Evaluation of Sulfuric Acid-Induced Degradation of Potassium Silicate Activated Metakaolin Geopolymers by Semi-Quantitative SEM-EDX Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
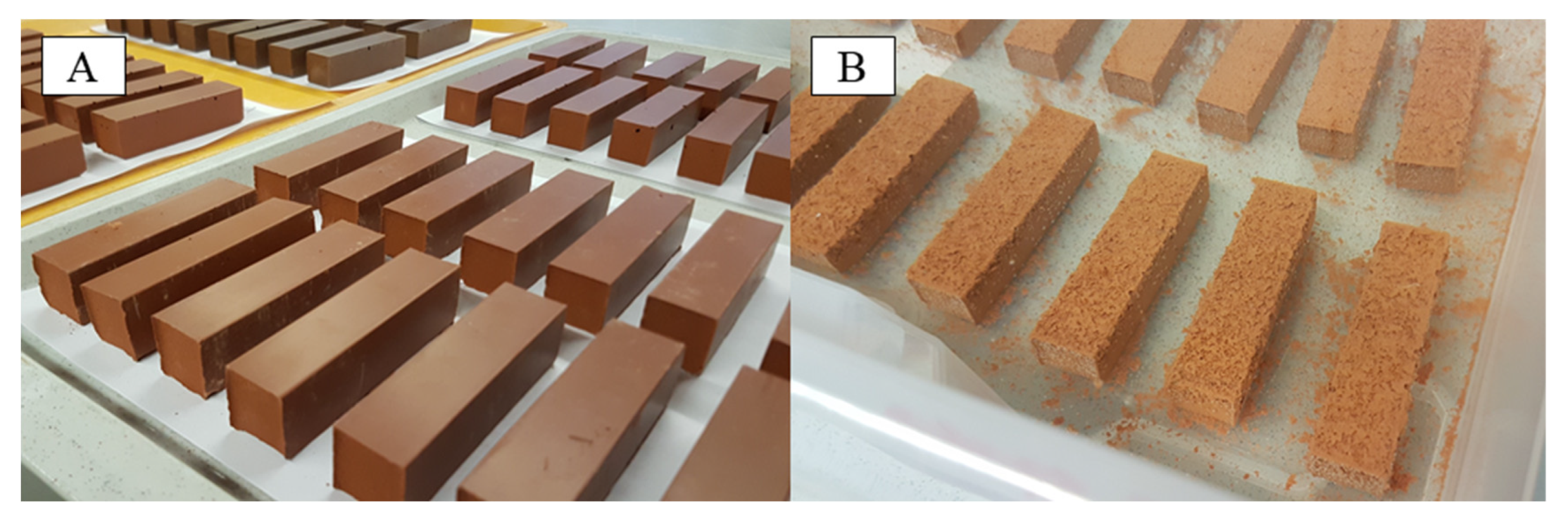
2.2. Geopolymer Samples
2.3. Characterization Methods of Unexposed Samples
2.4. Exposure to Sulfuric Acid
2.5. Sample Preparation after Sulfuric Acid Exposure
2.6. Characterization of Sulfuric Acid Exposed Samples
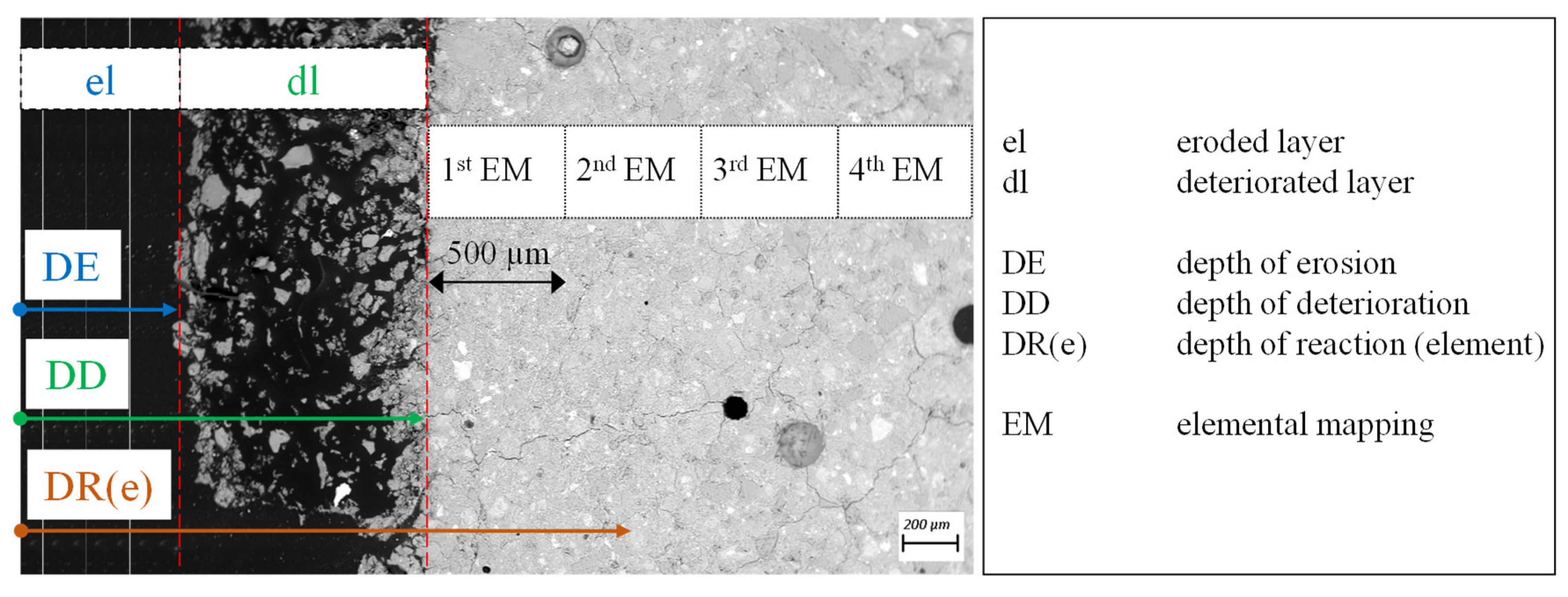
2.7. Position of Elemental Mappings and Terminology of Experimental Results
3. Results
3.1. Physical Properties of Unexposed Samples
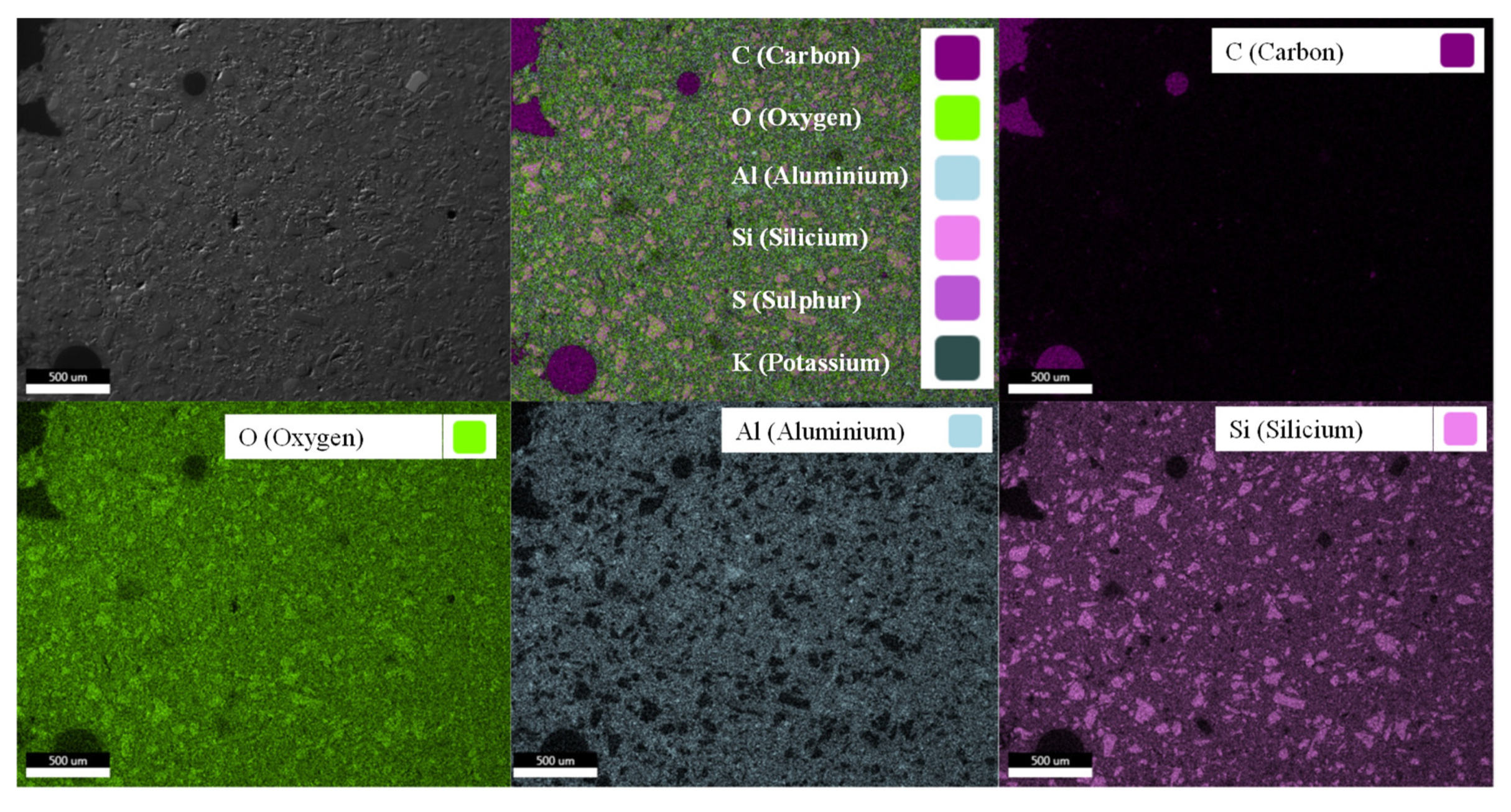
3.2. Elemental Mapping of Unexposed Geopolymers
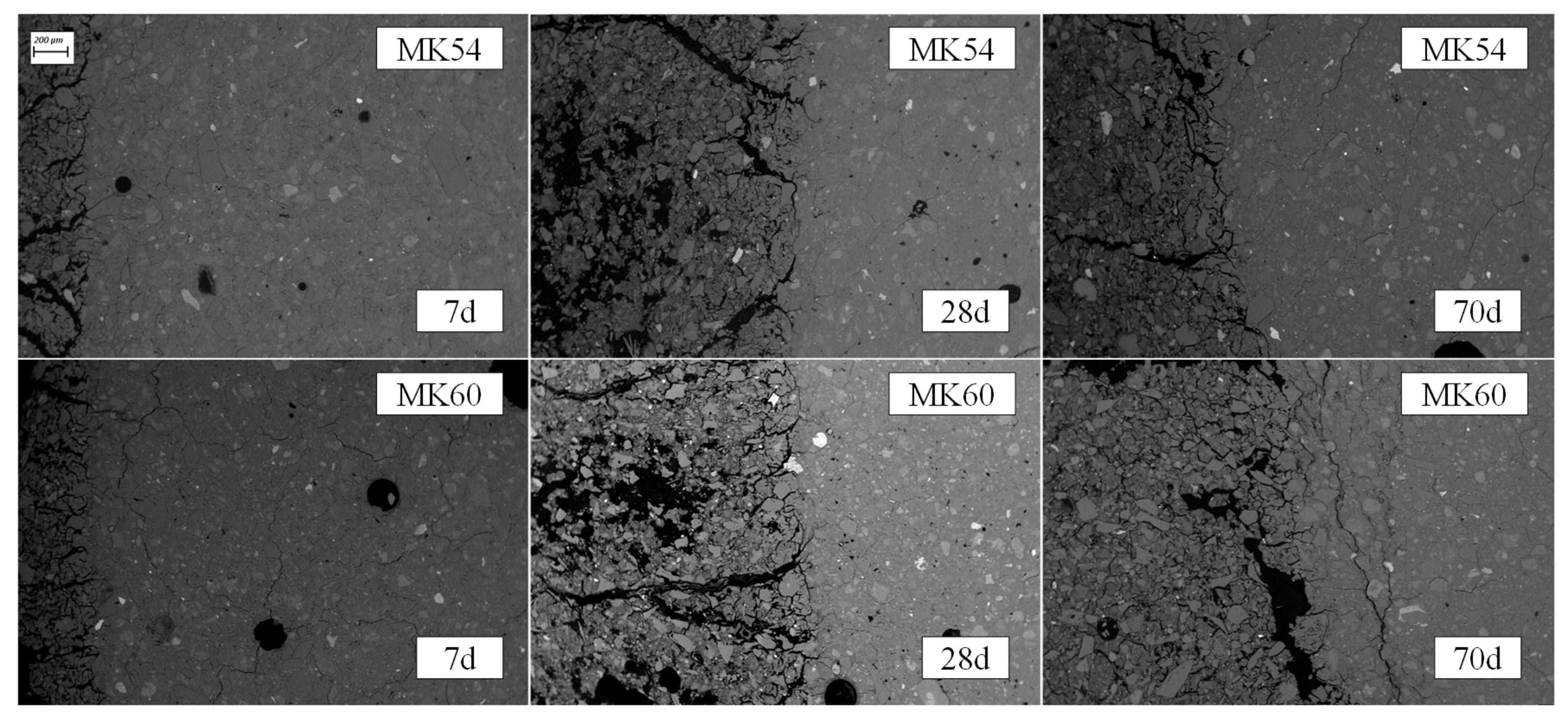
3.3. SEM Images of Sulfuric Acid Exposed Samples
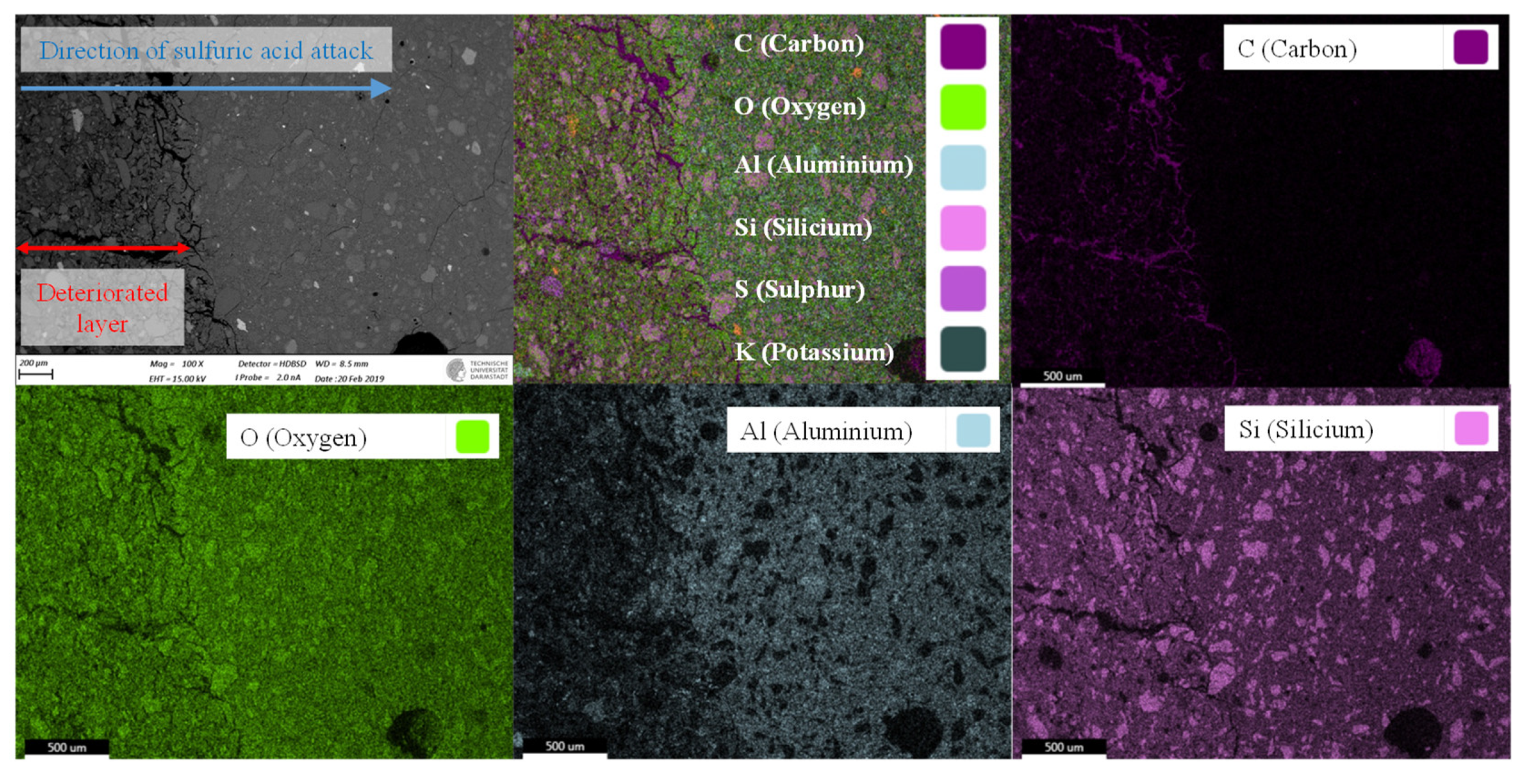
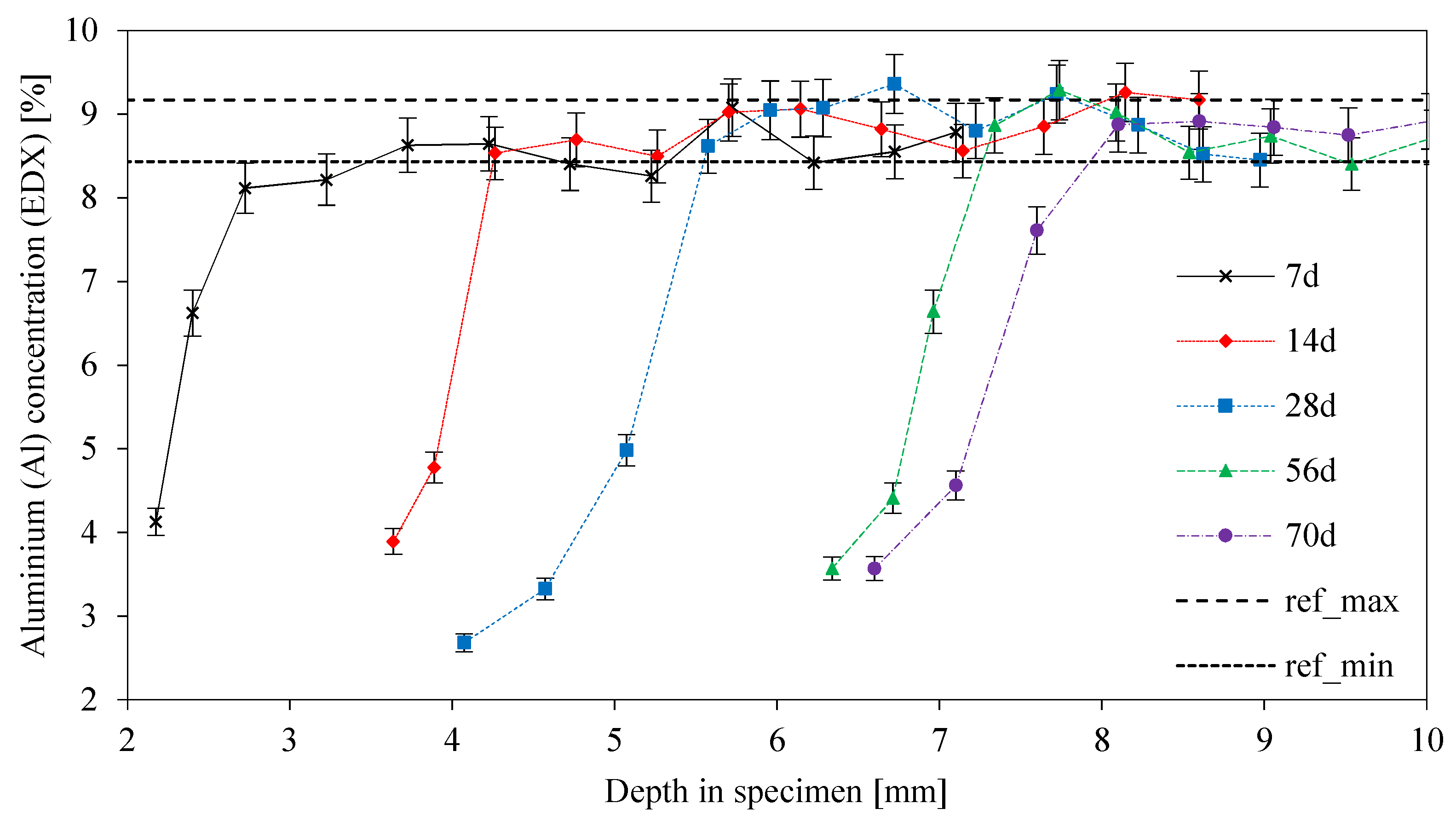
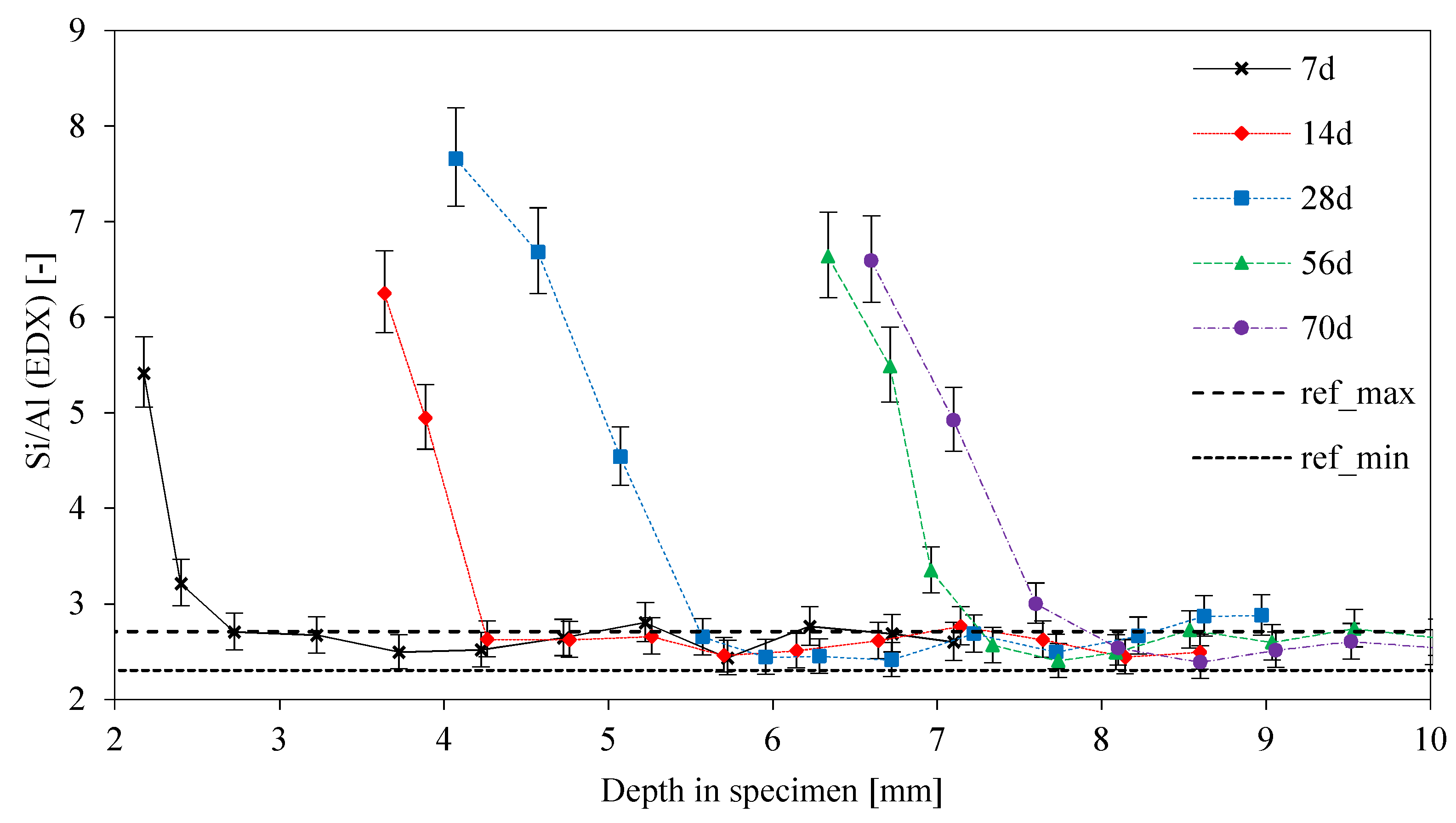
3.4. Elemental Mapping of Sulfuric Acid Exposed Geopolymers
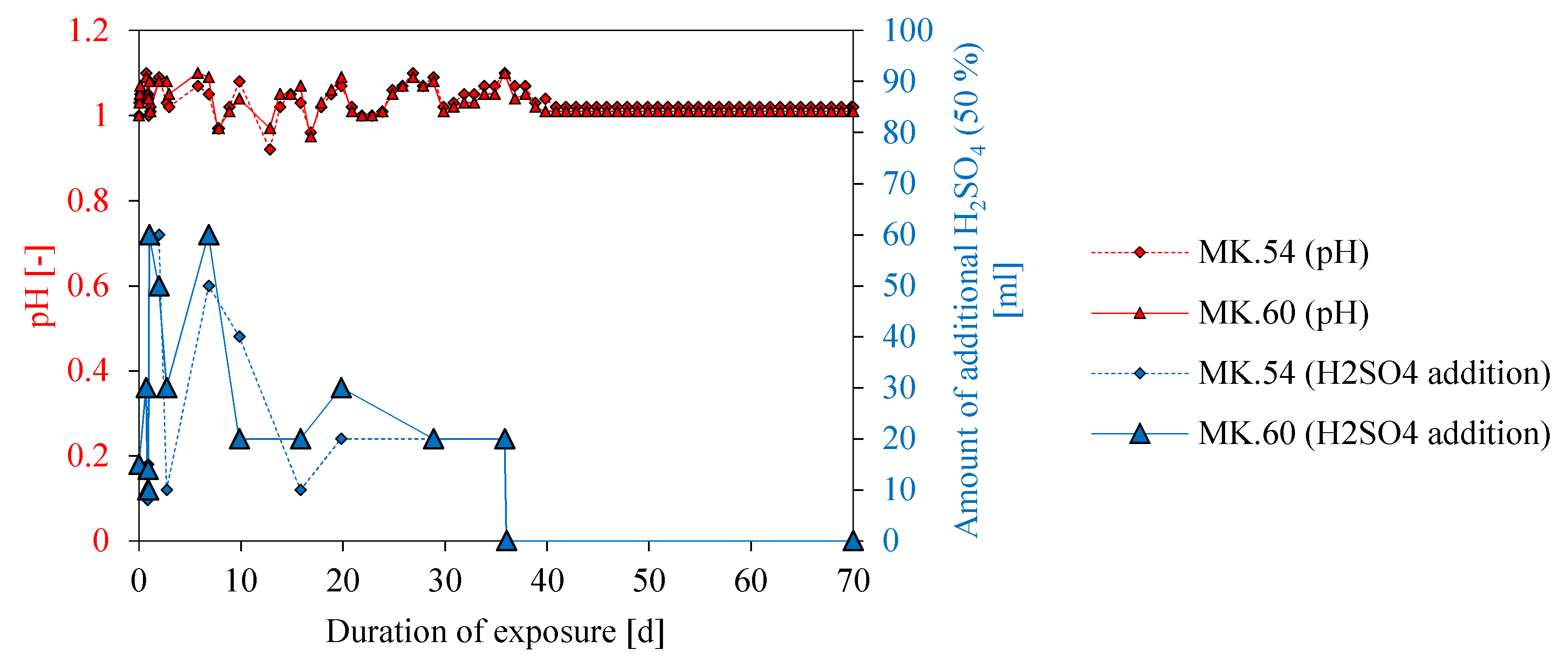
3.5. pH of Sulfuric Acid Solution
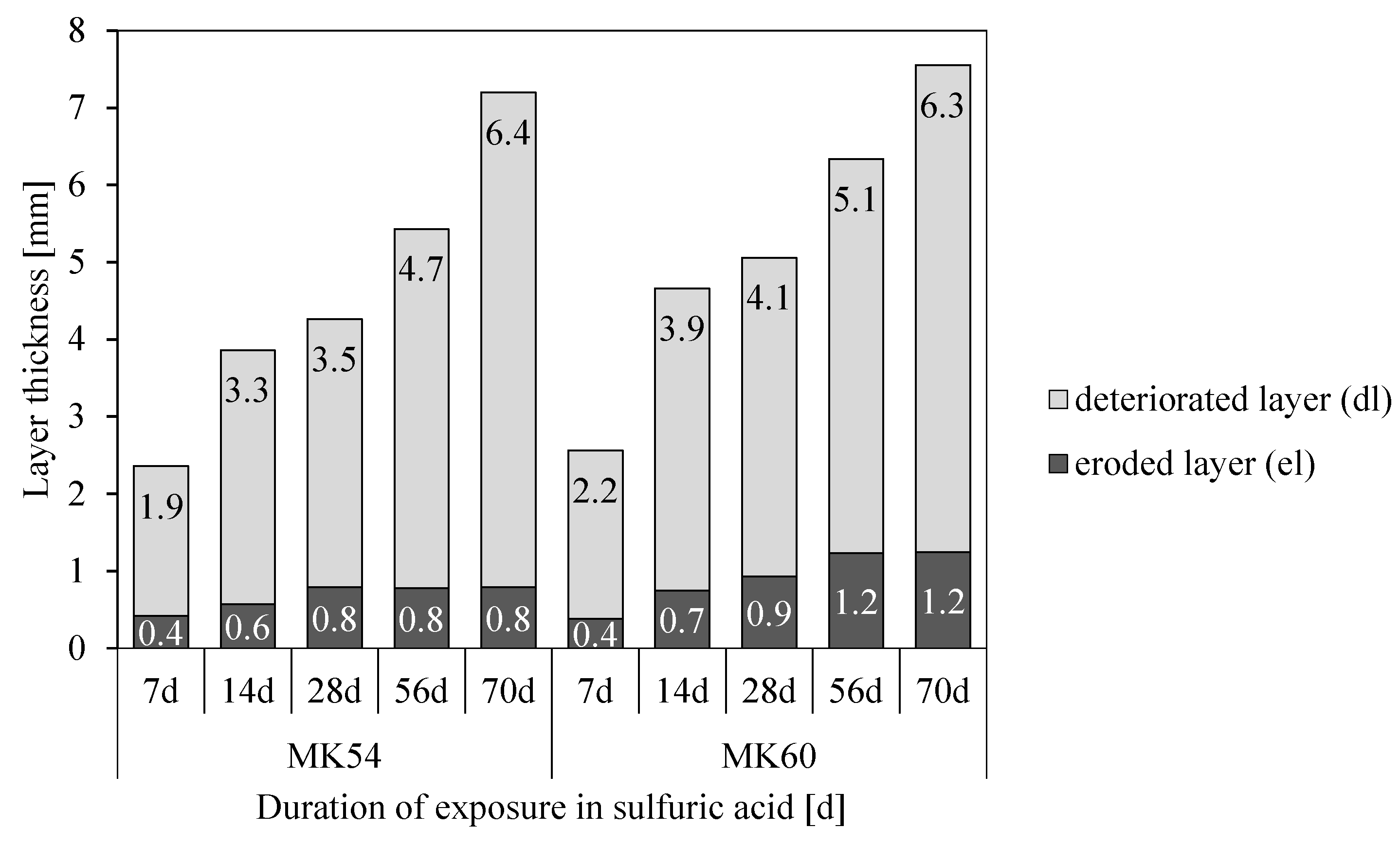
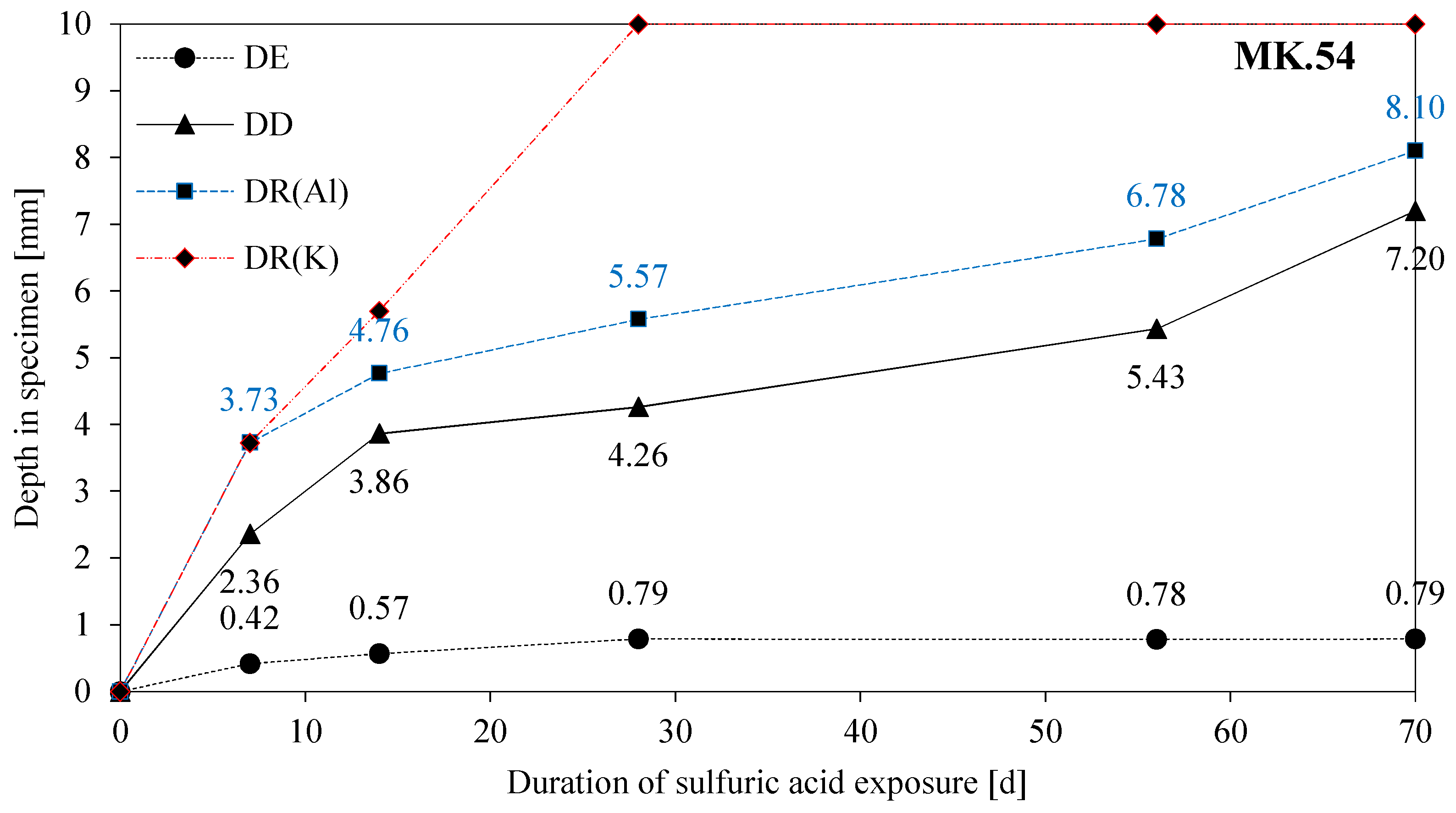
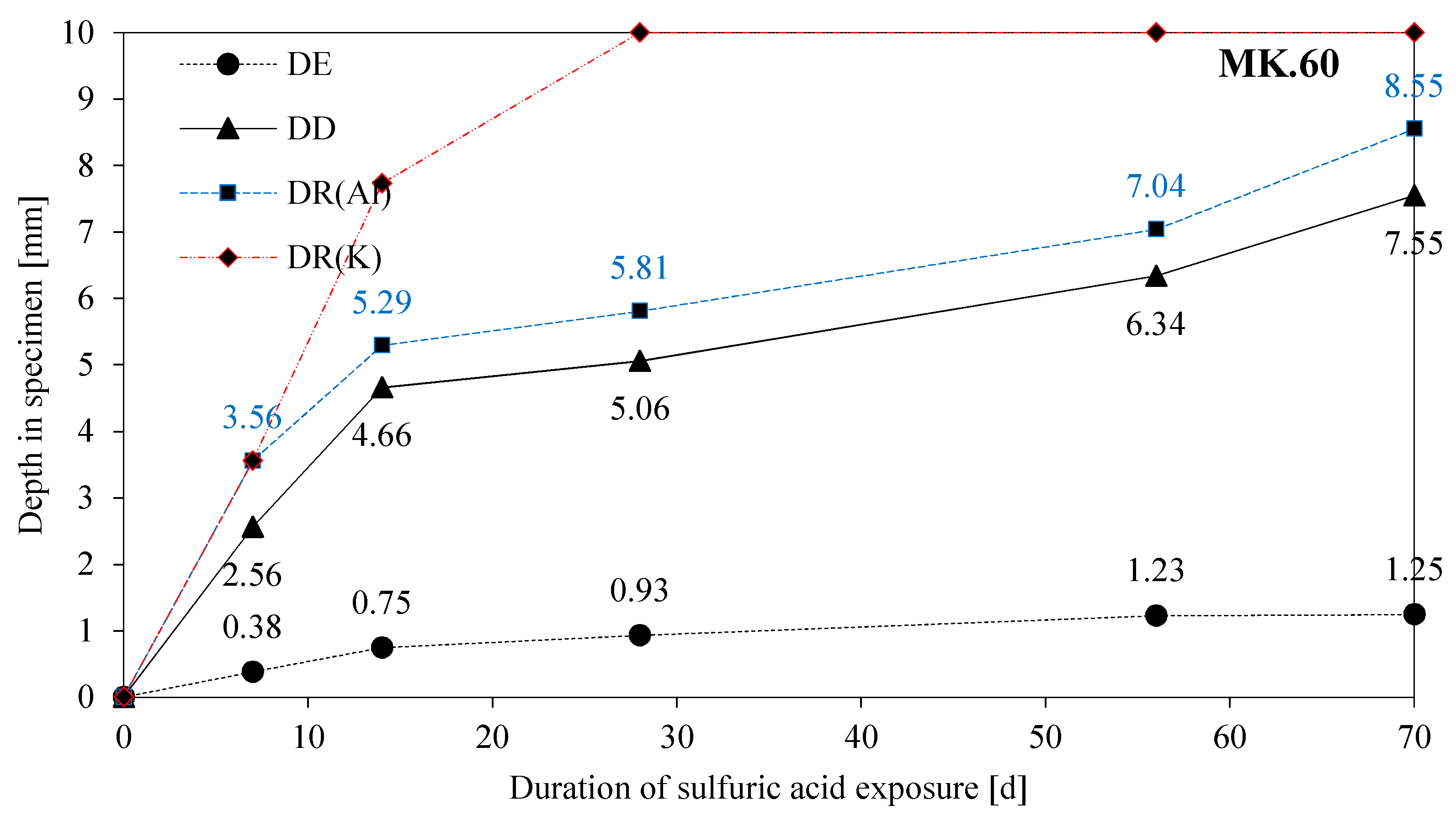
3.6. Eroded and Deteriorated Layer
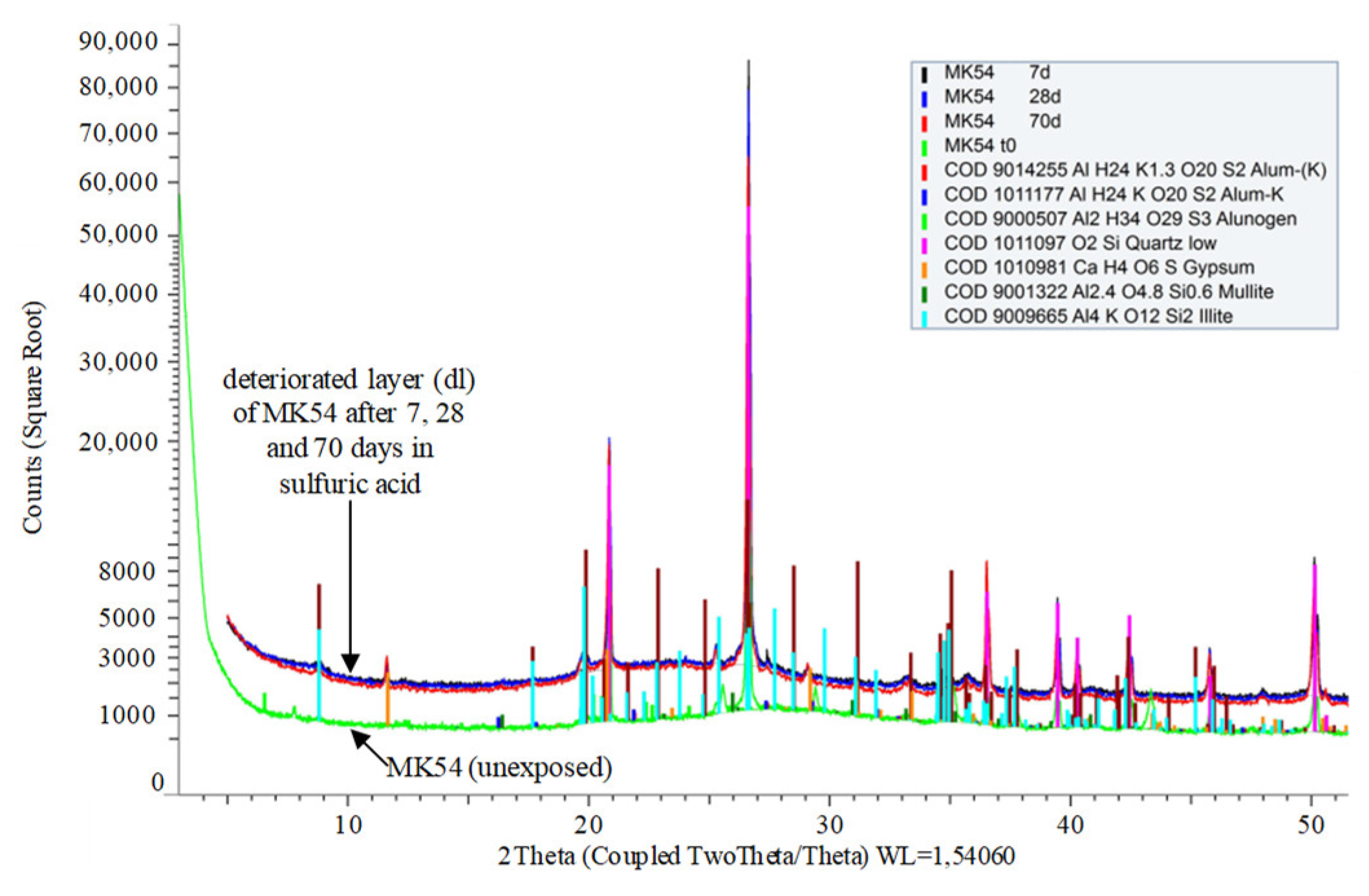
3.7. Powder X-ray Diffraction of Unexposed Specimen and Deteriorated Layer
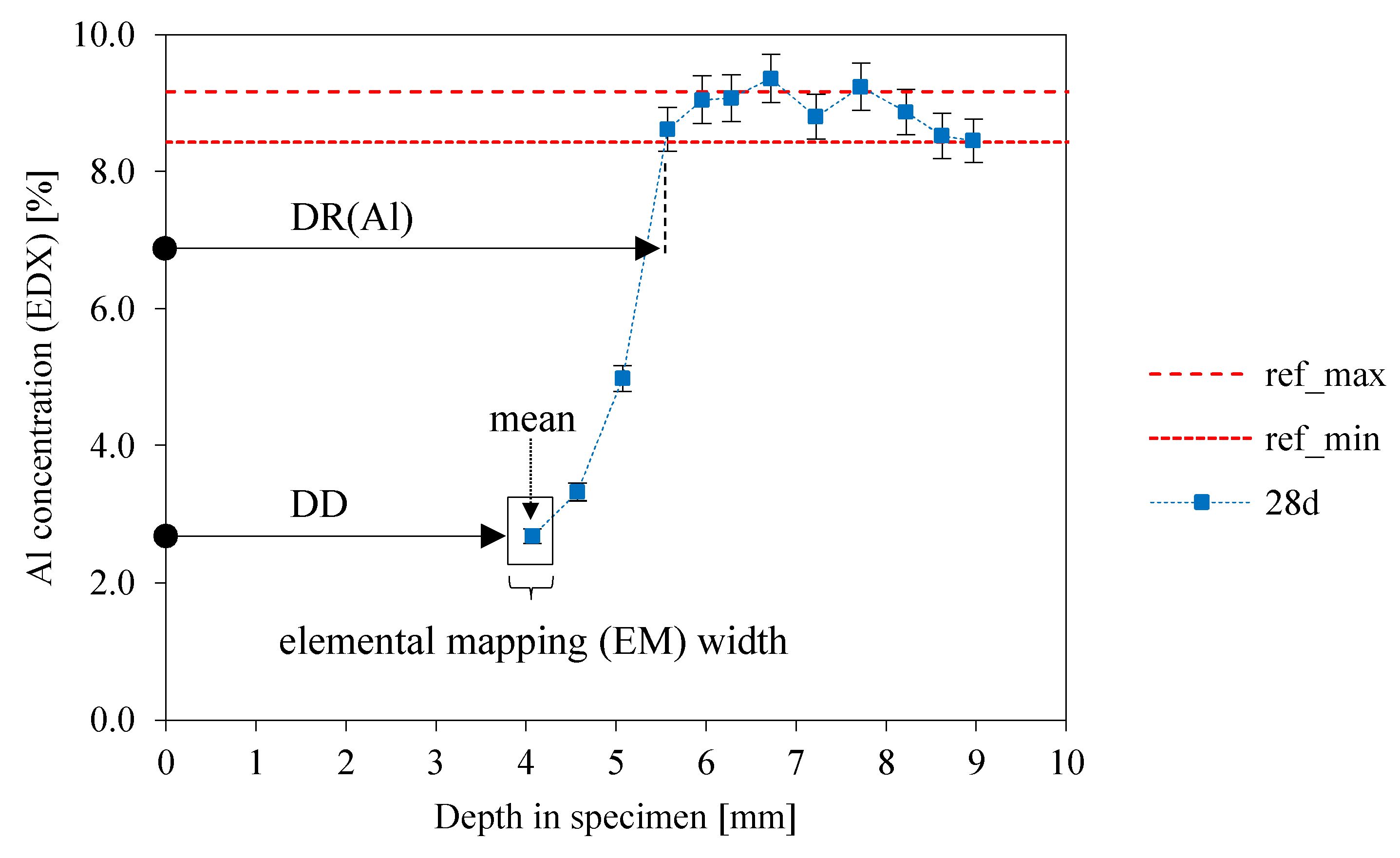
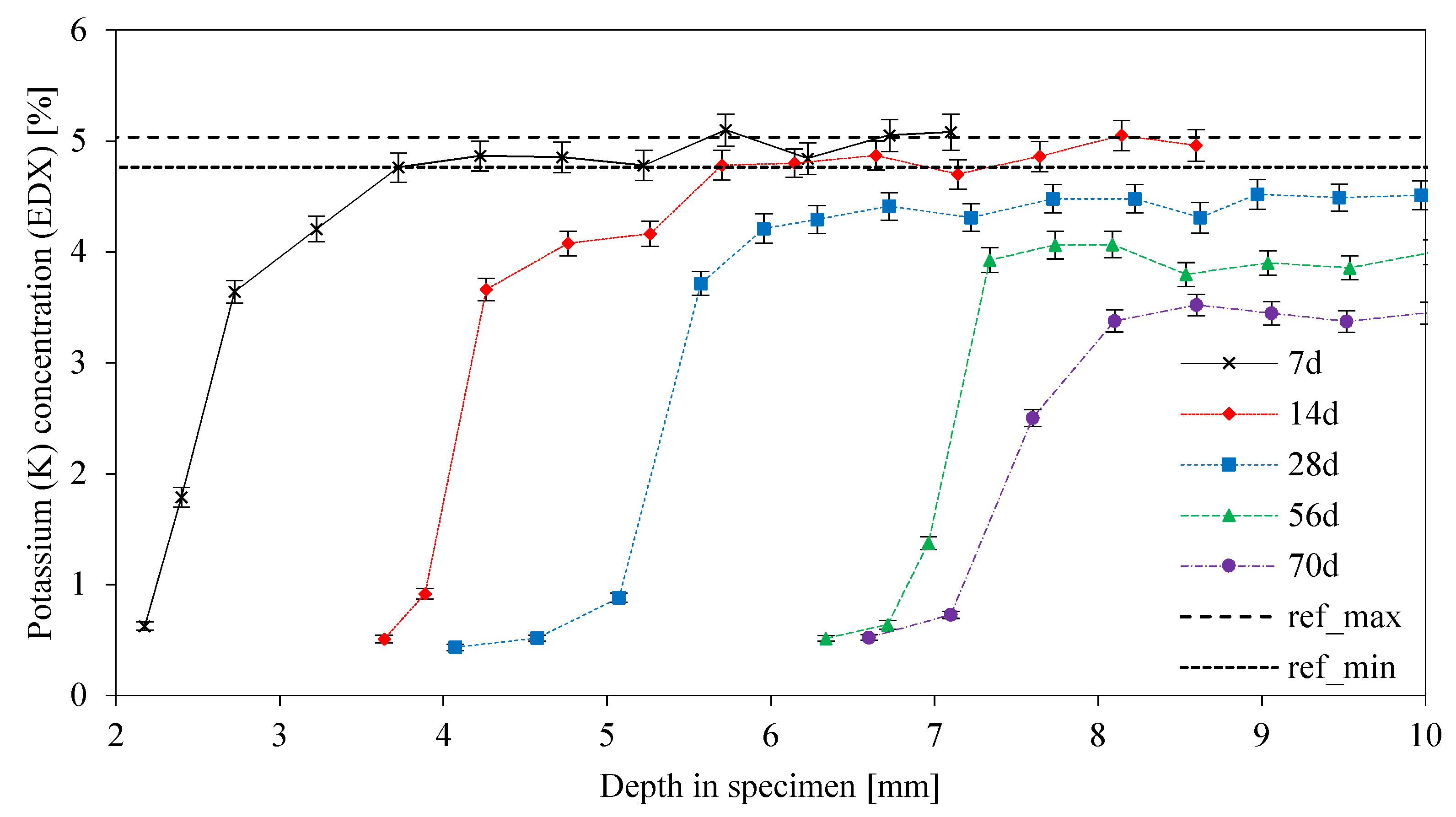
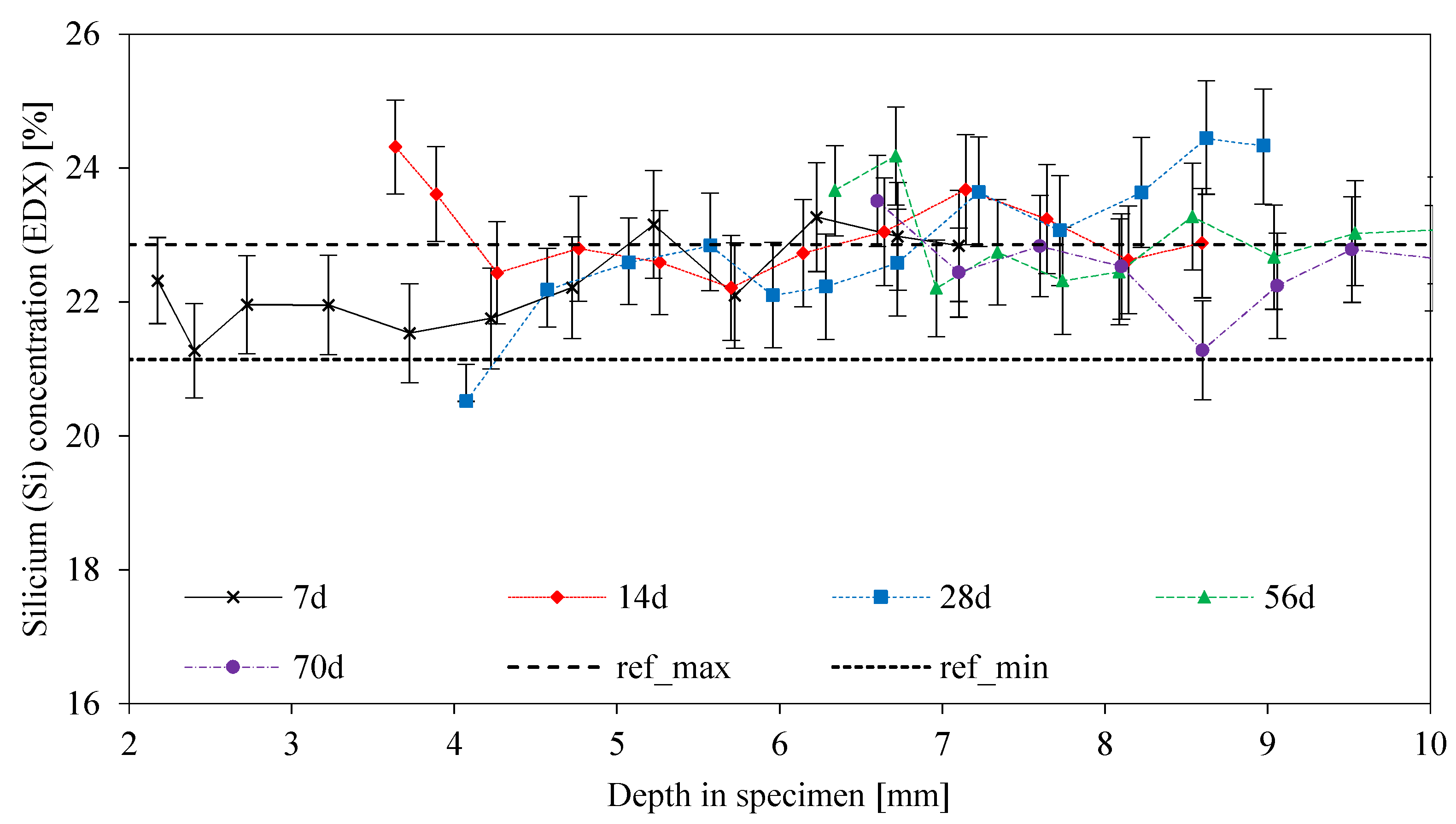
3.8. SEM-EDX: Depth of Reaction DR(e)
4. Discussion
5. Conclusions
- The depth of erosion (DE) of the acid exposed surface of specimens increases up to 28 days (MK54) and 56 days (MK60) without any relevant further increase in values after the aforementioned durations;
- The gradient of the DD-curve (depth of deterioration) is steep up to 14 days of exposure with a lower gradient after that point. The same effect can be observed for the depth of reaction for aluminum (DR(Al));
- The curve for the depth of reaction of potassium (DR(K)) is similar to the DR(Al) curve up to 7 days of exposure, with steeply rising values after 7 days;
- The gradients of DE, DD, and DR(Al) indicate a two-stage degradation mechanism (initially chemically followed by a diffusion-controlled), as already proposed by Lloyd et al. [40];
- The DR(K) and DR(Al) values indicate a leaching of potassium in the first step of degradation, followed by the dealumination and depolymerization of the geopolymer network;
- Stronger deterioration of the MK60 sample within the first 14 days of exposure might be induced by the higher porosity and coarser pore size distribution of MK60;
- After 14 days of exposure, the lower degradation rate (DR(Al) and DD) of MK60 compared to MK54 might result from the diffusive controlled degradation mechanism after that time point and the higher amount of alkalis in the MK60 sample;
- After sulfuric acid exposure, newly built (potassium) aluminum sulfate minerals in the deteriorated layer like alunogen and alum-(K) indicate a possible pore blocking effect.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2006, 42, 2917–2933. [Google Scholar] [CrossRef]
- Buchwald, A.; Zellmann, H.-D.; Kaps, C. Condensation of aluminosilicate gels—model system for geopolymer binders. J. Non-Cryst. Solids 2011, 357, 1376–1382. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.; Lin, W.; Liu, Z.-Y. In situ monitoring of the hydration process of K-PS geopolymer cement with ESEM. Cem. Concr. Res. 2004, 34, 935–940. [Google Scholar] [CrossRef]
- De Silva, P.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]
- Lloyd, R.R.; Provis, J.L.; Van Deventer, J.S. Pore solution composition and alkali diffusion in inorganic polymer cement. Cem. Concr. Res. 2010, 40, 1386–1392. [Google Scholar] [CrossRef]
- Loewenstein, W. The distribution of aluminum in the tetrahedra of silicates and aluminates. Am. Miner. J. Earth Planet. Mater. 1954, 39, 92–96. [Google Scholar]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- Dong, T.; Xie, S.; Wang, J.; Chen, Z.; Liu, Q. Properties and characterization of a metakaolin phosphate acid–based geopolymer synthesized in a humid environment. J. Aust. Ceram. Soc. 2020, 56, 175–184. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, H.; Yuan, P.; Deng, L.; Zhong, X.; Li, Y.; Wang, Q.; Liu, D. Novel acid-based geopolymer synthesized from nanosized tubular halloysite: The role of precalcination temperature and phosphoric acid concentration. Cem. Concr. Compos. 2020, 110, 103601. [Google Scholar] [CrossRef]
- Puertas, F.; Fernández-Jiménez, A. Mineralogical and microstructural characterisation of alkali-activated fly ash/slag pastes. Cem. Concr. Compos. 2003, 25, 287–292. [Google Scholar] [CrossRef]
- Yip, C.; Lukey, G.; Van Deventer, J. The coexistence of geopolymeric gel and calcium silicate hydrate at the early stage of alkaline activation. Cem. Concr. Res. 2005, 35, 1688–1697. [Google Scholar] [CrossRef]
- Shi, C.; Wu, X.; Tang, M. Hydration of alkali-slag cements at 150 °C. Cem. Concr. Res. 1991, 21, 91–100. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Palomo, A.; Fernández-Jiménez, A.; Criado, M. “Geopolymers”: Same basic chemistry, different microstructures. Mater. Constr. 2004, 54, 77–91. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F. Introduction to Handbook of Alkali-activated Cements, Mortars and Concretes. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Elsevier BV: Amsterdam, The Netherlandscity, 2015; pp. 1–16. [Google Scholar]
- Krivenko, P. Alkaline cements: Terminology, classification, aspects of durability. 1997. [Google Scholar]
- Hermmann, A.; König, A.; Dehn, F. Vorschlag zur Klassifizierung von alkalisch-aktivierten Bindemitteln und Geopolymer-Bindemitteln. Beton Fachz. Für Bau Tech. 2015, 7+8, 390–395. [Google Scholar]
- Xu, H.; Van Deventer, J.S. Microstructural characterisation of geopolymers synthesised from kaolinite/stilbite mixtures using XRD, MAS-NMR, SEM/EDX, TEM/EDX, and HREM. Cem. Concr. Res. 2002, 32, 1705–1716. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Synthesis and thermal behaviour of potassium sialate geopolymers. Mater. Lett. 2003, 57, 1477–1482. [Google Scholar] [CrossRef]
- Olmstead, W.; Hamlin, H. Converting portions of the Los Angeles outfall sewer into a septic tank. Eng. News 1900, 44, 317–318. [Google Scholar]
- Koenig, A.; Dehn, F. Biogenic acid attack on concretes in biogas plants. Biosyst. Eng. 2016, 147, 226–237. [Google Scholar] [CrossRef]
- Scheydt, J.C. Mechanismen der Korrosion bei Ultrahochfestem Beton; KIT Scientific Publishing: Karlsruhe, Germany, 2014; Volume 74. [Google Scholar]
- Hüttl, R.; Hillemeier, B. Hochleistungsbeton–Beispiel Säureresistenz. Betonw. Fertil.-Tech 2000, 1, 52–60. [Google Scholar]
- Mosebach, H.; Stengel, F. Hochleistungsbeton mit hoher Chemischer Beständigkeit; Beton: Erkrath, Germany, 2010. [Google Scholar]
- Roberts, D.; Nica, D.; Zuo, G.; Davis, J. Quantifying microbially induced deterioration of concrete: Initial studies. Int. Biodeterior. Biodegrad. 2002, 49, 227–234. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A. Chemical durability of geopolymers. In Geopolymers; Elsevier BV: Amsterdam, The Netherlands, 2009; pp. 167–193. [Google Scholar]
- Ukrainczyk, N.; Muthu, M.; Vogt, O.; Koenders, E. Geopolymer, Calcium Aluminate, and Portland Cement-Based Mortars: Comparing Degradation Using Acetic Acid. Materials 2019, 12, 3115. [Google Scholar] [CrossRef] [PubMed]
- Koenig, A.; Herrmann, A.; Overmann, S.; Dehn, F. Resistance of alkali-activated binders to organic acid attack: Assessment of evaluation criteria and damage mechanisms. Constr. Build. Mater. 2017, 151, 405–413. [Google Scholar] [CrossRef]
- Bakharev, T. Resistance of geopolymer materials to acid attack. Cem. Concr. Res. 2005, 35, 658–670. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2006, 42, 3055–3065. [Google Scholar] [CrossRef]
- Song, X.; Marosszeky, M.; Brungs, M.; Munn, R. Durability of fly ash based geopolymer concrete against sulphuric acid attack. In Proceedings of the International Conference on Durability of Building Materials and Components, Lyon, France, 17–20 April 2005; pp. 369–375. [Google Scholar]
- Okoye, F.N.; Prakash, S.; Singh, N.B. Durability of fly ash based geopolymer concrete in the presence of silica fume. J. Clean. Prod. 2017, 149, 1062–1067. [Google Scholar] [CrossRef]
- Kwasny, J.; Aiken, T.A.; Soutsos, M.N.; McIntosh, A.J.; Cleland, D.J. Comparison of Lithomarge and Cement-Based Mortars Performance in Aggressive Aqueous Environments. In Proceedings of the Sixth International Conference on Durability of Concrete Structures, Leeds, UK, 18–20 July 2018. [Google Scholar]
- Dutta, A.; Moharana, N.C. Mechanical and Durability Properties of Fly Ash-Based Geopolymer Concrete. In Recent Developments in Sustainable Infrastructure; Springer: Singapore, 2020; pp. 699–717. [Google Scholar]
- Grengg, C.; Ukrainczyk, N.; Koraimann, G.; Mueller, B.; Dietzel, M.; Mittermayr, F. Long-term in situ performance of geopolymer, calcium aluminate and Portland cement-based materials exposed to microbially induced acid corrosion. Cem. Concr. Res. 2020, 131, 106034. [Google Scholar] [CrossRef]
- Sturm, P.; Gluth, G.; Jäger, C.; Brouwers, H.; Kühne, H.-C. Sulfuric acid resistance of one-part alkali-activated mortars. Cem. Concr. Res. 2018, 109, 54–63. [Google Scholar] [CrossRef]
- Thokchom, S.; Ghosh, P.; Ghosh, S. Acid resistance of fly ash based geopolymer mortars. Int. J. Recent Trends Eng. 2009, 1, 36. [Google Scholar]
- Sun, P.; Wu, H.-C. Chemical and freeze–thaw resistance of fly ash-based inorganic mortars. Fuel 2013, 111, 740–745. [Google Scholar] [CrossRef]
- Thokchom, S. Fly ash geopolymer pastes in sulphuric acid. Int. J. Eng. Innov. Res. 2014, 3, 943–947. [Google Scholar]
- Lloyd, R.R.; Provis, J.L.; Van Deventer, J.S.J. Acid resistance of inorganic polymer binders. 1. Corrosion rate. Mater. Struct. 2012, 45, 1–14. [Google Scholar] [CrossRef]
- Ariffin, M.; Bhutta, M.; Hussin, M.; Tahir, M.M.; Aziah, N. Sulfuric acid resistance of blended ash geopolymer concrete. Constr. Build. Mater. 2013, 43, 80–86. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Zhang, G.; Mann, D.; Lumsden, K.; Tao, M. Durability of red mud-fly ash based geopolymer and leaching behavior of heavy metals in sulfuric acid solutions and deionized water. Constr. Build. Mater. 2016, 124, 373–382. [Google Scholar] [CrossRef]
- Palomo, A.; Blanco-Varela, M.; Granizo, M.; Puertas, F.; Vazquez, T.; Grutzeck, M. Chemical stability of cementitious materials based on metakaolin. Cem. Concr. Res. 1999, 29, 997–1004. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C. An investigation of the microstructure and durability of a fluidized bed fly ash–metakaolin geopolymer after heat and acid exposure. Mater. Des. 2015, 74, 125–137. [Google Scholar] [CrossRef]
- Zhuang, H.J.; Zhang, H.Y.; Xu, H. Resistance of geopolymer mortar to acid and chloride attacks. Procedia Eng. 2017, 210, 126–131. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Lee, M.; Chen-Tan, N.; Van Riessen, A. Characterisation of class F fly ash geopolymer pastes immersed in acid and alkaline solutions. Cem. Concr. Compos. 2011, 33, 1086–1091. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Strength and Durability in Acid Media of Alkali Silicate-Activated Metakaolin Geopolymers. J. Am. Ceram. Soc. 2012, 95, 2307–2313. [Google Scholar] [CrossRef]
- Monique, T.T.; Julien, S.; Sylvie, R. Durability of tubular geopolymer reinforced with silica sand. New J. Glass Ceram. 2012, 2012, 18601. [Google Scholar]
- Gao, X.X.; Michaud, P.; Joussein, E.; Rossignol, S. Behavior of metakaolin-based potassium geopolymers in acidic solutions. J. Non-Cryst. Solids 2013, 380, 95–102. [Google Scholar] [CrossRef]
- Bouguermouh, K.; Bouzidi, N.; Mahtout, L.; Pérez-Villarejo, L.; Martínez-Cartas, M.L. Effect of acid attack on microstructure and composition of metakaolin-based geopolymers: The role of alkaline activator. J. Non-Cryst. Solids 2017, 463, 128–137. [Google Scholar] [CrossRef]
- Aly, Z.; Vance, E.R.; Perera, D.S.; Hanna, J.; Griffith, C.; Davis, J.; Durce, D. Aqueous leachability of metakaolin-based geopolymers with molar ratios of Si/Al = 1.5–4. J. Nucl. Mater. 2008, 378, 172–179. [Google Scholar] [CrossRef]
- Baščarević, Z.; Komljenović, M.; Miladinović, Z.; Nikolić, V.; Marjanović, N.; Žujović, Z.; Petrović, R. Effects of the concentrated NH4NO3 solution on mechanical properties and structure of the fly ash based geopolymers. Constr. Build. Mater. 2013, 41, 570–579. [Google Scholar] [CrossRef]
- Vogt, O.; Ukrainczyk, N.; Ballschmiede, C.; Koenders, E. Vogt Reactivity and Microstructure of Metakaolin Based Geopolymers: Effect of Fly Ash and Liquid/Solid Contents. Materials 2019, 12, 3485. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung. DIN EN 196-1:2016-11, Prüfverfahren für Zement—Teil 1: Bestimmung der Festigkeit; Deutsche Fassung EN 196-1:2016; Deutsches Institut für Normung: Berlin, Germany, 2016. [Google Scholar]
- Koenig, A.; Dehn, F. Main considerations for the determination and evaluation of the acid resistance of cementitious materials. Mater. Struct. 2016, 49, 1693–1703. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-ray Microanalysis; Springer: New York City, NY, USA, 2017. [Google Scholar]
- Benavent, V.; Frizon, F.; Poulesquen, A. Effect of composition and aging on the porous structure of metakaolin-based geopolymers. J. Appl. Cryst. 2016, 49, 2116–2128. [Google Scholar] [CrossRef]
- Ko, M.-S.; Chen, H.-Y.; Lyu, S.-J.; Wang, T.; Ueng, T.-H. Permeation characteristics and impact factors of geopolymers made of kaolin. Constr. Build. Mater. 2015, 93, 301–308. [Google Scholar] [CrossRef]
- Tchakoute, H.; Rüscher, C.; Djobo, J.; Kenne, B.; Njopwouo, D. Influence of gibbsite and quartz in kaolin on the properties of metakaolin-based geopolymer cements. Appl. Clay Sci. 2015, 107, 188–194. [Google Scholar] [CrossRef]
- Schmücker, M.; MacKenzie, K.J. Microstructure of sodium polysialate siloxo geopolymer. Ceram. Int. 2005, 31, 433–437. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Aly, Z.; Vance, E.; Perera, D. Aqueous dissolution of sodium aluminosilicate geopolymers derived from metakaolin. J. Nucl. Mater. 2012, 424, 164–170. [Google Scholar] [CrossRef]
- Allahverdi, A.; Skvara, F. Nitric acid attack on hardened paste of geopolymeric cements, Part 1. Ceram. Silik. 2001, 45, 81–88. [Google Scholar]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; Krieger: Malabar, Florida, USA, 1984. [Google Scholar]
- Iler, R.K. The Colloid Chemistry of Silica and Silicates; LWW: Philadelphia, PA, USA, 1955; Volume 80. [Google Scholar]
- Latella, B.A.; Perera, D.; Durce, D.; Mehrtens, E.G.; Davis, J. Mechanical properties of metakaolin-based geopolymers with molar ratios of Si/Al ≈ 2 and Na/Al ≈ 1. J. Mater. Sci. 2008, 43, 2693–2699. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lorente, S.; Duhart-Barone, A.; LaMotte, H. Porous arrangement and transport properties of geopolymers. Constr. Build. Mater. 2018, 191, 853–865. [Google Scholar] [CrossRef]
- Steins, P.; Poulesquen, A.; Frizon, F.; Diat, O.; Jestin, J.; Causse, J.; Lambertin, D.; Rossignol, S. Effect of aging and alkali activator on the porous structure of a geopolymer. J. Appl. Cryst. 2014, 47, 316–324. [Google Scholar] [CrossRef]
- Berodier, E.; Bizzozero, J.; Muller, A. Mercury intrusion porosimetry. In A Practical Guide to Microstructural Analysis of Cementitious Materials; London, UK, 2016; Volume 419. [Google Scholar]
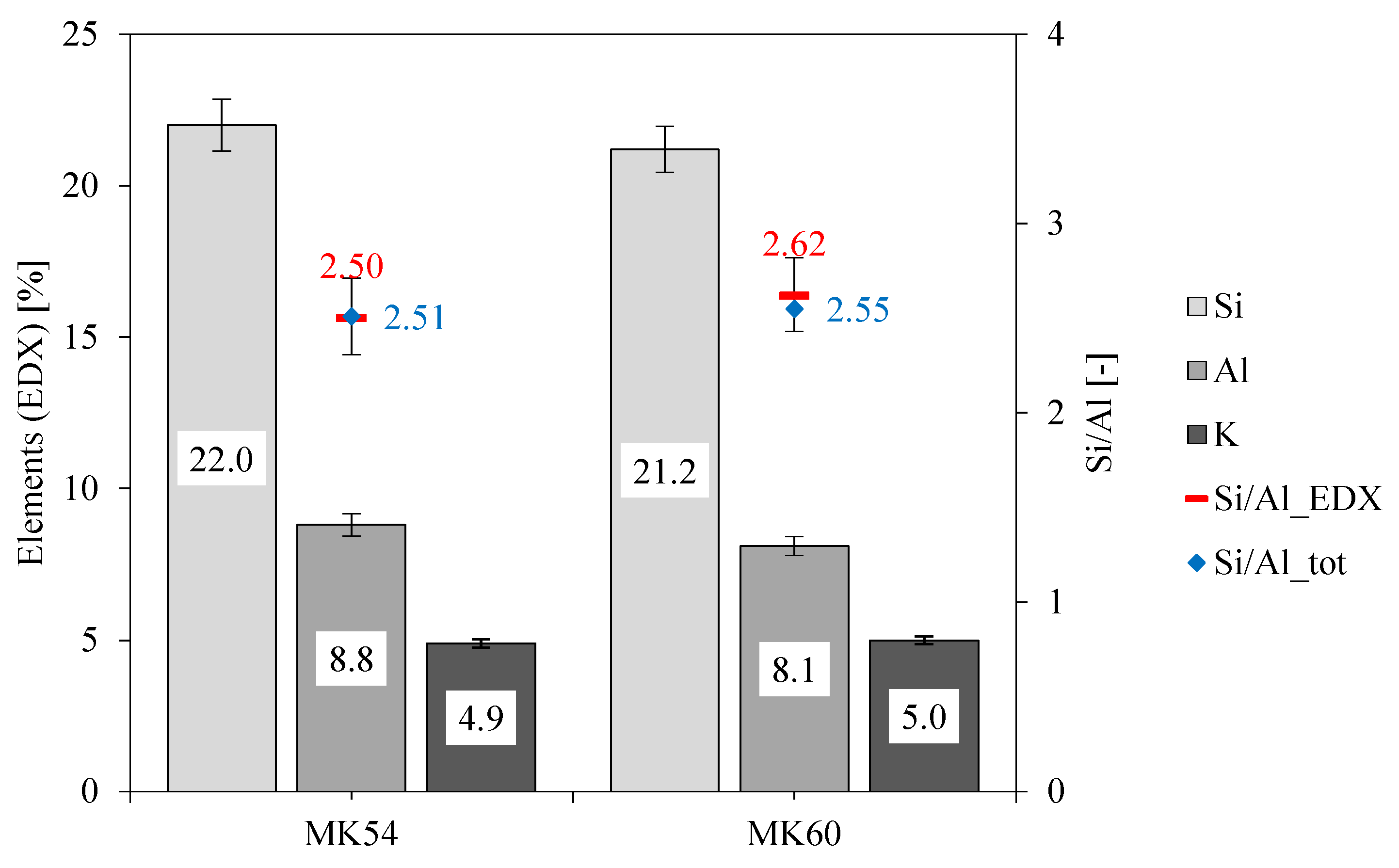

| Name | l/s [-] | Si/Alam [-] | Si/Altot [-] | K/Alam [-] | K/Altot [-] |
|---|---|---|---|---|---|
| MK54 | 0.54 | 1.37 | 2.51 | 0.64 | 0.50 |
| MK60 | 0.60 | 1.42 | 2.55 | 0.71 | 0.55 |
| Name | Total Porosity | Pore Size Distribution by Volume Ratio [%] | |||
|---|---|---|---|---|---|
| [%] | <10 nm | 10 nm–20 nm | 20 nm–50 nm | 50 nm–100 nm | |
| MK54 | 25.6 | 31.4 | 36.8 | 13.1 | 15.9 |
| MK60 | 26.4 | 28.3 | 30.6 | 27.3 | 13.6 |
| Name | Potassium Percentage from SEM-EDX Elemental Mapping [%] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unexposed | 7 d | 14 d | 28 d | 56 d | 70 d | |||||||
| refmin | refmax | Min | Max | Min | Max | Min | Max | Min | Max | Min | Max | |
| MK54 | 4.8 | 5.0 | 0.6 | 5.1 | 0.5 | 5.1 | 0.4 | 4.5 | 0.5 | 4.1 | 0.5 | 3.7 |
| MK60 | 4.9 | 5.1 | 0.5 | 5.1 | 0.6 | 5.0 | 0.6 | 5.1 | 0.6 | 4.6 | 0.5 | 3.7 |
| Name | 14 d Sample | Percentage Increase, Starting from 14 d Percentage [%] | ||||||
|---|---|---|---|---|---|---|---|---|
| [mm] | DD | DR(Al) | ||||||
| DD | DR(Al) | 28 d | 56 d | 70 d | 28 d | 56 d | 70 d | |
| MK54 | 3.9 | 4.8 | 10.4 | 40.7 | 86.5 | 17.0 | 42.4 | 70.2 |
| MK60 | 4.7 | 5.3 | 8.6 | 36.1 | 62.0 | 9.8 | 33.1 | 61.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogt, O.; Ballschmiede, C.; Ukrainczyk, N.; Koenders, E. Evaluation of Sulfuric Acid-Induced Degradation of Potassium Silicate Activated Metakaolin Geopolymers by Semi-Quantitative SEM-EDX Analysis. Materials 2020, 13, 4522. https://doi.org/10.3390/ma13204522
Vogt O, Ballschmiede C, Ukrainczyk N, Koenders E. Evaluation of Sulfuric Acid-Induced Degradation of Potassium Silicate Activated Metakaolin Geopolymers by Semi-Quantitative SEM-EDX Analysis. Materials. 2020; 13(20):4522. https://doi.org/10.3390/ma13204522
Chicago/Turabian StyleVogt, Oliver, Conrad Ballschmiede, Neven Ukrainczyk, and Eddie Koenders. 2020. "Evaluation of Sulfuric Acid-Induced Degradation of Potassium Silicate Activated Metakaolin Geopolymers by Semi-Quantitative SEM-EDX Analysis" Materials 13, no. 20: 4522. https://doi.org/10.3390/ma13204522
APA StyleVogt, O., Ballschmiede, C., Ukrainczyk, N., & Koenders, E. (2020). Evaluation of Sulfuric Acid-Induced Degradation of Potassium Silicate Activated Metakaolin Geopolymers by Semi-Quantitative SEM-EDX Analysis. Materials, 13(20), 4522. https://doi.org/10.3390/ma13204522







