Effect of Substrate Stiffness on Physicochemical Properties of Normal and Fibrotic Lung Fibroblasts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PDMS Substrates
2.2. Cell Culture
2.3. Force Spectroscopy
2.4. Time of Fight Secondary Ion Mass Spectrometry (ToF-SIMS)
2.5. Colorimetric MTS Assay
2.6. Fluorescence Imaging
3. Results
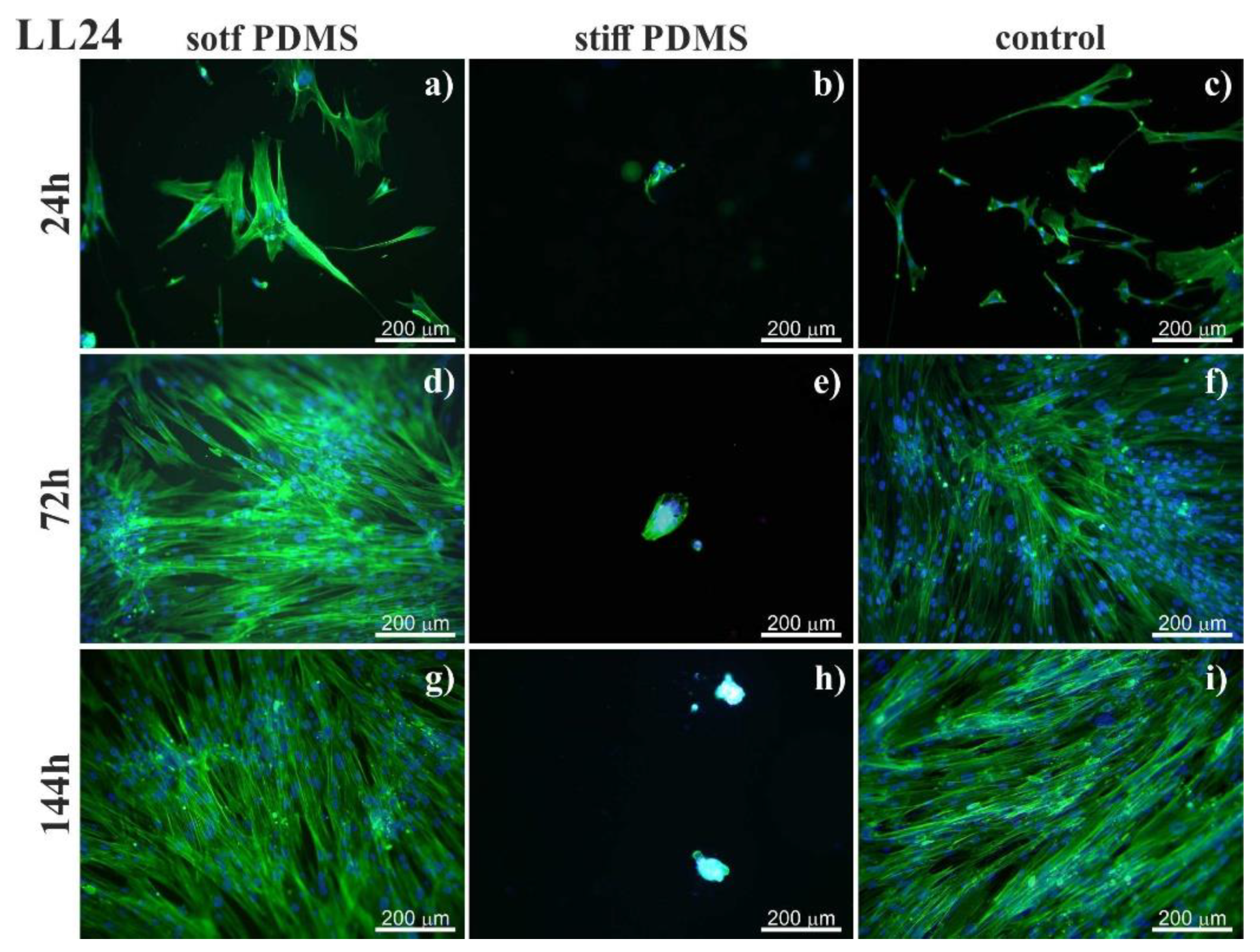
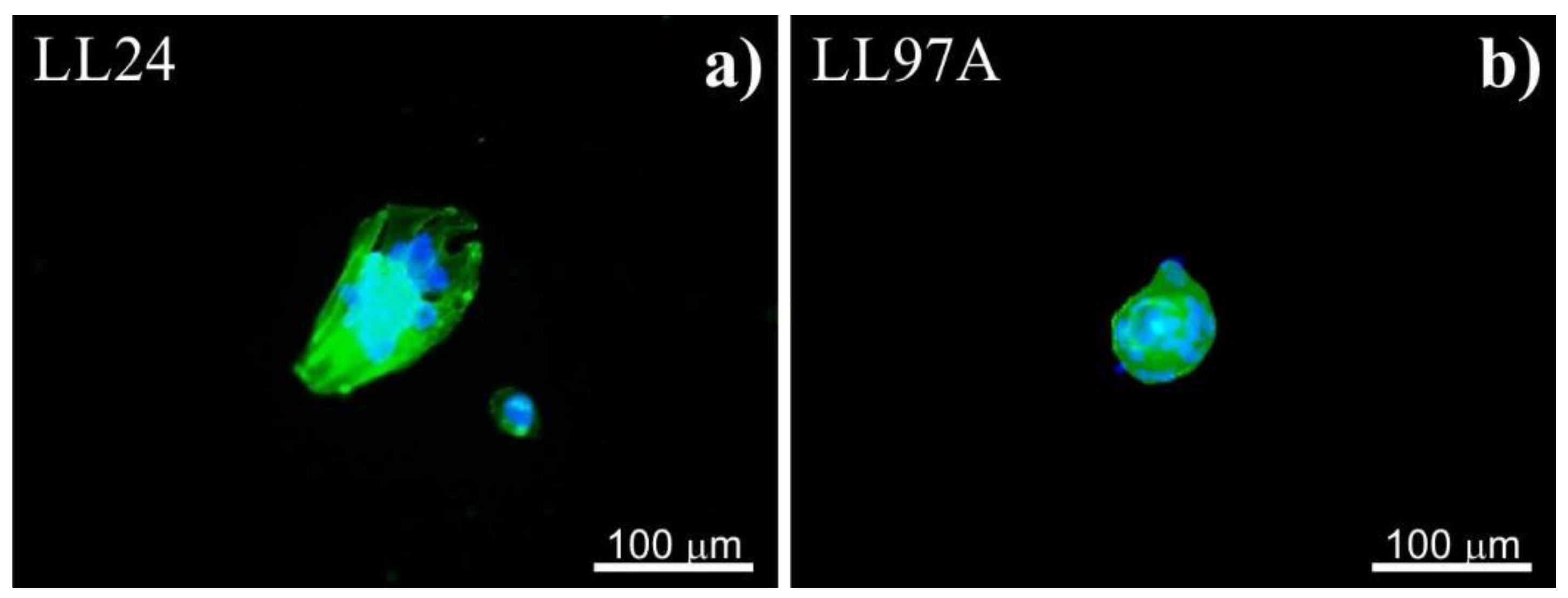
3.1. Fluorescence
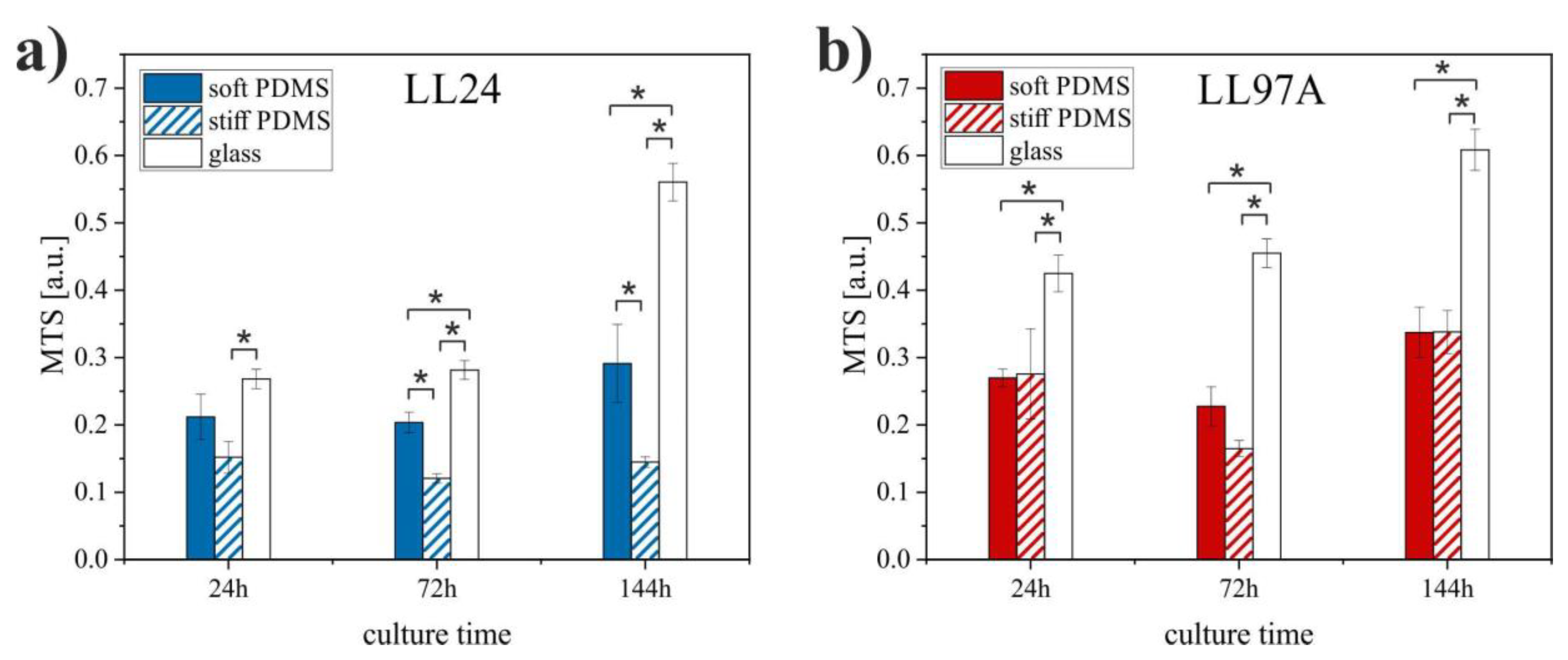
3.2. MTS
3.3. Elasticity
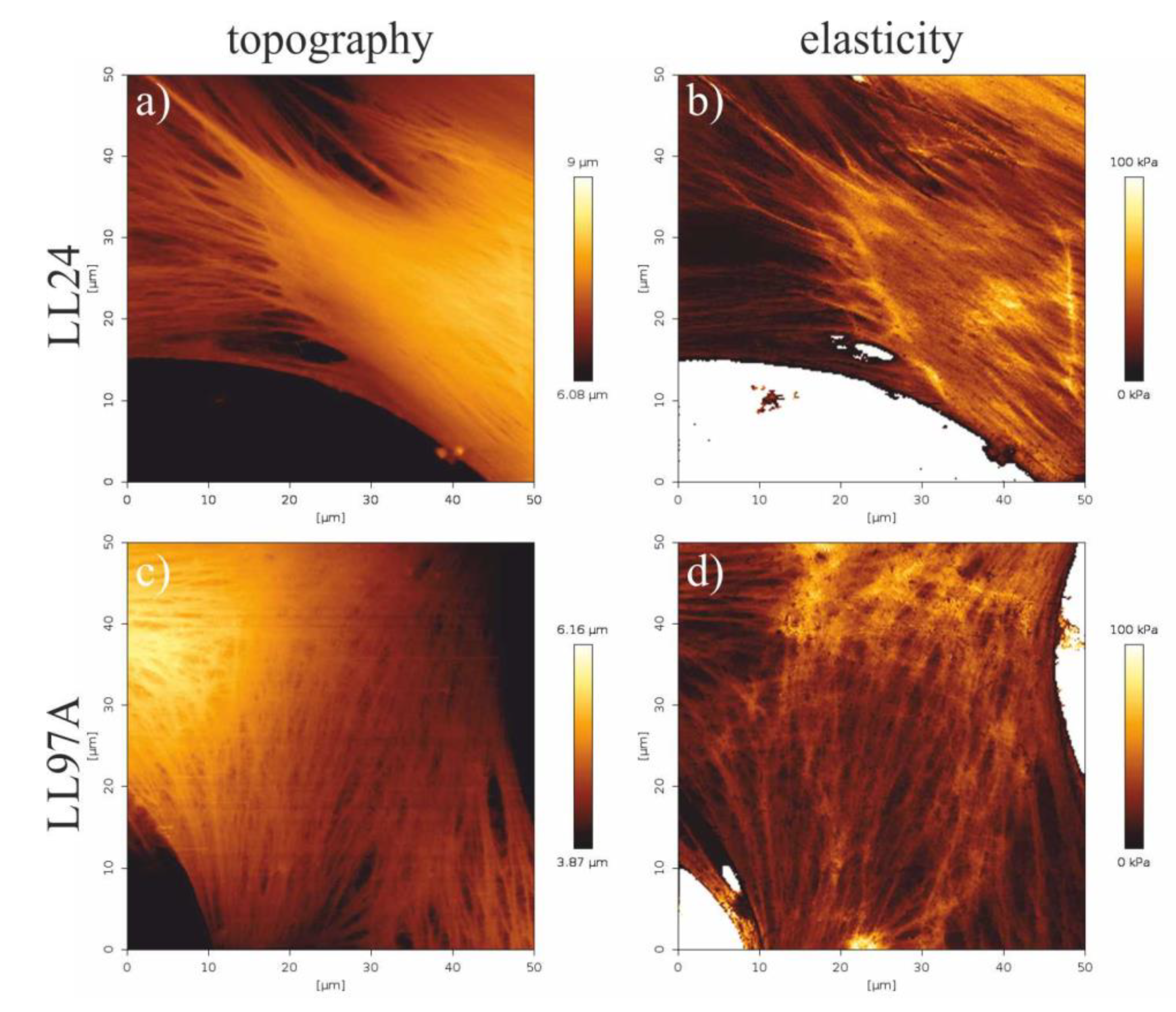
3.4. Elasticity Maps
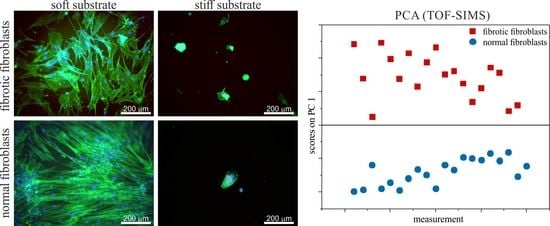
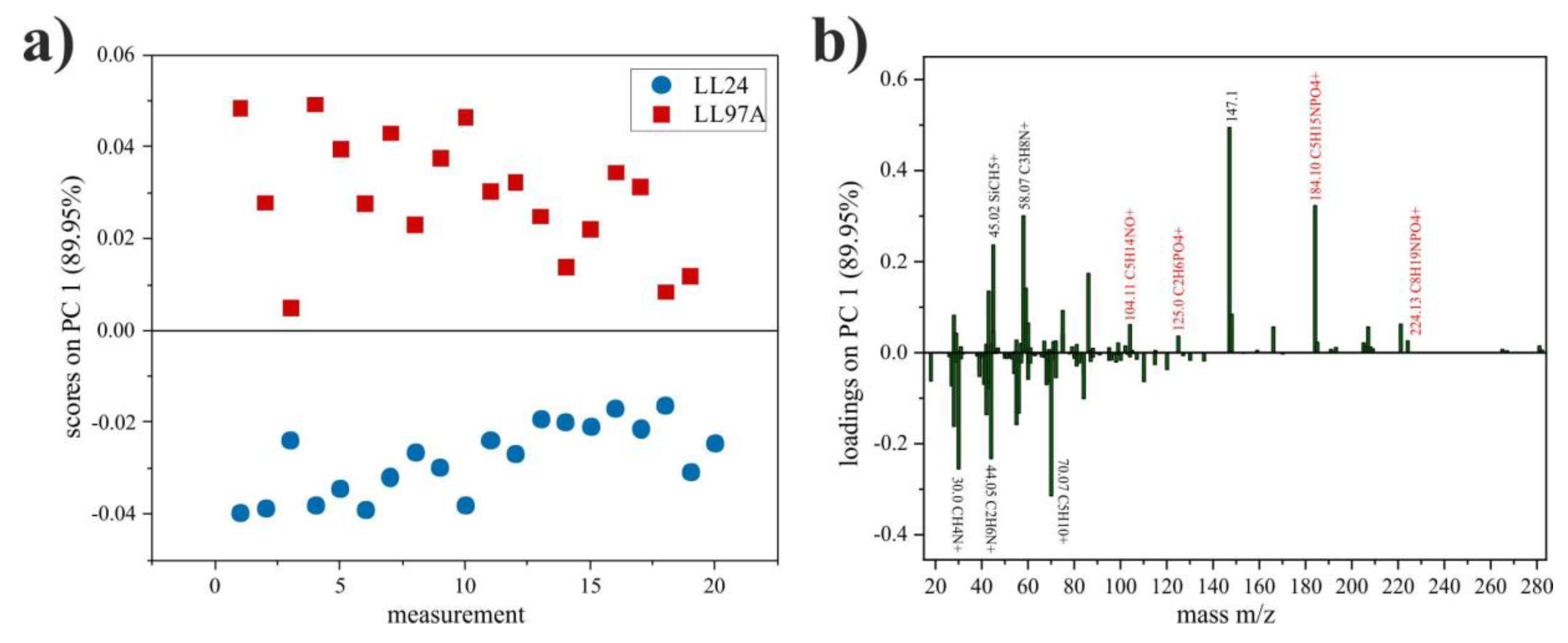
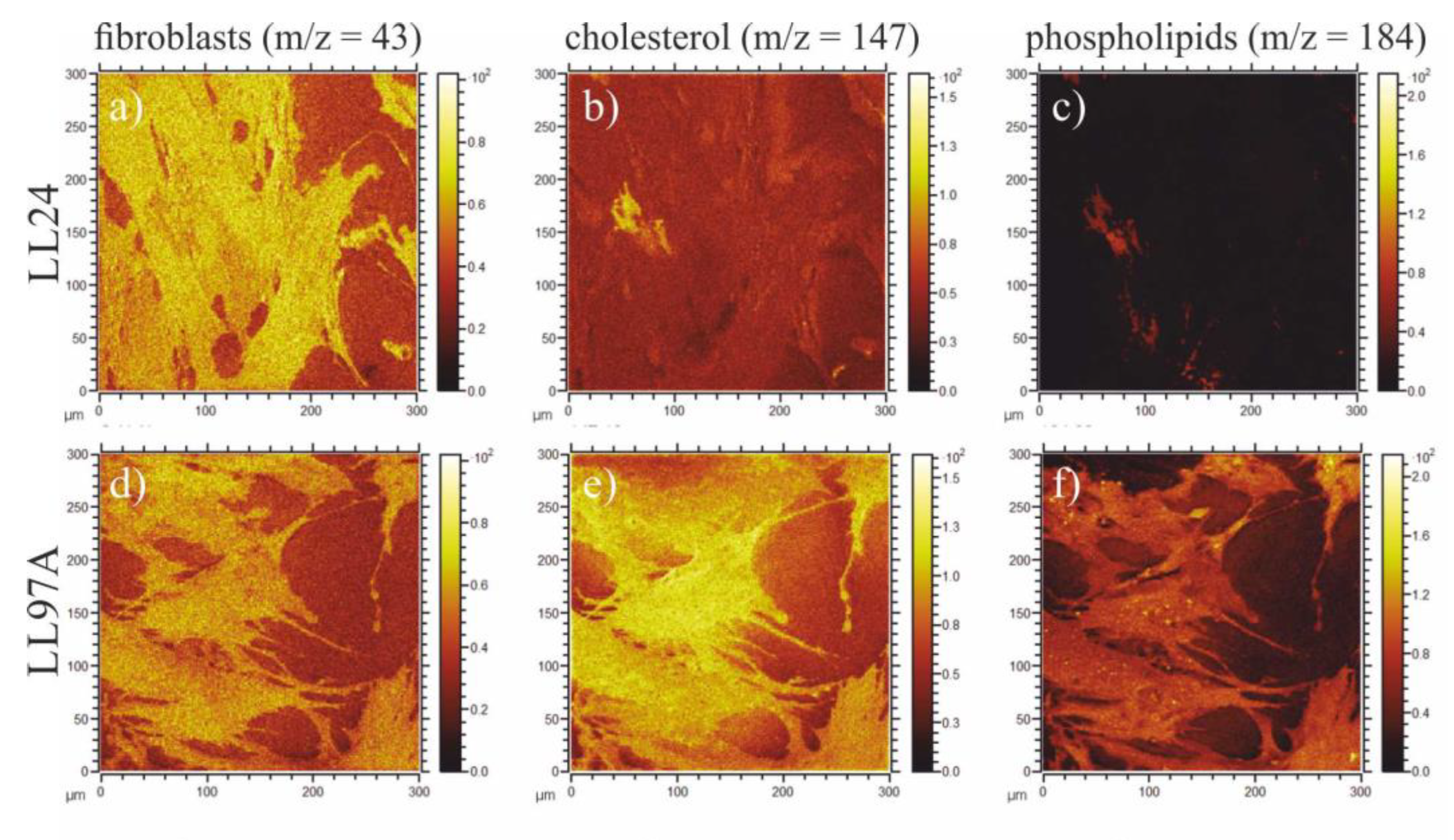
3.5. ToF-SIMS
4. Discussion
4.1. Fluorescence
4.2. MTS
4.3. Elasticity
4.4. Elasticity Maps
4.5. ToF-SIMS
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Prim. 2017, 3, 17075. [Google Scholar] [CrossRef] [PubMed]
- King, T.E. Clinical Advances in the Diagnosis and Therapy of the Interstitial Lung Diseases. Am. J. Respir. Crit. Care Med. 2005, 172, 268–279. [Google Scholar] [CrossRef] [PubMed]
- Vancheri, C.; Failla, M.; Crimi, N.; Raghu, G. Idiopathic pulmonary fibrosis: A disease with similarities and links to cancer biology. Eur. Respir. J. 2010, 35, 496–504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Travis, W.D.; Costabel, U.; Hansell, D.M.; King, T.E.; Lynch, D.A.; Nicholson, A.G.; Ryerson, C.J.; Ryu, J.H.; Selman, M.; Wells, A.U.; et al. An Official American Thoracic Society/European Respiratory Society Statement: Update of the International Multidisciplinary Classification of the Idiopathic Interstitial Pneumonias. Am. J. Respir. Crit. Care Med. 2013, 188, 733–748. [Google Scholar] [CrossRef]
- Nalysnyk, L.; Cid-Ruzafa, J.; Rotella, P.; Esser, D. Incidence and prevalence of idiopathic pulmonary fibrosis: Review of the literature. Eur. Respir. Rev. 2012, 21, 355–361. [Google Scholar] [CrossRef] [Green Version]
- Navaratnam, V.; Fleming, K.M.; West, J.; Smith, C.J.P.; Jenkins, R.G.; Fogarty, A.; Hubbard, R.B. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax 2011, 66, 462–467. [Google Scholar] [CrossRef] [Green Version]
- Raghu, G.; Collard, H.R.; Egan, J.J.; Martinez, F.J.; Behr, J.; Brown, K.K.; Colby, T.V.; Cordier, J.-F.; Flaherty, K.R.; Lasky, J.A.; et al. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: Evidence-based Guidelines for Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2011, 183, 788–824. [Google Scholar] [CrossRef]
- Ley, B.; Collard, H.R.; King, T.E. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Flaherty, K.; King, T.E.; Raghu, G.; Lynch, J.P.; Colby, T.V.; Travis, W.D.; Gross, B.H.; Kazerooni, E.A.; Toews, G.B.; Long, Q.; et al. Idiopathic Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2004, 170, 904–910. [Google Scholar] [CrossRef] [Green Version]
- Flaherty, K.; Andrei, A.-C.; King, T.E.; Raghu, G.; Colby, T.V.; Wells, A.; Bassily, N.; Brown, K.; Du Bois, R.; Flint, A.; et al. Idiopathic Interstitial Pneumonia. Am. J. Respir. Crit. Care Med. 2007, 175, 1054–1060. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Sgalla, G.; Iovene, B.; Calvello, M.; Ori, M.; Varone, F.; Richeldi, L. Idiopathic pulmonary fibrosis: Pathogenesis and management. Respir. Res. 2018, 19, 1–18. [Google Scholar] [CrossRef]
- Maher, T.M.; Strek, M.E. Antifibrotic therapy for idiopathic pulmonary fibrosis: Time to treat. Respir. Res. 2019, 20, 205–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.S. Acute Exacerbations in Patients With Idiopathic Pulmonary Fibrosis. Interstitial Lung Disease 2018, 14, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Müller, N.L.; A White, D.; Jiang, H.; Gemma, A. Diagnosis and management of drug-associated interstitial lung disease. Br. J. Cancer 2004, 91, S24–S30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Upagupta, C.; Shimbori, C.; Alsilmi, R.; Kolb, M. Matrix abnormalities in pulmonary fibrosis. Eur. Respir. Rev. 2018, 27, 180033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, C.; Montaño, M.; Garcıa-Alvarez, J.; Ruiz, V.; Uhal, B.D.; Selman, M.; Pardo, A. Fibroblasts from Idiopathic Pulmonary Fibrosis and Normal Lungs Differ in Growth Rate, Apoptosis, and Tissue Inhibitor of Metalloproteinases Expression. Am. J. Respir. Cell Mol. Boil. 2001, 24, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, S.; Hogaboam, C.M.; Jarai, G. Deficient repair response of IPF fibroblasts in a co-culture model of epithelial injury and repair. Fibrogenesis Tissue Repair 2014, 7, 7. [Google Scholar] [CrossRef] [Green Version]
- Zygmunt, V.V.; Estany, S.; Colom, A.; Montes-Worboys, A.; Machahua, C.; Sanabria, A.J.; Llatjos, R.; Escobar, I.; Manresa, F.; Dorca, J.; et al. Fibroblast viability and phenotypic changes within glycated stiffened three-dimensional collagen matrices. Respir. Res. 2015, 16, 82. [Google Scholar] [CrossRef] [Green Version]
- Booth, A.J.; Hadley, R.; Cornett, A.M.; Dreffs, A.A.; Matthes, S.A.; Tsui, J.L.; Weiss, K.M.; Horowitz, J.C.; Fiore, V.F.; Barker, T.H.; et al. Acellular Normal and Fibrotic Human Lung Matrices as a Culture System for In Vitro Investigation. Am. J. Respir. Crit. Care Med. 2012, 186, 866–876. [Google Scholar] [CrossRef] [Green Version]
- Jaffar, J.; Yang, S.-H.; Kim, S.Y.; Kim, H.-W.; Faiz, A.; Chrzanowski, W.; Burgess, J.K.; Yang, S.-H. Greater cellular stiffness in fibroblasts from patients with idiopathic pulmonary fibrosis. Am. J. Physiol. Cell. Mol. Physiol. 2018, 315, L59–L65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faffe, D.S.; Zin, W.A. Lung Parenchymal Mechanics in Health and Disease. Physiol. Rev. 2009, 89, 759–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enomoto, N.; Suda, T.; Kono, M.; Kaida, Y.; Hashimoto, D.; Fujisawa, T.; Inui, N.; Nakamura, Y.; Imokawa, S.; Funai, K.; et al. Amount of elastic fibers predicts prognosis of idiopathic pulmonary fibrosis. Respir. Med. 2013, 107, 1608–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabasa, M.; Duch, P.; Jorba, I.; Giménez, A.; Lugo, R.; Pavelescu, I.; Rodríguez-Pascual, F.; Molina-Molina, M.; Xaubet, A.; Pereda, J.; et al. Epithelial contribution to the profibrotic stiff microenvironment and myofibroblast population in lung fibrosis. Mol. Boil. Cell 2017, 28, 3741–3755. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Mih, J.D.; Shea, B.S.; Kho, A.T.; Sharif, A.S.; Tager, A.M.; Tschumperlin, D.J. Feedback amplification of fibrosis through matrix stiffening and COX-2 suppression. J. Cell Biol. 2010, 190, 693–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B. Mechanical Aspects of Lung Fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 137–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackwell, T.S.; Tager, A.M.; Borok, Z.; Moore, B.B.; Schwartz, D.A.; Anstrom, K.J.; Bar-Joseph, Z.; Bitterman, P.; Blackburn, M.R.; Bradford, W.; et al. Future Directions in Idiopathic Pulmonary Fibrosis Research. An NHLBI Workshop Report. Am. J. Respir. Crit. Care Med. 2014, 189, 214–222. [Google Scholar] [CrossRef]
- Miki, H.; Mio, T.; Nagai, S.; Hoshino, Y.; Nagao, T.; Kitaichi, M.; Izumi, T. Fibroblast Contractility. Am. J. Respir. Crit. Care Med. 2000, 162, 2259–2264. [Google Scholar] [CrossRef]
- Marinković, A.; Liu, F.; Tschumperlin, D.J. Matrices of Physiologic Stiffness Potently Inactivate Idiopathic Pulmonary Fibrosis Fibroblasts. Am. J. Respir. Cell Mol. Boil. 2013, 48, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Balestrini, J.L.; Chaudhry, S.; Sarrazy, V.; Koehler, A.; Hinz, B. The mechanical memory of lung myofibroblasts. Integr. Biol. 2012, 4, 410–421. [Google Scholar] [CrossRef]
- Giménez, A.; Duch, P.; Puig, M.; Gabasa, M.; Xaubet, A.; Alcaraz, J. Dysregulated Collagen Homeostasis by Matrix Stiffening and TGF-β1 in Fibroblasts from Idiopathic Pulmonary Fibrosis Patients: Role of FAK/Akt. Int. J. Mol. Sci. 2017, 18, 2431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, Y.; Sasaki, R.; Domura, R.; Okamoto, M. Cellular morphologies, motility, and epithelial–mesenchymal transition of breast cancer cells incubated on viscoelastic gel substrates in hypoxia. Mater. Today Chem. 2019, 13, 8–17. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Darnell, M.; Klumpers, D.; Bencherif, S.A.; Weaver, J.C.; Huebsch, N.; Mooney, D.J. Substrate stress relaxation regulates cell spreading. Nat. Commun. 2015, 6, 6364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, P.; Lu, H.; Zhang, X. Measuring the Young’s Relaxation Modulus of PDMS Using Stress Relaxation Nanoindentation. In MRS Online Proceedings Library Archive; Cambridge University Press (CUP): Cambridge, UK, 2009; Volume 1222, pp. 105–110. [Google Scholar] [CrossRef]
- Raczkowska, J.; Orzechowska, B. Effect of tuned elasticity and chemical modification of substrate on fibrotic and normal lung fibroblasts. Micron 2020, 102948. [Google Scholar] [CrossRef]
- Kuznetsova, T.G.; Starodubtseva, M.; Yegorenkov, N.; Chizhik, S.A.; Zhdanov, R.I. Atomic force microscopy probing of cell elasticity. Micron 2007, 38, 824–833. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–104. [Google Scholar] [CrossRef] [Green Version]
- Gostek, J.; Awsiuk, K.; Pabijan, J.; Rysz, J.; Budkowski, A.; Lekka, M. Differentiation between Single Bladder Cancer Cells Using Principal Component Analysis of Time-of-Flight Secondary Ion Mass Spectrometry. Anal. Chem. 2015, 87, 3195–3201. [Google Scholar] [CrossRef]
- Piehowski, P.D.; Carado, A.J.; Kurczy, M.E.; Ostrowski, S.G.; Heien, M.L.; Winograd, N.; Ewing, A.G. MS/MS Methodology To Improve Subcellular Mapping of Cholesterol Using TOF-SIMS. Anal. Chem. 2008, 80, 8662–8667. [Google Scholar] [CrossRef] [Green Version]
- Adams, K.J.; Debord, J.D.; Fernandez-Lima, F.A. Lipid specific molecular ion emission as a function of the primary ion characteristics in TOF-SIMS. J. Vac. Sci. Technol. B 2016, 34, 051804. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Jiang, L.-T.; Okada, R.; Fu, J. UV-Modulated Substrate Rigidity for Multiscale Study of Mechanoresponsive Cellular Behaviors. Langmuir 2012, 28, 10789–10796. [Google Scholar] [CrossRef] [Green Version]
- Palchesko, R.N.; Zhang, L.; Sun, Y.; Feinberg, A.W. Development of Polydimethylsiloxane Substrates with Tunable Elastic Modulus to Study Cell Mechanobiology in Muscle and Nerve. PLoS ONE 2012, 7, e51499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guvendiren, M.; Burdick, J.A. Stiffening hydrogels to probe short- and long-term cellular responses to dynamic mechanics. Nat. Commun. 2012, 3, 792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloxin, A.M.; Benton, J.A.; Anseth, K.S. In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 2010, 31, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhagat, A.A.S.; Jothimuthu, P.; Papautsky, I. Photodefinable polydimethylsiloxane (PDMS) for rapid lab-on-a-chip prototyping. Lab Chip 2007, 7, 1192. [Google Scholar] [CrossRef] [PubMed]
- Raczkowska, J.; Prauzner-Bechcicki, S.; Dąbczyński, P.; Szydlak, R. Elasticity patterns induced by phase-separation in polymer blend films. Thin Solid Film. 2017, 624, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Raczkowska, J.; Prauzner-Bechcicki, S.; Lukes, J.; Sepitka, J.; Bernasik, A.; Awsiuk, K.; Paluszkiewicz, C.; Pabijan, J.; Lekka, M.; Budkowski, A. Physico-chemical properties of PDMS surfaces suitable as substrates for cell cultures. Appl. Surf. Sci. 2016, 389, 247–254. [Google Scholar] [CrossRef]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Prauzner-Bechcicki, S.; Raczkowska, J.; Madej, E.; Pabijan, J.; Lukes, J.; Sepitka, J.; Rysz, J.; Awsiuk, K.; Bernasik, A.; Budkowski, A.; et al. PDMS substrate stiffness affects the morphology and growth profiles of cancerous prostate and melanoma cells. J. Mech. Behav. Biomed. Mater. 2015, 41, 13–22. [Google Scholar] [CrossRef]
- Arias, C.J.; Keller, T.C.S.; Schlenoff, J.B. Quasi-Spherical Cell Clusters Induced by a Polyelectrolyte Multilayer. Langmuir 2015, 31, 6436–6446. [Google Scholar] [CrossRef]
- Liu, Y.; Clem, B.; Zuba-Surma, E.K.; El-Naggar, S.; Telang, S.; Jenson, A.B.; Wang, Y.; Shao, H.; Ratajczak, M.Z.; Chesney, J.; et al. Mouse fibroblasts lacking RB1 function form spheres and undergo reprogramming to a cancer stem cell phenotype. Cell Stem Cell 2009, 4, 336–347. [Google Scholar] [CrossRef] [Green Version]
- Salmenperä, P.; Kankuri, E.; Bizik, J.; Sirén, V.; Virtanen, I.; Takahashi, S.; Leiss, M.; Fässler, R.; Vaheri, A. Formation and activation of fibroblast spheroids depend on fibronectin–integrin interaction. Exp. Cell Res. 2008, 314, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Seldin, M.; Fu, K.; Li, S.; Lam, L.; Wang, P.; Wang, Y.; Huang, D.; Nguyen, T.L.; Wei, B.; et al. Topological Arrangement of Cardiac Fibroblasts Regulates Cellular PlasticityNovelty and Significance. Circ. Res. 2018, 123, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Lekka, M.; Pabijan, J.; Orzechowska, B. Morphological and mechanical stability of bladder cancer cells in response to substrate rigidity. Biochim. Biophys. Acta (BBA) Gen. Subj. 2019, 1863, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Grady, M.E.; Composto, R.J.; Eckmann, D.M. Cell elasticity with altered cytoskeletal architectures across multiple cell types. J. Mech. Behav. Biomed. Mater. 2016, 61, 197–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pachenari, M.; Seyedpour, S.; Janmaleki, M.; Shayan, S.B.; Taranejoo, S.; Hosseinkhani, H. Mechanical properties of cancer cytoskeleton depend on actin filaments to microtubules content: Investigating different grades of colon cancer cell lines. J. Biomech. 2014, 47, 373–379. [Google Scholar] [CrossRef]
- Ebihara, T.; Venkatesan, N.; Tanaka, R.; Ludwig, M.S. Changes in Extracellular Matrix and Tissue Viscoelasticity in Bleomycin–induced Lung Fibrosis. Am. J. Respir. Crit. Care Med. 2000, 162, 1569–1576. [Google Scholar] [CrossRef]
- Brown, A.C.; Fiore, V.F.; Sulchek, T.A.; Barker, T.H. Physical and chemical microenvironmental cues orthogonally control the degree and duration of fibrosis-associated epithelial-to-mesenchymal transitions. J. Pathol. 2012, 229, 25–35. [Google Scholar] [CrossRef] [Green Version]
- Luo, Q.; Kuang, D.; Zhang, B.; Halim, A. Cell stiffness determined by atomic force microscopy and its correlation with cell motility. Biochim. Biophys. Acta (BBA) Gen. Subj. 2016, 1860, 1953–1960. [Google Scholar] [CrossRef]
- Gavara, N.; Chadwick, R.S. Relationship between cell stiffness and stress fiber amount, assessed by simultaneous atomic force microscopy and live-cell fluorescence imaging. Biomech. Model. Mechanobiol. 2015, 15, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Gowdy, K.M.; Fessler, M.B. Emerging roles for cholesterol and lipoproteins in lung disease. Pulm. Pharmacol. Ther. 2012, 26, 430–437. [Google Scholar] [CrossRef] [Green Version]
- Tsai, M.-J.; Chang, W.-A.; Liao, S.-H.; Chang, K.-F.; Sheu, C.-C.; Kuo, P.-L. The Effects of Epigallocatechin Gallate (EGCG) on Pulmonary Fibroblasts of Idiopathic Pulmonary Fibrosis (IPF)-A Next-Generation Sequencing and Bioinformatic Approach. Int. J. Mol. Sci. 2019, 20, 1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griese, M.; Kirmeier, H.G.; Liebisch, G.; Rauch, D.; Stückler, F.; Schmitz, G.; Zarbock, R. ILD-BAL working group of the Kids-Lung-Register Surfactant Lipidomics in Healthy Children and Childhood Interstitial Lung Disease. PLoS ONE 2015, 10, e0117985. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.Y.; Veldhuizen, R.; Neumann, A.W.; Petersen, N.O.; Possmayer, F. Current perspectives in pulmonary surfactant — Inhibition, enhancement and evaluation. Biochim. Biophys. Acta (BBA) Biomembr. 2008, 1778, 1947–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westergren-Thorsson, G.; Hedström, U.; Nybom, A.; Tykesson, E.; Åhrman, E.; Hornfelt, M.; Maccarana, M.; Van Kuppevelt, T.H.; Dellgren, G.; Wildt, M.; et al. Increased deposition of glycosaminoglycans and altered structure of heparan sulfate in idiopathic pulmonary fibrosis. Int. J. Biochem. Cell Biol. 2017, 83, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Egashira, R.; Jacob, J.; Kokosi, M.; Brun, A.-L.; Rice, A.; Nicholson, A.G.; Wells, A.U.; Hansell, D.M. Diffuse Pulmonary Ossification in Fibrosing Interstitial Lung Diseases: Prevalence and Associations. Radiol. 2017, 284, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Morales, D.V.; Welsh, C.H.; McDermott, M.T.; Schwarz, M.I. Calcium Deposition with or without Bone Formation in the Lung. Am. J. Respir. Crit. Care Med. 2002, 165, 1654–1669. [Google Scholar] [CrossRef] [Green Version]
- Almstrand, A.-C.; Josefson, M.; Bredberg, A.; Lausmaa, J.; Sjövall, P.; Larsson, P.; Olin, A.-C. TOF-SIMS analysis of exhaled particles from patients with asthma and healthy controls. Eur. Respir. J. 2011, 39, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Breitenstein, D.; Batenburg, J.J.; Hagenhoff, B.; Galla, H.-J. Lipid Specificity of Surfactant Protein B Studied by Time-of-Flight Secondary Ion Mass Spectrometry. Biophys. J. 2006, 91, 1347–1356. [Google Scholar] [CrossRef] [Green Version]
- Spickett, C.M.; Pitt, A.R. Oxidative lipidomics coming of age: Advances in analysis of oxidized phospholipids in physiology and pathology. Antioxidants Redox Signal. 2015, 22, 1646–1666. [Google Scholar] [CrossRef] [Green Version]
- Henry, S.A.; Kohlwein, S.D.; Carman, G.M. Metabolism and Regulation of Glycerolipids in the YeastSaccharomyces cerevisiae. Genetics 2012, 190, 317–349. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Dagan, S.; Somogyi, A.; Wysocki, V.H.; Scaraffia, P.Y. Low Mass MS/MS Fragments of Protonated Amino Acids Used for Distinction of Their 13C- Isotopomers in Metabolic Studies. J. Am. Soc. Mass Spectrom. 2013, 24, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byfield, F.J.; Aranda-Espinoza, H.; Romanenko, V.G.; Rothblat, G.H.; Levitan, I. Cholesterol Depletion Increases Membrane Stiffness of Aortic Endothelial Cells. Biophys. J. 2004, 87, 3336–3343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Oliveira Andrade, L. Understanding the role of cholesterol in cellular biomechanics and regulation of vesicular trafficking: The power of imaging. Biomed. Spectrosc. Imaging 2016, 5, S101–S117. [Google Scholar] [CrossRef] [Green Version]
- Levitan, I. Paradoxical impact of cholesterol on lipid packing and cell stiffness. Front. Biosci. 2016, 21, 1245–1259. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raczkowska, J.; Orzechowska, B.; Patryas, S.; Awsiuk, K.; Kubiak, A.; Kinoshita, M.; Okamoto, M.; Bobrowska, J.; Stachura, T.; Soja, J.; et al. Effect of Substrate Stiffness on Physicochemical Properties of Normal and Fibrotic Lung Fibroblasts. Materials 2020, 13, 4495. https://doi.org/10.3390/ma13204495
Raczkowska J, Orzechowska B, Patryas S, Awsiuk K, Kubiak A, Kinoshita M, Okamoto M, Bobrowska J, Stachura T, Soja J, et al. Effect of Substrate Stiffness on Physicochemical Properties of Normal and Fibrotic Lung Fibroblasts. Materials. 2020; 13(20):4495. https://doi.org/10.3390/ma13204495
Chicago/Turabian StyleRaczkowska, Joanna, Barbara Orzechowska, Sabina Patryas, Kamil Awsiuk, Andrzej Kubiak, Masaya Kinoshita, Masami Okamoto, Justyna Bobrowska, Tomasz Stachura, Jerzy Soja, and et al. 2020. "Effect of Substrate Stiffness on Physicochemical Properties of Normal and Fibrotic Lung Fibroblasts" Materials 13, no. 20: 4495. https://doi.org/10.3390/ma13204495
APA StyleRaczkowska, J., Orzechowska, B., Patryas, S., Awsiuk, K., Kubiak, A., Kinoshita, M., Okamoto, M., Bobrowska, J., Stachura, T., Soja, J., Sładek, K., & Lekka, M. (2020). Effect of Substrate Stiffness on Physicochemical Properties of Normal and Fibrotic Lung Fibroblasts. Materials, 13(20), 4495. https://doi.org/10.3390/ma13204495