AFM Analyses of 3XXX Series Al Alloy Reinforced with Different Hard Nanoparticles Produced in Liquid State
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Master Compound Samples Powder Characterization
3.2. XRD Characterization of Extruded Samples
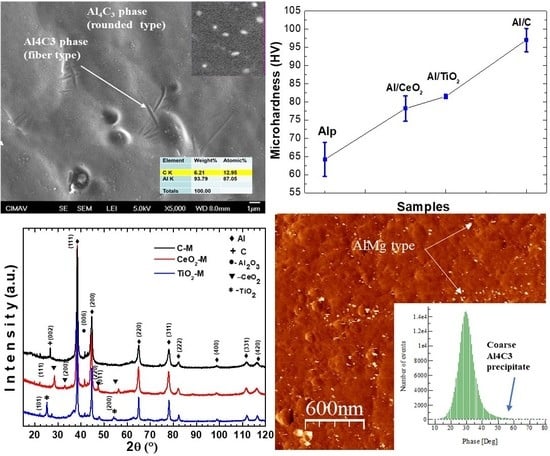
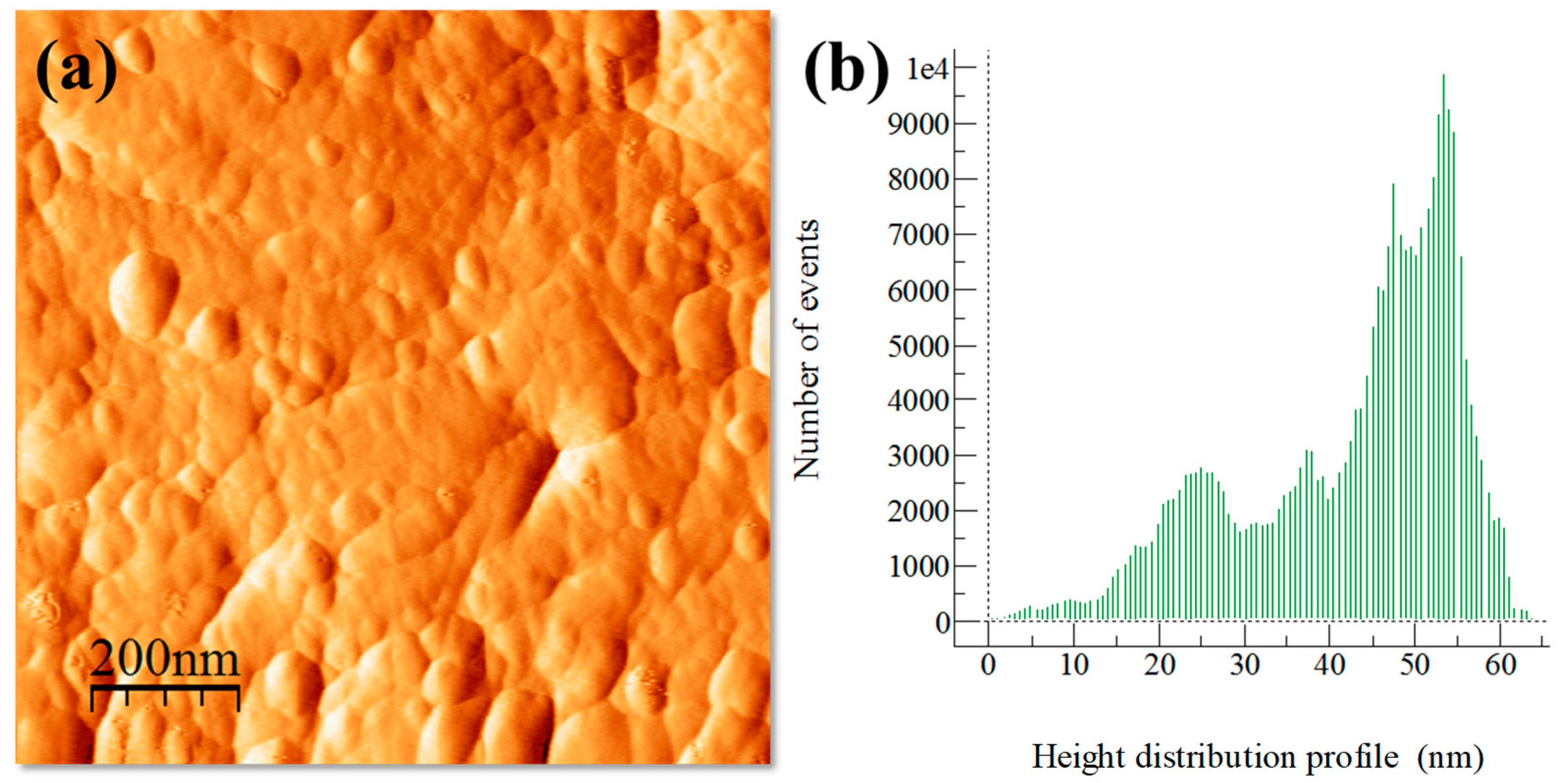
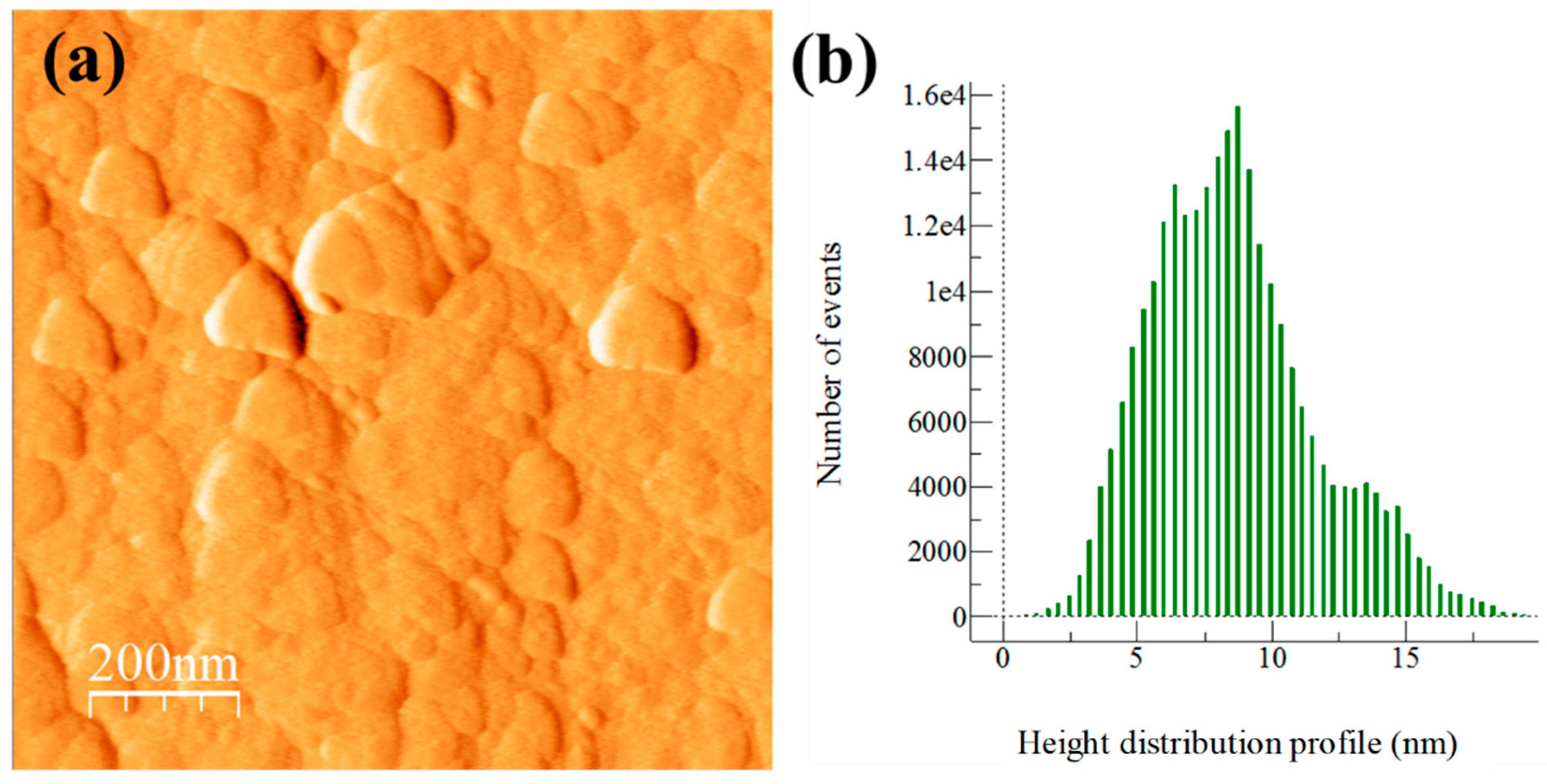
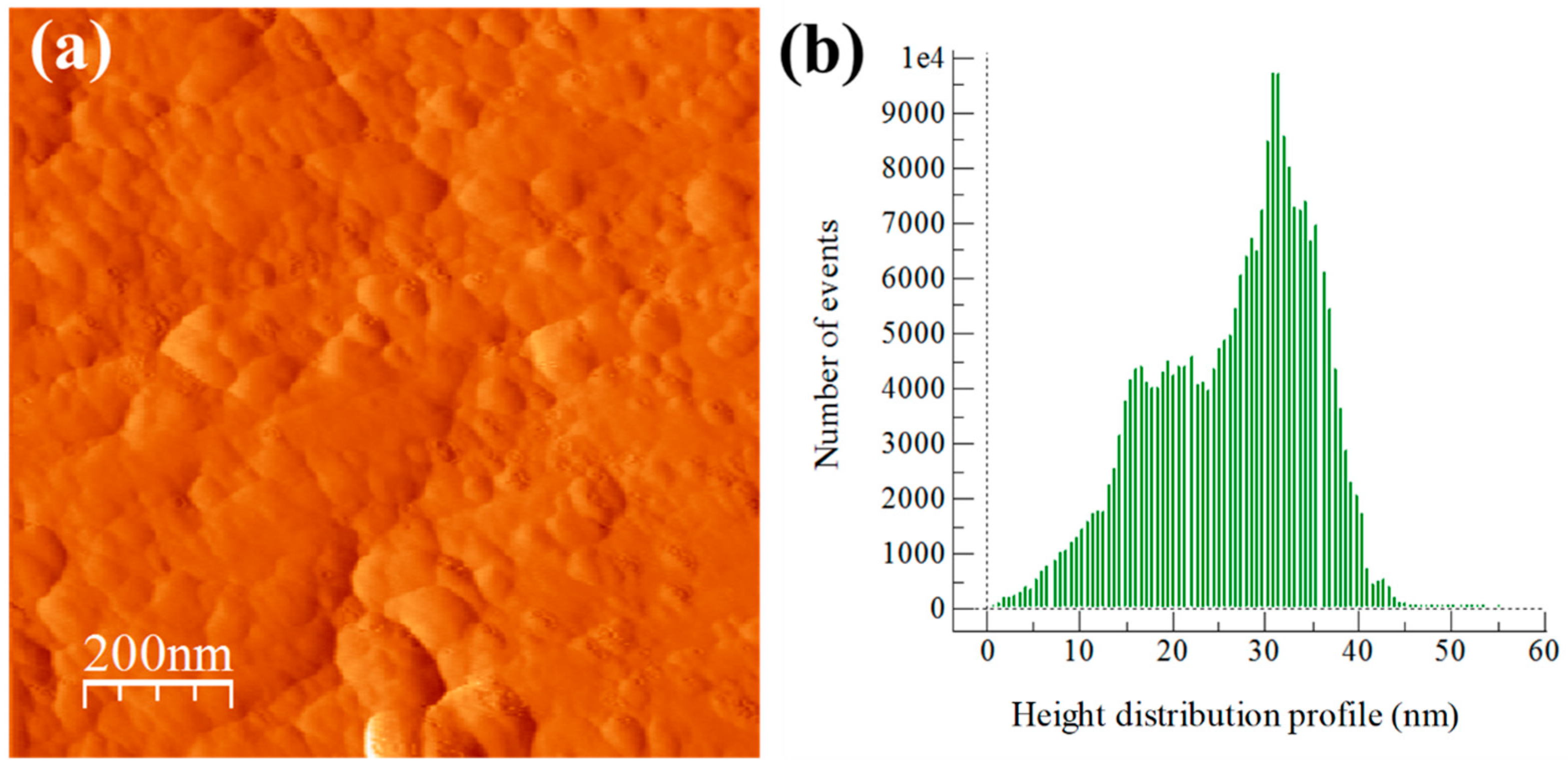
3.3. Extruded Samples AFM Topography Characterization
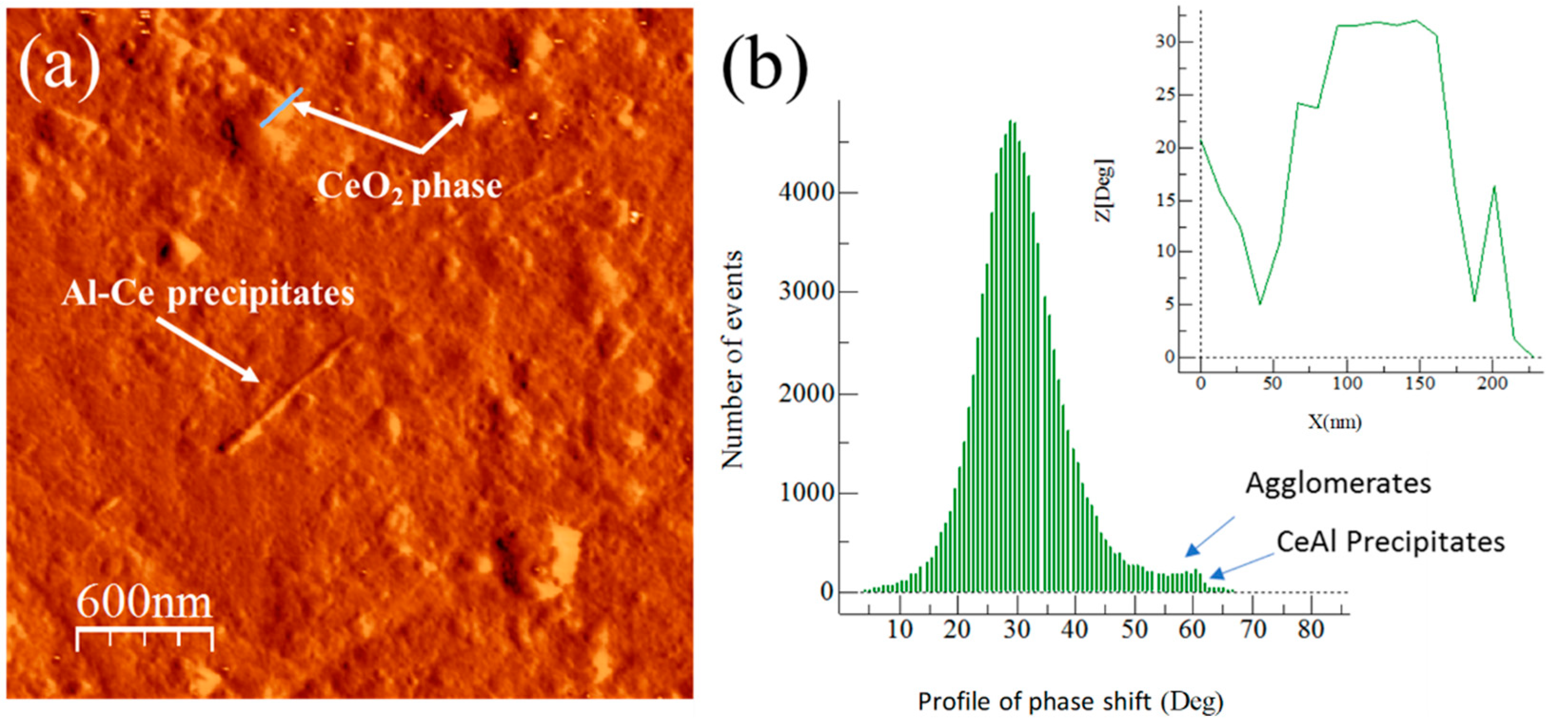
3.4. Master Compound Samples AFM Phase Characterization
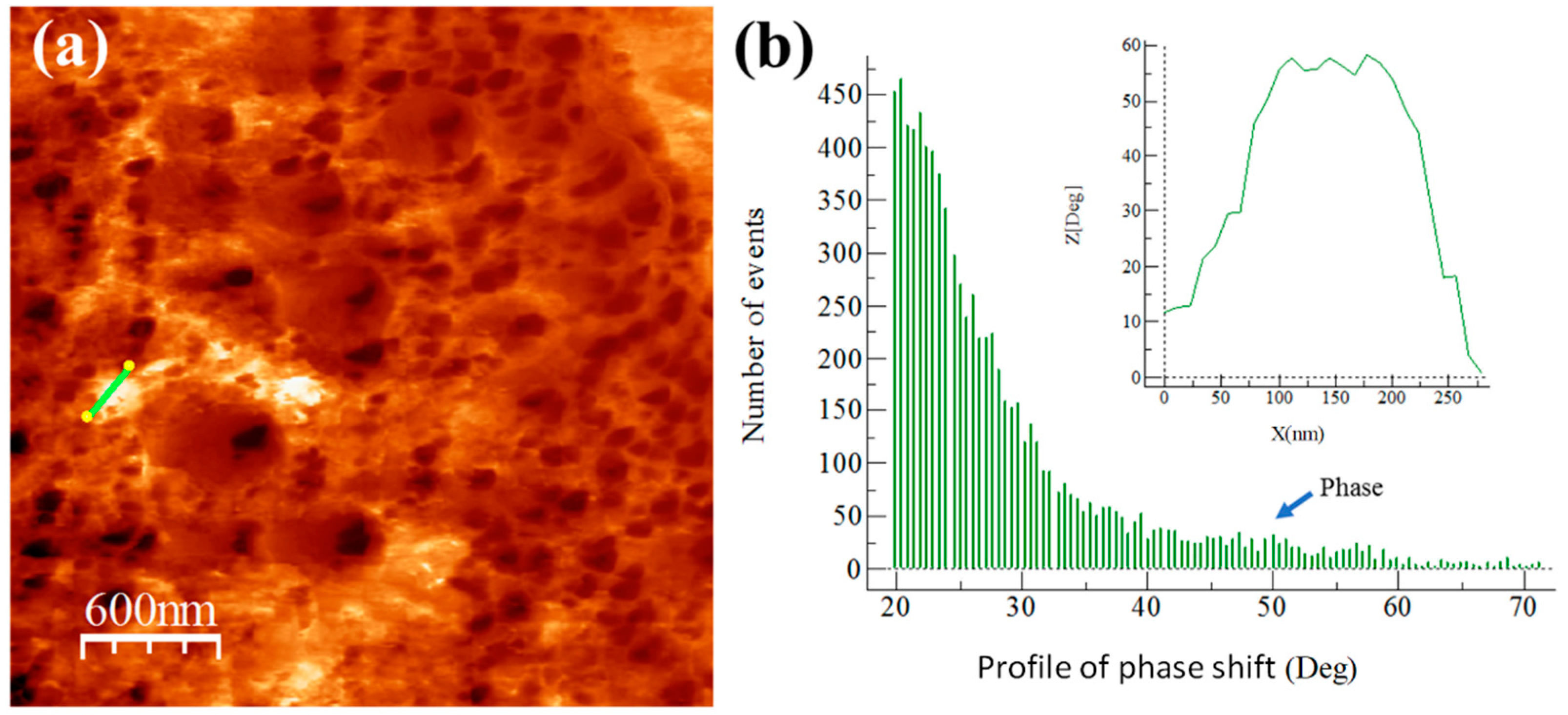
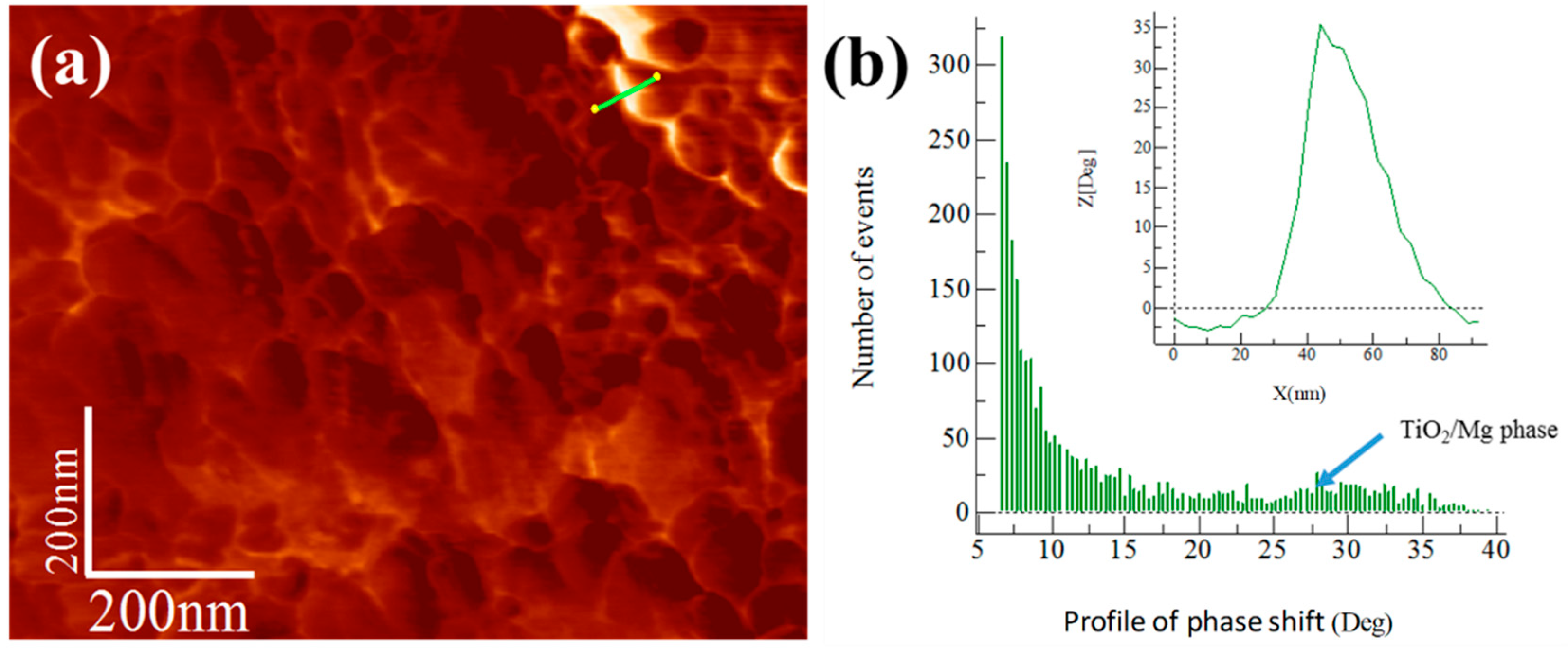
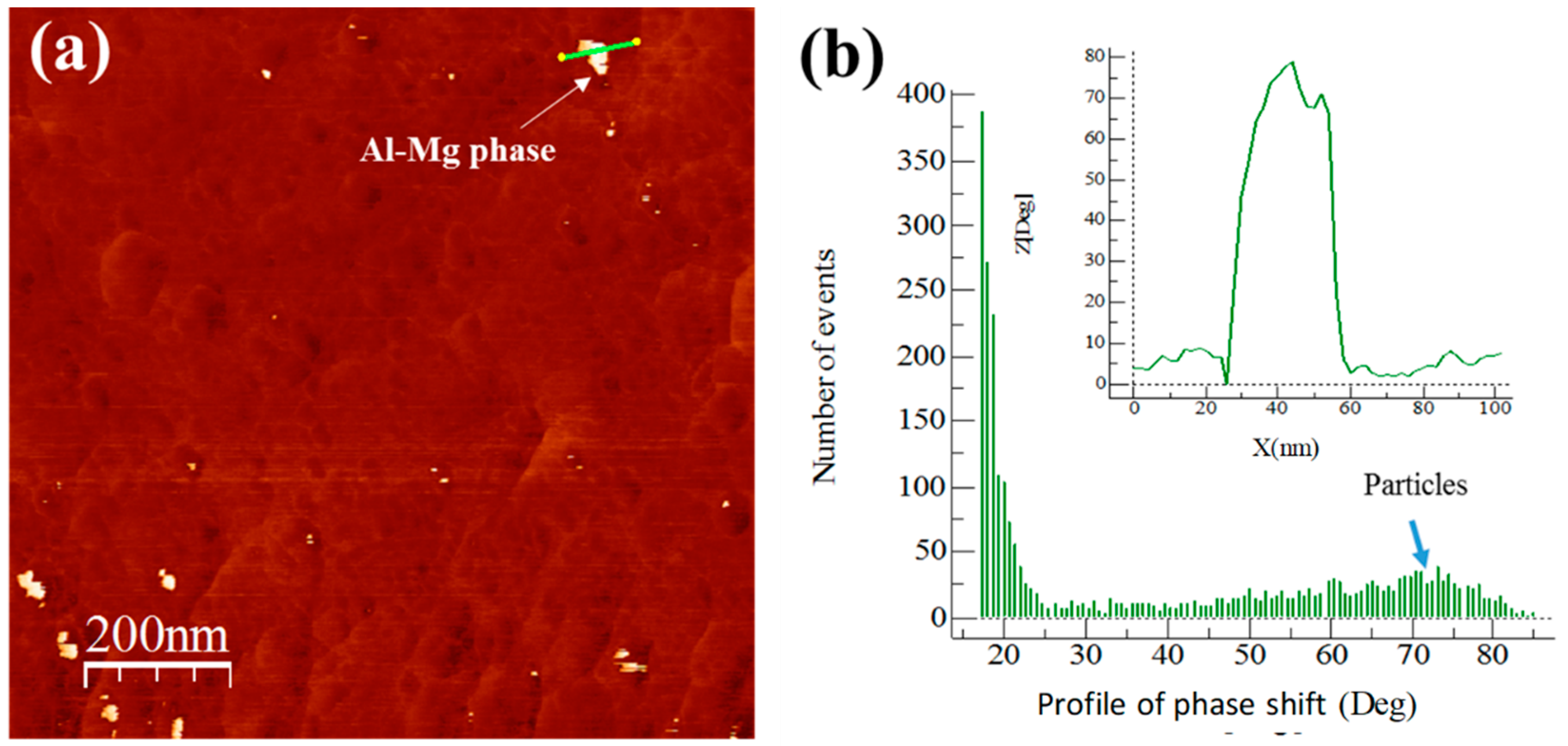
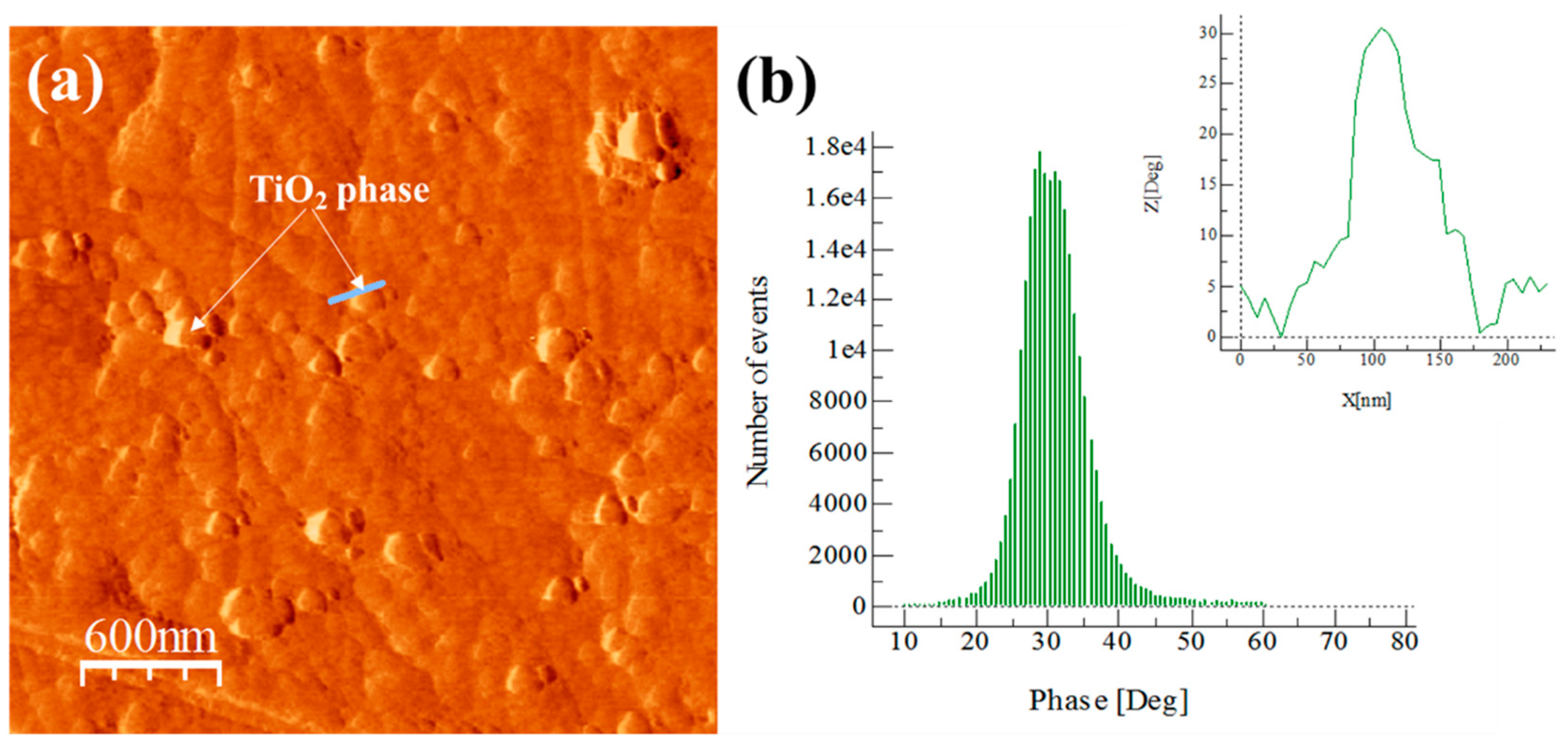
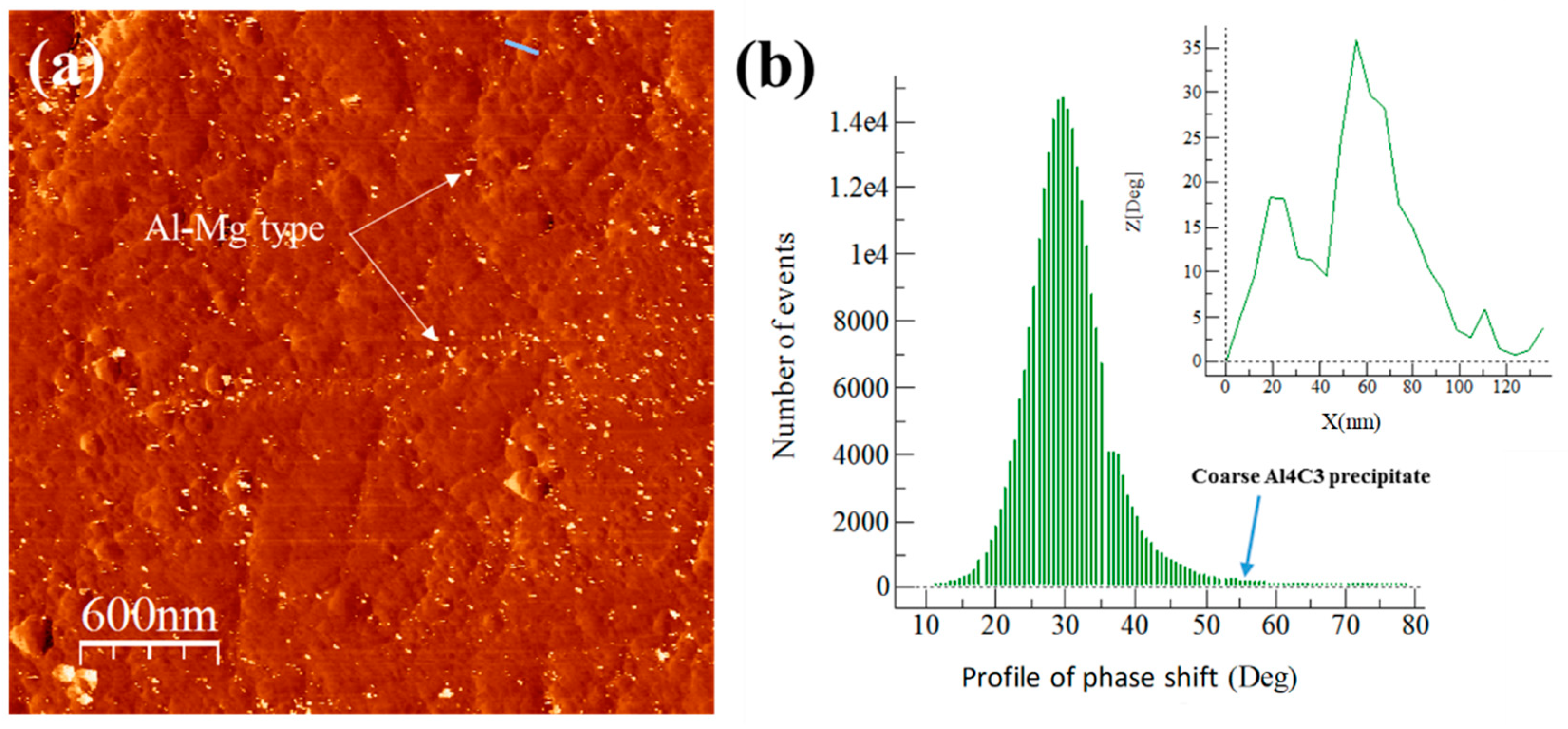
3.5. Extruded Samples AFM Phase Characterization
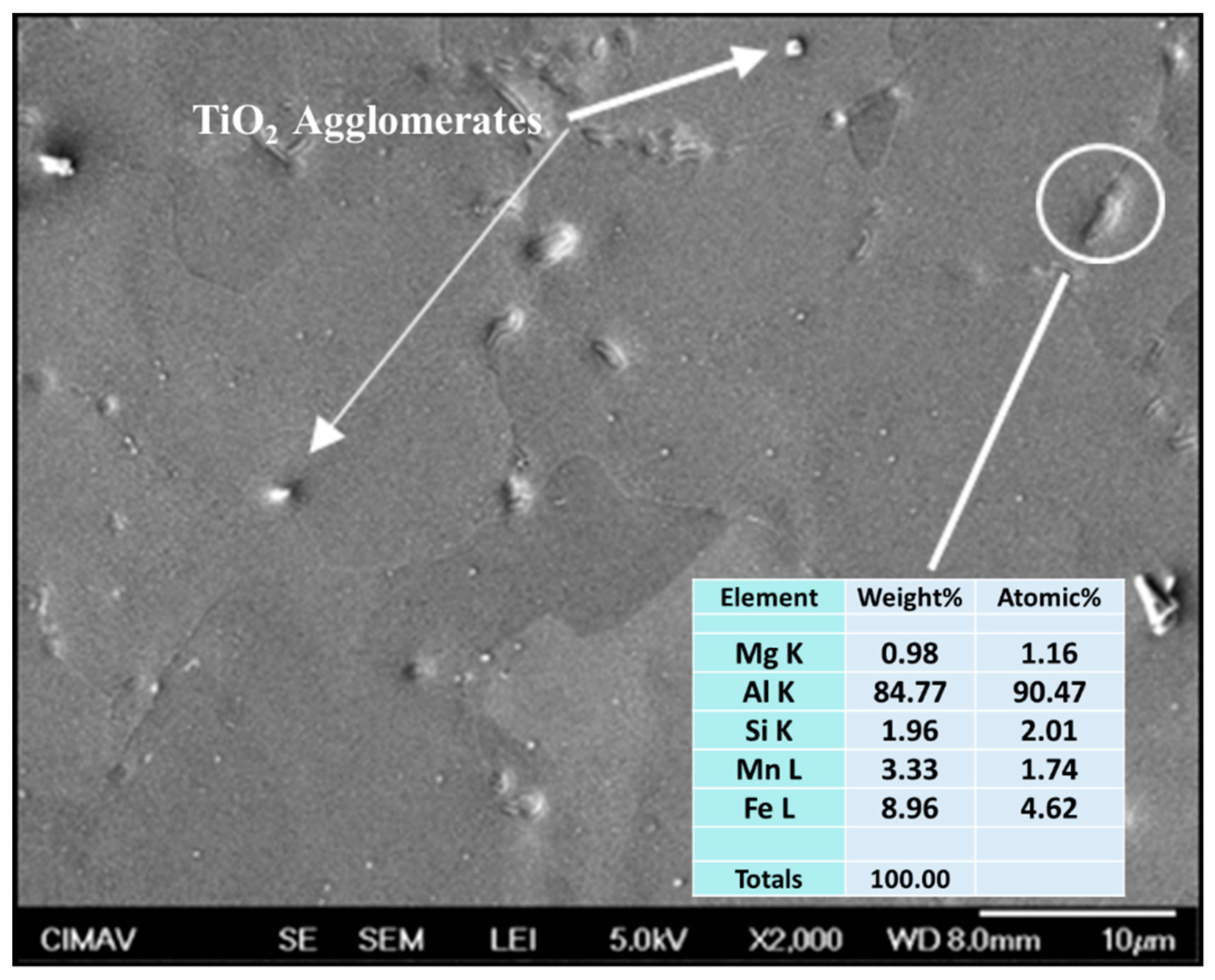
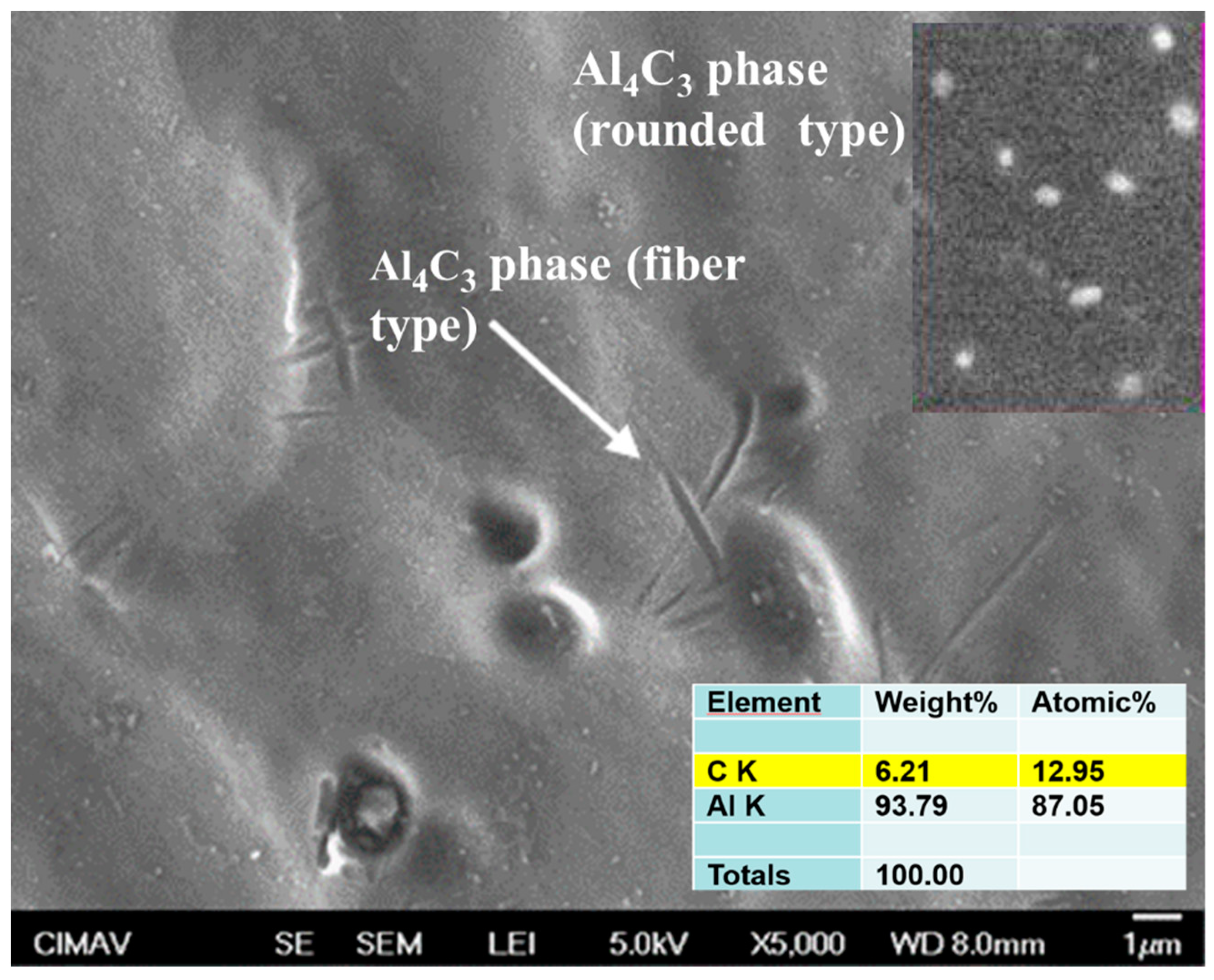
3.6. SEM Characterization
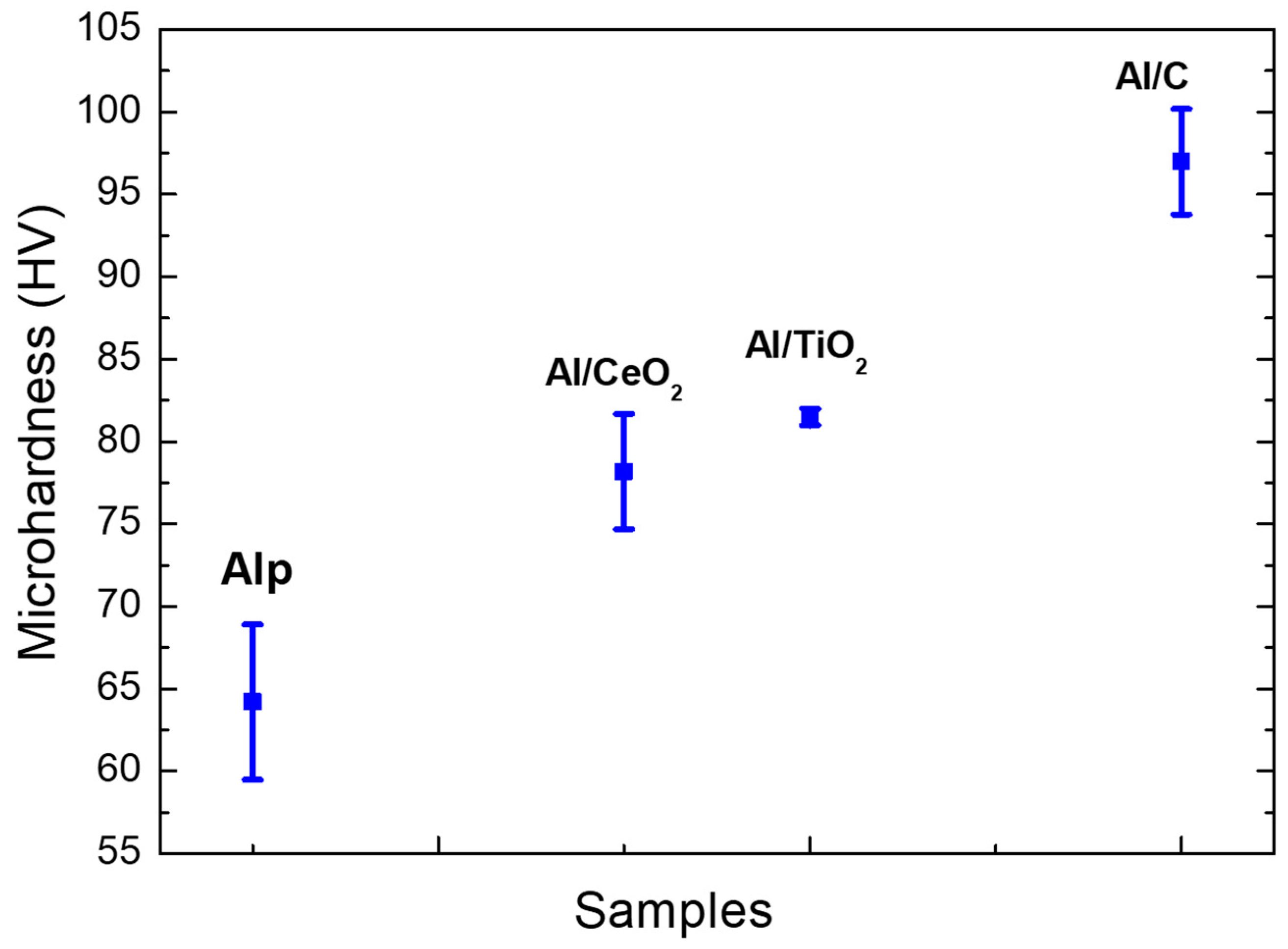
3.7. Mechanical Properties Characterization
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Khakbiz, M.; Akhlaghi, F. Synthesis and structural characterization of Al–B4C nano-composite powders by mechanical alloying. J. Alloy. Compd. 2009, 479, 334–341. [Google Scholar] [CrossRef]
- Abdoli, H.; Asgharzadeh, H.; Salahi, E. Sintering behavior of Al–AlN-nanostructured composite powder synthesized by high-energy ball milling. J. Alloy. Compd. 2009, 473, 116–122. [Google Scholar] [CrossRef]
- Birol, Y. Response to thermal exposure of the mechanically alloyed Al/C powder blends. J. Alloy. Compd. 2008, 460, L1–L5. [Google Scholar] [CrossRef]
- Arik, H. Effect of mechanical alloying process on mechanical properties of α-Si3N4 reinforced aluminum-based composite materials. Mater. Des. 2008, 29, 1856–1861. [Google Scholar] [CrossRef]
- Santos-Beltrán, A.; Goytia-Reyes, R.; Morales-Rodriguez, H.; Gallegos-Orozco, V.; Santos-Beltrán, M.; Baldenebro-Lopez, F.; Martínez-Sánchez, R. Characterization of Al–Al4C3 nanocomposites produced by mechanical milling. Mater. Charact. 2015, 106, 368–374. [Google Scholar] [CrossRef]
- Martínez-Sánchez, R.; Reyes-Gasga, J.; Caudillo, R.; García-Gutierrez, D.I.; Márquez-Lucero, A.; Estrada-Guel, I.; Mendoza-Ruiz, D.C.; José Yacaman, M. Mechanical and microstructural characterization of aluminum reinforced with carbon-coated silver nanoparticles. J. Alloy. Compd. 2007, 438, 195–201. [Google Scholar] [CrossRef]
- Casati, R.; Vedani, M. Metal Matrix Composites Reinforced by Nano-Particles—A Review. Mater. Today Proc. 2014, 4, 65–83. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Li, C.; Chou, T.-W. Nanocomposites in context. Compos. Sci. Technol. 2005, 65, 491–516. [Google Scholar] [CrossRef]
- Sajjadi, S.A.; Ezatpour, H.R.; Torabi Parizi, M. Comparison of microstructure and mechanical properties of A356 aluminum alloy/Al2O3 composites fabricated by stir and compo-casting processes. Mater. Des. 2012, 34, 106–111. [Google Scholar] [CrossRef]
- Santos-Beltrán, A.; Gallegos-Orozco, V.; Reyes, R.G.; Miki-Yoshida, M.; Estrada-Guel, I.; Martínez-Sánchez, R. Mechanical and microstructural characterization of dispersion strengthened Al–C system nanocomposites. J. Alloy. Compd. 2010, 489, 626–630. [Google Scholar] [CrossRef]
- Mazahery, A.; Abdizadeh, H.; Baharvandi, H.R. Development of high-performance A356/nano-Al2O3 composites. Mater. Sci. Eng. A 2009, 518, 61–64. [Google Scholar] [CrossRef]
- Chen, F.; Chen, Z.; Mao, F.; Wang, T.; Cao, Z. TiB2 reinforced aluminum based in situ composites fabricated by stir casting. Mater. Sci. Eng. A 2015, 625, 357–368. [Google Scholar] [CrossRef]
- Dehghan Hamedan, A.; Shahmiri, M. Production of A356–1 wt % SiC nanocomposite by the modified stir casting method. Mater. Sci. Eng. A 2012, 556, 921–926. [Google Scholar] [CrossRef]
- Ezatpour, H.R.; Sajjadi, S.A.; Sabzevar, M.H.; Huang, Y. Investigation of microstructure and mechanical properties of Al6061-nanocomposite fabricated by stir casting. Mater. Des. 2014, 55, 921–928. [Google Scholar] [CrossRef]
- Suprapto, W. Effect recycled aluminium structures of metallurgycal and melt efficiency. IOP Conf. Ser. Mater. Sci. Eng. 2019, 494, 012085. [Google Scholar] [CrossRef]
- Moses, J.J.; Dinaharan, I.; Sekhar, S.J. Prediction of influence of process parameters on tensile strength of AA6061/TiC aluminum matrix composites produced using stir casting. Trans. Nonferr. Met. Soc. China 2016, 26, 1498–1511. [Google Scholar] [CrossRef]
- Krishnan, P.K.; Christy, J.V.; Arunachalam, R.; Mourad, A.H.I.; Muraliraja, R.; Al-Maharbi, M.; Murali, V.; Chandra, M.M. Production of aluminum alloy-based metal matrix composites using scrap aluminum alloy and waste materials: Influence on microstructure and mechanical properties. J. Alloy. Compd. 2019, 784, 1047–1061. [Google Scholar] [CrossRef]
- Tahamtan, S.; Halvaee, A.; Emamy, M.; Zabihi, M.S. Fabrication of Al/A206–Al2O3 nano/micro composite by combining ball milling and stir casting technology. Mater. Des. 2013, 49, 347–359. [Google Scholar] [CrossRef]
- Elango, G.; Raghunath, B.K. Tribological Behavior of Hybrid (LM25Al + SiC+ TiO2) Metal Matrix Composites. Procedia Eng. 2013, 64, 671–680. [Google Scholar] [CrossRef]
- Irhayyim, S.S.; Hammood, H.S.; Abdulhadi, H.A. Effect of nano-TiO2 particles on mechanical performance of Al–CNT. AIMS Mater. Sci. 2019, 6, 1124–1134. [Google Scholar] [CrossRef]
- Malaki, M.; Xu, W.; Kasar, A.K.; Menezes, P.L.; Dieringa, H.; Varma, R.S.; Gupta, M. Advanced Metal Matrix Nanocomposites. Metals 2019, 9, 330. [Google Scholar] [CrossRef]
- Xue, J.; Wang, J.; Han, Y.; Chen, C.; Sun, B. Behavior of CeO2 additive in in-situ TiB2 particles reinforced 2014 Al alloy composite. Trans. Nonferr. Met. Soc. China 2012, 22, 1012–1017. [Google Scholar] [CrossRef]
- Mohammed, H.; Reddy, M.P.; Ubaid, F.; Shakoor, A.; Mohamed, A.M.A. Structural and mechanical properties of CeO2 reinforced Al matrix nanocomposites. Adv. Mater. Lett. 2018, 9, 602–605. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Habibnejad-Korayem, M.; Mahmudi, R.; Poole, W.J. Enhanced properties of Mg-based nano-composites reinforced with Al2O3 nano-particles. Mater. Sci. Eng. A 2009, 519, 198–203. [Google Scholar] [CrossRef]
- Zhu, J.-Q.; Liu, X.; Yang, Q.-S. Dislocation-blocking mechanism for the strengthening and toughening of laminated graphene/Al composites. Comput. Mater. Sci. 2019, 160, 72–81. [Google Scholar] [CrossRef]
- Khodabakhshi, F.; Simchi, A.; Kokabi, A.H.; Gerlich, A.P.; Nosko, M. Effects of post-annealing on the microstructure and mechanical properties of friction stir processed Al–Mg–TiO2 nanocomposites. Mater. Des. 2014, 63, 30–41. [Google Scholar] [CrossRef]
- Gadelmawla, E.S.; Koura, M.M.; Maksoud, T.M.A.; Elewa, I.M.; Soliman, H.H. Roughness parameters. J. Mater. Process. Technol. 2002, 123, 133–145. [Google Scholar] [CrossRef]




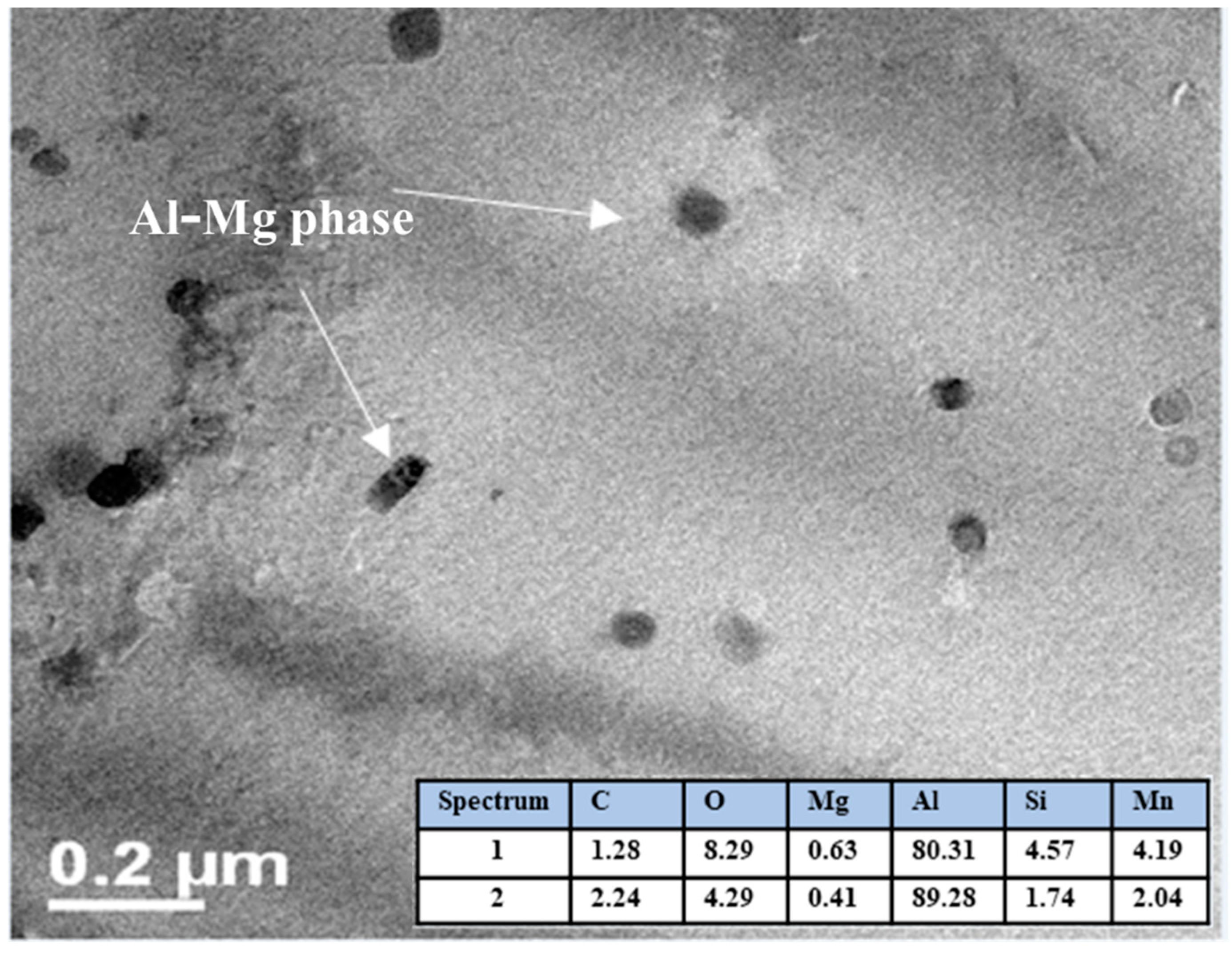
| Weight Percent of Elements | ||||||
|---|---|---|---|---|---|---|
| Al | Mn | Fe | V | Cu | Cr | Zn |
| Balance | 0.74 | 0.64 | 0.4 | 0.13 | 0.18 | 0.08 |
| Phase | Average Size (nm) | Standard Deviation (SD) |
|---|---|---|
| C | 64.9 | 0.18 |
| CeO2 | 40.6 | 0.25 |
| TiO2 | 18.2 | 0.63 |
| Composites | Micro-strains × 10−4 (%) | Topography Roughness RMS (nm) | RMS (SD, nm) | Event Number (EN) (At 30° angle phase) | EN (SD) |
|---|---|---|---|---|---|
| Al/Ref | 1.4 | 12.79 | ±4.88 | ~0 | - |
| Al/C | 5.1 | 8.54 | ±3.25 | 26,000 | ±3.78 |
| Al/CeO2 | 5.3 | 8.69 | ±3.86 | 4700 | ±3.88 |
| Al/TiO2 | 8.6 | 3.10 | ±1.29 | 17,800 | ±2.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallegos-Orozco, V.; Santos-Beltrán, A.; Santos-Beltrán, M.; Estrada-Guel, I.; Ronquillo-Ornelas, I.; Brito-Chaparro, J.; Carbajal-Sanchez, R. AFM Analyses of 3XXX Series Al Alloy Reinforced with Different Hard Nanoparticles Produced in Liquid State. Materials 2020, 13, 272. https://doi.org/10.3390/ma13020272
Gallegos-Orozco V, Santos-Beltrán A, Santos-Beltrán M, Estrada-Guel I, Ronquillo-Ornelas I, Brito-Chaparro J, Carbajal-Sanchez R. AFM Analyses of 3XXX Series Al Alloy Reinforced with Different Hard Nanoparticles Produced in Liquid State. Materials. 2020; 13(2):272. https://doi.org/10.3390/ma13020272
Chicago/Turabian StyleGallegos-Orozco, Verónica, Audel Santos-Beltrán, Miriam Santos-Beltrán, Ivanovich Estrada-Guel, Iza Ronquillo-Ornelas, José Brito-Chaparro, and Ricardo Carbajal-Sanchez. 2020. "AFM Analyses of 3XXX Series Al Alloy Reinforced with Different Hard Nanoparticles Produced in Liquid State" Materials 13, no. 2: 272. https://doi.org/10.3390/ma13020272
APA StyleGallegos-Orozco, V., Santos-Beltrán, A., Santos-Beltrán, M., Estrada-Guel, I., Ronquillo-Ornelas, I., Brito-Chaparro, J., & Carbajal-Sanchez, R. (2020). AFM Analyses of 3XXX Series Al Alloy Reinforced with Different Hard Nanoparticles Produced in Liquid State. Materials, 13(2), 272. https://doi.org/10.3390/ma13020272