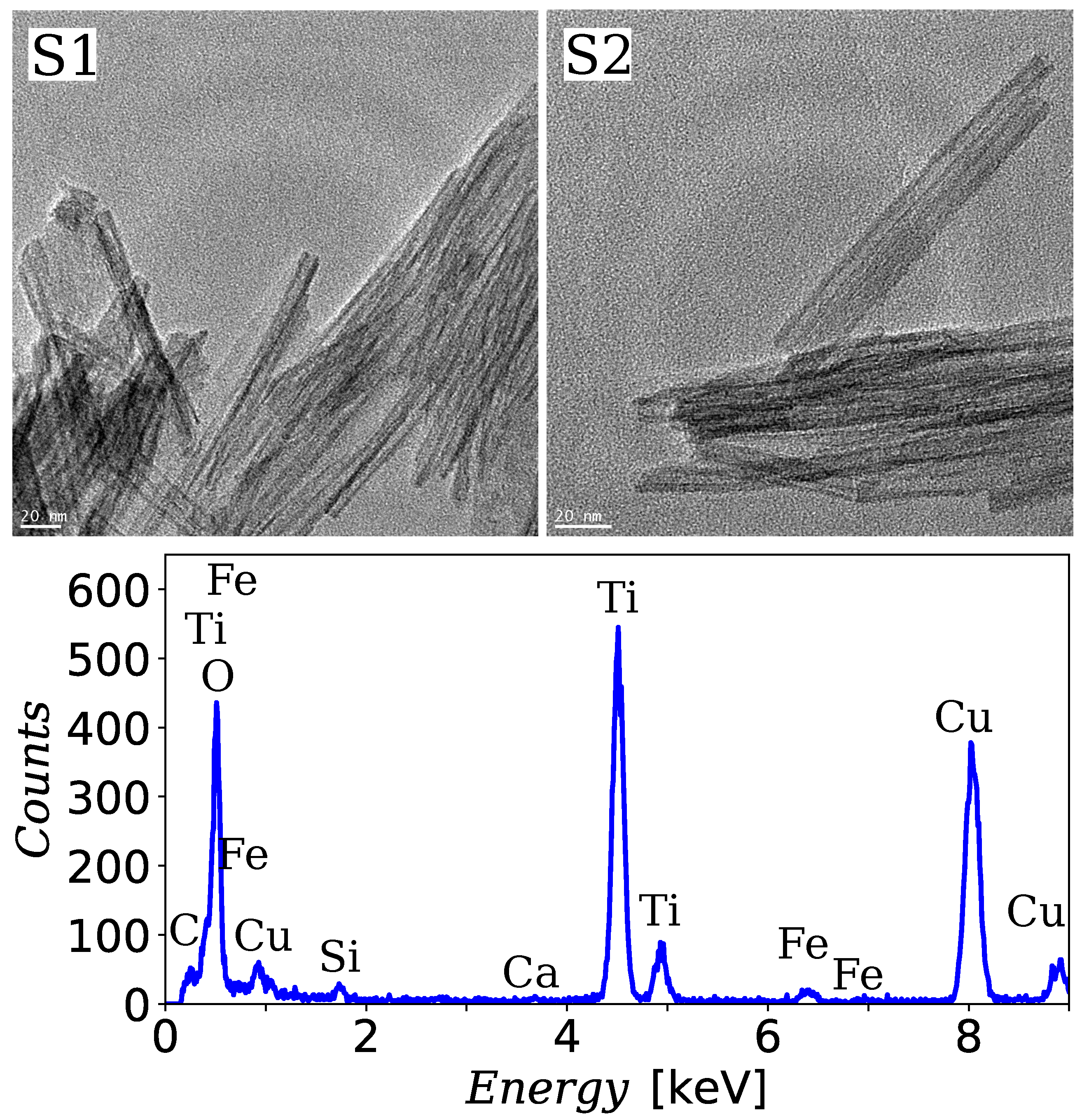
3.1. TEM Analysis
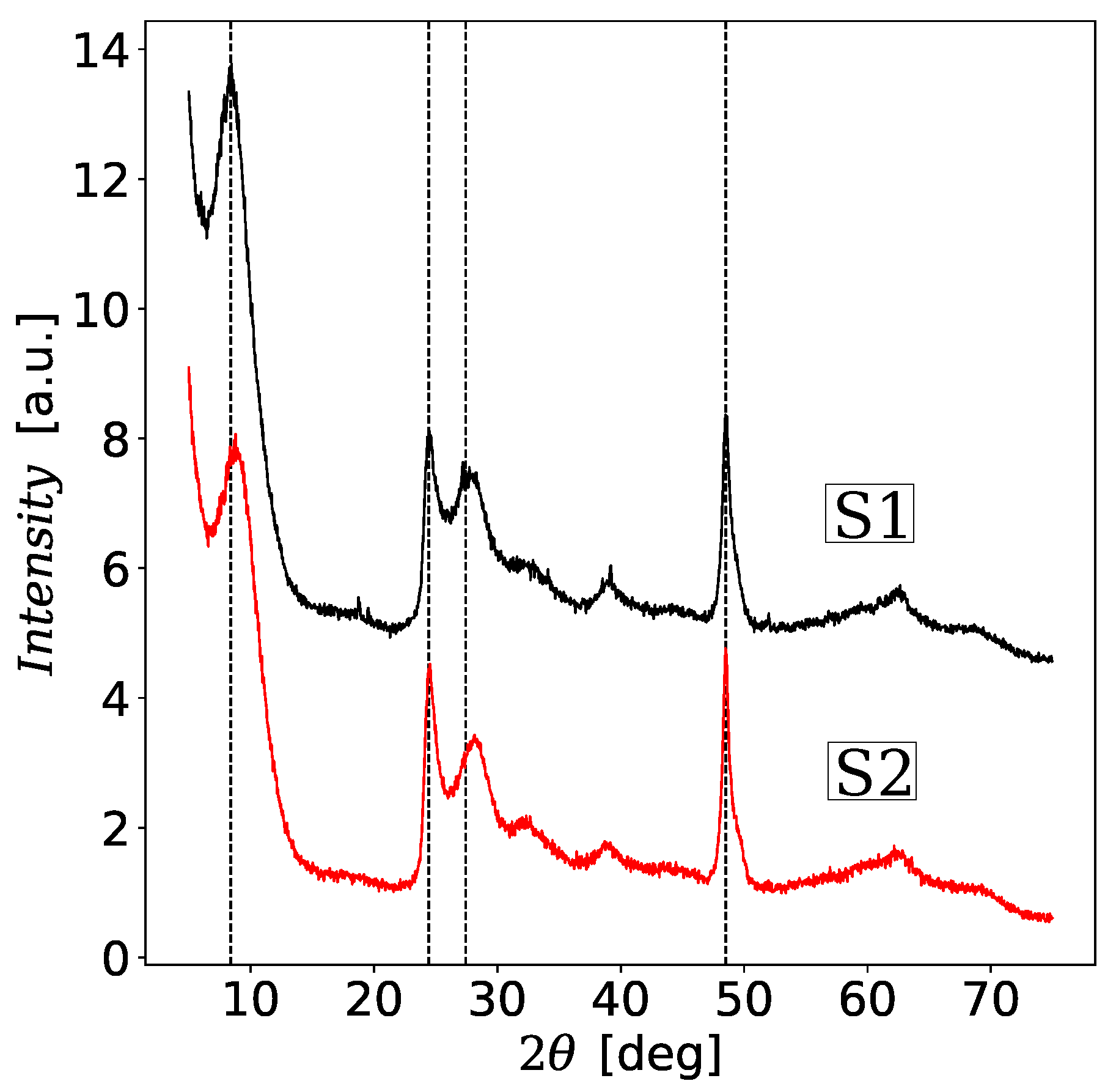
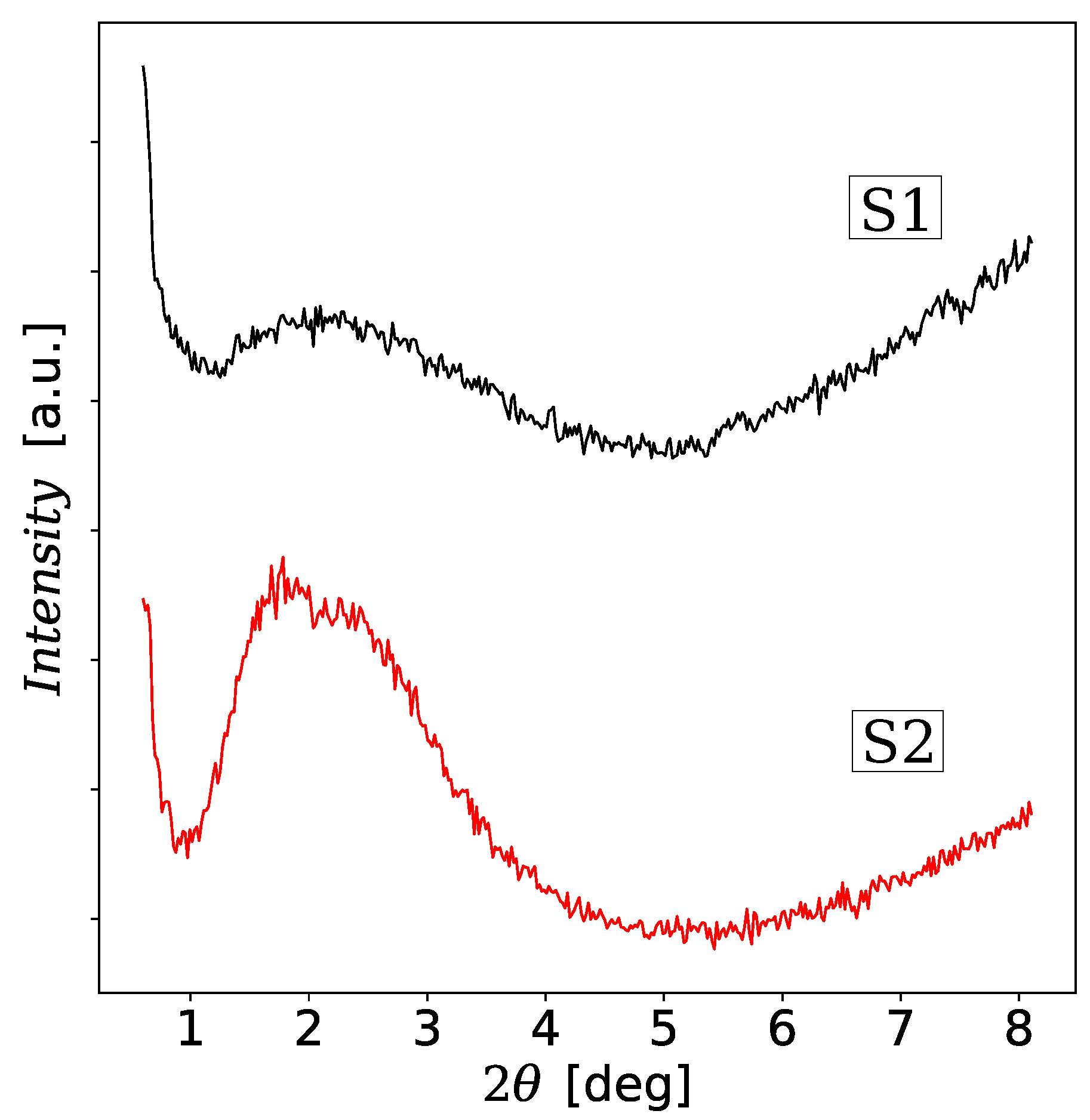
3.2. Powder XRD Analysis
3.3. EPR Analysis
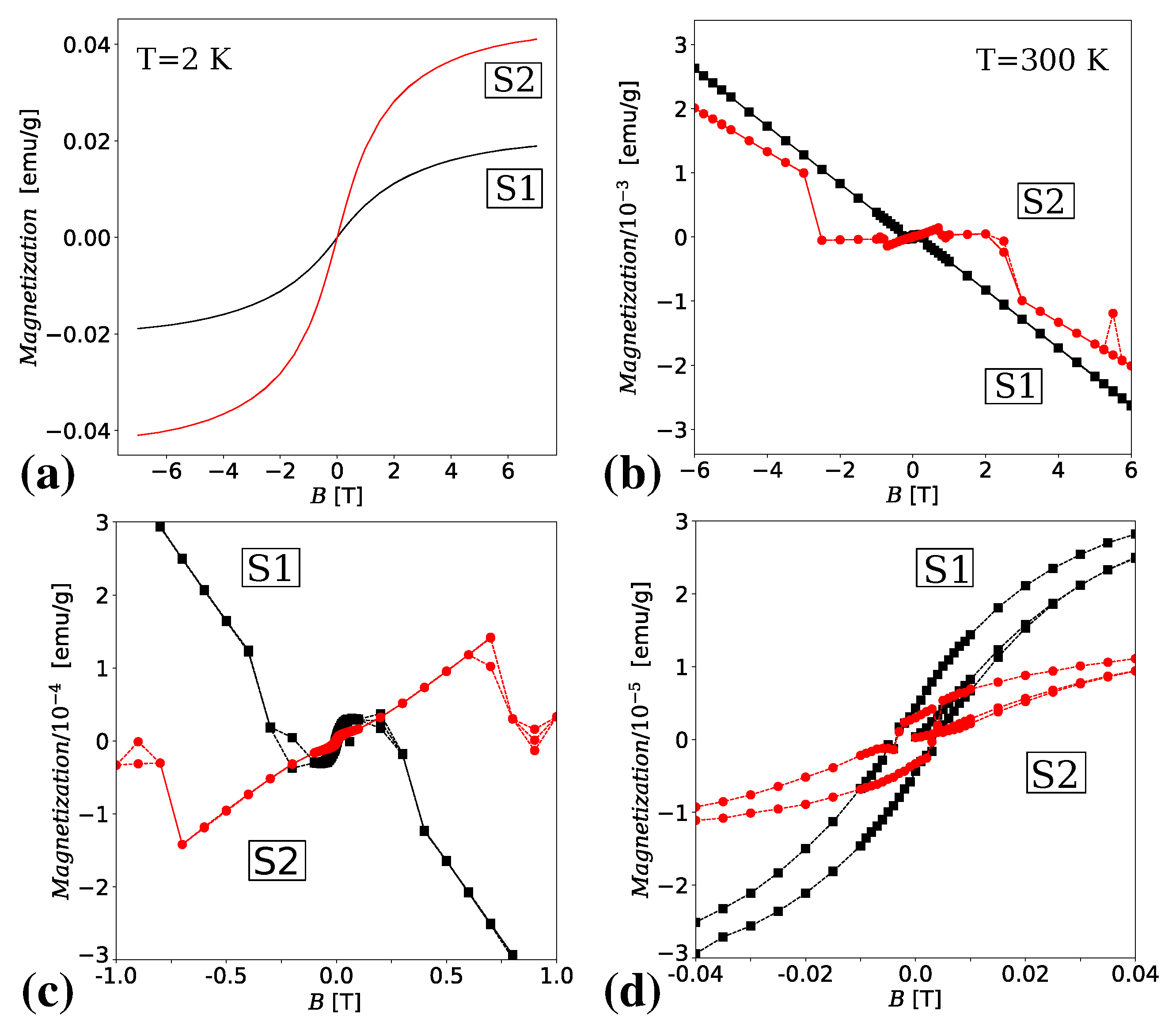
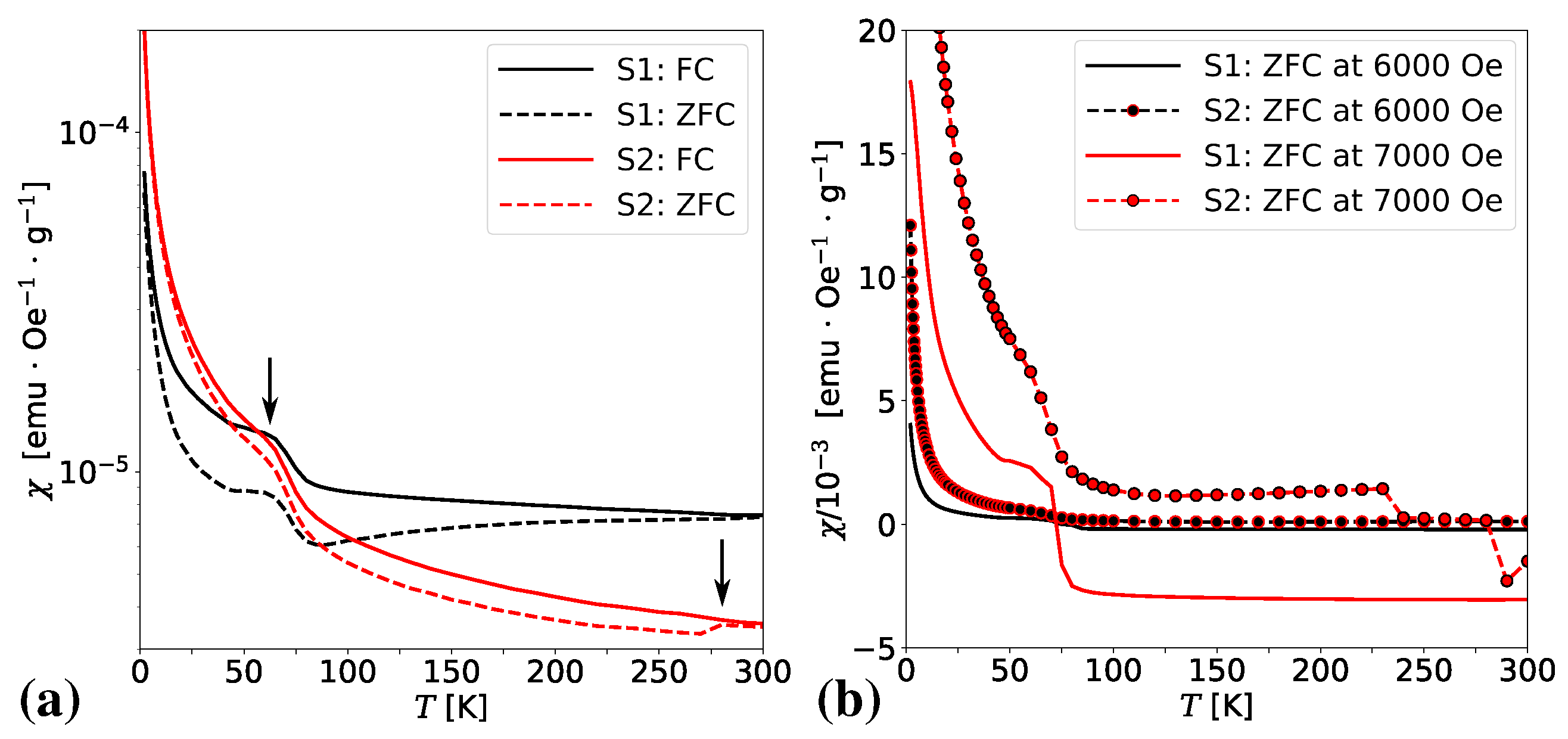
3.4. Magnetic Properties
3.5. Photocatalytic Effect
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EDS | Energy Dispersive X-ray spectroscopy |
| MB | Methylene Blue |
| MCM-41 | Mobil Composition of Matter No. 41 |
| DFT | Density Functional Theory |
| EPR | Electron Paramagnetic Resonance |
| TEM | Transmission Electron Microscopy |
| TNT | Titanate Nanotubes |
| XRD | X-ray Powder Diffraction |
| UV-Vis | Ultraviolet - Visible |
| ZFC/FC | Zero Field Cooled/Field Cooled |
References
- Çeşmeli, S.; Biray Avci, C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J. Drug Target. 2019, 27, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, L.; Pettit, S.L.; Zhao, W.; Michaels, J.T.; Kuhn, J.N.; Alcantar, N.A.; Ergas, S.J. Oxidation of off flavor compounds in recirculating aquaculture systems using UV-TiO2 photocatalysis. Aquaculture 2019, 502, 32–39. [Google Scholar] [CrossRef]
- Pignanelli, F.; Fernández-Werner, L.; Romero, M.; Mombrú, D.; Tumelero, M.A.; Pasa, A.A.; Germán, E.; Faccio, R.; Mombrú, Á.W. Hydrogen titanate nanotubes for dye sensitized solar cells applications: Experimental and theoretical study. Mater. Res. Bull. 2018, 106, 40–48. [Google Scholar] [CrossRef]
- Chen, H.; Wu, Y.H.; Ma, H.; Shi, J.B.; Pan, X.W.; Lei, B.X.; Sun, Z.F. Dye-sensitized titanium dioxide nanotube array solar cells with superior performance induced by ferroelectric barium titanate. Thin Solid Films 2020, 709, 138205. [Google Scholar] [CrossRef]
- Sang, N.X.; Minh, V.C. Thermal annealing-induced self-junction of hydrothermal titanate nanotubes/TiO2 nanoparticles with enhanced photocatalytic activity. Nanotechnology 2020, 31, 435703. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, J.; Wu, P.; Luo, D.; Yan, R.; Zhang, H.; Jiang, W. Simultaneous Removal of Tetracycline and Cu (II) in Hybrid Wastewater through Formic-Acid Assisted TiO2 Photocatalysis. Ind. Eng. Chem. Res. 2020, 59, 15098–15108. [Google Scholar] [CrossRef]
- Beketova, D.; Motola, M.; Sopha, H.; Michalicka, J.; Cicmancova, V.; Dvorak, F.; Hromadko, L.; Frumarova, B.; Stoica, M.; Macak, J.M. One-Step Decoration of TiO2 Nanotubes with Fe3O4 Nanoparticles: Synthesis and Photocatalytic and Magnetic Properties. ACS Appl. Nano Mater. 2020, 3, 1553–1563. [Google Scholar] [CrossRef]
- Zhao, X.; Du, P.; Cai, Z.; Wang, T.; Fu, J.; Liu, W. Photocatalysis of bisphenol A by an easy-settling titania/titanate composite: Effects of water chemistry factors, degradation pathway and theoretical calculation. Environ. Pollut. 2018, 232, 580–590. [Google Scholar] [CrossRef]
- Dudefoi, W.; Moniz, K.; Allen-Vercoe, E.; Ropers, M.H.; Walker, V.K. Impact of food grade and nano-TiO2 particles on a human intestinal community. Food Chem. Toxicol. 2017, 106, 242–249. [Google Scholar] [CrossRef]
- Muniyappan, S.; Solaiyammal, T.; Sudhakar, K.; Karthigeyan, A.; Murugakoothan, P. Conventional hydrothermal synthesis of titanate nanotubes: Systematic discussions on structural, optical, thermal and morphological properties. Mod. Electron. Mater. 2017, 3, 174–178. [Google Scholar] [CrossRef]
- Xu, X.; Ding, X.; Chen, Q.; Peng, L.M. Electronic, optical, and magnetic properties of Fe-intercalated H2Ti3O7 nanotubes: First-principles calculations and experiments. Phys. Rev. B 2006, 73, 165403. [Google Scholar] [CrossRef]
- Asahi, R.; Taga, Y.; Mannstadt, W.; Freeman, A.J. Electronic and optical properties of anatase TiO2. Phys. Rev. B 2000, 61, 7459. [Google Scholar] [CrossRef]
- Roose, B.; Pathak, S.; Steiner, U. Doping of TiO2 for sensitized solar cells. Chem. Soc. Rev. 2015, 44, 8326–8349. [Google Scholar] [CrossRef]
- Hoye, R.L.; Musselman, K.P.; MacManus-Driscoll, J.L. Research update: Doping ZnO and TiO2 for solar cells. APL Mater. 2013, 1, 060701. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Al-Shahry, M.; Ingler, W.B. Efficient photochemical water splitting by a chemically modified n-TiO2. Science 2002, 297, 2243–2245. [Google Scholar] [CrossRef]
- Faughnan, B.; Kiss, Z. Photoinduced Reversible Charge-Transfer Processes in Transition-Metal-Doped Single-Crystal SrTiO3 and TiO2. Phys. Rev. Lett. 1968, 21, 1331. [Google Scholar] [CrossRef]
- Wu, Q.; Van De Krol, R. Selective photoreduction of nitric oxide to nitrogen by nanostructured TiO2 photocatalysts: Role of oxygen vacancies and iron dopant. J. Am. Chem. Soc. 2012, 134, 9369–9375. [Google Scholar] [CrossRef]
- Yang, C.K. A metallic graphene layer adsorbed with lithium. Appl. Phys. Lett. 2009, 95, 163115. [Google Scholar] [CrossRef]
- Liau, L.C.K.; Lin, C.C. Fabrication and characterization of Fe3+-doped titania semiconductor electrodes with p–n homojunction devices. Appl. Surf. Sci. 2007, 253, 8798–8801. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Yu, J.; Wu, Z.; Gong, C.; Xiao, W.; Sun, L.; Lin, C. Fe3+-doped TiO2 nanotube arrays on Ti-Fe alloys for enhanced photoelectrocatalytic activity. Nanomaterials 2016, 6, 107. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Zhang, J.; Hu, Y.; Bai, Z.; Wei, C. Dual-template synthesis of mesoporous TiO2 nanotubes with structure-enhanced functional photocatalytic performance. Appl. Catal. B Environ. 2019, 250, 301–312. [Google Scholar] [CrossRef]
- Zavala, M.Á.L.; Morales, S.A.L.; Ávila-Santos, M. Synthesis of stable TiO2 nanotubes: Effect of hydrothermal treatment, acid washing and annealing temperature. Heliyon 2017, 3, e00456. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Q.; Peng, L.M. Structure and formation of H2Ti3O7 nanotubes in an alkali environment. Phys. Rev. B 2005, 71, 014104. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Li, G.; Meng, J.; Tong, W. Synthesis of titanate-based nanotubes for one-dimensionally confined electrical properties. J. Phys. Chem. C 2009, 113, 16996–17001. [Google Scholar] [CrossRef]
- Ferreira, V.; Nunes, M.; Silvestre, A.J.; Monteiro, O. Synthesis and properties of Co-doped titanate nanotubes and their optical sensitization with methylene blue. Mater. Chem. Phys. 2013, 142, 355–362. [Google Scholar] [CrossRef]
- Szirmai, P.; Stevens, J.; Horváth, E.; Ćirić, L.; Kollár, M.; Forró, L.; Náfrádi, B. Competitive ion-exchange of manganese and gadolinium in titanate nanotubes. Catal. Today 2017, 284, 146–152. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, Q.; Li, Z.; Liu, J.; Zhang, H.; Li, R.; Wang, J. Efficient extraction of uranium from aqueous solution using an amino-functionalized magnetic titanate nanotubes. J. Hazard. Mater. 2018, 353, 9–17. [Google Scholar] [CrossRef]
- Marć, M.; Dudek, M.R.; Kozioł, J.J.; Zapotoczny, B. Adsorption of methylene blue on titanate nanotubes synthesized with ultra-small Fe3O4 nanoparticles. Nano 2018, 13, 1850142. [Google Scholar] [CrossRef]
- Marques, T.M.; Sales, D.A.; Silva, L.S.; Bezerra, R.D.; Silva, M.S.; Osajima, J.A.; Ferreira, O.P.; Ghosh, A.; Silva Filho, E.C.; Viana, B.C.; et al. Amino-functionalized titanate nanotubes for highly efficient removal of anionic dye from aqueous solution. Appl. Surf. Sci. 2020, 512, 145659. [Google Scholar] [CrossRef]
- Sallem, F.; Boudon, J.; Heintz, O.; Séverin, I.; Megriche, A.; Millot, N. Synthesis and characterization of chitosan-coated titanate nanotubes: Towards a new safe nanocarrier. Dalton Trans. 2017, 46, 15386–15398. [Google Scholar] [CrossRef]
- Sruthi, S.; Loiseau, A.; Boudon, J.; Sallem, F.; Maurizi, L.; Mohanan, P.; Lizard, G.; Millot, N. In vitro interaction and biocompatibility of titanate nanotubes with microglial cells. Toxicol. Appl. Pharm. 2018, 353, 74–86. [Google Scholar] [CrossRef] [PubMed]
- Gusmão, S.B.; Ghosh, A.; Marques, T.M.; Gusmão, G.O.; Oliveira, T.G.; Cavalcante, L.C.D.; Vasconcelos, T.L.; Abreu, G.J.; Guerra, Y.; Peña-Garcia, R.; et al. Structural and magnetic properties of titanate nano-heterostructures decorated with iron based nanoparticles. J. Phys. Chem. Solids 2020, 145, 109561. [Google Scholar] [CrossRef]
- Deng, L.; Wang, S.; Liu, D.; Zhu, B.; Huang, W.; Wu, S.; Zhang, S. Synthesis, characterization of Fe-doped TiO2 nanotubes with high photocatalytic activity. Catal. Lett. 2009, 129, 513–518. [Google Scholar] [CrossRef]
- Han, W.Q.; Wen, W.; Yi, D.; Liu, Z.; Maye, M.M.; Lewis, L.; Hanson, J.; Gang, O. Fe-doped trititanate nanotubes: Formation, optical and magnetic properties, and catalytic applications. J. Phys. Chem. C 2007, 111, 14339–14342. [Google Scholar] [CrossRef]
- Liu, W.; Zhao, X.; Borthwick, A.G.; Wang, Y.; Ni, J. Dual-enhanced photocatalytic activity of Fe-deposited titanate nanotubes used for simultaneous removal of As (III) and As (V). ACS Appl. Mater. Interfaces 2015, 7, 19726–19735. [Google Scholar] [CrossRef]
- Zaki, A.; Hafiez, M.A.; El Rouby, W.M.; El-Dek, S.; Farghali, A. Novel magnetic standpoints in Na2Ti3O7 nanotubes. J. Magn. Magn. Mater. 2019, 476, 207–212. [Google Scholar] [CrossRef]
- Wang, X.; Gao, X.; Li, G.; Gao, L.; Yan, T.; Zhu, H.Y. Ferromagnetism of Co-doped TiO2 (B) nanotubes. Appl. Phys. Lett. 2007, 91, 143102. [Google Scholar] [CrossRef]
- Lu, C.; Guan, W.; Hoang, T.K.; Guo, J.; Gou, H.; Yao, Y. Visible-light-driven catalytic degradation of ciprofloxacin on metal (Fe, Co, Ni) doped titanate nanotubes synthesized by one-pot approach. J. Mater. Sci. Mater. Electron. 2016, 27, 1966–1973. [Google Scholar] [CrossRef]
- Huang, C.; Liu, X.; Kong, L.; Lan, W.; Su, Q.; Wang, Y. The structural and magnetic properties of Co-doped titanate nanotubes synthesized under hydrothermal conditions. Appl. Phys. A 2007, 87, 781–786. [Google Scholar] [CrossRef]
- Vranješ, M.; Jakovljević, J.K.; Milošević, M.; Ćirić Marjanović, G.; Stoiljković, M.; Konstantinović, Z.; Pavlović, V.; Milivojević, D.; Šaponjić, Z. Hydrothermal synthesis of Mn2+ doped titanate nanotubes: Investigation of their structure and room temperature ferromagnetic behavior. Solid State Sci. 2019, 94, 155–161. [Google Scholar] [CrossRef]
- Hafez, H.S.; Saif, M.; McLeskey, J.T.; Abdel-Mottaleb, M.; Yahia, I.; Story, T.; Knoff, W. Hydrothermal Preparation of Gd3+-Doped Titanate Nanotubes: Magnetic Properties and Photovoltaic Performance. Int. J. Photoenergy 2009, 2009, 240402. [Google Scholar] [CrossRef]
- Marques, T.M.; Luz-Lima, C.; Sacilloti, M.; Fujisawa, K.; Perea-Lopez, N.; Terrones, M.; Silva, E.N.; Ferreira, O.P.; Viana, B.C. Photoluminescence enhancement of titanate nanotubes by insertion of rare earth ions in their interlayer spaces. J. Nanomater. 2017, 2017, 3809807. [Google Scholar] [CrossRef]
- Bavykin, D.V.; Lapkin, A.A.; Plucinski, P.K.; Torrente-Murciano, L.; Friedrich, J.M.; Walsh, F.C. Deposition of Pt, Pd, Ru and Au on the surfaces of titanate nanotubes. Top. Catal. 2006, 39, 151–160. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 photocatalysis: Mechanisms and materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef] [PubMed]
- Bahnemann, D.W. Current challenges in photocatalysis: Improved photocatalysts and appropriate photoreactor engineering. Res. Chem. Intermediat. 2000, 26, 207–220. [Google Scholar] [CrossRef]
- Wang, C.y.; Pagel, R.; Bahnemann, D.W.; Dohrmann, J.K. Quantum yield of formaldehyde formation in the presence of colloidal TiO2-based photocatalysts: Effect of intermittent illumination, platinization, and deoxygenation. J. Phys. Chem. B 2004, 108, 14082–14092. [Google Scholar] [CrossRef]
- Hufschmidt, D.; Bahnemann, D.; Testa, J.J.; Emilio, C.A.; Litter, M.I. Enhancement of the photocatalytic activity of various TiO2 materials by platinisation. J. Photochem. Photobiol. A 2002, 148, 223–231. [Google Scholar] [CrossRef]
- Kokorin, A.; Amal, R.; Teoh, W.; Kulak, A. Studies of Nanosized Iron-Doped TiO2 Photocatalysts by Spectroscopic Methods. Appl. Magn. Reson. 2017, 48, 447–459. [Google Scholar] [CrossRef]
- Papa, A.L.; Maurizi, L.; Vandroux, D.; Walker, P.; Millot, N. Synthesis of titanate nanotubes directly coated with USPIO in hydrothermal conditions: A new detectable nanocarrier. J. Phys. Chem. C 2011, 115, 19012–19017. [Google Scholar] [CrossRef]
- Grzechulska-Damszel, J.; Morawski, A.W.; Grzmil, B. Thermally modified titania photocatalysts for phenol removal from water. Int. J. Photoenergy 2006, 2006. [Google Scholar] [CrossRef]
- Mozia, S.; Tomaszewska, M.; Morawski, A.W. Decomposition of nonionic surfactant in a labyrinth flow photoreactor with immobilized TiO2 bed. Appl. Catal. B Environ. 2005, 59, 155–160. [Google Scholar] [CrossRef]
- Zapotoczny, B.; Guskos, N.; Kozioł, J.; Dudek, M. Preparation of the narrow size distribution USPIO in mesoporous silica for magnetic field guided drug delivery and release. J. Magn. Magn. Mater. 2015, 374, 96–102. [Google Scholar] [CrossRef]
- Zhang, D.; Cong, T.; Xia, L.; Pan, L. Growth of black TiO2 nanowire/carbon fiber composites with dendritic structure for efficient visible-light-driven photocatalytic degradation of methylene blue. J. Mater. Sci. 2019, 54, 7576–7588. [Google Scholar] [CrossRef]
- Rahman, N.R.A.; Muniandy, L.; Adam, F.; Iqbal, A.; Ng, E.P.; Lee, H.L. Detailed photocatalytic study of alkaline titanates and its application for the degradation of methylene blue (MB) under solar irradiation. J. Photochem. Photobiol. A 2019, 375, 219–230. [Google Scholar] [CrossRef]
- Chen, Q.; Du, G.; Zhang, S.; Peng, L.M. The structure of trititanate nanotubes. Acta Crystallogr. B 2002, 58, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Gerritsen, H.; Harrison, S.; Lewis, H.; Wittke, J. Fine Structure, Hyperfine Structure, and Relaxation Times of Cr3+ in TiO2 (Rutile). Phys. Rev. Lett. 1959, 2, 153. [Google Scholar] [CrossRef]
- Chester, P. Electron spin resonance in semiconducting rutile. J. Appl. Phys. 1961, 32, 2233–2236. [Google Scholar] [CrossRef]
- Golden, E.M.; Giles, N.C.; Yang, S.; Halliburton, L.E. Interstitial silicon ions in rutile TiO2 crystals. Phys. Rev. B 2015, 91, 134110. [Google Scholar] [CrossRef]
- Carriazo, J.; Ensuncho-Muñoz, A.; Almanza, O. Electron Paramagnetic Resonance (EPR) investigation of TiO2-delaminated clays. Rev. Mex. Ing. Quim. 2014, 13, 473–481. [Google Scholar]
- Naresh, N.; Bhowmik, R.; Ghosh, B.; Banerjee, S. Study of surface magnetism, exchange bias effect, and enhanced ferromagnetism in α-Fe1.4Ti0.6O3 alloy. J. Appl. Phys. 2011, 109, 093913. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Murakami, M.; Shono, T.; Hasegawa, T.; Fukumura, T.; Kawasaki, M.; Ahmet, P.; Chikyow, T.; Koshihara, S.Y.; Koinuma, H. Room-temperature ferromagnetism in transparent transition metal-doped titanium dioxide. Science 2001, 291, 854–856. [Google Scholar] [CrossRef]
- Hong, N.H.; Sakai, J.; Poirot, N.; Brizé, V. Room-temperature ferromagnetism observed in undoped semiconducting and insulating oxide thin films. Phys. Rev. B 2006, 73, 132404. [Google Scholar] [CrossRef]
- Yoon, S.D.; Chen, Y.; Yang, A.; Goodrich, T.L.; Zuo, X.; Arena, D.A.; Ziemer, K.; Vittoria, C.; Harris, V.G. Oxygen-defect-induced magnetism to 880 K in semiconducting anatase TiO2-δ films. J. Phys. Condens. Matter 2006, 18, L355. [Google Scholar] [CrossRef]
- Pemmaraju, C.D.; Sanvito, S. Ferromagnetism driven by intrinsic point defects in HfO2. Phys. Rev. Lett. 2005, 94, 217205. [Google Scholar] [CrossRef]
- Sharma, S.; Chaudhary, S.; Kashyap, S.C.; Sharma, S.K. Room temperature ferromagnetism in Mn doped TiO2 thin films: Electronic structure and Raman investigations. J. Appl. Phys. 2011, 109, 083905. [Google Scholar] [CrossRef]
- Choudhury, B.; Choudhury, A. Room temperature ferromagnetism in defective TiO2 nanoparticles: Role of surface and grain boundary oxygen vacancies. J. Appl. Phys. 2013, 114, 203906. [Google Scholar] [CrossRef]
- Tang, A.S.; Pelliciari, J.; Song, Q.; Song, Q.; Ning, S.; Freeland, J.W.; Comin, R.; Ross, C.A. XMCD study of magnetism and valence state in iron-substituted strontium titanate. Phys. Rev. Mater. 2019, 3, 054408. [Google Scholar] [CrossRef]
- Li, D.-X.; Qin, X.-B.; Zheng, L.-R.; Li, Y.-X.; Cao, X.-Z.; Li, Z.-X.; Yang, J.; Wang, B.-Y. Defect types and room-temperature ferromagnetism in undoped rutile TiO2 single crystals. Chin. Phys. B 2013, 22, 037504. [Google Scholar] [CrossRef]
- Soltani, T.; Entezari, M.H. Photolysis and photocatalysis of methylene blue by ferrite bismuth nanoparticles under sunlight irradiation. J. Mol. Catal. A Chem. 2013, 377, 197–203. [Google Scholar] [CrossRef]
- Benhabiles, O.; Mahmoudi, H.; Lounici, H.; Goosen, M.F. Effectiveness of a photocatalytic organic membrane for solar degradation of methylene blue pollutant. Desalination Water Treat. 2016, 57, 14067–14076. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; Ahmed, E.; Farghali, A.; Zaki, A.; Barakat, N.A. Synthesis of Fe/Co-doped titanate nanotube as redox catalyst for photon-induced water splitting. Mater. Chem. Phys. 2018, 217, 125–132. [Google Scholar] [CrossRef]
- Jang, J.S.; Kim, D.H.; Choi, S.H.; Jang, J.W.; Kim, H.G.; Lee, J.S. In-situ synthesis, local structure, photoelectrochemical property of Fe-intercalated titanate nanotube. Int. J. Hydrog. Energy 2012, 37, 11081–11089. [Google Scholar] [CrossRef]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]


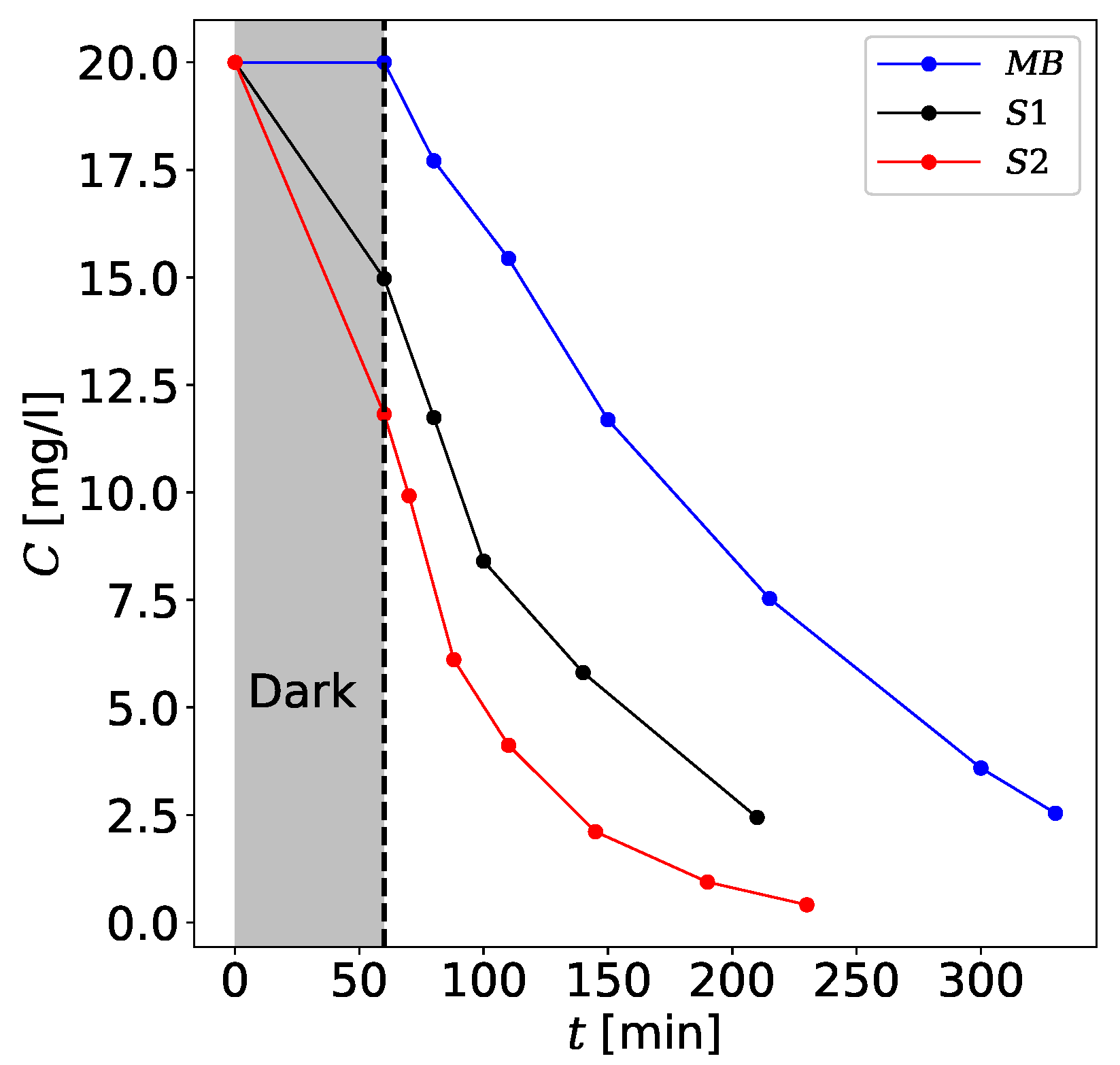
| Sample | [] | |
|---|---|---|
| MB | 7.549 | 0.990 |
| S1 | 11.933 | 0.996 |
| S2 | 19.549 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marć, M.; Najder-Kozdrowska, L.; Guskos, N.; Żołnierkiewicz, G.; Montero, A.M.; Dudek, M.R. The Use of Ultra-Small Fe3O4 Magnetic Nanoparticles for Hydrothermal Synthesis of Fe3+-Doped Titanate Nanotubes. Materials 2020, 13, 4612. https://doi.org/10.3390/ma13204612
Marć M, Najder-Kozdrowska L, Guskos N, Żołnierkiewicz G, Montero AM, Dudek MR. The Use of Ultra-Small Fe3O4 Magnetic Nanoparticles for Hydrothermal Synthesis of Fe3+-Doped Titanate Nanotubes. Materials. 2020; 13(20):4612. https://doi.org/10.3390/ma13204612
Chicago/Turabian StyleMarć, Maciej, Lidia Najder-Kozdrowska, Nikos Guskos, Grzegorz Żołnierkiewicz, Ana Maria Montero, and Mirosław Roman Dudek. 2020. "The Use of Ultra-Small Fe3O4 Magnetic Nanoparticles for Hydrothermal Synthesis of Fe3+-Doped Titanate Nanotubes" Materials 13, no. 20: 4612. https://doi.org/10.3390/ma13204612
APA StyleMarć, M., Najder-Kozdrowska, L., Guskos, N., Żołnierkiewicz, G., Montero, A. M., & Dudek, M. R. (2020). The Use of Ultra-Small Fe3O4 Magnetic Nanoparticles for Hydrothermal Synthesis of Fe3+-Doped Titanate Nanotubes. Materials, 13(20), 4612. https://doi.org/10.3390/ma13204612





