Abstract
SnS2 as a high energy anode material has attracted extensive research interest recently. However, the fast capacity decay and low rate performance in alkaline-ion batteries associated with repeated volume variation and low electrical conductivity plague them from practical application. Herein, we propose a facile method to solve this problem by synthesizing porous SnS2 microflowers with in-situ formed sulfur vacancies. The flexible porous nanosheets in the three-dimensional flower-like nanostructure provide facile strain relaxation to avoid stress concentration during the volume changes. Rich sulfur vacancies and porous structure enable the fast and efficient electron transport. The porous SnS2−x microflowers exhibit outstanding performance for lithium ion battery in terms of high capacity (1375 mAh g−1 at 100 mA g−1) and outstanding rate capability (827 mA h g−1 at high rate of 2 A g−1). For sodium ion battery, a high capacity (~522 mAh g−1) can be achieved at 5 A g−1 after 200 cycles for SnS2−x microflowers. The rational design in nanostructures, as well as the chemical compositions, might create new opportunities in designing the new architecture for highly efficient energy storage devices.
1. Introduction
The energy crisis brings about a challenge to human life and sustainable and clean energy storage devices play a key role to overcome these issues [1]. Lithium-ion batteries (LIBs) dominate the commercial portable energy storage devices, including cell phones. However, their low energy density hinders them from some emerging applications such as electric vehicle (EVs) [2]. In commercial LIBs, graphite is commercialized as an anode electrode and its limited theoretical capacity (372 mAh g−1) is far from the increasing demand [3,4,5]. Thus, high capacity anode development becomes crucial. Additionally, sodium-ion batteries (SIBs) have become promising alternative energy storage devices for large-scale applications due to the low cost and earth-abundance of sodium resources [6]. Nevertheless, due to the narrow lattice spacing of graphite and large radius of the Na+ ions (0.97 Å), graphite usually exhibits sluggish kinetics for Na+ ions and inferior performance as anode for SIBs [7,8,9]. Therefore, development of high energy and long life anode materials is urgently needed.
Tin-based materials have received more and more research interest due to the high theoretical capacity and low charging/discharging potential platform [10,11]. Despite the high lithium/sodium storage capability, the fast capacity loss during repetitive huge volume expansion–contraction remains a major challenge to tin-based materials [12]. As a typical layered metal dichalcogenide material, SnS2 is a typical two-dimensional layered structure and the large interlayer spacing could not only store the intercalated Li+ or Na+ ions but also release the stress induced by volume change [13]. However, as a ceramic semiconductor, tin disulfide suffers from low electronic conductivities [14,15,16,17,18,19]. Different highly conductive materials have been added to composite with SnS2, such as grapheme [20,21,22,23,24,25], PPy [18], and so on [26,27]. Another method is to decrease the feature size of SnS2 and build nanostructures in order to reduce the length of ion transportation. Sulfur vacancies have been recently reported to increase the electrical conductivity of SnS2 [28,29,30,31,32,33,34]. Thus, rational design of hierarchical structures with sulfur vacancies would improve the electrochemical performance of SnS2.
Herein, we designed three-dimensional hierarchical porous SnS2−x microflowers with interconnected porous nanosheets as anode for LIBs and SIBs. The SnS2−x microflowers exhibit excellent electrochemical properties for LIBs and SIBs. The excellent electrochemical performance of SnS2−x microflowers can be attributed to the porous structure of SnS2−x nanosheets, which provide more accessible active sites and shorten the length for alkaline ion diffusion and rich sulfur vacancy. The rich sulfur vacancy would increase the electric conductivity and the formation of porous structure would lead to the facile strain relaxation during battery cycling. In this way, the porous SnS2−x microflowers exhibit promising performance in both lithium and sodium ion storage.
2. Results and Discussion
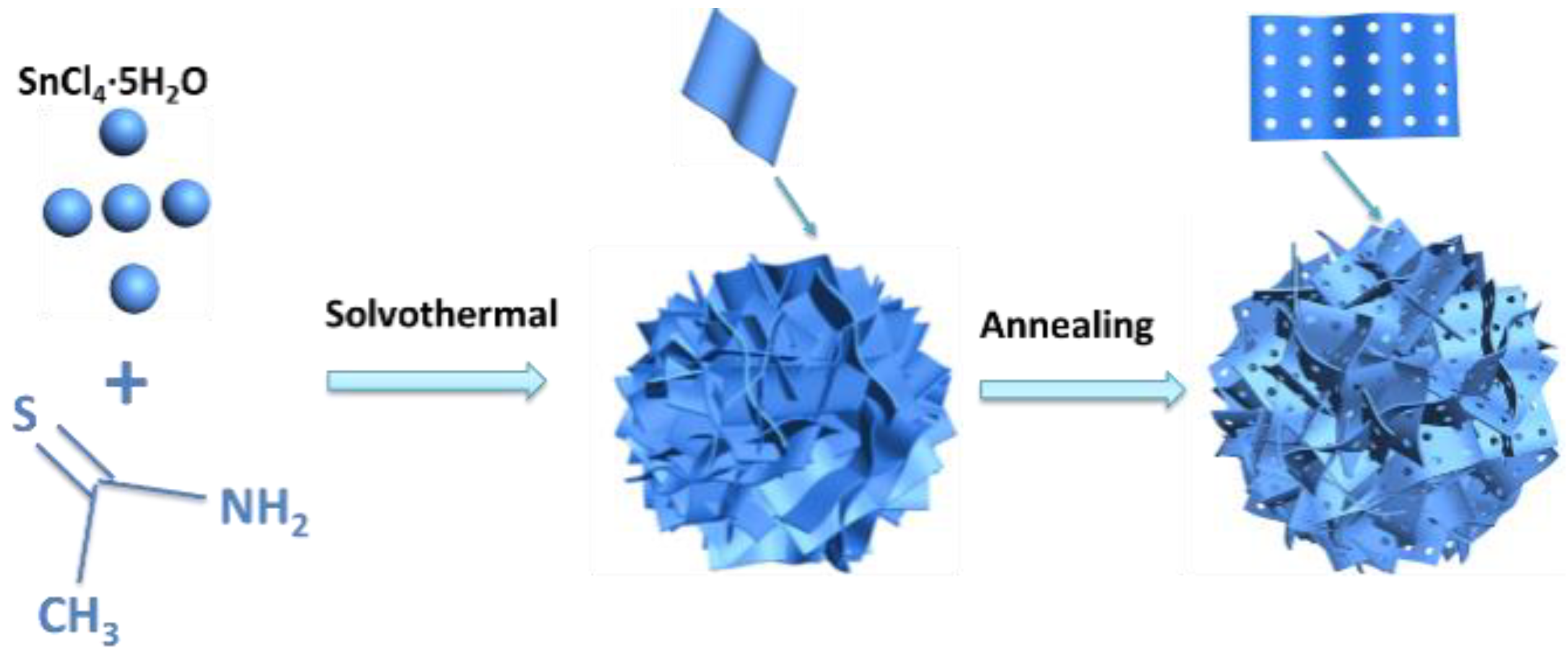
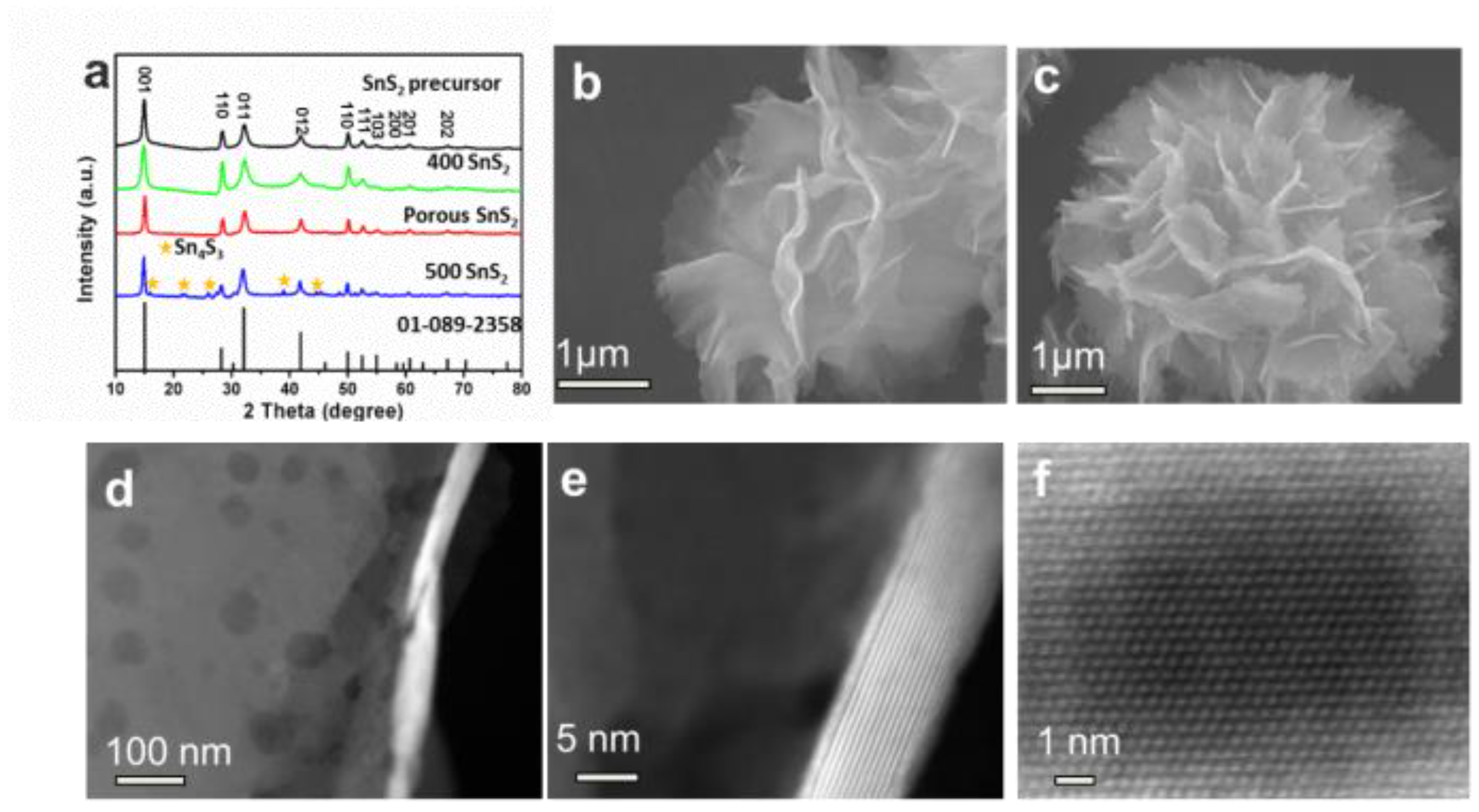
SnS2−x microflowers were synthesized via a hydrothermal reaction and this was followed by annealing process (Figure 1). After the hydrothermal reaction, SnS2 microflowers were formed. The microflowers are composed of interconnected flexible thin SnS2 nanosheets. The additional annealing process not only increases the crystallinity of the SnS2 microflowers but also introduces sulfur vacancies in the SnS2−x nanosheets. XRD spectra were carried out to investigate the crystal structure of the products (Figure 2a). All the diffraction peaks of the SnS2, SnS2-400, and SnS2-450 are well indexed to the hexagonal structure of SnS2 (JCPDS No.01-089-2358). All of these well-defined identified diffraction peaks at 15.0°, 28.2°, 32.1°, and 41.9° can be well assigned to (001), (100), (011), and (012) planes of hexagonal SnS2, respectively. When the annealing temperature was increased to 500 °C, the impurity phase Sn4S3 (Orthorhombic, JCPDS No.00-030-1379) is generated accompanying with the main phase SnS2. Furthermore, the morphologies of the as-prepared samples were characterized by SEM and TEM. The SnS2 microflowers are composed of thin and smooth nanosheets (Figure 2b). The SEM morphology of microflowers kept unchanged for SnS2−x-400 and SnS2−x-450 (Figure 2c and Figure S1), while it became a mixture of microflowers and bulks for SnS2−x-500 (Figure S2). The energy dispersive spectrometer (EDS) mapping confirms the homogeneous distribution of Sn and S elements in the entire sample of SnS2−x microflowers (Figure S2). In order to further identify the pore size and crystallographic structure of the SnS2−x microflowers, scanning transmission electron microscopy (STEM) and high resolution scanning transmission electron microscopy (HRSTEM) were further carried out. There are no obvious pores observed on SnS2 nanosheets (Figure S3). Pores become distinct on the nanoflakes of SnS2−x-400 and SnS2−x-450, while the pore size of the SnS2−x-450 nanosheets is larger than that in SnS2−x-400 nanosheets (Figure 2c, Figure S3). However, further increasing the annealing temperature, no pores were observed on SnS2−x-500 due to the phase evolution to Sn3S4 (Figure S3). HRTEM image in Figure 2d shows that the pore size is in the range of 2–20 nm. The side view of the nanosheet suggests that the nanosheet is composed of 15 layers (Figure 2e). The HRSTEM image reveals the typical 2H phase of SnS2 and no obvious lattice shift was observed, suggesting that the formation of pores does not affect the crystal structure.
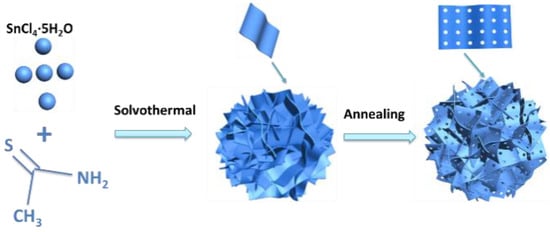
Figure 1.
Schematic of the synthesis of SnS2 microflowers.
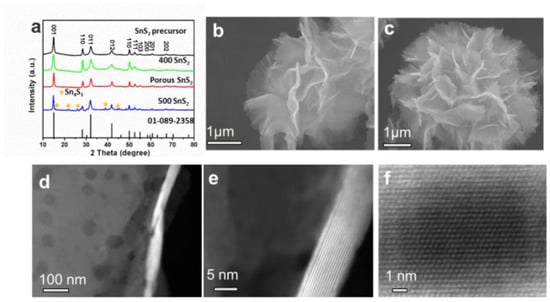
Figure 2.
(a) XRD patterns of SnS2, SnS2−x-400, SnS2−x-450, and SnS2−x-500. (b,c) SEM images of SnS2 microflowers and SnS2−x-450 microflowers. (d) TEM image and (e,f) canning transmission electron microscopy (STEM) images of SnS2−x-450 microflowers, showing the atomic resolution of the 2H-SnS2.
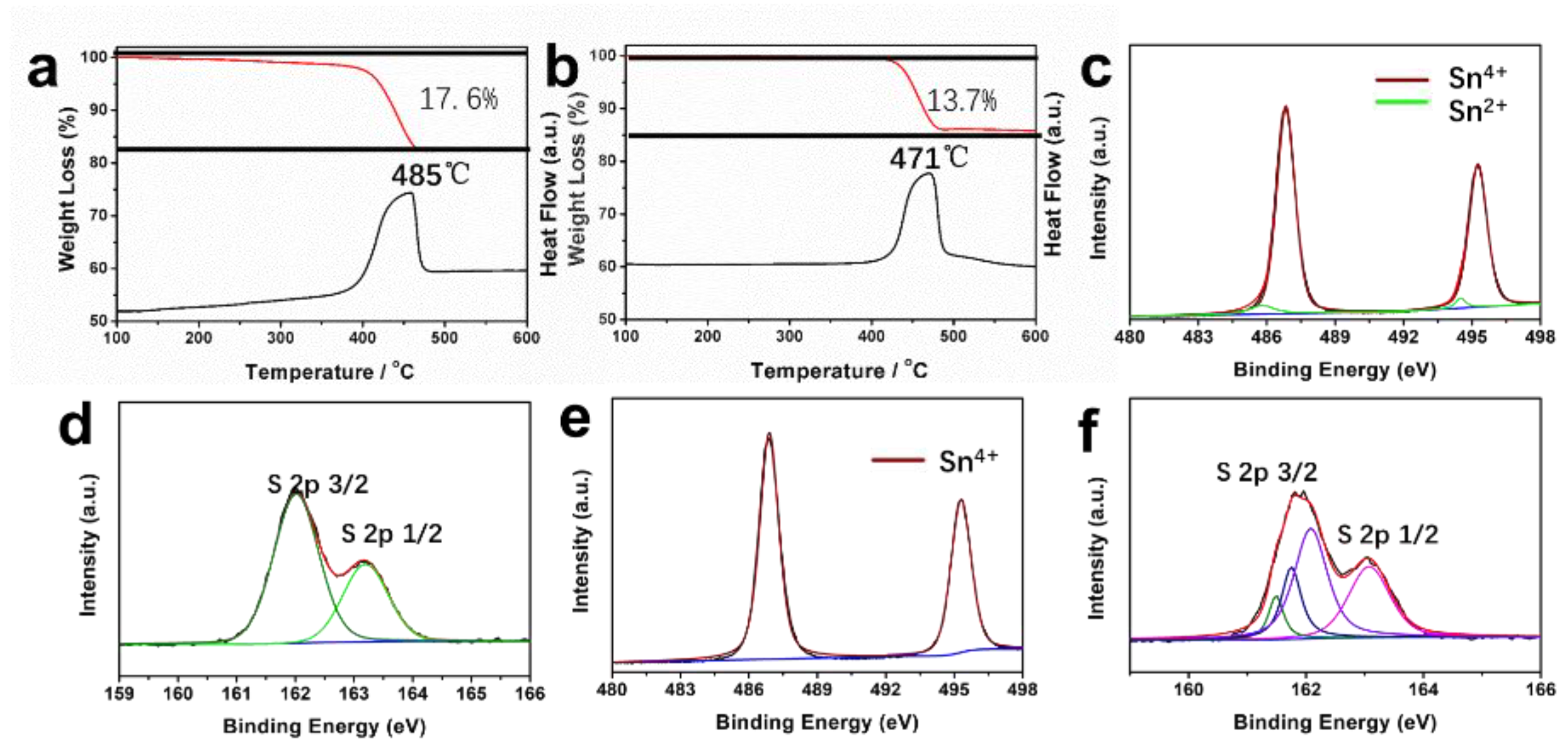
In order to investigate the contents of sulfur vacancies in SnS2−x samples, thermogravimetric (TG) analysis was carried out in the air (Figure 3a,b). For SnS2 microflowers, the total weight loss is 17.6%, while the total weight loss of SnS2−x microflowers is only 13.7%. During the annealing in air, the SnS2 is oxided to SnO2. The much lower weight loss of SnS2−x microflowers corresponds to the rich sulfur vacancies in SnS2−x microflowers, which is formed during the annealing process under sulfur-deficient environment.
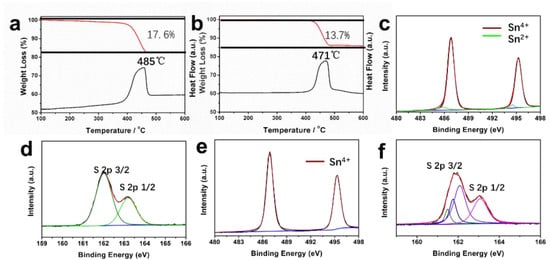
Figure 3.
(a,b) Thermogravimetric (TG) curves and their corresponding DSC curves of the SnS2 and SnS2−x microflowers. (c,d) Sn 3d spectrum and S 2p spectrum for SnS2−x microflowers. (e,f) Sn 3d spectrum and S 2p spectrum for SnS2 microflowers.
To further study the formation of sulfur vacancies in SnS2−x microflowers during annealing process, XPS measurements were conducted on the samples before and after the annealing (Figure 3). In Figure 3c–f, both Sn 3d and S 2p peaks in XPS spectra were shifted to lower binding energies after annealing, suggesting the formation of low-valance Sn2+ and sulfur vacancies [32]. In addition, the Sn 3d spectrum shown in Figure 3c can be further deconvoluted into four peaks. The two dominating peaks at 495.3 eV and 487.0 eV correspond to Sn4+, while the two small peaks at 485.8 eV and 494 eV correspond to Sn2+, indicating the coexistence of Sn4+ and Sn2+ in SnS2−x microflowers [35]. However, there are only two peaks (Figure 3e) for the SnS2 microflowers (495.3 eV, 487.0 eV), suggesting the presence of Sn4+ in the SnS2 microflowers. The N2 adsorption–desorption isotherms of SnS2 microflowers and SnS2−x microflowers are shown in Figure S4. The specific surface area of porous SnS2−x-450 microflowers is 20.1 m2 g–1, which is much higher than SnS2 microflowers (5.8 m2 g−1), SnS2−x-400 (9.8 m2 g−1), and SnS2−x-500 (6.6 m2 g−1). The higher surface area is attributed to a greater number of pores, as evidenced by the STEM images in Figure 2d.
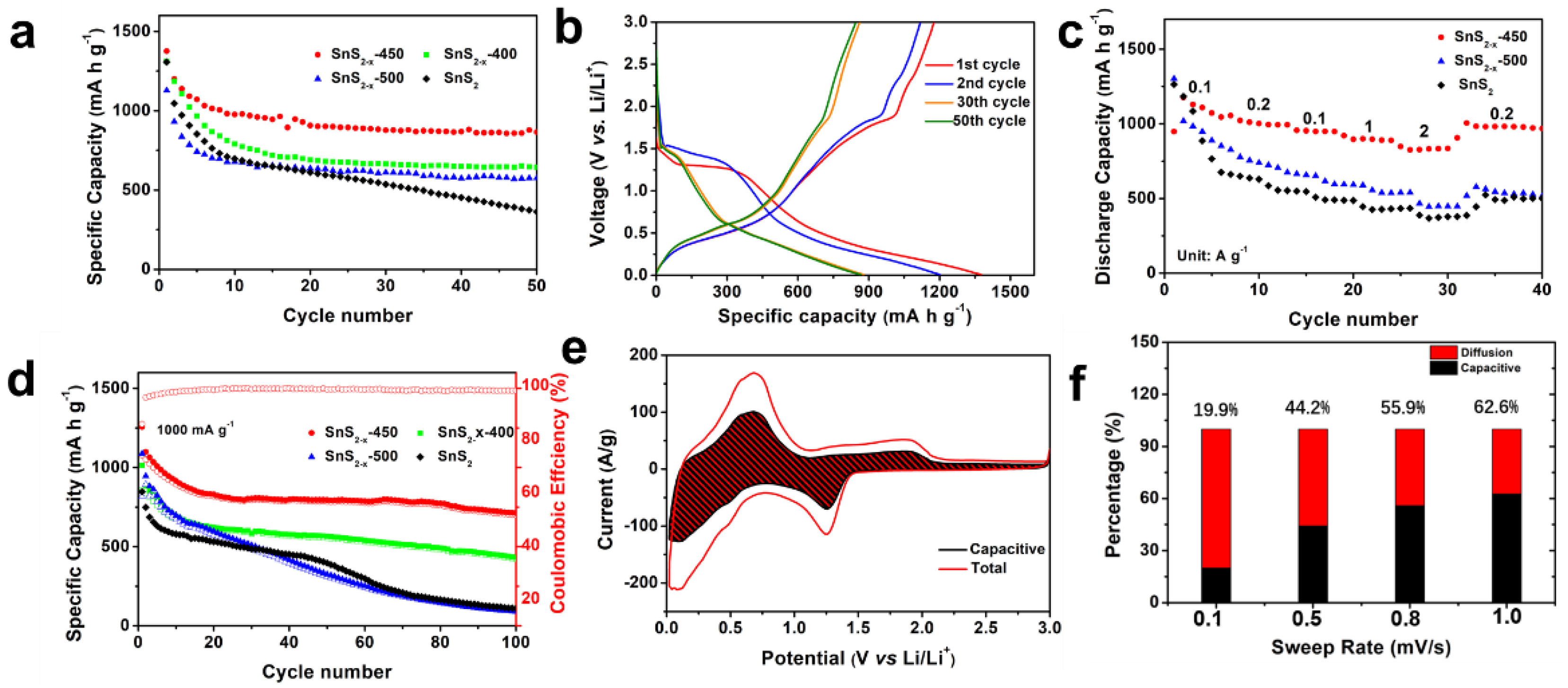
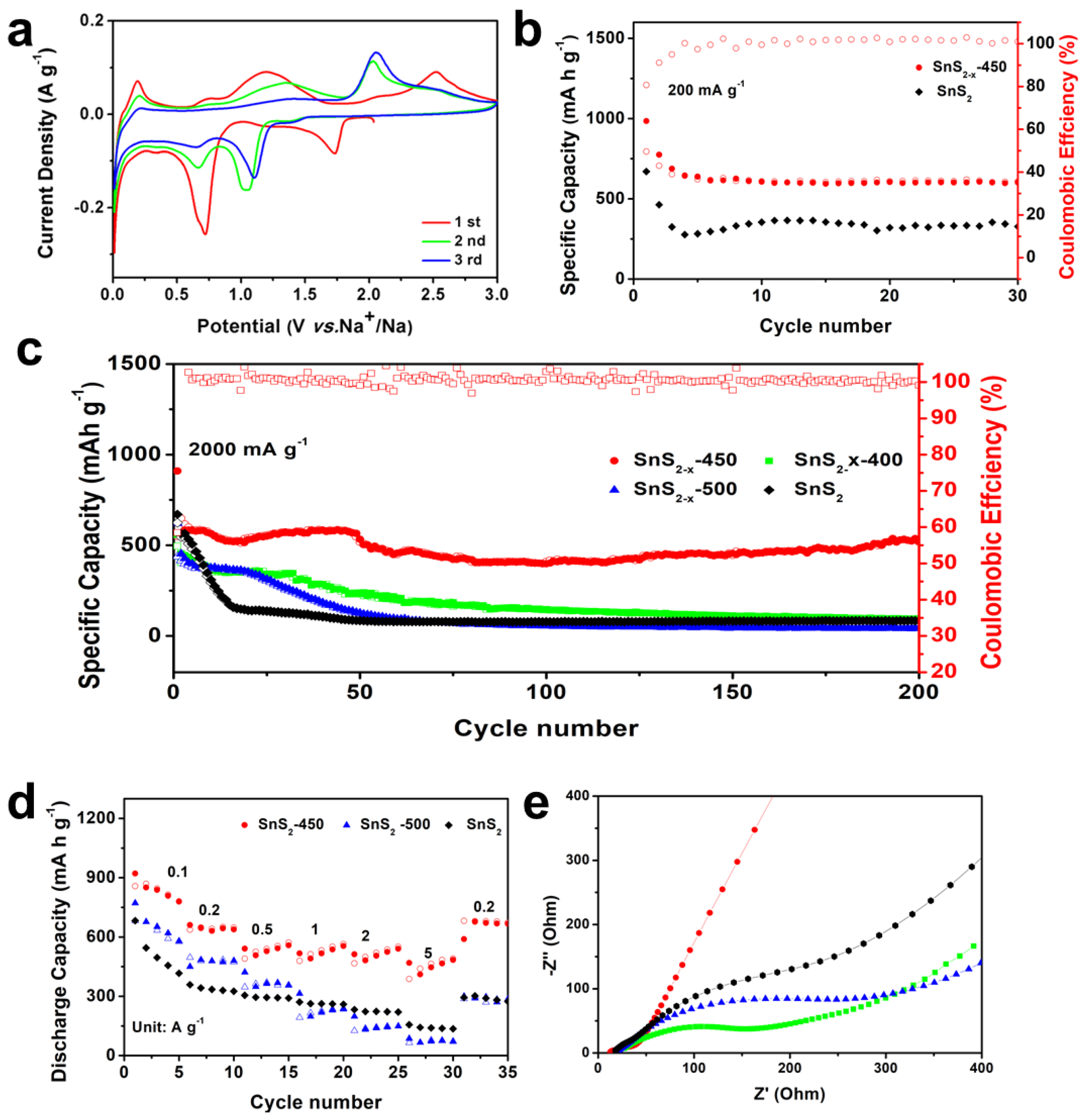
Sulfur vacancies are believed to stabilize this material for alkaline ion storage. Cyclic voltammetry (CV) of SnS2−x microflower sample was measured at 0.1 mV s−1 in the potential range of 0.01–3 V vs. Li/Li+, which is shown in Figures S5 and S6. In the initial sweeping process, the CV curves show two broad peaks located at ~1.4 V and ~0.2 V, ascribing to the conversion reaction of SnS2 into metallic Sn and Li2S (reaction 1) and the alloying reaction of Li-Sn alloy (reaction 2), respectively. Cycling performances of the samples are one of the most important parameters for batteries and are evaluated by a galvanostatic charging/discharging test as in Figure 4a. SnS2−x-450 exhibits much better cycling stability than those of SnS2, SnS2-400, and SnS2-500. At 100 mA g−1 (corresponding to 0.06C), the initial discharging capacity of SnS2−x-450 is 1375 mA h g−1, surpassing those of SnS2 (1311 mAh g−1), SnS2-400 (1128 mAh g−1), and SnS2-500 (1306 mAh g−1). The 50th capacity of SnS2−x-450 is 865 mA h g−1 corresponding to a capacity retention of 62.9%, which is much higher than that of SnS2 (362 mA h g−1, 27.8%), SnS2−x-400 (643 mA h g−1, 57.0%), and SnS2−x-500 (575 mA h g−1, 44.0%), manifesting the increased capacity and enhanced cycling stability. Figure 4b displays the galvanostatic charging/discharging curves of the SnS2−x-450 at 100 mA g−1. The initial discharging capacity is 1375 mA h g−1, and the initial charging capacity is 1176 mA h g−1. Figure 4c summarizes the rate performances of all samples. At rates of 100, 200, 500, 1000, 2000, and 200 mA g−1 (Figure 4d), the discharging capacities of the SnS2−x-450 microflowers were 1130, 1010, 954, 899, 827, and 1005 mA h g−1, respectively. Notably, even at a high current density of 2000 mAh g−1, the SnS2−x-450 microflowers still retain a high capacity of 827 mAh g−1. Besides, the capacity at each current density was far higher than that of the SnS2, SnS2−x-400, and SnS2−x-500. The cycling stability at 1000 mA g−1 was further measured. (Figure 4c). After 100 cycles, the SnS2−x-450 still maintained a high capacity of 712 mA h g−1, surpassing those of SnS2 (111 mA h g−1, 19.0%), SnS2−x-400 (431 mA h g−1, 57%), and SnS2−x-500 (91 mA h g−1, 13.0%). The capacity of SnS2−x-450 is still more than twice that of graphite.
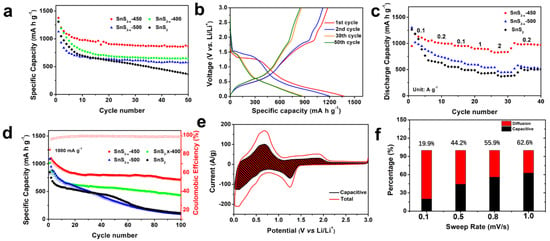
Figure 4.
(a) Cycling performances at 100 mA g−1. (b) Charge and discharge curves of SnS2−x-450. (c) Rate performances and (d) Cycling performances at 1000 mA g−1. (e) Capacitive and diffusion-controlled contributions to charge storage in SnS2−x-450 at different scan rates of 0.1, 0.5, 0.8, and 1 mV s−1. (f) Capacitive contributions of SnS2−x-450 to charge storage at 1 mV s−1. The shaded region is the pseudocapacitive contribution in SnS2−x-450.
To further investigate the charge storage mechanism in SnS2−x microflowers, the kinetics of the charging process was studied to gain more quantitative charge storage processes [36]. Figure 4e shows the contributions from the diffusion-controlled process. When the scan rates were increased from 0.1 to 1.0 mV s−1, the proportions of the capacitive contribution increased. The capacitive contribution reaches 62.6% at 1.0 mV s−1. Figure 4f shows the capacitive and diffusion-controlled contributions to charge storage in the SnS2−x-450-based lithium-ion battery at 1 mV s−1. The diffusion-controlled regions are mainly located around the peaks of the CV, revealing that the redox peaks are governed by the diffusion, while the rest of the regions are capacitive-controlled. In order to further investigate the electrochemical kinetics, electrochemical impedance spectra (EIS) in Figure S7 shows that SnS2−x-450 holds enhanced kinetics for Li-ion insertion/extraction. The TEM images and the EDS mapping of the sample after 100 cycles are carried out to reveal the stability of the SnS2−x nanostructure in Figure S8. It is found that the microflowers tend to aggregate together, while the nanosheet morphology keeps stable. The EDS mapping in Figure S8 shows the homogeneous distribution of the Sn and S elements in the area, which confirms the excellent stability of SnS2−x nanostructure.
In addition, we studied the electrochemical performance of SnS2−x microflower electrodes for sodium ion storage. The Na-storage behavior of the SnS2−x microflower electrode was measured based on the half cell with Na metal anode and SnS2−x microflower cathodes in the range of 0.01–3 V. CV curves of SnS2−x microflower-based SIB were first tested at 0.1 mV s−1. As shown in Figure 5a, the cathodic peak located at 1.74 V is attributed to the sodium intercalation into SnS2 host, while the peak at 0.73 V to the conversion reaction, alloying reaction, and solid electrolyte interface (SEI) film formation [35]. Figure S9 shows the galvanostatic charging/discharging profiles of the SnS2−x microflower electrode at a current density of 200 mA g−1 at different cycles. The initial discharge capacity is 984 mAh g−1, and the first charge capacity is 793 mAh g−1. The observed potential plateaus in the charging/discharging curves correspond to the redox peaks in the CV curves. Figure 5b shows the cycling performance of SnS2−x microflower-based SIB at 200 mAh g−1. The initial capacity of SnS2−x-450 and SnS2 is 984, 641 mAh g−1, respectively. After 30 cycles, the SnS2−x-450 still retained a capacity of 608 mAhg−1, which was much higher than SnS2 (340 mAh g−1), demonstrating the improved capacity and cycling stability. Furthermore, the rate performances were investigated in Figure 5d. Notably, even at a high current density of 5000 mA g−1, the SnS2−x-450 still keeps a high capacity of 491 mAh g−1, showing an excellent rate capability. Besides, the capacity of SnS2−x-450 was much higher than that of the SnS2, SnS2−x-400, and SnS2−x-500 at each current density. As displayed in Figure 5c, at current density of 1000 mA g−1, SnS2−x-450 could maintain a capacity of 522 mA h g−1 after 200 cycles, with a capacity retention of 89.5% from 3 to 200 cycles, surpassing those of SnS2 (84 mAh g−1, 14.8%), SnS2−x-400 (96 mAh g−1, 21.6%), and SnS2−x-500 (43 mAh g−1, 7.0%).
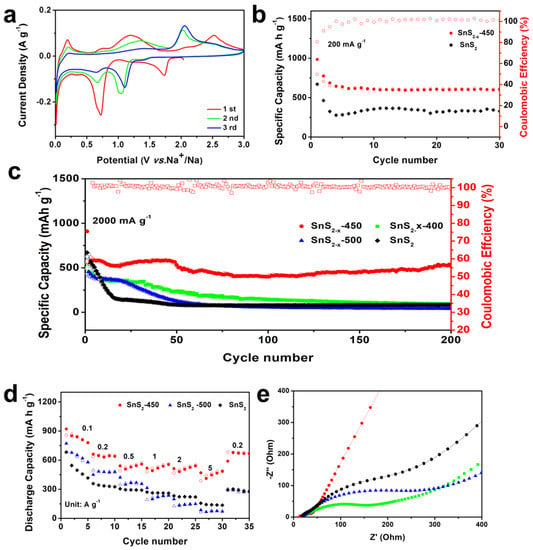
Figure 5.
(a) Cyclic voltammetry (CV) curves of of SnS2−x microflowers. (b) Cycling performances of SnS2 and SnS2−x microflowers at 200 mA g−1. (c) Cycling performances of SnS2, SnS2−x-400, SnS2−x-450, and SnS2−x-500 at 2000 mA g−1 (d) Rate performances of SnS2, SnS2−x-450 and SnS2−x-500 at current densities ranging from 200 to 5000 mA g−1. (e) EIS spectra of the SnS2, SnS2−x-400, SnS2−x-450, and SnS2−x-500.
3. Conclusions
In summary, we have successfully fabricated porous SnS2−x microflower structure through a facile solvothermal method, followed by the thermal treatment. The porous structure and sulfur vacancies are formed mainly due to the partial loss of sulfur during the annealing under sulfur-deficient environment. The rich sulfur vacancy increases the electric conductivity and the formation of porous structure leads to the facile strain relaxation during battery cycling. In this way, the porous SnS2−x microflowers exhibit promising performance as the anode for LIBs in terms of high capacity (1375 mAh g−1 at 100 mA g−1) and outstanding rate capability (827 mA h g−1 at 2 A g−1). For SIBs, a high capacity of ~522 mAh g−1 is achieved at 5 A g−1 after 200 cycles for SnS2−x microflowers. The simultaneous rational structural design and chemical composition control of SnS2−x microflowers in this work brings new insight to the synthesis of advanced functional electrode materials and offers great potential for next-generation energy storage devices.
Supplementary Materials
The following are available online at https://www.mdpi.com/1996-1944/13/2/443/s1, Figure S1: SEM images of SnS2 (a, b), SnS2−x-400 (c, d), SnS2−x-450 (e, f) and SnS2−x-500 (g, h). Figure S2: SEM image of a representive SnS2−x nanoflower and its elemental mapping images of S (b), and Sn (c). Figure S3. TEM images of SnS2 (a, b), SnS2−x-400 (c, d), and SnS2−x-500 (e, f). Figure S4. Nitrogen adsorption-desorption isotherms and pore size distributions (insets) of SnS2 (a), SnS2−x-400 (b), SnS2−x-450 (c) and SnS2−x-500 (d). Figure S5. CV curves of the LIB based on SnS2−x-450 nanoflowers. Figure S6. CV curves of SnS2−x-450 at different scan rates ranging from 0.1 to 1 mV s−1. Figure S7. EIS of of LIBs based on SnS2, SnS2−x-400, SnS2−x-450 and SnS2−x-500 nanoflowers. Figure S8. Charge and discharge curves of SnS2−x-450 at different cycles as SIBs.
Author Contributions
Data curation, L.Z., B.Y. and C.S.; Formal analysis, B.Y. and S.S.; Funding acquisition, K.Z.; Investigation, C.S. and K.Z.; Methodology, L.Z.; Project administration, K.Z.; Software, L.Z., S.S. and W.X.; Writing—original draft, L.Z. and K.Z.; Writing—review & editing, C.S., W.X. and K.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (21905169). The S/TEM work was performed at the Nanostructure Research Center (NRC), which is supported by the Fundamental Research Funds for the Central Universities (WUT: 2019III012GX), the State Key Laboratory of Advanced Technology for Materials Synthesis and Processing, and the State Key Laboratory of Silicate Materials for Architectures (all of the laboratories are at Wuhan University of Technology).
Conflicts of Interest
The authors declare no conflict of interest
References
- Yao, B.; Chandrasekaran, S.; Zhang, J.; Xiao, W.; Qian, F.; Zhu, C.; Duoss, E.B.; Spadaccini, C.M.; Worsley, M.A.; Li, Y. Efficient 3D printed pseudocapacitive electrodes with ultrahigh MnO2 loading. Joule 2018, 3, 459–470. [Google Scholar] [CrossRef]
- Vernardou, D.; Kazas, A.; Apostolopoulou, M.; Katsarakis, N.; Koudoumas, E. Cationic effect on the electrochemical characteristics of the hydrothermally grown manganese dioxide. J. Electr. Mater. 2017, 46, 2232–2240. [Google Scholar] [CrossRef]
- Zhao, K.; Sun, C.; Yu, Y.; Dong, Y.; Zhang, C.; Wang, C.; Voyles, P.M.; Mai, L.; Wang, X. Surface Gradient Ti-Doped MnO2 Nanowires for High-Rate and Long-Life Lithium Battery. Acs Appl. Mater. Interfaces 2018, 10, 44376–44384. [Google Scholar] [CrossRef] [PubMed]
- Cabana, J.; Monconduit, L.; Larcher, D.; Palacín, M.R. Beyond Intercalation-Based Li-Ion Batteries: The State of the Art and Challenges of Electrode Materials Reacting Through Conversion Reactions. Adv. Mater. 2010, 22, E170–E192. [Google Scholar] [CrossRef] [PubMed]
- Jeong, M.G.; Du Hoang, L.; Islam, M.; Lee, J.K.; Sun, Y.; Jung, O. Self-rearrangement of silicon nanoparticles embedded in micro-carbon sphere framework for high-energy and long-life lithium-ion batteries. Nano Lett. 2017, 17, 5600–5606. [Google Scholar] [CrossRef]
- Zhao, K.; Wang, C.; Yu, Y.; Yan, M.; Wei, Q.; He, P.; Dong, Y.; Zhang, Z.; Wang, X.; Mai, L. Ultrathin Surface Coating Enables Stabilized Zinc Metal Anode. Adv. Mater. Interfaces 2018, 5, 1800848. [Google Scholar] [CrossRef]
- Shi, S.; Li, Z.; Sun, Y.; Wang, B.; Liu, Q.; Hou, Y.; Huang, S.; Huang, J.; Zhao, Y. A covalent heterostructure of monodisperse Ni2P immobilized on N, P-co-doped carbon nanosheets for high performance sodium/lithium storage. Nano Energy 2018, 48, 510. [Google Scholar] [CrossRef]
- Lu, J.; Zhao, S.; Fan, S.; Lv, Q.; Li, J.; Lv, R. Hierarchical SnS/SnS2 heterostructures grown on carbon cloth as binder-free anode for superior sodium-ion storage. Carbon 2019, 148, 525–531. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, R.; Chen, B.; Wu, L.; Zou, C.; Ai, W.; Zhang, H.; Huang, W.; Yu, T. Progressively Exposing Active Facets of 2D Nanosheets toward Enhanced Pseudocapacitive Response and High-Rate Sodium Storage. Adv. Mater. 2019, 31, 1900526. [Google Scholar] [CrossRef]
- Wu, Y.; Nie, P.; Wu, L.; Dou, H.; Zhang, X. 2D MXene/SnS2 composites as high-performance anodes for sodium ion batteries. Chem. Eng. J. 2018, 334, 932. [Google Scholar] [CrossRef]
- Zheng, J.; Xiong, X.; Wang, G.; Lin, Z.; Ou, X.; Yang, C.; Liu, M. SnS2 nanoparticles anchored on three-dimensional reduced graphene oxide as a durable anode for sodium ion batteries. Chem. Eng. J. 2018, 339, 78. [Google Scholar] [CrossRef]
- Li, W.; Chou, S.-L.; Wang, J.-Z.; Kim, J.H.; Liu, H.-K.; Dou, S.-X. Sn4+xP3@ Amorphous Sn-P Composites as Anodes for Sodium-Ion Batteries with Low Cost, High Capacity, Long Life, and Superior Rate Capability. Adv. Mater. 2014, 26, 4037–4042. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Zhao, K.; He, Y.; Zheng, J.; Xu, W.; Zhang, C.; Wang, X.; Guo, M.; Mai, L.; Wang, C.; et al. Interconnected Vertically Stacked 2D-MoS2 for Ultrastable Cycling of Rechargeable Li-Ion Battery. ACS Appl. Mater. Interfaces 2019, 11, 20762. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, X.-Y.; Fang, Y.; Zhu, X.; Bao, J.; Zhou, X.; Lou, X.W.D. Confining SnS2 ultrathin nanosheets in hollow carbon nanostructures for efficient capacitive sodium storage. Joule 2018, 2, 725–735. [Google Scholar] [CrossRef]
- Jiang, Y.; Song, D.; Wu, J.; Wang, Z.; Huang, S.; Xu, Y.; Chen, Z.; Zhao, B.; Zhang, J. Sandwich-like SnS2/Graphene/SnS2 with Expanded Interlayer Distance as High-Rate Lithium/Sodium-Ion Battery Anode Materials. ACS Nano 2019, 13, 9100–9111. [Google Scholar] [CrossRef]
- Ou, X.; Cao, L.; Liang, X.; Zheng, F.; Zheng, H.-S.; Yang, X.; Wang, J.-H.; Yang, C.; Liu, M. Fabrication of SnS2/Mn2SnS4/Carbon Heterostructures for Sodium-Ion Batteries with High Initial Coulombic Efficiency and Cycling Stability. ACS Nano 2019, 13, 3666–3676. [Google Scholar] [CrossRef]
- Shi, X.; Chen, S.-L.; Fan, H.; Chen, X.-H.; Yuan, D.; Tang, Q.; Hu, A.; Luo, W.; Liu, H. Metallic State SnS2 Nanosheets with Expanded Lattice Spacing for High Performance Sodium-ion Battery. ChemSusChem 2019, 2, 4046–4053. [Google Scholar] [CrossRef]
- Wu, L.; Zheng, J.; Wang, L.; Xiong, X.; Shao, Y.; Wang, G.; Wang, J.-H.; Zhong, S.; Wu, M. PPy-encapsulated SnS2 Nanosheets Stabilized by Defects on a TiO2 Support as a Durable Anode Material for Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2019, 58, 811–815. [Google Scholar] [CrossRef]
- Wang, T.; Qu, J.; Legut, D.; Qin, J.; Li, X.; Zhang, Q. Unique Double-Interstitialcy Mechanism and Interfacial Storage Mechanism in the Graphene/Metal Oxide as the Anode for Sodium-Ion Batteries. Nano Lett. 2019, 19, 3122. [Google Scholar] [CrossRef]
- Wang, M.; Huang, Y.; Zhu, Y.; Wu, X.; Zhang, N.; Zhang, H. Binder-free flower-like SnS2 nanoplates decorated on the graphene as a flexible anode for high-performance lithium-ion batteries. J. Alloys Compounds 2019, 774, 601–609. [Google Scholar] [CrossRef]
- Yin, L.; Cheng, R.; Song, Q.; Yang, J.; Kong, X.; Huang, J.; Lin, Y.; Ouyang, H. Construction of nanoflower SnS2 anchored on g-C3N4 nanosheets composite as highly efficient anode for lithium ion batteries. Electrochim. Acta 2019, 293, 408–418. [Google Scholar] [CrossRef]
- Dashairya, L.; Sharma, M.; Basu, S.; Saha, P. SnS2/RGO based nanocomposite for efficient photocatalytic degradation of toxic industrial dyes under visible-light irradiation. J. Alloys Compounds 2019, 774, 625–636. [Google Scholar] [CrossRef]
- Bin, D.-S.; Duan, S.-Y.; Lin, X.-J.; Liu, L.; Liu, Y.; Xu, Y.-S.; Sun, Y.-G.; Tao, X.-S.; Cao, A.-M.; Wan, L.-J. Structural engineering of SnS2/Graphene nanocomposite for high-performance K-ion battery anode. Nano Energy 2019, 60, 912–918. [Google Scholar] [CrossRef]
- Xia, J.; Jiang, K.; Xie, J.; Guo, S.; Liu, L.; Zhang, Y.; Nie, S.; Yuan, Y.; Yan, H.; Wang, X. Tin disulfide embedded in N-, S-doped carbon nanofibers as anode material for sodium-ion batteries. Chem. Eng. J. 2019, 359, 1244–1251. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, M.; Wang, X.; Zeng, P.; Li, Y.; Liu, H.; Li, X.; Huang, C.; Shu, H.; Luo, Z.; et al. A tin disulfide nanosheet wrapped with interconnected carbon nanotube networks for application of lithium sulfur batteries. Electrochim. Acta 2019, 313, 151–160. [Google Scholar] [CrossRef]
- Choi, J.H.; Park, S.-K.; Kang, Y.C. Superior lithium-ion storage performances of SnO2 powders consisting of hollow nanoplates. J. Alloys Compounds 2019, 797, 380–389. [Google Scholar] [CrossRef]
- Wang, L.; Yuan, J.; Zhao, Q.; Wang, Z.; Zhu, Y.; Ma, X.; Cao, C. Supported SnS2 nanosheet array as binder-free anode for sodium ion batteries. Electrochim. Acta 2019, 308, 174–184. [Google Scholar] [CrossRef]
- Lian, S.; Sun, C.; Xu, W.; Huo, W.; Luo, Y.; Zhao, K.; Yao, G.; Xu, W.; Zhang, Y.; Li, Z.; et al. Built-in oriented electric field facilitating durable Zn-MnO2 battery. Nano Energy 2019, 62, 79. [Google Scholar] [CrossRef]
- Hawkins, C.G.; Whittaker-Brooks, L. Controlling Sulfur Vacancies in TiS2-x Cathode Insertion Hosts via the Conversion of TiS3 Nanobelts for Energy-Storage Applications. Acs Appl. Nano Mater. 2018, 1, 851–859. [Google Scholar] [CrossRef]
- Sun, G.; Li, F.; Wu, T.; Cong, L.; Sun, L.; Yang, G.; Xie, H.; Mauger, A.; Julien, C.M.; Liu, J. O-2 Adsorption Associated with Sulfur Vacancies on MoS2 Microspheres. Inorg. Chem. 2019, 58, 2169–2176. [Google Scholar] [CrossRef]
- Ni, J.; Fu, S.; Wu, C.; Maier, J.; Yu, Y.; Li, L. Self-Supported Nanotube Arrays of Sulfur-Doped TiO2 Enabling Ultrastable and Robust Sodium Storage. Adv. Mater. 2016, 28, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Lai, F.; He, G.; Feng, J.; Wang, W.; Lian, R.; Miao, Y.-E.; Wang, G.-C.; Parkin, I.P.; Liu, T. Sulfur-Deficient Bismuth Sulfide/Nitrogen-Doped Carbon Nanofibers as Advanced Free-Standing Electrode for Asymmetric Supercapacitors. Small 2018, 14, 1801562. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wei, Z.; Wang, C.; Ma, J. Vacancy-induced sodium-ion storage in N-doped carbon Nanofiber@MoS2 nanosheet arrays. Electrochim. Acta 2018, 285, 301–308. [Google Scholar] [CrossRef]
- Hawkins, C.G.; Whittaker-Brooks, L. Vertically oriented TiS2-x nanobelt arrays as binder- and carbon-free intercalation electrodes for Li- and Na-based energy storage devices. J. Mater. Chem. A 2018, 6, 21949–21960. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, L.; Xia, R.; Dong, Y.; Xu, W.; Niu, C.; He, L.; Yan, M.; Qu, L.; Mai, L. SnO2 Quantum Dots@Graphene Oxide as a High-Rate and Long-Life Anode Material for Lithium-Ion Batteries. Small 2016, 12, 588–594. [Google Scholar] [CrossRef]
- Zhao, K.; Liu, F.; Niu, C.; Xu, W.; Dong, Y.; Zhang, L.; Xie, S.; Yan, M.; Wei, Q.; Zhao, D.; et al. Graphene Oxide Wrapped Amorphous Copper Vanadium Oxide with Enhanced Capacitive Behavior for High-Rate and Long-Life Lithium-Ion Battery Anodes. Adv. Sci. 2015, 2, 1500154. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).